Abstract
Objective
To evaluate the prognostic value of baseline red cell distribution width (RDW) in patients with coronary artery diseases (CADs) undergoing percutaneous coronary intervention (PCI) by conducting a meta-analysis.
Design
Systematic review and meta-analysis.
Data source
PubMed, Embase, Wanfang, CNKI and VIP databases were searched from their inceptions to 19 June 2019.
Eligible criteria
Studies investigating the value of baseline RDW for predicting all-cause mortality, cardiovascular mortality and major adverse cardiac events (MACEs) in patients with CAD undergoing PCI were included.
Data extraction and synthesis
Two authors independently extracted the data and evaluated the methodological quality using the Newcastle–Ottawa Scale. STATA V.12.0 software was applied to produce the forest plots using a random-effect model.
Results
Twelve studies (13 articles) involving 17 113 patients were included and analysed. Comparison between the highest and lowest RDW category indicated that the pooled risk ratio (RR) was 1.77 (95% CI 1.32 to 2.37) for all-cause mortality, 1.70 (95% CI 1.25 to 2.32) for cardiovascular mortality and 1.62 (95% CI 1.21 to 2.18) for MACEs. The predictive effect of elevated RDW for all-cause mortality was stronger in the subgroup of patients without anaemia (RR 4.59; 95% CI 3.07 to 6.86) than with anaemia.
Conclusions
This meta-analysis indicated that elevated RDW was associated with higher risk of mortality and adverse cardiac events in patients with CAD undergoing PCI. The value of elevated RDW for predicting all-cause mortality appears to be stronger in patients without anaemia. RDW may be served as a promising prognostic biomarker in patients undergoing PCI.
Keywords: red cell distribution width, major adverse cardiac events, all-cause mortality, percutaneous coronary intervention, meta-analysis
Strengths and limitations of this study.
This meta-analysis summarised the most up-to-date data on the prognostic value of red cell distribution width (RDW) in patients with coronary artery disease undergoing percutaneous coronary intervention.
Literature search, study selection, data extraction and quality assessments were performed by two independent reviewers.
The majority of included studies were considered to be of higher methodological quality.
There was statistically significant heterogeneity when pooling all-cause mortality and major adverse cardiac events outcome.
The optimal cut-off value of RDW could not be established in the current meta-analysis.
Introduction
Red cell distribution width (RDW) is a parameter reflecting variability in circulating erythrocyte size. As a component of the complete blood count, RDW is routinely determined by automated haematology analysers. RDW is elevated in patients with anaemia, the presence of iron deficiency or who underwent blood transfusion.1 Traditionally, RDW was almost exclusively used for anaemia evaluation. RDW is also of interest for its predictive role in patients with cardiovascular disease.2 Therefore, RDW determination can improve the risk stratification of these high-risk patients.
Coronary artery disease (CAD) is usually caused by the build up of plaque, a waxy substance, inside the lining of large coronary arteries. A well-designed meta-analysis has demonstrated that increased RDW strongly predicted the major adverse cardiac events (MACEs) and mortality risk in patients with CAD.3 Percutaneous coronary intervention (PCI) is widely used in treating patients with CAD. Increased attention has been paid to the prognostic utility of RDW in patients undergoing PCI. High preprocedural RDW has been identified as an independent predictor of in-stent restenosis among patients with CAD.4 Several epidemiological studies5–13 have been reported that elevated RDW level was associated with adverse outcomes in patients undergoing PCI. However, the prognostic value of RDW on this particular subset of patients remains controversial.14 Nevertheless, the magnitude of prognostic values of RDW varied between studies. Anaemia is a well-known predictor of adverse prognosis in cardiovascular diseases. The predictive role of RDW is affected by the status of anaemia.3
No previous meta-analysis has been evaluated for the impact of elevated RDW on the adverse prognosis among patients with CAD undergoing PCI. To address these knowledge gaps, we conducted this meta-analysis to investigate the value of elevated RDW in predicting adverse clinical outcomes in this specific population.
Materials and methods
Search strategy
This study followed the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses.15 We comprehensively searched PubMed, Embase, Wanfang, CNKI and VIP databases for studies published from their inceptions to 19 June 2019 using the following search strategy: ‘red cell distribution width’ OR ‘RDW’ AND ‘percutaneous coronary intervention’ OR ‘angioplasty’ AND ‘major adverse cardiac events’ OR ‘cardiovascular mortality’ OR ‘all-cause mortality’ OR ‘death’ AND ‘follow-up’ (online supplementary text S1). In addition, a manual search was conducted in reference lists of the relevant studies. No language restrictions were applied in the literature search.
bmjopen-2019-033378supp003.pdf (29.9KB, pdf)
Study selection
The inclusion criteria were as follows: (1) prospective or retrospective observational study that recruited patients with CAD undergoing PCI; (2) baseline RDW as exposure; (3) all-cause mortality, cardiovascular mortality or MACEs (defined as death, target vessel revascularisation and reinfarction) as outcome measures and (4) reported adjusted risk ratio (RR) or HR with their 95% CI of outcomes with higher versus lower RDW. Anaemia was defined as the baseline haemoglobin level of less than 13 g/L in men and 12 g/L in women. The exclusion criteria were as follows: (1) reported unadjusted risk estimate; (2) follow-up duration less than 6 months; (3) without interesting outcome measures and (4) patients in other specific diseases’ populations (apart from CAD). For multiple articles from the same population, we only selected studies with larger sample sizes and the longest follow-up.
Data extraction and quality assessment
Two reviewers independently extracted the following relevant data and abstracted them in a standardised form: first author’s surname, year of publication, study design, country of origin, sample size, gender and mean age or age range; outcome measures, follow-up duration, most fully adjusted RR or HR, and adjustments for confounding factors. Where discrepancies were identified, two authors resolved the discrepancies through discussion. A 9-point Newcastle–Ottawa Scale (NOS) for cohort study16 was applied to evaluate the quality of the included studies, which judged the selection of study groups (4 points), comparability of groups (2 points) and ascertainment of outcomes (3 points). Studies awarded with a score of seven points or more were considered to be of high methodological quality.
Statistical analysis
STATA V.12.0 software (Stata Corporation, College Station, Texas, USA) was applied to produce the forest plots by using ‘Metan’ command. We pooled the adjusted risk estimate for the higher versus lower RDW category. Significant heterogeneity across studies was determined using the I2 statistics ≥50% and Cochrane’s Q test with a significance set at p<0.1. Given the various sources of heterogeneity, we selected the random-effects analyses for all outcomes. To observe the influence of any single study on the overall risk estimate, we performed a sensitivity analysis by omitting one study each time. For subgroup analysis, the eligible studies (more than five studies analysed) were grouped according to subtype of patients (ST-segment elevation myocardial infarction (STEMI) vs all CADs), sample size (≥800 vs <800), duration of follow-up (>24 months vs ≤24 months) and study quality (NOS ≥7 points vs NOS <7 points). Begg’s test,17 Egger test,18 funnel plot and Galbraith plot were used to detect publication bias (p<0.10 level of significance) when the outcomes were reported in more than six studies.
Patient and public involvement
Neither patients nor the public were directly involved in the design, conduct, reporting or dissemination of this research.
Results
Search results and studies’ characteristics
After the application of search strategy, a total of 845 potentially relevant articles were identified during our initial literature search. After reviewing the titles or abstracts, 832 articles were removed for various reasons. Finally, 12 studies (13 articles5–14 19–21) involving 17 113 patients undergoing PCI were included (figure 1).
Figure 1.
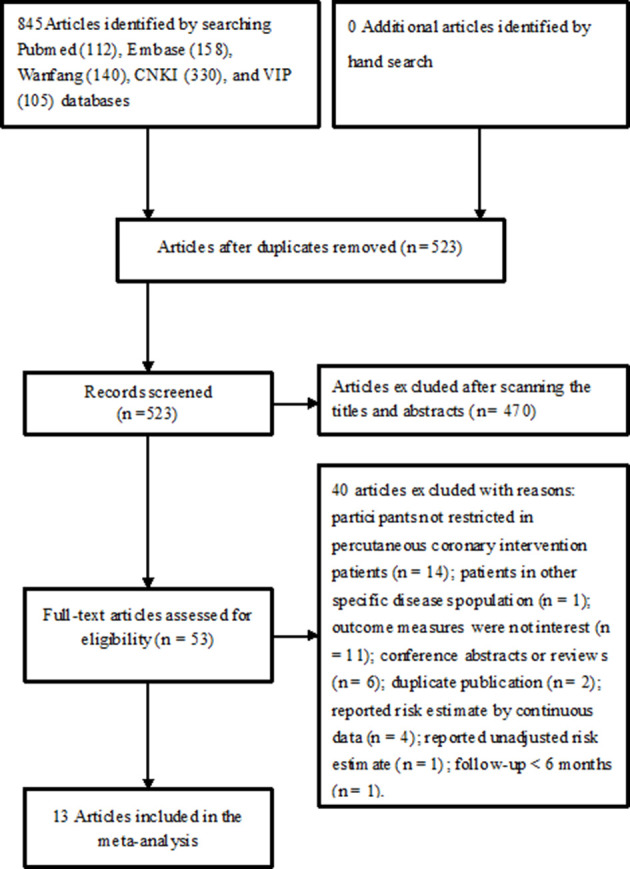
Flow chart of studies’ selection process.
The main features of the included studies are summarised in table 1. These studies were published between 2009 and 2019, with sample sizes ranging from 100 to 6046. There were six articles7 8 10 13 19 20 recruiting STEMI patients, two12 21 enrolling acute coronary syndrome (ACS) patients and others recruiting all CAD patients. Three articles9 10 21 included patients who treated with drug-eluting stents. The mean age of patients ranged from 56.6 to 66.6 years old. Of these 13 articles, four6 8 13 20 had a prospective design and others were retrospective in nature. The follow-up duration varied from 6 months to 5 years. The cut-off value of RDW ranged from 12.1% to 15.7%. For the quality assessment, eight articles were considered to be of higher methodological quality (online supplementary table S1).
Table 1.
Table 1Main features of the included studies
| Author/year | Country | Study design | Population (% male) | Age (years) | RDW cut-off | MACEs definition | Endpoints and RR/HR (95% CI) | Follow-up (months) |
Adjustment for covariates | Quality scores |
| Poludasu et al5 | USA | Retrospective | CAD 859 (74.3) | 62.2±10.5 | ≥15.7% vs<13.3% | — | Total deaths: 95 3.52 (1.01 to 12.3)† 6.40 (3.10 to 13.2)*† |
48 | Age, gender, BMI, hypertension, DM, non-STEMI, CRI, end-stage renal disease, haemoglobin, LVEF and outcome of PCI | 8 |
| Cavusoglu et al6 | USA | Prospective | CAD 370 (100) | 66.6±9.7 | ≥14.4% vs<14.4% | — | Total deaths: 51 2.69 (1.50 to 4.84) 4.73 (2.06 to 10.86)* |
24 | Age, haemoglobin, chronic HF on presentation, number of lesion arteries, creatinine and left ventricular systolic function | 6 |
| Uyarel et al7 | Turkey | Retrospective | STEMI 2506 (82.8) | 56.6±11.8 | >14.8% vs≤14.8% | — | CV deaths: 129 1.83 (1.03 to 3.24) |
21 | Sex, time to reperfusion, hypertension, smoking, eGFR, multivessel disease, unsuccessful of the procedure, anterior MI and blood transfusion | 7 |
| Isiket al‡8 | Turkey | Prospective | STEMI 100 (77) | 61.3±12.8 | ≥14% vs<14% | — | CV deaths: 14 5.89 (1.63 to 21.2) |
6 | Age, sex, hypertension, CAD, haemoglobin, CK-MB, hs-CRP, use of acetyl salicylic acid and β-blockers | 6 |
| Yao et al9 | China | Retrospective | CAD 2,169 (67.7) | 60.1±10.9 | ≥13% vs<13% | — | Total deaths: 68 1.82 (1.11 to 2.94) |
29 | Age, gender, DM, hypertension, peripheral vascular disease, number of vessels treated, multivessel disease, prior MI, eGFR, LVEF, number of stents implanted, total stent length and stent diameter | 8 |
| Wang et al10 | China | Retrospective | STEMI 484 (77.1) | 62.8±12.3 | ≥13.51% vs<12.3% | — | Total deaths: 68 1.58 (1.20 to 2.08) |
31.9 | Age, hypertension, haemoglobin, DM, PAD, previous MI, cerebralvascular disease, smoking, LVEF, length of stent and use of statins and clopidogrel | 7 |
| Liu et al11 | China | Retrospective | CAD 2732 (88.0) | 58.4±10.6 | ≥12.1% vs<12.1% | — | Total deaths: 61 3.93 (1.60 to 9.66)* |
18 | Age, DM, acute STEMI, LVEF, eGFR, TC, multivessel disease and number of lesion arteries | 7 |
| Li and Hua12 | China | Retrospective | ACS 438 (82.8) | 63.5±10.6 | ≥13.8% vs<13.8% | Death, TVR and reinfarction | MACEs: 61 1.38 (1.08 to 1.76) |
60 | Age, hypertension, pulse pressure, WBC, hs-CRP and mean platelet volume | 6 |
| Wei et al21 | China | Retrospective | NSTE-ACS 181 (75.1) | 66.3±10.9 | ≥13.3% vs<13.3% | Death, TVR and reinfarction | CV deaths: 14 3.69 (1.03 to 13.2) MACEs: 32 2.84 (1.32 to 6.15) |
14.7 | Age, platelet, LVEF and β-blockers | 6 |
| Isik et al‡13 | Turkey | Prospective | STEMI 96 (77.1) | 60.6±12.5 | ≥13.85% vs<13.85% | CV death, TVR, re-infarction | MACEs: 55 5.26 (1.71 to 16.10) |
48 | Age, hs-CRP, left anterior descending artery lesion, CK-MB, heart rate after PCI, ACEI, discontinuation of clopidogrel, electrocardiographic no-reflow and angiographic failure | 7 |
| Bozorgi et al19 | Iran | Retrospective | STEMI 838 (79.1) | 57.3±12.2 | ≥13.6% vs<13.6% | — | Total deaths: 75 2.91 (1.17 to 7.26) 2.81 (1.05 to 7.55)* |
6 | Age, gender, smoking, multi-vessel disease, LVEF and creatinine | 6 |
| Chang et al20 | China | Prospective | STEMI 390 (75.6) | 61.8±11.3 | ≥13.25% vs<13.25% | CV death and non-fatal MI | MACEs: 126 1.74 (1.44 to 2.09); CV deaths:5 4 1.56 (1.17 to 2.08) |
33.5 | Age, current smoker, hs-CRP, Killip class, anterior infarction, angina history and LDL | 7 |
| Wu et al14 | China | Retrospective | CAD 6046 (74.3) | 59.4±10.8 | ≥13.1% vs<13.1% | Death, TVR and reinfarction | Total deaths: 309 1.20 (0.94 to 1.54) CV deaths: 251 1.33 (1.01 to 1.76) MACEs: 845 1.16 (0.99 to 1.34) |
35.9 | Age, sex, DM, hypertension, smoking, drinking, SBP, heart rate, BUN, creatinine, FBG and use of statins, aspirin and clopidogrel | 8 |
*Patients without anaemia.
†Combined from subgroup using a random-effect model.
‡From the same population.
ACEI, ACE inhibitor; ACS, acute coronary syndrome; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary artery disease; CK, creatine kinase; CRI, chronic renal insufficiency; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HF, heart failure; hs-CRP, high-sensitivity C reactive protein; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; MACEs, major adverse cardiac events; MI, myocardial infarction; NOS, Newcastle–Ottawa Scale; NSTE, non ST-segment elevation; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RDW, red cell distribution width; RR, risk ratio; SBP, systolic blood pressure; STEMI, ST-segment elevation myocardial infarction; TC, total cholesterol; TVR, target vessel revascularisation; WBC, white blood cell.
bmjopen-2019-033378supp002.pdf (39.4KB, pdf)
All-cause mortality
Six studies5 6 9 10 14 19 reported the outcome of all-cause mortality in overall patients. Meta-analysis indicated that elevated RDW was associated with an increased risk of all-cause mortality (RR 1.77; 95% CI 1.32 to 2.37; figure 2A) in a random-effect model. There was significant heterogeneity (I2=56.3%; p=0.043). Sensitivity analyses indicated that the pooled RR ranged from 1.60 to 1.97 and low 95% CI ranged from 1.21 to 1.49 when omitting any single study each time. Table 2 lists the results of subgroup analysis. Publication bias may be present according to the result of Egger’s test (p=0.022) but not in the Begg’s test (p=0.133). In addition, visual inspection of the funnel plot (online supplementary figure S1) and Galbraith plot (online supplementary figure S2) indicated the presence of publication bias.
Figure 2.
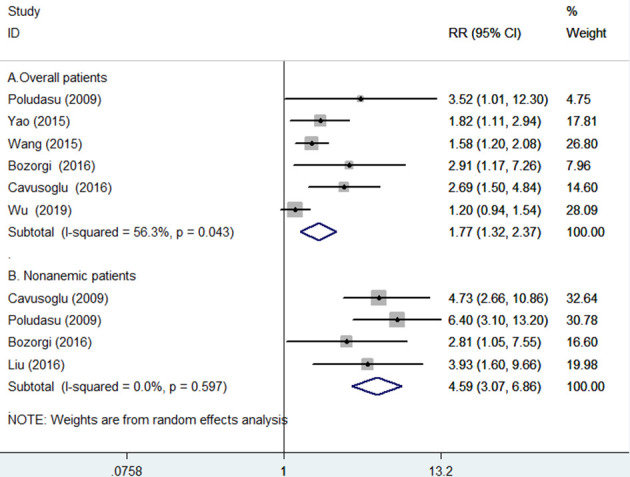
Forest plots showing pooled risk ratio with 95% CI of all-cause mortality for the higher versus lower red cell distribution width group in overall (A) and without anaemia (B) patients.
Table 2.
Subgroup analysis on all-cause mortality
| Subgroup | Number of studies | Pooled RR | 95% CI | Heterogeneity between studies |
| Type of patients | ||||
| All CAD | 4 | 1.84 | 1.16 to 2.93 | p=0.026; I2=67.7% |
| STEMI | 2 | 1.82 | 1.10 to 3.03 | p=0.209; I2=36.6% |
| Follow-up duration | ||||
| >24 months | 4 | 1.51 | 1.16 to 1.96 | p=0.146; I2=44.3% |
| ≤24 months | 2 | 2.75 | 1.68 to 4.51 | p=0.887; I2=0.0% |
| Sample sizes | ||||
| ≥800 | 4 | 1.77 | 1.12 to 2.79 | p=0.067; I2=58.2% |
| <800 | 2 | 1.93 | 1.16 to 3.20 | p=0.107; I2=61.5% |
| Country | ||||
| China | 3 | 1.44 | 1.14 to 1.82 | p=0.187; I2=40.3% |
| Others | 3 | 2.85 | 1.80 to 4.50 | p=0.928; I2=0.0% |
| Study quality | ||||
| NOS ≥7 | 4 | 1.51 | 1.16 to 1.96 | p=0.146; I2=44.3% |
| NOS <7 | 2 | 2.75 | 1.68 to 4.51 | p=0.887; I2=0.0% |
CAD, coronary artery disease; NOS, Newcastle–Ottawa Scale; RR, risk ratio; STEMI, ST-segment elevation myocardial infarction.
bmjopen-2019-033378supp001.pdf (76.9KB, pdf)
For the subgroup of patients without anaemia,5 6 11 19 the pooled RR of all-cause mortality was 4.59 (95% CI 3.07 to 6.86), without evidence of significant heterogeneity (I2=0.0%; p=0.597; figure 2B).
Cardiovascular mortality
Five studies7 8 14 20 21 reported the cardiovascular mortality as an outcome. Meta-analysis showed that elevated RDW was associated with an increased risk of cardiovascular mortality (RR 1.70; 95% CI 1.25 to 2.32; figure 3), without evidence of significant heterogeneity (I2=46.2%; p=0.115). Sensitivity analyses indicated that the pooled RR ranged from 1.51 to 2.06 and low 95% CI ranged from 1.17 to 1.31 when omitting any single study each time.
Figure 3.
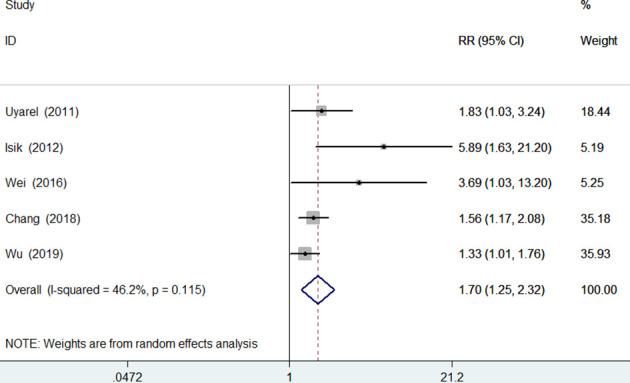
Forest plots showing pooled risk ratio with 95% CI of cardiovascular mortality for the higher versus lower red cell distribution width group in overall patients.
Major adverse cardiac events
Five studies12–14 20 21 provided data on the MACEs outcome. Meta-analysis indicated that elevated RDW was associated with an increased risk of MACEs (RR 1.62; 95% CI 1.21 to 2.18; figure 4) in a random-effect model, with evidence of statistically significant heterogeneity (I2=79.8%; p=0.001). Sensitivity analyses indicated that the pooled RR ranged from 1.50 to 1.84 and low 95% CI ranged from 1.11 to 1.33 when excluding any single study each time.
Figure 4.
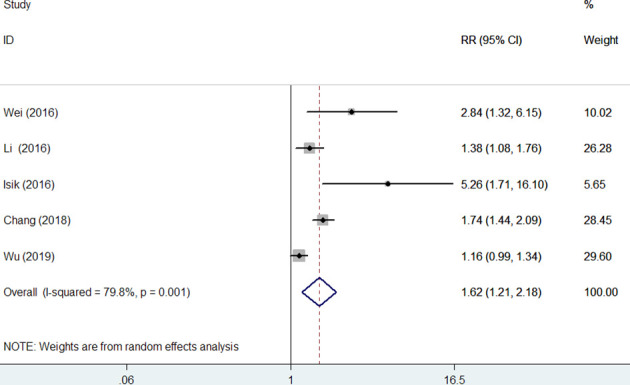
Forest plots showing pooled risk ratio with 95% CI of major adverse cardiac events for the higher versus lower red cell distribution width group in overall patients.
Discussion
Summary of main findings
The main findings of this meta-analysis were that elevated RDW at baseline was associated with increased risk of all-cause mortality, cardiovascular mortality and MACEs in patients with CAD undergoing PCI. The patients with elevated RDW level exhibited a 77%, 70% and 62% higher risk of all-cause mortality, cardiovascular mortality and MACEs, respectively. In patients without anaemia undergoing PCI, elevated RDW level significantly increased the risk of all-cause mortality by 4.59-fold.
Comparing with previous meta-analyses
Previous meta-analyses have evaluated the prognostic value of RDW in patients with CAD and ACS. Patients with CAD patients exhibiting elevated RDW had 2.2-fold and 2.13-fold higher risk of all-cause mortality and fatal/non-fatal events, respectively.3 Low RDW level was associated with 44% decreased risk of MACEs and 65% decreased risk of cardiovascular or all-cause mortality in patients with ACS.22 Our meta-analysis focused on the specific subpopulation of CAD to investigate the prognostic value of elevated baseline RDW in patients undergoing PCI.
Additional evidence
Elevated RDW as a predictor of all-cause mortality in patients undergoing PCI was supported by continuous variable analysis.23 24 Each percentage RDW elevation increased by approximately 70% high risk of MACEs.25 Apart from the long-term prognosis, elevated RDW also independently predicted contrast-induced acute kidney injury,26 27 stent restenosis28 29 and bleeding.30 Given these findings, determining RDW before PCI in patients with CAD may improve risk stratification.
Mechanisms underlying the prognostic value of RDW
Potential mechanisms underlying the association of RDW with adverse outcomes have not been clearly defined. Inflammatory markers are associated with the severity and extent of CAD.31 High level of RDW is linked with inflammatory markers.32 Inflammation can increase RDW level by impairing iron metabolism and modulating the bone marrow’s response to erythropoietin.33 Moreover, oxidative stress also injuries erythrocytes and reduces erythrocyte survival, thereby leading to RDW elevation.34 35 Elevated RDW level may reflect chronic inflammation and oxidative stress, which might result in increased adverse outcomes.
Implications for practice and research
Anaemia was independently associated with adverse outcomes among patients undergoing PCI.36 Treatment with PCI plays a crucial role in postsurgery anaemia due to arterial vessel wall injury and receiving antiplatelet or antithrombotic medication during the PCI procedure. Our subgroup analysis indicated that the association between elevated RDW and all-cause mortality risk was even stronger in patients without anaemia. This finding revealed that the predictive role of RDW is most useful in patients without anaemia. However, the reasons for the differences between anaemia and without anaemia were unclear. In addition, the RDW value for predicting all-cause mortality appeared to be weakened by the lengthening of the follow-up period in our subgroup analysis. This finding suggested that the prognostic utility of RDW may be more suitable for predicting middle-term outcome. Measuring RDW before PCI added valuable clinical prognosis information.
Study limitations
Our meta-analysis has several potential limitations. First, the cut-off value for elevated RDW level varied between studies and we could not establish the optimal cut-off value of RDW elevation. Second, nutritional deficiencies are closely associated with RDW. The lack of adjustment for some residual confounding factors such as iron, folate or vitamin B12, and antiplatelet and antithrombotic drugs may have led to overestimation of the pooling results. Furthermore, pooling the most fully adjusted risk estimate may have resulted in underestimation of risk summary. Third, we only analysed the prognostic value of elevated RDW level by categorical analysis and not by continuous variables due to insufficient such data. Fourth, statistically significant heterogeneity was found when pooling all-cause mortality and MACEs outcome. Different MACEs definition, duration of follow-up, subtype of CAD patients, cut-off value of RDW and levels of adjustment may be potential sources of heterogeneity. Finally, publication bias has been observed when pooling all-cause mortality outcome. However, results of publication bias test are potentially unreliable due to the number of included studies is less than the recommended arbitrary minimum number of 10.37
Conclusions
This meta-analysis indicated that elevated RDW is associated with higher risk of all-cause/cardiovascular mortality and adverse cardiac events in patients undergoing PCI. The value of elevated RDW for predicting all-cause mortality is stronger in patients without anaemia. RDW may serve as a promising risk stratification biomarker for this specific CAD population. Future well-designed prospective studies are required to verify these findings.
Supplementary Material
Footnotes
Contributors: DB contributed to the study design and interpretation of results. GL and FK searched the literature, abstracted data and assessed the study quality. XW and JL conducted the data analysis. CJ drafted the manuscript and GL revised the manuscript. All authors had full access to the data in the study and took responsibility for the integrity of the data and the accuracy of the data.
Funding: This work was supported by Zhejiang Science Research Fund of Traditional Chinese Medicine (Category A; 2018ZA128).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. No additional data are available.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors 2019;45:507–16. 10.1002/biof.1518 [DOI] [PubMed] [Google Scholar]
- 2.Uyarel H, Isik T, Ayhan E, et al. Red cell distrubition width (RDW): a novel risk factor for cardiovascular disease. Int J Cardiol 2012;154:351–2. 10.1016/j.ijcard.2011.10.126 [DOI] [PubMed] [Google Scholar]
- 3.Su C, Liao L-Z, Song Y, et al. The role of red blood cell distribution width in mortality and cardiovascular risk among patients with coronary artery diseases: a systematic review and meta-analysis. J Thorac Dis 2014;6:1429–40. 10.3978/j.issn.2072-1439.2014.09.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian H, Luo Z, Xiao C, et al. Red cell distribution width in coronary heart disease: prediction of restenosis and its relationship with inflammatory markers and lipids. Postgrad Med J 2018;94:489–94. 10.1136/postgradmedj-2018-135806 [DOI] [PubMed] [Google Scholar]
- 5.Poludasu S, Marmur JD, Weedon J, et al. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost 2009;102:581–7. 10.1160/TH09-02-0127 [DOI] [PubMed] [Google Scholar]
- 6.Cavusoglu E, Chopra V, Gupta A, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol 2010;141:141–6. 10.1016/j.ijcard.2008.11.187 [DOI] [PubMed] [Google Scholar]
- 7.Uyarel H, Ergelen M, Cicek G, et al. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis 2011;22:138–44. 10.1097/MCA.0b013e328342c77b [DOI] [PubMed] [Google Scholar]
- 8.Isik T, Kurt M, Ayhan E, et al. The impact of admission red cell distribution width on the development of poor myocardial perfusion after primary percutaneous intervention. Atherosclerosis 2012;224:143–9. 10.1016/j.atherosclerosis.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 9.Yao HM, Shen DL, Fu X, et al. Prognostic value of red cell distribution width for patients treated with drug-eluting stent. Clinical Focus 2015;30:919–23. [Google Scholar]
- 10.Wang J, Hua Q, Wang L, et al. Effect of red blood cell distribution width on the prognosis of patients with acute ST segment elevation myocardial infarction undergoing percutaneous coronary intervention with drug eluting stent. Chin J Intervent Cardiol 2015;23:156–62. [Google Scholar]
- 11.Liu XM, Dong JZ, Liu XH, et al. [The impact of red blood cell distribution width on outcome of elective percutaneous coronary intervention in non-anemia patients]. Zhonghua Nei Ke Za Zhi 2016;55:937–40. 10.3760/cma.j.issn.0578-1426.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 12.Li J, Hua Q. Value of red blood cell distribution width in predicting long-term outcome of patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Shanxi Med Univ 2016;47:905–9. [Google Scholar]
- 13.Isik T, Kurt M, Tanboga IH, et al. The impact of admission red cell distribution width on long-term cardiovascular events after primary percutaneous intervention: a four-year prospective study. Cardiol J 2016;23:281–8. 10.5603/CJ.a2015.0080 [DOI] [PubMed] [Google Scholar]
- 14.Wu T-T, Zheng Y-Y, Hou X-G, et al. Red blood cell distribution width as long-term prognostic markers in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis 2019;18:140. 10.1186/s12944-019-1082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O'Connell D, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses.. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 28 Jul 2019].
- 17.Begg CB, Mazumdar M. Operating characteristics of a RANK correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozorgi A, Khaki S, Mortazavi SH, et al. Effect of baseline red blood cell distribution width on short- and intermediate-term mortality of patients under primary percutaneous coronary intervention: a survival analysis. Crit Pathw Cardiol 2016;15:69–74. 10.1097/HPC.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 20.Chang X-W, Zhang S-Y, Wang H, et al. Combined value of red blood cell distribution width and global registry of acute coronary events risk score on predicting long-term major adverse cardiac events in STEMI patients undergoing primary PCI. Oncotarget 2018;9:13971–80. 10.18632/oncotarget.24128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei MQ, GX L, Lin TW. The impact of RDW on the long-term survival rate of patients who suffered NSTE-ACS and got a percutaneous des implantation. Genomics Appl Biol 2016;35:1306–13. [Google Scholar]
- 22.Abrahan LL, Ramos JDA, Cunanan EL, et al. Red cell distribution width and mortality in patients with acute coronary syndrome: a meta-analysis on prognosis. Cardiol Res 2018;9:144–52. 10.14740/cr732w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.JK W, Wen ZY, Jiao YD, et al. The predictive value of red cell distribution width in long-term prognosis of patients with acute non ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Zhonghua Xin Xue Guan Bing Za Zhi 2019;17:125–9. [Google Scholar]
- 24.Zhao N, Mi L, Liu X, et al. Combined value of red blood cell distribution width and global registry of acute coronary events risk score for predicting cardiovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. PLoS One 2015;10:e0140532. 10.1371/journal.pone.0140532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatemi O, Paranilam J, Rainow A, et al. Red cell distribution width is a predictor of mortality in patients undergoing percutaneous coronary intervention. J Thromb Thrombolysis 2013;35:57–64. 10.1007/s11239-012-0767-x [DOI] [PubMed] [Google Scholar]
- 26.Akin F, Celik O, Altun I, et al. Relation of red cell distribution width to contrast-induced acute kidney injury in patients undergoing a primary percutaneous coronary intervention. Coron Artery Dis 2015;26:289–95. 10.1097/MCA.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 27.Nichols EL, Brown JR. Clinical evaluation of red cell distribution width and contrast-induced acute kidney injury in percutaneous coronary interventions. Coron Artery Dis 2015;26:283–5. 10.1097/MCA.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 28.Zhao K, Li Y, Jin Z, et al. The association of red blood cell distribution width with drug-eluting stent restenosis in unstable angina pectoris patients. Int J Cardiol 2015;191:1–3. 10.1016/j.ijcard.2015.04.237 [DOI] [PubMed] [Google Scholar]
- 29.Zhao K, Li Y-J, Gao S. Role of red blood cell distribution in predicting drug-eluting stent restenosis in patients with stable angina pectoris after coronary stenting. Coron Artery Dis 2015;26:220–4. 10.1097/MCA.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 30.Fatemi O, Torguson R, Chen F, et al. Red cell distribution width as a bleeding predictor after percutaneous coronary intervention. Am Heart J 2013;166:104–9. 10.1016/j.ahj.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 31.Drakopoulou M, Toutouzas K, Stefanadi E, et al. Association of inflammatory markers with angiographic severity and extent of coronary artery disease. Atherosclerosis 2009;206:335–9. 10.1016/j.atherosclerosis.2009.01.041 [DOI] [PubMed] [Google Scholar]
- 32.Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628–32. 10.1043/1543-2165-133.4.628 [DOI] [PubMed] [Google Scholar]
- 33.Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86–105. 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z, Liu T, Li J, et al. Elevated red cell distribution width level is associated with oxidative stress and inflammation in a canine model of rapid atrial pacing. Int J Cardiol 2014;174:174–6. 10.1016/j.ijcard.2014.03.189 [DOI] [PubMed] [Google Scholar]
- 35.Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the women's health and aging study I. Clin Nutr 2010;29:600–4. 10.1016/j.clnu.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwok CS, Tiong D, Pradhan A, et al. Meta-analysis of the prognostic impact of anemia in patients undergoing percutaneous coronary intervention. Am J Cardiol 2016;118:610–20. 10.1016/j.amjcard.2016.05.059 [DOI] [PubMed] [Google Scholar]
- 37.Lau J, Ioannidis JPA, Terrin N, et al. The case of the misleading funnel plot. BMJ 2006;333:597–600. 10.1136/bmj.333.7568.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033378supp003.pdf (29.9KB, pdf)
bmjopen-2019-033378supp002.pdf (39.4KB, pdf)
bmjopen-2019-033378supp001.pdf (76.9KB, pdf)


