Abstract
Sleep is hypothesized to play an integral role in brain plasticity. This has traditionally been investigated using behavioral assays. In the last 10–15 years, studies combining sleep measurements with in vitro and in vivo models of synaptic plasticity have provided exciting new insights into how sleep alters synaptic strength. In addition, new theories have been proposed that integrate older ideas about sleep function and recent discoveries in the field of synaptic plasticity. There remain, however, important challenges and unanswered questions. For example, sleep does not appear to have a single effect on synaptic strength. An unbiased review of the literature indicates that the effects of sleep vary widely depending on ontogenetic stage, the type of waking experience (or stimulation protocols) that precede sleep and the type of neuronal synapse under examination. In this review, I discuss these key findings in the context of current theories that posit different roles for sleep in synaptic plasticity.
Keywords: Hebbian, Synaptic scaling, Homeostasis, Function, Ontogeny, Synaptic remodeling
1 Introduction
Sleep has long been suspected to play important roles in brain plasticity. Historically, scientists conceptualized and investigated the problem in terms of what was known about long-term synaptic potentiation (LTP) and depression (LTD) [reviewed in (Benington and Frank 2003; Frank and Benington 2006)]. LTP and LTD refer to use-dependent, persistent alterations in synaptic weights that strengthen (LTP) or weaken (LTD) specific synapses, respectively (Malenka and Bear 2004). These forms of plasticity are considered Hebbian because they are associative, input (synapse) specific, and require coordinated pre and postsynaptic activity (Markram et al. 2011). Although these effects were originally identified in vitro and involved what were at the time considered nonphysiological stimulus protocols, LTP and LTD can be induced and may occur naturally in vivo. These and related forms of synaptic plasticity are now widely considered to be cellular correlates (if not the substrates) of memory (Bear and Malenka 1994; Malenka and Bear 2004; Markram et al. 2011).
In the late 1990s, a non-Hebbian type of plasticity was described that adjusted all synapses in a neuron or network of neurons upward or downward in response to global changes in activity. This type of plasticity was dubbed “synaptic scaling” or “homeostatic synaptic plasticity” (Pozo and Goda 2010; Turrigiano 1999, 2008) and was proposed to offset pure Hebbian mechanisms in the brain. In the following sections, I discuss our current knowledge concerning the potential role of sleep in both Hebbian and non-Hebbian synaptic plasticity in the developing and adult brain. I also discuss these findings in the context of recent theories of sleep function that incorporate Hebbian and non-Hebbian forms of plasticity in different ways.
2 Sleep and Developmental Plasticity
In most of the mammalian species studied in detail, sleep amounts are highest during the neonatal period, the phase of life that is characterized by rapid brain development and synaptic plasticity (Frank and Heller 1997; Jouvet-Mounier et al. 1970; Roffwarg et al. 1966). Therefore, if sleep contributes to synaptic plasticity one would expect this to be especially true in developing animals. This possibility has been investigated in the visual system which is highly plastic during a critical period of development. The critical period refers to a developmental window of time when the brain is exquisitely sensitive to changes in experience. It has been traditionally investigated by closing an eye [monocular deprivation (MD)], which alters cortical responses in favor of the open eye [reviewed in (Sengpiel et al. 1998; Singer 1979)]. These changes in vivo are temporally associated with a form of an in vitro cortical LTP. In this type of LTP, high frequency white matter stimulation in cortical slices prepared from postnatal (P) day 28–30 rats produces synaptic potentiation in cortical layers II/III. This form of LTP is not observed in cortical slices from adult rats (Kirkwood et al. 1995). In the last decade, several in vitro and in vivo studies suggest an important role for sleep in these types of plasticity [for additional discussion, see (Frank 2011)].
2.1 Sleep and Developmentally Regulated LTP In Vitro
While both nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep may play a role in synaptic plasticity, REM sleep in particular may be important for developmentally regulated plasticity. Studies in rats show that 1 week of REM sleep deprivation prolongs the critical period for the developmentally regulated form of LTP in vitro (Shaffery et al. 2002). That is, after REM sleep deprivation, LTP can be induced at ages when this form of plasticity is normally no longer present. A similar extension of the critical period was not seen in cortical slices from control rats. Conversely, REM sleep deprivation had no effect on a nondevelopmentally regulated form of LTP evoked by layer IV stimulation. Subsequent studies from these investigators showed that this plasticity could be partially rescued if REM sleep deprivation was administered near (or overlapping) the end of the critical period (Shaffery et al. 2005; Shaffery and Roffwarg 2003). More recent findings show that the effects of REM sleep deprivation can be prevented by chronically infusing brain-derived neurotrophic factor (BDNF) into the visual cortex. This indicates that REM sleep may normally promote BDNF synthesis (Shaffery et al. 2012). This idea is supported by work in the developing cat, demonstrating that sleep is accompanied by increased cortical synthesis of BDNF (Seibt et al. 2012).
2.2 Sleep and Ocular Dominance Plasticity (ODP)
ODP refers to electrophysiological and anatomical changes in visual cortical circuits in vivo triggered by MD or other changes in patterned vision. These changes include a shift in evoked electrical potentials and an expansion of thalamocortical efferent axonal arbors in favor of the seeing eye. Although originally described in developing animals (Hubel and Wiesel 1970; Wesel and Hubel 1963), ODP also occurs in the adult brain (Sato and Stryker 2008; Sawtell et al. 2003) and shares in common numerous mechanisms that mediate Hebbian and non-Hebbian plasticity in the hippocampus and nonsensory cortex. ODP is considered physiological for the following reasons. First, it occurs in the intact, unanesthetized brain in response to changes in sensory input that animals actually experience. Second, while the experimental procedure of MD is artificial, as is the case for many laboratory manipulations, this change in visual input occurs naturally in amblyopia. Amblyopia occurs in humans (and other mammals with binocular vision) when patterned vision is reduced in one eye during early life, often as a result of a cataract. Third, the resulting plasticity involves naturally occurring changes in synaptic proteins and molecules. Fourth, the underlying plasticity is present, with or without MD, as it governs cortical adjustments to visual input that normally occur during the critical period. For these reasons, ODP is recognized as a canonical model of physiological plasticity in vivo [for review see, (Espinosa and Stryker 2012; Tropea et al. 2009)].
A role for sleep in ODP has been demonstrated in the cat. During the peak of the critical period, as little as 6 h of sleep significantly enhances the effects of MD on cortical neurons; a process which does not occur when animals are instead sleep-deprived (Frank et al. 2001). Subsequent investigations have shown that this form of sleep-dependent plasticity requires cortical neuronal activity (Frank et al. 2006; Jha et al. 2005). For example, after MD reversible inactivation of the sleeping visual cortex with gamma-aminobutyric acid (GABA) receptor agonists or the cation channel blocker lidocaine inhibits the enhancement of ODP normally observed after sleep. These results were not due to abnormal sleep or visual processing upon testing—as sleep architecture and basic visual response properties were unchanged in infused animals. Interestingly, additional sleep with cortical activity restored did not rescue ODP. This indicates that sleep immediately following waking experience is critical for the consolidation of this type of plasticity (Jha et al. 2005).
Although the precise activity-dependent mechanisms engaged in the sleeping brain are unknown, they include synaptic potentiation. For example, both acute (Aton et al. 2009) and chronic recording (Aton et al. 2013) of single neurons show that responses to the nondeprived eye become stronger after sleep. In comparison, sleep has little to no effect on the magnitude of depression observed in the deprived-eye pathway. Infusing NMDAR antagonists, protein kinase inhibitors, or blocking protein synthesis (Fig. 1) in the visual cortex during post-MD sleep completely abolishes this potentiated response (Aton et al. 2009; Seibt et al. 2012). In addition, post-MD sleep is accompanied by activation of several kinases implicated in LTP [extracellular regulated kinase [ERK] and calcium calmodulin kinase II (CaMKII)] and phosphorylation of glutamate AMPA receptors (AMPAR) that lead to trafficking and insertion of this receptor into the postsynaptic membrane (Aton et al. 2009). Post-MD sleep also promotes the synthesis of several proteins implicated in LTP [e.g., BDNF and postsynaptic density (PSD)-95] (Seibt et al. 2012).
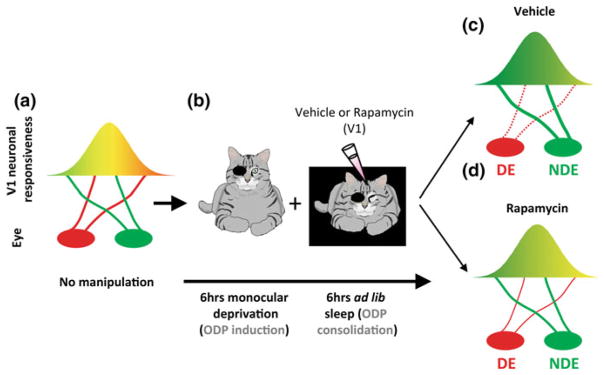
Fig. 1.
Ocular dominance plasticity in the cat requires protein synthesis during sleep. a In developing cats with normal vision, most neurons in the primary visual cortex (V1) are binocular (i.e., equally responsive to inputs from either eye, represented as the yellow area). b When animals are deprived of patterned visual input in one eye (i.e., monocular deprivation) most neurons in V1 become responsive only to stimulation of the nondeprived eye (NDE). This canonical form of physiological plasticity is known as ocular dominance plasticity (ODP). It is induced very rapidly in awake cats (6 h) and is enhanced/consolidated by subsequent sleep (6 h). To test the role of protein synthesis in sleep-dependent ODP, visual cortices are infused with vehicle or the selective mammalian target of rapamycin (mTOR) inhibitor during the post-MD sleep period. c Sleep-dependent ODP is intact in the vehicle infused hemispheres and includes a maintenance of depression of the DE visual input (dotted red line) and potentiation of the NDE input (thick red line). d Inhibition of protein synthesis in V1 with rapamycin during post-MD sleep blocks sleep-dependent ODP. This essentially halts plastic changes at a stage induced by waking experience alone. Reproduced with permission from (Seibt and Frank 2012)
A particular striking set of observations in the cat is that plasticity in wakefulness and sleep appear to be governed by distinct mechanisms. In addition to a difference in the direction of plastic change (weakening in wakefulness, strengthening in sleep), the effects of MD in the awake brain do not require protein synthesis. In contrast to sleep-dependent plasticity, intracortical blockade of protein synthesis has no effect on circuit weakening in wakefulness. In addition, while a number of plasticity-related mRNAs are upregulated by visual experience, they are not translated into proteins until sleep occurs (Seibt et al. 2012). This suggests that the transcription and translation of plasticity-related mRNAs are divided across sleep and wake (Fig. 2).
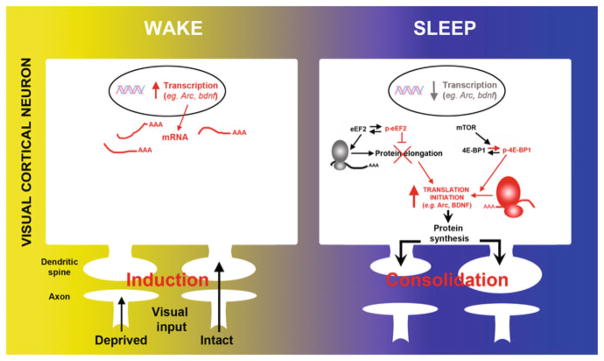
Fig. 2.
The transcription and translation of plasticity-related mRNAs are divided across wake and sleep. During wake, monocular deprivation triggers activity-dependent transcription of immediate-early and neurotrophin genes (e.g., arc, bdnf, c-fos) in V1. Subsequent sleep activates a cascade of translational events (increased translation initiation via 4E-BP1 phosphorylation and reduced global elongation via eEF2 phosphorylation) leading to a net increase in translation initiation of subsets of mRNA. Arc and bdnf are two examples of important plasticity-related genes where transcription is decreased and translation is increased during sleep. Reproduced with permission from (Seibt et al. 2012)
A two-stage process in ODP is further demonstrated by a recent study of single neuron activity in freely behaving animals. Aton et al. (2013) used chronic, stereotrode recording of single visual cortical neurons to track their activity and interactions before, during, and after a period of MD. In contrast to previous studies employing similar longitudinal recording (Mioche and Singer 1989), neuronal activity was also recorded across the sleep-wake cycle. MD in the awake animal caused a profound reduction in firing rate in fast-spiking neurons (i.e., putative GABAergic cells) in the visual cortex. This decrease in activity was maintained during the first 6 h of post-MD sleep and accompanied by an increase in firing in regular-spiking neurons (i.e., putative excitatory neurons). The decrease in fast-spiking activity was also proportional to plastic changes in regular-spiking neurons observed after sleep. This suggests that in addition to changes in the deprived eye pathway, MD alters intracortical inhibition which contributes to sleep-dependent changes in excitatory circuits. Together, these results clearly demonstrate that sleep promotes cortical potentiation in the developing cat cortex.
The above results cannot be ascribed to nonphysiological processes resulting from MD as recently asserted (Tononi and Cirelli 2014). For example, it was claimed that the effect of short-term MD used in these studies (6 h) is nonphysiological because “wake is accompanied not by the usual net potentiation but by massive synaptic depression” (Tononi and Cirelli 2014). This is incorrect in three aspects. First, there is no “massive” synaptic depression after only 6 h of MD (Aton et al. 2009; Dumoulin et al. 2013; Frank et al. 2001; Seibt et al. 2012). Several days of MD in cats are required to produce what might constitute “massive” (i.e., saturating) weakening effects in deprived eye pathways (Crair et al. 1997; Olson and Freeman 1975, 1980). However, 6 h of MD in the awake cat only produces a small change in these circuits. Second, there are many examples of waking experience that do not produce “net potentiation”, but instead synaptic weakening [for discussion, see (Frank 2012)]. While it is true that motor learning commonly leads to potentiation in motor cortex, this is not universally true in other parts of the brain or with other types of experience (Frank 2012). Therefore, the mere direction of plastic change does not determine whether the underlying plasticity is “nonphysiological”. Third, although MD eventually leads to large changes in thalamocortical circuitry, other manipulations of experience in early life do so as well (de Villers-Sidani and Merzenich 2011; Foeller and Feldman 2004). The fact that the developing brain is more plastic than the adult brain does not mean that developmental plasticity is “nonphysiological”. It was further claimed that short-term MD is “followed by a 40 %decrease in slow waves during subsequent sleep” (Miyamoto et al. 2003). The 40 %reduction in NREM slow wave activity (SWA) in the Miyamoto study was not induced by MD, but by months of dark rearing from birth (Miyamoto et al. 2003), which is a completely different experimental paradigm with very different effects on developing circuits (Buisseret and Imbert 1976; Fagiolini et al. 1994). In fact, short-term MD used in studies from the Stryker and Frank laboratories does not significantly reduce visual cortical SWA (Seibt et al. 2012) or alter in any way basic visual processing in cortical neurons, except the expected loss of response to the deprived eye. More specifically, visual responses in the intact visual pathway are completely normal, which is to be expected if the underlying processes are physiological (Frank et al. 2001). In addition, the blockade of ODP by sleep deprivation is unlikely explained by increases in stress hormone concentration. This is because ODP is remarkably resistant to the effects of the principal stress hormone corticosterone (Daw et al. 1991), and corticosterone levels after MD (or sleep deprivation) are tenfold lower than those reported to reduce ODP (unpublished observations).
3 Sleep and Plasticity in the Adult Brain
3.1 LTP and LTD: In Vitro and In Vivo Studies in Adult Animals
The role of sleep in adult synaptic plasticity has historically been investigated using classic Hebbian LTP and LTD. Beginning in the late 1980s, several investigators showed that sleep states influence tetany-induced LTP in animal models [reviewed in (Benington and Frank 2003; Hennevin et al. 2007)]. Overall, it appears that hippocampal LTP can be induced during REM sleep, whereas similar stimulus protocols during NREM sleep have no effect or produce LTD. Subsequent investigations have shown that sleep and sleep loss can affect the induction or maintenance of LTP in vivo and in vitro. Romcy-Pereira and Pavlides (Romcy-Pereira and Pavlides 2004) found that REM sleep deprivation and total sleep deprivation impair the maintenance of LTP in the dentate gyrus, but enhance this process in the medial prefrontal cortex (mPFC). Marks and Wayner found that sleep disruption also reduces hippocampal LTP in anesthetized rats (Marks and Wayner 2005). Kim et al., also employed the “flower pot” REM sleep deprivation technique for 5 days in rats, after which tetany was applied to the hippocampus while the animals were awake (Kim et al. 2005). In contrast to Marks and Wayner, these investigators report a delayed effect of REM sleep deprivation on LTP; reductions in LTP were observed 24 h after the termination of REM sleep deprivation. A large number of studies also show that in vitro hippocampal LTP (either the incidence or maintenance) is reduced in rodents that undergo varying amounts of REM sleep deprivation, total sleep deprivation, or sleep restriction (Arrigoni et al. 2009; Campbell et al. 2002; Chen et al. 2006; Davis et al. 2003; Florian et al. 2011; Ishikawa et al. 2006; Kopp et al. 2006; McDermott et al. 2003, 2006; Ravassard et al. 2006, 2009; Tartar et al. 2006; Vecsey et al. 2009).
The underlying mechanisms mediating the effects of sleep loss on LTP and LTD are not well understood. However, they do not appear to be simply due to indirect effects of the sleep deprivation procedures. For example, these deficits can be dissociated from changes in stress hormones (Kopp et al. 2006; Ravassard et al. 2009). Diminished plasticity may instead be linked to decrements in hippocampal NMDA receptor function (Chen et al. 2006; Kopp et al. 2006; Longordo et al. 2009; McDermott et al. 2005) and ERK/MAPK activation (Ravassard et al. 2009) combined with reductions in plasticity-related mRNAs or proteins (Davis et al. 2006; Guzman-Marin et al. 2006), and elevated concentrations of PDE4 (Vecsey et al. 2009) and extracellular adenosine (Arrigoni et al. 2009; Florian et al. 2011).
Most studies of LTP and sleep have focused on the adult hippocampus. Consequently, very little is known about how sleep and sleep loss impact adult cortical plasticity. Aton et al., provide some of the first unequivocal evidence that sleep directly promotes naturally occurring LTP in the adult cortex in vivo (Aton et al. 2014). In adult nonanesthetized mice, brief exposure to a visual stimulus (phase-reversing, oriented gratings) results in an enhancement of cortical responses to stimuli of the same orientation (orientation-specific response potentiation: OSRP). OSRP is considered an in vivo form of LTP because it involves many of the same mechanisms governing Hebbian LTP in vitro (Cooke and Bear 2010). For example, tetany-induced cortical LTP occludes subsequent OSRP (and vice versa). Occlusion is a classic means of determining if two processes share the same intracellular mechanisms. Occlusion occurs because one process depletes substrates (e.g., phosphorylated proteins) that are also used for the second process. The role of sleep in adult OSRP was investigated by examining the effects of experience alone, or in combination with a subsequent period of sleep, or sleep deprivation. Chronic stereotrodes were used to chronically record the activity of single cortical neurons in the visual cortex in unanesthetized adult mice before, during, and after presentation of a specifically orientated grating (the training stimulus). OSRP developed several hours after the stimulus presentation, but only after the mice slept. OSRP was prevented when the animals were instead kept awake after the stimulus (Aton et al. 2014).
3.2 Replay-Reactivation of Waking Experience in the Sleeping Adult Brain
In the mid-1990s, Matt Wilson and Bruce McNaughton demonstrated that hippocampal place cells in rats replay patterns of activity during sleep originally present during prior waking experience (Wilson and McNaughton 1994). This work extended previous findings from Pavlides and Winson (1989), who found that place cells active during exploration showed increased activity in subsequent sleep. Collectively, these findings led to the theory that replay may be a means of transferring information (or memories) from the hippocampus to the neocortex (Buzsaki 1996; Diekelmann and Born 2010). On a synaptic level, this transfer likely involves LTP as it occurs during rapid bursts of hippocampal activity among specific sets of circuits [ripples and sharp waves] (Buzsaki 1996; Schwindel and McNaughton 2011). Evidence of replay has been found in an impressive number of animal species, ranging from birds (Dave and Margoliash 2000) to primates(Hoffman and McNaughton 2002) and based on brain imaging, humans (Deuker et al. 2013; Maquet et al. 2000; Peigneux et al. 2004). The animal studies are also embedded in well-established paradigms of behavior, cellular physiology, and plasticity [reviewed in (Girardeau and Zugaro 2011; Schwindel and McNaughton 2011)].
The phenomenon appears quite robust, as variants have been found in the rodent hippocampus, ventral striatum, and cortex (Ji and Wilson 2007; Kudrimoti et al. 1999; Lee and Wilson 2002; Louie and Wilson 2001; Pennartz et al. 2004; Skaggs and McNaughton 1996; Wilson and McNaughton 1994). Although there is some evidence that forms of replay occur during REM sleep (Louie and Wilson 2001; Poe et al. 2000), communication between the hippocampus and cortex is generally conjectured to occur during NREM sleep. This is because during this state activity in the hippocampus is consistent with outflow, rather than inflow (Buzsaki 1996; Diekelmann and Born 2010; Graves et al. 2001; Hasselmo 1999). There are indeed interesting correlations between ripples and sharp waves (hippocampal events when replay is reported) and thalamocortical spindles and delta waves consistent with this hypothesis (Battaglia et al. 2004; Siapas and Wilson 1998; Sirota et al. 2003). In addition, though quite rare, there are instances when hippocampal and cortical replay occur simultaneously (Ji and Wilson 2007; Qin et al. 1997).
3.3 Re-examining Replay-Reactivation
To summarize, there is little doubt about the presence of neuronal replay during sleep. The basic findings of Wilson and McNaughton have been replicated and extended in the rodent model and similar forms of reactivation are reported in other vertebrates including, possibly, humans. Historically, however, there has been some doubt about the significance of neuronal replay.
First, replay is not unique to sleep. Replay can be detected during periods of waking immobility and even during active exploration (Foster and Wilson 2006; Kudrimoti et al. 1999; O’Neill et al. 2006). This in turn suggests that replay may have little to do with central functions of sleep and is instead one of many phenomena that are peripherally modulated by sleep. While it remains possible that replay in sleep is qualitatively different than replay in wake, this has yet to be fully determined. Therefore sleep is sufficient but not necessary for replay.
Second, replay in sleep is generally not detected during learning but well after the animal learns the task. For example, most studies require that the animals be pretrained on a maze for several days to weeks before replay can be detected (Frank 2007; Peyrache et al. 2009). The slow appearance of replay might reflect a gradually developing engram that appears after initial learning and contributes to the transfer of memories from short-term stores (hippocampus) to long-term stores (neocortex). However, it could also indicate that replay is only a decaying reverberation of a very well-ingrained pattern of neural activity present during wakefulness. This may explain the ephemeral nature of replay. It is typically detectable only within the first 20–30 min of sleep and then fades away. In some measures, it also accounts for only a fraction of total variance in neuronal activity [reviewed in (Frank 2007)].
Unfortunately, very little data exist on the effects of novel experience on replay which might distinguish between these two possibilities. Some studies do show that neuronal activity patterns associated with novel maze running or motor behavior can be detected in sleep within a few days (as opposed to weeks for familiar mazes), but the novel tasks are often very similar to familiar tasks. For example, in one study there was substantial overlap in cells active in the familiar versus novel maze configurations [between 70 and 77 % (Kudrimoti et al. 1999)]. This issue seemed to be resolved by studies reporting novelty induced reactivation of waking activity patterns in the sleeping rat forebrain (Ribeiro 2004, 2007), but these findings have been challenged on technical and methodological grounds (Tatsuno et al. 2006). Recent findings from Peyrache et al., however, provide more compelling evidence that replay can occur following novel experience. In this study, rats were exposed to novel learning rules, and medial prefrontal cortex ensemble recordings showed that patterns of activity induced by learning “replayed” in subsequent NREM sleep (Peyrache et al. 2009).
A final consideration is that until quite recently, there has been no convincing evidence that replay in sleep has any function. Two independent studies in rodents provide evidence that interrupting the hippocampal bursts that convey replay impairs critically important behavior [learning and memory] (Ego-Stengel and Wilson 2010; Girardeau et al. 2009). These studies must be cautiously interpreted because they involved disruption of the hippocampal ripples and sharp waves, and not replay per se. It is also not clear if similar results would obtain if disruption were restricted to replay in wakefulness versus sleep. More recently, it has been shown that hippocampal replay during sleep can be triggered by presentation of auditory tones present during experience—which suggests that replay represents a memory trace (Bendor and Wilson 2012). Interestingly, similar experiments in humans lead to greater performance on memory tasks (Schönauer et al. 2013), and spontaneous replay can predict future performance (Deuker et al. 2013). These results strongly suggest that replay induces adaptive, functional plastic changes in the brain.
3.4 The Synaptic Homeostasis Hypothesis
Numerous theories have been proposed to explain how sleep may influence synaptic plasticity in the adult brain. In the last decade, SHY has received particular attention. Its hypothesized roles for sleep are not unique. Several theories proposing a synaptic weakening effect in either NREM or REM sleep predate SHY (Crick and Mitchison 1983, 1995; Giuditta 1995). Other scientists have also proposed mechanisms by which synapses might be weakened in sleep (Benington and Frank 2003; Poe et al. 2000). SHY, however, encompasses a number of perennial ideas about sleep function in one theory (e.g., metabolism, plasticity and homeostasis). It also attempts to integrate Hebbian and non-Hebbian forms of plasticity across the sleep-wake cycle.
In the original description of SHY, learning occurs during wakefulness through synaptic potentiation which in turn drives sleep need. This form of synaptic potentiation appeared to be Hebbian LTP. First of all, it was described interchangeably as “long-term potentiation,” “LTP,” and “LTP-like” (Tononi and Cirelli 2003, 2006). The former terms classically refer to Hebbian mechanisms (Markram et al. 2011). Second, the synaptic potentiation of wakefulness involved “strong presynaptic firing…accompanied by postsynaptic depolarization” (Tononi and Cirelli 2006), which is a hallmark of correlation-based, or Hebbian LTP (Markram et al. 2011).
According to SHY, sleep weakens synapses through a process originally called downscaling (Tononi and Cirelli 2003, 2006), and more recently renamed as “synaptic renormalization” (Tononi and Cirelli 2012). The mechanisms governing renormalization are unspecified. However, the core features of synaptic renormalization are very similar to forms of non-Hebbian plasticity identified in the late 1990s. As originally described by Turrigiano et al. (1998), synaptic scaling (or homeostasis) is characterized by a global adjustment of synaptic weights in a neuron or network which is proportional to the strength at each synapse. Synaptic downscaling is proposed to offset Hebbian LTP, which if left unchecked would result in run-away synaptic strengthening that would saturate a neuron or neuronal network’s ability to form new synapses, or further strengthen existing ones (Turrigiano 1999, 2007; Turrigiano et al. 1998; Turrigiano and Nelson 2000). Therefore, the key concept of synaptic scaling is a global adjustment of synaptic weights that allows the network to retain past information, make new connections, and avoid network instability (i.e., a saturation of synaptic strength).
Synaptic renormalization has the same basic properties. In SHY, downscaling (or “renormalization”), affects all or most synapses, it offsets LTP (or LTP-like plasticity), and thereby allows more potentiation to occur (i.e., more learning). It involves a form of synaptic weakening (originally called “downscaling”) that is also proportional at each synapse (Tononi and Cirelli 2003, 2006). The consequences of unchecked synaptic potentiation in SHY are also similar to the network instability described in synaptic scaling, as sleep prevents “synaptic overload” (Tononi and Cirelli 2006). There appear to be only two principle differences between synaptic scaling proper and synaptic renormalization. First, in SHY LTP (or LTP-like plasticity) and downscaling are divided across wakefulness and sleep, respectively. Second, the cellular mechanisms governing synaptic scaling are not integrated into SHY. There are additional components to SHY not explicitly discussed in the original descriptions of synaptic scaling (e.g., metabolism, “synaptic space”). These, however, represent relatively minor differences or an emphasis on different outcomes of the same basic process.
3.5 Re-examining SHY
The theoretical underpinnings and evidence for SHY have been extensively reviewed elsewhere (Frank 2012; Tononi and Cirelli 2014). There is little doubt that under certain conditions and within some brain regions, synapses appear to be weaker after prolonged sleep periods. These findings are consistent with SHY and other theories that posit a similar synaptic weakening effect of sleep (Crick and Mitchison 1983; Giuditta 1995). Very little is known, however, about the mechanisms that drive this weakening process and whether they truly require sleep (Frank 2012, 2013).
For example, a central role was originally given to NREM slow wave activity (SWA), which was proposed to directly downscale synapses (Tononi and Cirelli 2003, 2006). This role has become obscure over the years, as SWA is sometimes also considered an “index” (Tononi 2009) or “sensor” of synaptic potentiation (Tononi and Cirelli 2012). Regardless of the particular role ascribed to SWA in SHY, there is currently no direct evidence that SWA in vivo weakens synapses (Frank 2012; Steriade and Timofeev 2003), and some findings indicating quite the opposite. For example, Tsanov and Manahan-Vaughan showed that when measured during the rodent light phase (when rodents sleep), evoked cortical excitatory postsynaptic potentials (EPSPs) do not decline across the sleep period, and peaks in SWA precede increases in EPSPs. These results suggest that in the adult visual cortex, sleep and possibly SWA, might promote synaptic strengthening. This is consistent with Tsanov and Manahan-Vaughan’s own conclusions, i.e., that the light (sleep) phase “…leads to synaptic potentiation” (Tsanov and Manahan-Vaughan 2007).
More direct evidence that SWA promotes synaptic potentiation comes from Chauvette et al. (2012) who showed that cortical postsynaptic potentials in vivo are potentiated after a period of NREM SWA, but not wakefulness. They also showed that periods of wakefulness did not result in synaptic potentiation. Intriguingly, experiments in vitro which simulated SWA specifically led to synaptic potentiation, while simulations of waking activity did not. It has been suggested that this is due to sleep inertia in the waking periods following sleep (Tononi and Cirelli 2014), but this is unlikely for several reasons. First, there were no significant differences in membrane potential in wake before or after NREM sleep (Timofeev personal communication). Second, the initial enhancement of the electrophysiological response persisted over several sleep-wake cycles, which would be unlikely if this was due to a transient inertial effect. Third, sleep inertia cannot explain the results of the experiments in vitro where membrane potential was controlled (Chauvette et al. 2012).
It also appears that synaptic scaling, as presently understood, does not operate in a manner consistent with SHY. As discussed above, the concepts of synaptic scaling are incorporated to a large degree into SHY. It is therefore reasonable to ask whether mechanisms known to govern synaptic downscaling might also govern “synaptic renormalization” in sleep. As recently reviewed (Frank 2012), many molecular and electrophysiological changes reported across the sleep-wake cycle are inconsistent with only global synaptic downscaling during sleep. For example, it has been suggested that global decreases in cortical activity (down-states) that occur during NREM sleep might downscale synapses. However, the basic principle of synaptic scaling is that global decreases in neuronal activity upscale synapses, while increases in neuronal activity downscale synapses. Consequently, downstates in NREM sleep should upscale, not downscale synapses (Frank 2012).
An additional consideration is that non-Hebbian synaptic scaling might be state-independent and thus occurs without sleep (Frank 2012). This possibility is supported by findings in the visual cortex in vivo. In one study, changes in cortical firing rates were chronically recorded after manipulations known to induce non-Hebbian synaptic scaling in the monocular segment of the visual cortex. There was no evidence of state-dependent alterations in scaling; rather decreases and increases in cortical activity were similar in wake and sleep (Hengen et al. 2013). Whether downscaling and upscaling both occur equally independently of vigilance state is presently unknown. Collectively, however, the above findings do not support a unique role for sleep in synaptic homeostasis.
Given the above considerations, it is not clear if the synaptic changes reported after sleep in support of SHY reflect an active sleep-dependent mechanism. They may instead result from other physiological processes that coincide with sleep, but are themselves not sleep-dependent (Frank 2012). These include circadian rhythms in brain temperature and in mammal’s glucocorticoid secretion.
3.6 Independent Factors that Alter Synapses: Brain Temperature
In mammals, the biological clock produces rhythms in brain temperature with a peak in the active phase and a trough in the sleep phase (Glotzbach and Heller 2000). Therefore, the effects of elevated brain temperature on synapses will accumulate during the normal waking period and decline during sleep. What might these effects be? A number of studies in vitro show that cooler temperatures cause a number of synaptic changes similar to those reported after sleep. These include a reduction in dendritic spines (Roelandse and Matus 2004) and concentrations of proteins that make up the postsynaptic density (Roelandse and Matus 2004). Cooling also reversibly reduces excitatory postsynaptic field potentials (EPSPs), and reverses (de-potentiates) LTP (Bittar and Muller 1993) and reduces cortical synaptic strength as measured by mini-EPSPs (Simkus and Stricker 2002).
Strong effects of naturally occurring brain temperature gradients on EPSPs are also reported in freely behaving rodents (Moser et al. 1993). Prior to Moser’s now classic studies, field recordings from the hippocampus (EPSPs) in vivo were thought to exclusively reflect plastic changes associated with learning or novel experience. They were higher after rodents engaged their environments during exploration, but not when they were less mobile. However, motor activity alone increases hippocampal temperature and EPSPs in a manner unrelated to learning-related plasticity. It is instead caused by the normal rise in brain temperature associated with waking movement and dissipates as the brain naturally cools. Similar temperature gradients across the subjective day and night have been reported in rodent cortex (Franken et al. 1991, 1993). It is also interesting that reports of heightened synaptic potentiation in wake versus sleep based on electrophysiological recordings rely on various forms of novel experience to maintain wakefulness (Hanlon et al. 2011; Vyazovskiy et al. 2008). Therefore reports of heightened cortical excitability or responsiveness in mammals after prolonged wakefulness or sleep deprivation (relative to sleep) may reflect circadian rhythms in brain temperature and secondary increases associated with motor activity— rather than vigilance state per se.
The effects of temperature may be even more extreme in insects commonly used in sleep studies. Insects are ectotherms and do not internally generate heat as do mammals. Temperature is instead behaviorally regulated either by selecting warmer environments or through activity (Stevenson 1985). Temperature gradients as small as ≈8°C are sufficient to alter synaptic structures in Drosophila (Peng et al. 2007; Zhong and Wu 2004). These include increased axonal arborization in mushroom body neurons (Peng et al. 2007) and motor nerve terminals in vivo (Zhong and Wu 2004) and neurite extension in vitro (Peng et al. 2007). Intriguingly, these temperature effects are mediated by signaling pathways shared by activity-dependent synaptic plasticity [e.g., cAMP] (Peng et al. 2007). Whether similar temperature gradients exist across Drosophila wake and sleep is unknown, but similar gradients in ambient temperature are encountered under natural conditions (Vanin et al. 2012). They may even occur in insects housed under constant ambient temperatures. This is because core temperature tracks motor/muscle activity in small terrestrial insects (Stevenson 1985); processes which are strongly influenced by the biological clock. Interestingly, this may be especially true for flying insects which expend considerable energy to bring thoracic flight muscles to high temperatures prior to flight [a phenomenon called “pre-flight warm-up”] (Heinrich 1974). Although, as discussed by Heinrich, small flying insects in flight will dissipate increases in core temperature due to convection, this may not be true under housing conditions typically used in sleep studies of Drosophila. Typically, Drosophila are housed in glass tubes that prevent flight and the convection that would occur as the animal flies through a large space. These tubes, however, do not prevent nonflight motor activity, including presumably, movements of the wings which are part of the pre-flight warm-up.
3.7 Independent Factors that Alter Synapses: Glucocorticoids
In rodents and humans, glucocorticoid (corticosterone and cortisol) concentrations rise and fall in parallel with the circadian wake and sleep phases of the 24 h day (Van Cauter 2005). They also are higher during wakefulness (relative to sleep), when waking is enforced during the normal sleep phase. There is considerable evidence that outputs of the Hypothalamic-Pituitary-Axis (HPA) profoundly influence synaptic efficacy and morphology. Glucocorticoid effects are diverse and dependent upon different classes of receptors (Joels et al. 2008). They have also been chiefly explored in the hippocampus rather than the neocortex. Nevertheless, increases in corticosterone during the normal waking period or after sleep deprivation may generally promote glutamatergic neurotransmission and neuronal excitability (Joels et al. 2008). Acute increases in corticosterone (or stress) increase the frequency (Olijslagers et al. 2008) and amplitude of mEPSCs in the hippocampus (Karst and Joels 2005), strengthen glutamatergic synapses onto dopamine neurons (Daftary et al. 2009), and increase glutamatergic release/calcium mobilization in cortical synaptoneurosomes (Satoh and Shimeki 2010). Acute increases in corticosterone also promote AMPAR synaptic transmission, AMPAR trafficking and insertion into cortical and hippocampal synapses, and cortical dendritic spine turnover (Conboy and Sandi 2009; Groc et al. 2008; Liu et al. 2010; Yuen et al. 2011). More recently it has been shown that even relatively small, transient increases in exogenous corticosterone can lead to rapid spinogenesis in vivo, which slowly decline over 5 h (Komatsuzaki et al. 2012).
Perhaps the most compelling evidence to date that normal circadian cycles of HPA activity influence cortical synaptic plasticity comes from Liston et al. (2013). In this study, it was shown that the normal peaks in glucocorticoid concentrations (during the rodent active phase) directly promote cortical dendritic spine formation that accompanies motor learning. Interestingly, they also found that the normal troughs (which correspond to the sleep phase) had dual effects; they promote the stabilization of newly formed spines associated with learning, and the pruning of preexisting spines, not associated with learning. These findings are consistent with previously reported biphasic effects of glucocorticoids which are comprised of rapid increases in synaptic efficacy (and spine formation) followed by a slower, time-dependent normalization of synapses to baseline levels [for discussion, see (Joels et al. 2008; Tse et al. 2012)]. These biphasic and prolonged synaptic changes are strikingly similar to those ascribed to wakefulness and sleep in SHY. They are, however, ultimately driven by the biological clock and are thus not state-dependent.
4 Discussion
There has been impressive progress in our understanding of how sleep and sleep loss impact brain plasticity. There also remain a number of unresolved issues. For example, while abundant evidence exists to support a general synaptic weakening after sleep, it is not at all clear that these changes are caused by sleep. Many findings cited in support of one theory of sleep-dependent plasticity (SHY) can be explained by circadian rhythms in brain temperature and HPA activity. There is also strong evidence that under certain conditions, sleep may instead strengthen synapses. These include changes in sensory input during early life that lead to cortical re-mapping. I discuss these topics in more detail in the following sections.
4.1 Brain Plasticity in Adult and Developing Brains: Difference in Degree or Kind?
An important unanswered question is whether sleep-dependent plasticity in the developing and adult brain is different. It has been suggested, for example, that synaptic downscaling as described in SHY is even more important during early life. For example, during times of overall synaptogenesis (Tononi and Cirelli 2012). However, if these developmental ages are characterized by an overall gain of synapses (Aghajanian and Bloom 1967; Sur and Leamey 2001), and animals at these ages spend most of their time in sleep [e.g., rats ≈75 % (Frank and Heller 1997), ferrets (Thurber et al. 2008) ≈85 % total recording time], then it follows that sleep cannot be a time for net synaptic weakening. This could only be true if all the synaptogenesis is compressed into the tiny fraction of time spent awake. This seems highly unlikely. Indeed, recent work in infant rodents indicates that bursts of activity during sleep are well-suited for forming sensory/motor circuits, a process known to involve synapse formation (Khazipov et al. 2004; Tiriac et al. 2012).
It also appears that a global downscaling function for sleep cannot fully explain experience-dependent plasticity occurring at later developmental ages (Fig. 3). On the contrary, investigations into a classic, physiological form of developmental plasticity (ODP) show unequivocally that sleep potentiates responses in some circuits, while maintaining depression in others. In addition, sleep appears to play no special role in at least one type of synaptic scaling in vivo in rodents at similar ages (Hengen et al. 2013). Reconciling these findings with SHY is complicated by the fact that while progress has been made identifying the cellular mechanisms governing sleep-dependent ODP (from receptors to circuits (Frank 2011), very little is known about the mechanisms governing SHY. It is also unclear if synaptic changes ascribed to sleep in SHY are in fact sleep-dependent (Frank 2012). Nevertheless, it remains possible that both Hebbian and non-Hebbian forms of plasticity operate during sleep in early life. For example, the effects of sleep on ODP suggest that synaptic weakening and strengthening both occur during sleep, but at different time points. The first few hours of post-MD sleep are accompanied by activation of several kinases and the synthesis of proteins implicated in LTP (Aton et al. 2009; Seibt et al. 2012). However, these events are transient, and by 6 h they return to baseline or even drop below baseline values. This is consistent with a “Boom and Bust” model shown in Fig. 3, according to which sleep first leads to synaptic potentiation, and then a general synaptic downscaling. Similar models that integrate synaptic strengthening and weakening across sleep have been proposed for the adult brain (Genzel et al. 2014; Ribeiro 2011).
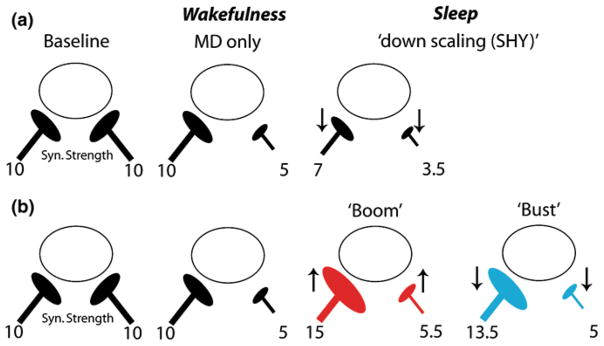
Fig. 3.
A “Boom and Bust” model of sleep-dependent plasticity explains the effects of sleep on ocular dominance plasticity. The initial effects of Monocular Deprivation (MD) in the cat are a weakening of responses to the deprived eye during wakefulness. After sleep, there is no further weakening in deprived eye circuits and instead responses to the nondeprived eye become stronger. a According to the Synaptic Homeostasis Hypothesis (SHY), sleep globally downscales synaptic strength in a manner proportionate to the strength at each synapse. This produces no net potentiation in the nondeprived circuits and increases depression in the deprived eye pathways. b According to the Boom and Bust model, sleep immediately after experience leads to synaptic potentiation (“Boom”). This is likely Hebbian, but may involve heterosynaptic changes due to synaptic tagging and capture of plasticity-related proteins in neighboring synapses (Redondo et al. 2010). As sleep progresses, global downscaling ensues, which reduces synaptic strength proportionately at each synapse (“Bust”). The net result is potentiation in the nondeprived eye pathways, and no further modifications in the deprived eye circuits, which fits empirical data. For illustration purposes, arbitrary units of synaptic strength are shown
One might further speculate that sleep-dependent downscaling only appears at a certain stage of development and then persists into adulthood. The appearance of this downscaling function may be tied to widespread synaptic pruning which is developmentally regulated and occurs after an earlier explosive period of synaptogenesis. Interestingly, the only age when sleep appears to eliminate dendritic spines (in mammal’s) is during this one specific window of developmental time (Maret et al. 2011; Yang and Gan 2011). This particular stage of development is also closely associated with the appearance of adult-like sleep regulation and sleep architecture (Alfoldi et al. 1990; Frank et al. 1998).
4.2 Making and Breaking Synapses in Sleep: Future Directions
A major challenge to the field is reconciling SHY with findings that show that sleep also increases synaptic strength (Aton et al. 2009, 2014; Chauvette et al. 2012; Dumoulin et al. 2013; Seibt et al. 2012). One possibility is that “replay-reactivation” occurs against a background of global downscaling. For example, sleep during the early part of the rest phase may express high levels of replay (leading to synaptic potentiation) that then declines. Coincident with replay is a slower, non-Hebbian scaling event which progressively asserts greater influence as replay fades. As this downscaling affects all synapses in proportion to their strength, the relative differences in strength are preserved. This is consistent with the time course of replay during sleep and properties of non-Hebbian synaptic scaling as originally described by Turrigiano. This is also predicted by the “Boom and Bust” model shown in Fig. 3 and other theories that posit dual effects of sleep on synaptic strength (Genzel et al. 2014; Giuditta 1995; Ribeiro 2011). It also leads to testable predictions. For example, if early sleep is essential for synaptic strengthening, then sleep deprivation, or manipulations of signaling pathways during early sleep, should predominantly interfere with synaptic potentiation. This is obviously an oversimplification of what might actually occur in the sleeping brain, as changes in one sleep state might set in motion changes in another (Benington and Heller 1994; Giuditta 1995). However, it is generally supported by several results in the developing cat. Sleep deprivation in the first 2 h of post-MD sleep reduces kinase activation and protein synthesis. NMDAR blockage during the first few hours of post-MD sleep inhibits sleep-dependent plasticity, but this inhibition does not occur when blockage occurs later in the sleep period (Aton et al. 2009). Similar results are obtained when all cortical activity is silenced during post-MD sleep (Jha et al. 2005).
However, any attempt to integrate theories like SHY with findings in the developing and adult brain requires further identification of the cellular mechanisms governing SHY. It is essential that circadian factors be eliminated as variables in measurements of plasticity. This is a formidable, unaddressed problem in studies that use laboratory animals with strong circadian rhythms in hormone release and brain temperature. A very simple means of controlling for circadian cycles in glucocorticoid release is to combine adrenalectomy with hormone pellets. This results in a flat, continuous release of stress hormones and allows for the isolation of biological changes due to sleep and wake, versus those driven by circadian rhythms in HPA activity. As recently shown by Mongrain et al., this technique has already been used to identify state-driven molecular changes in the brain (Mongrain et al. 2010). Therefore, a similar approach could determine which of the reported changes in synapses observed after sleep or wake are truly state-dependent, or instead driven by the HPA. Controlling for circadian changes in brain temperature in mammals is technically more challenging, but this is feasible in ectothermic insects such as the fruit fly. It would be interesting to track core temperature in these animals across the normal sleep-wake cycle and determine if natural fluctuations under typical laboratory conditions are within the range known to influence synaptic morphology in this species.
It is also critical to conduct direct tests of hypothesized relationships between synaptic plasticity and sleep function. If sleep need arises from synaptic potentiation, then mutations in fruit flies or mice that reduce synaptic potentiation should also reduce sleep need. There are a number of mutant mouse lines with profound reductions in LTP, but these mice have not been examined with respect to sleep (Frank 2012). These mutations can also now be experimentally induced, particularly in fruit flies, with increasingly fine temporal precision. These techniques thus do not suffer from limitations of constitutive mutations (i.e., developmental compensation in embryonic “knock-outs”) and can provide potentially powerful and direct tests of current theory. There is now no reasonable objection to pursuing these direct tests of SHY (or similar theories).
A second set of important experiments to conduct are those aimed at ascertaining the function of synaptic changes observed after sleep. More specifically, there is currently no empirical evidence that downscaling of synapses reported after sleep in rodents has any function (Tononi and Cirelli 2014). In addition, many findings cited in support of SHY are derived from nonphysiological manipulations (i.e., forms of stimulation not naturally experienced by the intact brain, or measurement conditions that do not reproduce the conditions of the intact brain (Albensi et al. 2007; Holscher 1999)). These include exogenous, transcallosal electrical stimulation (Vyazovskiy et al. 2008), intracranial infusions of chemicals that cause cortical spreading depression (Faraguna et al. 2010), intracortical infusions of neurotrophins and antibodies (Faraguna et al. 2008), and transcranial electromagnetic fields (Huber 2007). None of these conditions occur naturally in animals.
In contrast, synaptic changes noted after MD, OSRP, and sleep are part of an endogenous response to changes in sensory experience in vivo. There is also increasing evidence that hippocampal replay of waking experience in sleep leads to adaptive changes in the brain. Therefore, direct tests of how “down-scaled” synapses in sleep lead to adaptive changes (behaviorally or otherwise) are now needed. One promising approach along these lines is recent work in Drosophila (Donlea et al. 2011). It has been shown in fruit flies that certain forms of experience can saturate synapse number which prevents certain forms of learning. Learning can be rescued after a period of sleep, which also reduces synapses. It will therefore be important to determine if these findings generalize to other circuits in Drosophila, and to other species.
References
- Aghajanian GK, Bloom FE. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967;6:716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Oliver DR, Toupin J, Odero G. Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp Neurol. 2007;204:1–13. doi: 10.1016/j.expneurol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Alfoldi P, Tobler I, Borbely AA. Sleep regulation in rats during early development. Am J Physiol. 1990;258:R634–R644. doi: 10.1152/ajpregu.1990.258.3.R634. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Lu J, Vetrivelan R, Saper CB. Long-term synaptic plasticity is impaired in rats with lesions of the ventrolateral preoptic nucleus. Eur J Neurosci. 2009;30:2112–2120. doi: 10.1111/j.1460-9568.2009.07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, Frank MG. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc Natl Acad Sci USA. 2013;110:3101–3106. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Suresh A, Broussard C, Frank MG. Sleep promotes cortical response potentiation following visual experience. Sleep. 2014 doi: 10.5665/sleep.3830. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69:77–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Does the function of REM sleep concern non-REM sleep or waking? Prog Neurobiol. 1994;44:433–449. doi: 10.1016/0301-0082(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bittar P, Muller D. Time-dependent reversal of long-term potentiation by brief cooling shocks in rat hippocampal slices. Brain Res. 1993;620:181–188. doi: 10.1016/0006-8993(93)90154-f. [DOI] [PubMed] [Google Scholar]
- Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in the rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75:1105–1113. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Hardy M, Zhang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2009;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Ruthazer ES, Gillespie DC, Stryker MP. Relationship between the ocular dominance and orientation maps in visual cortex of monocularly deprived cats. Neuron. 1997;19:307–318. doi: 10.1016/s0896-6273(00)80941-1. [DOI] [PubMed] [Google Scholar]
- Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- Crick F, Mitchison G. REM sleep and neural nets. Behav Brain Res. 1995;69:147–155. doi: 10.1016/0166-4328(95)00006-f. [DOI] [PubMed] [Google Scholar]
- Dadvand L, Stryker MP, Frank MG. Sleep does not enhance the recovery of deprived eye responses in developing visual cortex. Neuroscience. 2006;143:815–826. doi: 10.1016/j.neuroscience.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftary SS, Panksepp J, Dong Y, Saal DB. Stress-induced, glucocorticoid-dependent strengthening of glutamatergic synaptic transmission in midbrain dopamine neurons. Neurosci Lett. 2009;452:273–276. doi: 10.1016/j.neulet.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules of sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Meighan PC, Taishi P, Krueger JM, Harding JW, Wright JW. REM sleep deprivation attenuates actin-binding protein cortactin: a link between sleep and hippocampal plasticity. Neurosci Lett. 2006;400:191–196. doi: 10.1016/j.neulet.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Daw NW, Sato H, Fox K, Carmichael T, Gingerich R. Cortisol reduces plasticity in the kitten visual cortex. J Neurobiol. 1991;22:158–168. doi: 10.1002/neu.480220206. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Merzenich MM. Chapter 8—Lifelong plasticity in the rat auditory cortex: basic mechanisms and role of sensory experience. In: Chapman CE, Kalaska JF, Green AM, Franco L, editors. Progress in brain research. Elsevier; 2011. pp. 119–131. [DOI] [PubMed] [Google Scholar]
- Deuker L, Olligs J, Fell J, Kranz TA, Mormann F, Montag C, Reuter M, Elger CE, Axmacher N. Memory consolidation by replay of stimulus-specific neural activity. J Neurosci. 2013;33:19373–19383. doi: 10.1523/JNEUROSCI.0414-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin MC, Aton S, Watson A, Coleman T, Frank MG. ERK activity during sleep is necessary for the consolidation of cortical plasticity. Cerebral cortex. 2013 doi: 10.1093/cercor/bht250. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Faraguna U, Nelson A, Vyazovskiy VV, Cirelli C, Tononi G. Unilateral cortical spreading depression affects sleep need and induces molecular and electrophysiological signs of synaptic potentiation in vivo. Cereb Cortex. 2010;20:2939–2947. doi: 10.1093/cercor/bhq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian CD, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci. 2011;31:6956–6962. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr Opin Neurobiol. 2004;14:89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frank MG. Hippocampal dreams, cortical wishes: a closer look at neuronal replay and the hippocampal-neocortical dialogue during sleep. Cell Sci Rev. 2007;3:161–171. [Google Scholar]
- Frank MG. Sleep and developmental brain plasticity: not just for kids. Prog Brain Res. 2011;193:221–232. doi: 10.1016/B978-0-444-53839-0.00014-4. [DOI] [PubMed] [Google Scholar]
- Frank MG. Erasing synapses in sleep: is it time to be SHY? Neural Plasticity. 2012;2012:264–378. doi: 10.1155/2012/264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG. Why I’m not SHY: a reply to Tononi and Cirelli. Neural plasticity. 2013;2013:394–946. doi: 10.1155/2013/394946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Benington J. The role of sleep in brain plasticity: dream or reality? Neurosci. 2006;12:477–488. doi: 10.1177/1073858406293552. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Frank MG, Jha SK, Coleman T. Blockade of postsynaptic activity in sleep inhibits developmental plasticity in visual cortex. NeuroReport. 2006;17:1459–1463. doi: 10.1097/01.wnr.0000233100.05408.e4. [DOI] [PubMed] [Google Scholar]
- Frank MG, Morrissette R, Heller HC. Effects of sleep deprivation in neonatal rats. Am J Physiol. 1998;275:R148–R157. doi: 10.1152/ajpregu.1998.275.1.R148. [DOI] [PubMed] [Google Scholar]
- Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- Franken P, Tobler I, Borbely AA. Effects of 12-h sleep deprivation and of 12-h cold exposure on sleep regulation and cortical temperature in the rat. Physiolo Behav. 1993;54:885–894. doi: 10.1016/0031-9384(93)90297-s. [DOI] [PubMed] [Google Scholar]
- Genzel L, Kroes MCW, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 2014;37:10–19. doi: 10.1016/j.tins.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Zugaro M. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol. 2011;252:452–459. doi: 10.1016/j.conb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Giuditta A. The sequential hypothesis of the function of sleep. Behav Brain Res. 1995;69:157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. Temperature regulation. In: Roth C, Kryger M, Dement WC, editors. Principles and practice of sleep medicine. Saunders; Philadelphia: 2000. pp. 289–304. [Google Scholar]
- Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends Neurosci. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation. J Physiol (Lond) 2006;575:807–819. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Vyazovskiy VV, Faraguna U, Tononi G, Cirelli C. Synaptic potentiation and sleep need: clues from molecular and electrophysiological studies. Curr Top Med Chem. 2011;11:2472–2482. doi: 10.2174/156802611797470312. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Thermoregulation in endothermic insects. Science. 1974;185:747–756. doi: 10.1126/science.185.4153.747. [DOI] [PubMed] [Google Scholar]
- Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013;80:335–342. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennevin E, Huetz C, Edeline J-M. Neural representations during sleep: from sensory processing to memory traces. Neurobiol Learn Mem. 2007;87:416–440. doi: 10.1016/j.nlm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate cortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Holscher C. Synaptic plasticity and learning and memory: LTP and beyond. J Neurosci Res. 1999;58:62–75. [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2006;24:243–248. doi: 10.1111/j.1460-9568.2006.04874.x. [DOI] [PubMed] [Google Scholar]
- Jha SK, Jones BE, Coleman T, Steinmetz N, Law C, Griffin G, Hawk J, Frank MG. Sleep-dependent plasticity requires cortical activity. J Neurosci. 2005;25:9266–9274. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–106. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Joels M, Krugers H, Karst H. Stress-induced changes in hippocampal function. Prog Brain Res. 2008;167:3–15. doi: 10.1016/S0079-6123(07)67001-0. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Karst H, Joels M. Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. J Neurophysiol. 2005;94:3479–3486. doi: 10.1152/jn.00143.2005. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Kim E, Mahmoud GS, Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett. 2005;388:163–167. doi: 10.1016/j.neulet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Komatsuzaki Y, Hatanaka Y, Murakami G, Mukai H, Hojo Y, Saito M, Kimoto T, Kawato S. Corticosterone induces rapid spinogenesis via synaptic glucocorticoid receptors and kinase networks in hippocampus. PLoS ONE. 2012;7:e34124. doi: 10.1371/journal.pone.0034124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan W-B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yuen EY, Yan Z. The stress hormone corticosterone increases synaptic amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors via serum- and glucocorticoid-inducible kinase (SGK) regulation of the GDI-Rab4 complex. J Biol Chem. 2010;285:6101–6108. doi: 10.1074/jbc.M109.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longordo F, Kopp C, Mishina M, Lujan R, Luthi A. NR2A at CA1 synapses is obligatory for the susceptibility of hippocampal plasticity to sleep loss. J Neurosci. 2009;29:9026–9041. doi: 10.1523/JNEUROSCI.1215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, Luxen A, Franck G, Van Der Linden M, Smith C, Cleeremans A. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci. 2011 doi: 10.1038/nn.2934. (advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Gerstner W, Sjostrom PJ. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2011;3:1–24. doi: 10.3389/fnsyn.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks CA, Wayner MJ. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res Bull. 2005;66:114–119. doi: 10.1016/j.brainresbull.2005.03.018. [DOI] [PubMed] [Google Scholar]
- McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep-deprivation induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol (Lond) 2005 doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol (Lond) 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol. 1989;62:185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 2010;33:1147–1157. doi: 10.1093/sleep/33.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Mathiesen I, Andersen P. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science. 1993;259:1324–1326. doi: 10.1126/science.8446900. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Senior T, Csicsvari J. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron. 2006;49:143–155. doi: 10.1016/j.neuron.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Olijslagers JE, de Kloet ER, Elgersma Y, van Woerden GM, Joels M, Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci. 2008;27:2542–2550. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- Olson C, Freeman RD. Progressive changes in kitten striate cortex during monocular deprivation. J Neurophysiol. 1975;38:26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- Olson C, Freeman RD. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39:17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, Luxen A, Maquet P. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Peng IF, Berke BA, Zhu Y, Lee W-H, Chen W, Wu C-F. Temperature-dependent developmental plasticity of drosophila neurons: cell-autonomous roles of membrane excitability, Ca2+ influx, and cAMP signaling. J Neurosci. 2007;27:12611–12622. doi: 10.1523/JNEUROSCI.2179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. J Neurosci. 2004;24:6446–6456. doi: 10.1523/JNEUROSCI.0575-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci. 2009;12:919–926. doi: 10.1038/nn.2337. [DOI] [PubMed] [Google Scholar]
- Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–180. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in cortiocortical and hippocampalcortical neuronal ensembles. Philos Trans R Soc Lond B Biol Sci. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte J, Gay N, Touret M, Luppi P, Salin PA. Paradoxical sleep amount modulates neuronal plasticity in adult rat hippocampus. J Sleep Res. 2006;15:191. [Google Scholar]
- Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scote-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Okuno H, Spooner PA, Frenguelli BG, Bito H, Morris RGM. Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci. 2010;30:4981–4989. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol. 2004;2:e24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S. Novel experience induces persistent sleep-dependent plasticity in the cortex but not in the hippocampus. Front Neurosci. 2007;1:43–55. doi: 10.3389/neuro.01.1.1.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S. Sleep and plasticity. Pflugers Archiv Eur J Physiol. 2011:1–10. doi: 10.1007/s00424-011-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelandse M, Matus A. Hypothermia-associated loss of dendritic spines. J Neurosci. 2004;24:7843–7847. doi: 10.1523/JNEUROSCI.2872-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Romcy-Pereira R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci. 2004;20:3453–3462. doi: 10.1111/j.1460-9568.2004.03808.x. [DOI] [PubMed] [Google Scholar]
- Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28:10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh E, Shimeki S. Acute restraint stress enhances calcium mobilization and glutamate exocytosis in cerebrocortical synaptosomes from mice. Neurochem Res. 2010;35:693–701. doi: 10.1007/s11064-009-0120-8. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Schönauer M, Geisler T, Gais S. Strengthening procedural memories by reactivation in sleep. J Cogn Neurosci. 2013;26:143–153. doi: 10.1162/jocn_a_00471. [DOI] [PubMed] [Google Scholar]
- Schwindel DC, McNaughton B. Hippocampal-cortical interactions and the dynamics of memory trace activation. Prog Brain Res. 2011;193:163–177. doi: 10.1016/B978-0-444-53839-0.00011-9. [DOI] [PubMed] [Google Scholar]
- Seibt J, Dumoulin M, Aton SJ, Naidoo J, Watson A, Coleman T, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibt J, Frank MG. Translation regulation in sleep: making experience last. Communicative and integrative biology. 2012 doi: 10.4161/cib.21010. (in Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengpiel F, Godecke I, Stawinski P, Hubener M, Lowel S, Bonhoffer T. Intrinsic and environmental factors in the development of functional maps in cat visual cortex. Neuropharmacology. 1998;37:607–621. doi: 10.1016/s0028-3908(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Shaffery JP, Lopez J, Bissette G, Roffwarg HP. Rapid eye movement sleep deprivation revives a form of developmentally regulated synaptic plasticity in the visual cortex of post-critical period rats. Neurosci Lett. 2005;391:131–135. doi: 10.1016/j.neulet.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Shaffery JP, Lopez J, Roffwarg HP. Brain-derived neurotrophic factor (BDNF) reverses the effects of rapid eye movement sleep deprivation (REMSD) on developmentally regulated, long-term potentiation (LTP) in visual cortex slices. Neurosci Lett. 2012;513:84–88. doi: 10.1016/j.neulet.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffery JP, Roffwarg HP. Rapid eye-movement sleep deprivation does not ‘rescue’ developmentally regulated long-term potentiation in visual cortex of mature rats. Neurosci Lett. 2003;342:196–200. doi: 10.1016/s0304-3940(03)00279-9. [DOI] [PubMed] [Google Scholar]
- Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110:431–443. doi: 10.1016/s0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interations between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Simkus CRL, Stricker C. Properties of mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:509–520. doi: 10.1113/jphysiol.2002.022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neuronal mechanisms in experience dependent modification of visual cortex function. In: Cuenod M, Kreutzberg GW, Bloom FE, editors. Development and chemical sensitivity of neurons. Elsevier/North-Holland Biomedical Press; Amsterdam: 1979. pp. 457–477. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. PNAS. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–576. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Stevenson RD. Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am Nat. 1985;125:102–117. [Google Scholar]
- Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuno M, Lipa P, McNaughton BL. Methodological considerations on the use of template matching to study long-lasting memory trace replay. J Neurosci. 2006;26:10727–10742. doi: 10.1523/JNEUROSCI.3317-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurber A, Jha SK, Coleman T, Frank MG. A preliminary study of sleep ontogenesis in the ferret (Mustela putorius furo) Behav Brain Res. 2008;189:41–51. doi: 10.1016/j.bbr.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med. 2009;5:S16–S19. [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Time to be SHY? some comments on sleep and synaptic homeostasis. Neural plasticity. 2012 doi: 10.1155/2012/415250. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Van Wart A, Sur M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos Trans R Soc B Biol Sci. 2009;364:341–355. doi: 10.1098/rstb.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. The adult visual cortex expresses dynamic synaptic plasticity that is driven by the light/dark cycle. J Neurosci. 2007;27:8414–8421. doi: 10.1523/JNEUROSCI.1101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Bagot RC, Wong TP. Dynamic regulation of NMDAR function in the adult brain by the stress hormone corticosterone. Front Cell Neurosci. 2012;6:9. doi: 10.3389/fncel.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. In: Cull-Candy S, Klein R, editors. Curr Opin Neurobiol Signal mech. Vol. 17. 2007. pp. 318–324. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Van Cauter E. Endocrine physiology. In: Kryger M, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Elsevier; Philadelphia: 2005. pp. 266–282. [Google Scholar]
- Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–375. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li X-Y, Descalzi G, Kim SS, Chen T, Shang Y-Z, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;28:1029–1040. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–682. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Yang G, Gan W-B. Sleep contributes to dendritic spine formation and elimination in the developing mouse somatosensory cortex. Dev Neurobiol. 2011;72:1391–1398. doi: 10.1002/dneu.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wu C-F. Neuronal activity and adenylyl cyclase in environment-dependent plasticity of axonal outgrowth in Drosophila. J Neurosci. 2004;24:1439–1445. doi: 10.1523/JNEUROSCI.0740-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]