Abstract
Objective:
To determine if there are differences in overall (OS) or event free survival (EFS) in patients with and without concomitant extra-adrenal metastases undergoing adrenal metastasectomy.
Background:
There is growing interest in the use of local therapies in patients with oligometastatic disease. Previously published series have indicated that long-term survival is possible with resection. Adrenalectomy has been used to treat adrenal metastases in select patients.
Methods:
Patients who underwent adrenal metastasectomy from 1994–2015 were identified from a prospectively maintained institutional database of adrenalectomy patients, excluding adrenalectomies due to tumor extension or for palliation. Sites of disease, treatment history, and survival data were extracted from chart review.
Results:
One hundred and seventy-four patients were included. Tumor histology included 68 non-small cell lung, 34 renal, 18 colorectal, 11 melanoma, 10 hepatocellular, 8 sarcoma, and 25 other cancers. The median follow-up among survivors was 5.2 (1-21) years. OS at 3 and 5-years was 50% and 40%, respectively. Patients with (n=83) and without (n=91) extra-adrenal metastases did not differ with respect to age, adrenal tumor size, or margin status. Median OS (3.3 years for patients with concomitant extra-adrenal metastases and 3.0 years for patients with isolated adrenal metastases, p=0.816) and EFS (9.39 months versus 9.59 months, p=0.87) were similar. Factors negatively associated with OS included adrenal tumor size (p<0.01), renal primary versus other (p<0.01), and adrenal margin status (p<0.01).
Conclusions:
In selected patients undergoing adrenal metastasectomy, there were no significant differences in OS or EFS between patients with and without concomitant extra-adrenal metastases.
Mini-Abstract:
This study describes our institution’s experience with adrenal metastasectomy from 1994-2015. We compared the outcomes of patients with and without a history or presence of extra-adrenal metastatic disease. We found no significant difference in overall or event free survival between these two groups of patients.
Introduction
In 1995, Hellman and Weichselbaum introduced the concept of “oligometastasis”, referring to a tumor state which is intermediate between purely localized lesions and widely metastatic disease. In this state, it is hypothesized that there is a stepwise progression with intermediate stages of metastatic capacity that may be limited to specific organs and metastases might be present in limited numbers1,2. Since the introduction of this concept, there has been growing recognition that patients with oligometastatic disease represent a distinct group. The implication of oligometastases is that there may be local modalities, some of which may even represent the standard of care, that are effective and may even result in cure for patients with oligometastatic disease2. Recommending and choosing the best local therapies are critical for these patients. Aside from surgical resection, the use of stereotactic radiotherapy, radiofrequency ablation, and MRI-guided focused ultrasound offer less invasive methods of regional therapy that may offer curative potential in the treatment of oligometastases2. At our high-volume cancer center, surgery is often favored based on our institutional outcomes of resected metastatic disease 3–6 and the advantage of surgery providing more precise staging, tissue, and extent of disease assessment.
Isolated adrenal metastases most commonly occur in patients with cancers from the lung, kidney, breast, melanoma, and colon6–9. The management of patients with adrenal metastases is multidisciplinary. Multiple facets of the patient’s presentation, disease burden, and performance status must be evaluated prior to considering surgical intervention5. Treatment options for patients with metastatic disease include chemotherapy, local ablation, and surgical resection. Previously published series have indicated that long-term survival is possible with resection10, and that patients with a longer disease-free interval and smaller tumor size fare more favorably 3,11.
Patients with adrenal metastases may present with synchronous or metachronous extra-adrenal metastatic disease. Patients with extra-adrenal disease may have had previous systemic or local treatments or may undergo a combined resection at the time of adrenalectomy. Subgroup analysis of several recent series shows an inconsistent correlation between extra-adrenal disease and survival. Some series suggest reduced survival12 whereas others show no difference in survival13, 14. While most patients presenting with multiple sites of resectable metastatic disease are appropriately referred for chemotherapy, many may never undergo multidisciplinary evaluation. However, with the increasing efficacy of systemic agents and the evolving chronicity of some metastatic cancers, multi-organ metastasectomy is increasingly being considered as a treatment option. The goal of this study is to describe our institution’s experience with adrenal metastasectomy in patients with a history of extra-adrenal metastases, and to compare the outcome of this group with the outcome of patients undergoing adrenal metastasectomy for isolated adrenal disease.
Methods
Since 1994, a prospective database has been maintained for patients undergoing adrenalectomy at Memorial Sloan-Kettering Cancer Center. The decision to perform adrenalectomy was informed by multiple factors, including extent of disease, performance status, response to previous treatment, and tumor biology. In many cases, patients were referred for surgical evaluation following treatment limiting chemotherapy toxicity or chemotherapy intolerance. Patients were excluded if they had palliative resections or if adrenalectomy was performed due to direct extension of tumor from another site. Patient records were reviewed to determine the clinical course, pathology, and outcome.
Definitions
Metastatic disease was defined most commonly by CT imaging and/or PET scan but given the time range over which this study took place there was no uniform imaging protocol to determine extent of disease. Patients with extra-adrenal metastatic disease were defined as those who had either known sites of extra-adrenal metastases at the time of adrenalectomy (“presence of” extra-adrenal metastases) or a previously known site of extra-adrenal metastatic disease that had undergone treatment prior to adrenalectomy (“history of” extra-adrenal metastases; which were no longer detectable at the time of resection). Patients whose extra-adrenal metastatic disease was present at the time of adrenalectomy underwent selective debulking surgery; extra-adrenal disease was stable, resected along with adrenal metastases, or responsive to other systemic treatments. Those patients with previously known sites were treated with a variety of modalities including radiation therapy, chemotherapy, ablation, and resection. Whether resection of extra-adrenal metastases was appropriate was determined on a case-by-case basis, considering response to previous treatment, tumor size, and location of disease. Specific systemic treatments were not included in the analysis due to the variability of histologic primary diagnoses, chemotherapeutic agents, and timing of administration. At our institution, a multidisciplinary approach is typically carried out for these patients.
Patients who presented with both a primary tumor and metastatic disease, either adrenal or extra-adrenal, were considered to have synchronous disease and those who had an interval of time between the diagnosis and detection of their primary and metastatic disease were considered metachronous. In cases of metachronous disease, regional nodal metastatic disease related to the primary tumor was not considered extra-adrenal metastatic disease. Patients in whom known, stable extra-adrenal disease remained after surgery were considered to be “alive with disease” (AWD) at the conclusion of their adrenalectomies. All other patients, whether they had isolated adrenal metastatic disease or a history of successfully treated extra-adrenal metastatic disease, were considered to have “no evidence of disease” (NED) at the conclusion of their operations.
Length of stay was defined as the number of days from operation to discharge. Overall survival was the interval from the date of adrenalectomy to the date of last known follow up or death. Recurrence and progression-free survival were calculated based on the interval between the date of adrenalectomy to the date of the first recurrence or progression, as defined by biopsy or imaging. Because several patients with extra-adrenal disease had known, stable extra-adrenal disease at the time of adrenalectomy, an event in these patients was considered progression rather than recurrence. Recurrence was used for the remainder of the patients in the study. For event free survival (EFS), an event was defined as recurrence for patients who were NED after surgery, progression for those who were AWD, or death.
Statistical Methods:
Sociodemographic and disease characteristics by extra-adrenal metastases status were summarized using the frequency and percentage for categorical factors, and median and interquartile (IQR) range for continuous variables. Overall survival (OS) and event-free survival (EFS) were examined from the time of adrenalectomy until the time of death (for OS) or until the first recurrence or progression, as defined by biopsy or imaging or death, whichever came first (for EFS). Patients who did not have an event by the end of the study were censored at the time of the last available follow-up. OS and EFS were estimated using the Kaplan-Meier method and compared between patients and disease characteristics using the log-rank test. A Cox proportional hazards model was used to examine the association between extra-adrenal metastases status and other disease characteristics and outcomes. Hazard ratios (HR) and 95% confidence interval (CI) were reported.
All P values were based on 2-tailed statistical analysis and P<.05 was considered to indicate statistical significance. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Among all 174 study patients, the median age was 62 and the majority were male (Table 1). Metastatic non-small cell lung cancers (NSCLCs) were the most common primary tumors (39%), followed by renal, colorectal, melanoma, hepatocellular carcinoma, sarcoma, and other. Laparoscopy has been routinely used at our center to perform adrenalectomy since the early 2000’s and 41% of patients in this study underwent laparoscopic resection. We observed a total of 114 deaths and the median follow-up among survivors was 5.2 years (range 1-21). OS at 3 and 5-years were 50% (95% CI: 42-57%), and 40% (95% CI: 32-46%), respectively (Figure 1). We did not detect any significant difference in OS between patients who presented with a history or presence of extra-adrenal metastases (3.3 years, 95% CI: 2.3-5.1) compared to patients with isolated adrenal disease (3.0 years, 95% CI: 1.9-4.9; p=0.816). A subgroup analysis of all patients in the extra-adrenal metastatic disease group was done to specifically compare patients with a history of (n=75) versus presence of (n=8) extra-adrenal metastatic disease at the time of adrenalectomy. In the latter group, extra-adrenal metastases (usually in 1 or 2 other locations, most often bone, liver, lung, and brain) remained following resection for a number of reasons. In 7 patients, resection of other metastases was not attempted because they were responding to systemic therapy, inoperable, and/or stable. In 1 patient, some tumor was assumed to remain following gross resection of multiple tumors across the abdomen (Supplemental Table). This subgroup analysis did not detect any significant difference in OS between these two groups of patients (p=0.87). Factors that were negatively associated with OS for the entire cohort included tumor size (p<0.01), renal versus other primary tumor site (p<0.01), and positive margin status (p<0.01; Table 2).
Table 1:
Demographic, clinical, and outcome data for all patients
| Extra-Adrenal Metastases (n=83) | Isolated Adrenal Metastases (n=91) | Total (n=174) | |
|---|---|---|---|
| Age, yrs (Median, IQR) | 60.0 (51.0 – 67.0) | 65.0 (54.0 – 71.0) | 62.0 (53-70) |
| Gender | |||
| Female | 38 (46%) | 35 (38%) | 73 (42%) |
| Male | 45 (54%) | 56 (62%) | 101 (58%) |
| Primary Site | |||
| NSCLC | 20 (24%) | 48 (53%) | 68 (39%) |
| Renal | 11 (13%) | 23 (25%) | 34 (20%) |
| Colorectal | 18 (22%) | 0 (0%) | 18 (10%) |
| Melanoma | 6 (7%) | 5 (5%) | 11 (6%) |
| HCC | 3 (4%) | 7 (8%) | 10 (6%) |
| Sarcoma | 7 (8%) | 1 (1%) | 8 (5%) |
| Other | 18 (22%) | 7 (8%) | 25 (14%) |
| Operation time (Median, IQR) | 178.0 (140-235) | 162.0 (121-230) | 172.5 (126.5-231.5) |
| Operation Approach | |||
| Laparoscopic | 34 (41%) | 37 (41%) | 71 (41%) |
| Open | 49 (59%) | 54 (59%) | 103 (59%) |
| Median LOS (Median, IQR) | 4.00 (2-7) | 4.00 (2-7) | 4.00 (2.0-7.0) |
| Margin | |||
| Negative | 20 (24%) | 16 (18%) | 36 (21%) |
| Positive | 62 (75%) | 75 (82%) | 137 (79%) |
| Unknown | 1 (1%) | 0 (0%) | 1 (1%) |
| Synchronous Disease | |||
| Yes | 33 (40%) | 29 (32%) | 62 (36%) |
| No | 50 (60%) | 62 (68%) | 112 (64%) |
| Median Tumor size (cm) (Median,IQR) | 4.00 (3.0-6.0) | 4.60 (2.8-7.6) | 4.50 (3.0-7.0) |
Figure 1:
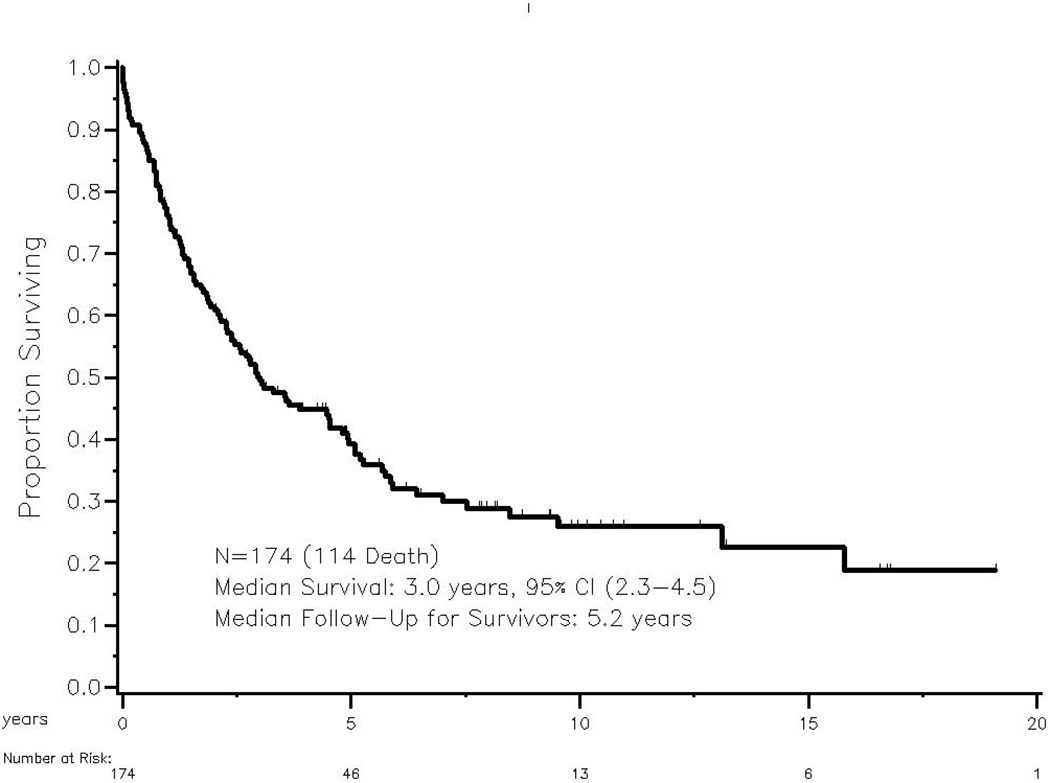
Overall survival of patients undergoing adrenal metastasectomy
Table 2:
Univariate analysis of factors associated with overall survival
| HR (95%CI) | p-value | |
|---|---|---|
| Extra-Adrenal Mets | 0.816 | |
| Yes | 0.95 (0.66-1.38 | |
| No | Reference | |
| Age | 0.782 | |
| >62 yr | 1.05 (0.73-1.51) | |
| <=62 yr | Reference | |
| Gender | 0.96 | |
| Male | 1.008 (0.69-1.46) | |
| Female | Reference | |
| Tumor size | <0.01 | |
| >4.5cm | 1.78 (1.22-2.58) | |
| <=4.5 cm | Reference | |
| Primary Site | ||
| NSCLC | 1.00 (0.67-1.49) | 0.973 |
| Renal | 0.42 (0.23-0.75) | <0.01 |
| Others | Reference | |
| Operation Approach | 0.19 | |
| Laparoscopic | 1.28 (0.87-1.88) | |
| Open | 49 (59%) | |
| Margin | <0.01 | |
| Positive | 2.1 (1.36-3.14) | |
| Negative | Reference | |
| Synchronous Disease | 0.825 | |
| No | 1.05 (0.70-1.53) | |
| Yes | Reference |
We observed 152 events. We did not detect any significant difference in event free survival (EFS) between patients with a history or presence of extra-adrenal metastatic disease versus those with isolated adrenal metastatic disease (9 months versus 10 months, respectively; p=0.87; Figure 2).
Figure 2:
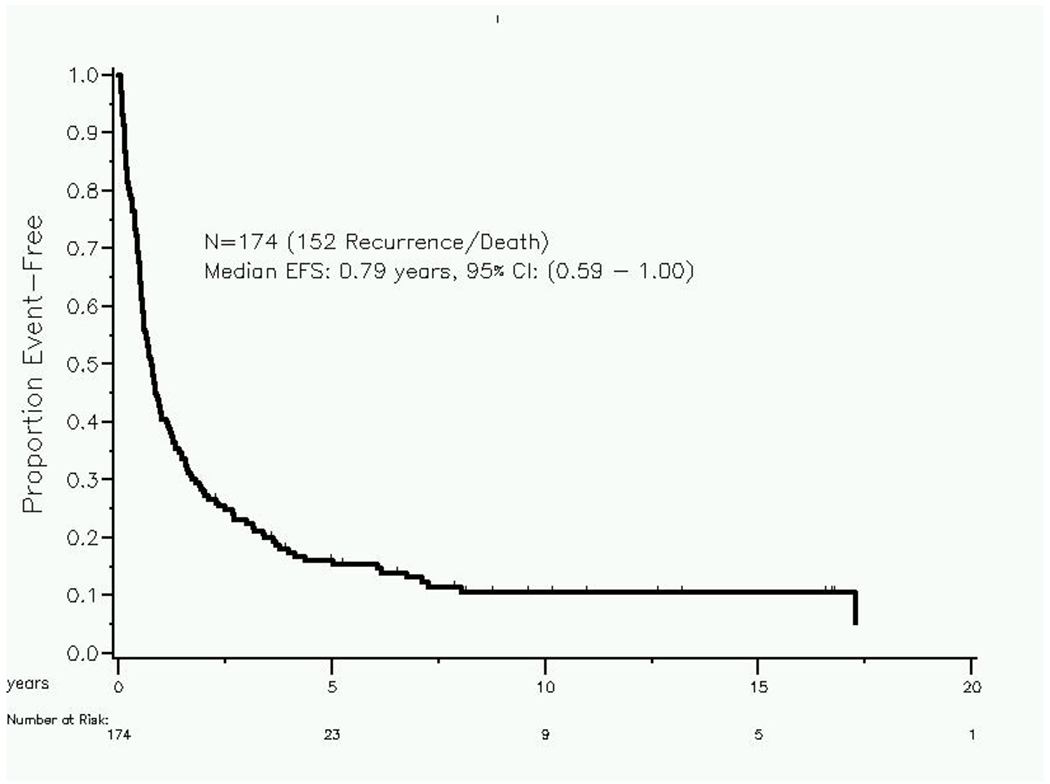
Event free survival in patients undergoing adrenal metastasectomy
The most common primary site encountered in this series was NSCLCs (n=68). As such, survival analysis was performed on this subgroup comparing patients with extra-adrenal metastatic disease (n=20) to those with isolated adrenal metastatic disease (n=48). We did not detect any significant association with OS between patients with extra-adrenal metastatic disease compared to those with isolated adrenal disease (log-rank p=0.30). Similarly, we did not detect any significant association with EFS between these two groups of patients with NSCLC (log-rank p=0.34).
Discussion
Adrenal metastasectomy has been an option for the treatment of adrenal metastases from a variety of primary tumor types. This study specifically focuses on patients with a history or presence of extra-adrenal metastases compared to those with isolated adrenal metastatic disease. The results of this study support the notion that these two groups of patients have similar long-term outcomes and that the presence or history of extra-adrenal metastatic disease should not be a contraindication for adrenalectomy.
The most common primary site in this series was non-small cell lung cancer followed by renal and colon. This is consistent with some series14 but not others12,13, where patients with primary renal malignancies are more likely to undergo adrenal metastasectomy than patients with primary lung malignancies. These differences likely represent treatment and referral biases, as well as the interdisciplinary care approach at our center. The prevalence of different primary sites is important to consider when comparing studies because metastatic non-small cell lung cancers and renal cancers have different natural histories. In this study, the median survival of patients with non-small cell lung cancers and renal cancers was 2.3 years and 7.5 years, respectively. Differences in the natural history of different primary cancer types make comparison of adrenal metastasectomy studies quite difficult, as these studies represent a selected and heterogeneous group of patients.
A diagnosis of lung cancer was more common in the isolated adrenal metastases group as compared to those with extra-adrenal metastases. The most common site of extra-adrenal metastases in this series was the liver and a majority of these patients had a colon primary. The frequent use of liver resection for colorectal metastases and the option for synchronous hepatic resection and adrenalectomy is likely responsible for the fact that 22% of patients in the extra-adrenal group had primary colon lesions. It should be noted that a greater degree of selection is likely responsible for the observed survival outcomes in the extra-adrenal metastases group as a whole. Many of the patients with extra-adrenal metastatic disease had undergone previous successful treatment of their metastatic disease, which enriches this patient population for longer survival. Nonetheless, median and event-free survivals were similar between the two groups. This finding was also true among individuals with non-small cell lung cancers, the most common primary site in this series. The results from this study support the feasibility of an operative approach to the treatment of adrenal metastatic disease in patients with both isolated adrenal and extra-adrenal metastatic disease.
Several recent studies have examined the association of extra-adrenal disease and survival. Vazquez et al. demonstrated reduced survival in patients with extra-adrenal disease12. In their cohort of 166 patients, they reported that 30% of patients had persistent disease after adrenalectomy. In our study, only 8% of patients were not rendered disease-free following resection. As such, it is difficult to compare the survival outcomes of these two studies. Another study of 62 patients, 9 of whom had extra-adrenal disease, did not find a difference in overall survival of patients with oligometastatic disease provided that the primary cancer and additional metastatic lesions were adequately controlled and amenable to resection12. Similar to our study, non-small cell lung cancers were the most common primary cancer in the cohort. Finally, a study by Zerrweck et al. reviewed 65 patients undergoing adrenal metastasectomy, 22 of whom had extra-adrenal disease. Patients with extra-adrenal disease had been successfully treated prior to adrenalectomy and one third of these patients were treated with simultaneous resection at the time of adrenalectomy or with resection prior to adrenalectomy, rendering all patients disease free after adrenalectomy13. They found no difference in overall survival based on the presence or history of extra-adrenal disease, consistent with our study. This data further supports the fact that adrenal metastasectomy can be offered to appropriately selected patients with advanced stage disease, even when not isolated to the adrenal gland.
Our study is the largest series to date to address this clinical question. The findings support the observation that selected patients with synchronous or a history of successfully treated extra-adrenal metastases can achieve similar overall survival outcomes when compared to patients with isolated adrenal disease. Critical to the selection process is the status of the patients’ extra-adrenal disease at the time of adrenalectomy. Most of the patients in our study had either undergone successful treatment of their extra-adrenal disease prior to adrenalectomy or underwent a planned combination resection at the time of adrenalectomy, with the ultimate goal of yielding “no evidence of disease” status following their operation. Of the 83 patients with a history or presence of extra-adrenal metastatic disease, there were 8 patients who underwent adrenalectomy for progressively enlarging adrenal lesions in the setting of known, stable, and indolent extra-adrenal disease. These patients were considered “alive with disease” at the conclusion of their operation. There was no significant difference in OS between patients who were disease-free versus alive with disease following operation, with disease status being a direct reflection of the presence or absence of extra-adrenal metastatic disease at the time of adrenal metastasectomy.
Previous publications from our group have established tumor size > 4.5 cm as a factor associated with shorter survival time3. In this series, both tumor size and margin status were significantly associated with overall survival.
This study has several limitations. We were unable to assess the impact of systemic therapy before and after adrenalectomy. In addition, this study did not include a comparison group of patients who were managed without surgery. While the ideal study would be a randomized trial to test whether continued chemotherapy is better than surgical intervention, a trial of this nature would be very challenging since the cases vary significantly. Finally, combining multiple primary sites of cancer introduces heterogeneity into the analysis and broadly applying outcomes data to a given patient should be performed cautiously and with multidisciplinary discussion. It should be noted that this study is not intended as a guide to select patients for adrenalectomy, since the heterogeneity of the primary disease types does not allow for a comparison. This study does, however, aim to show that in a highly selected group of patients whose functional status and disease biology made them good candidates for surgery, there is no apparent harm or hastening of disease progression with adrenalectomy. Additionally, many of the patients included in this study presented for surgical resection after experiencing chemotherapy toxicity or chemotherapy intolerance, further limiting their nonsurgical options and making an operation more preferable.
Conclusions
This study demonstrates that in selected patients, long-term survival is possible after adrenalectomy for patients with both isolated adrenal metastases and history or presence of extra-adrenal metastatic disease. Adrenal metastasectomy does not seem to hasten progression of disease for selected patients with oligometastatic disease. Multidisciplinary care and evaluation are critical for these and all patients.
Supplementary Material
Supplemental Table: Extra-adrenal metastases in patients AWD following resection
Acknowledgments
Source of Funding: Supported by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant funded by the National Cancer Institute of the National Institutes of Health (NIH). Grant Number P30-CA008748
Footnotes
Conflict of Interest: No conflicts of interest
References:
- 1.Hellman S, Weichselbaum RR Oligometastases. J Clin Oncol January 1995; 1(13), 8–10. [DOI] [PubMed] [Google Scholar]
- 2.Weichselbaum RR, Hellman S Oligometastases revisited Nat Rev Clin Oncol June 2011;(8), 378–382. [DOI] [PubMed] [Google Scholar]
- 3.Strong VE, D’Angelica M, Tang L, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol December 2007;14(12):3392–3400. [DOI] [PubMed] [Google Scholar]
- 4.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg September 1999; 230(3), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downey RJ, Ng KK. The management of non-small-cell lung cancer with oligometastases. Chest Surg Clin N Am February 2001;11(1):121–132, ix. [PubMed] [Google Scholar]
- 6.Kim SH, Brennan MF, Russo P, et al. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. Jan 15 1998;82(2):389–394. [PubMed] [Google Scholar]
- 7.Gittens PR Jr., Solish AF, Trabulsi EJ. Surgical management of metastatic disease to the adrenal gland. Semin Oncol April 2008;35(2):172–176. [DOI] [PubMed] [Google Scholar]
- 8.Sarela AI, Murphy I, Coit DG, et al. Metastasis to the adrenal gland: the emerging role of laparoscopic surgery. Ann Surg Oncol December 2003;10(10):1191–1196. [DOI] [PubMed] [Google Scholar]
- 9.Lo CY, van Heerden JA, Soreide JA, et al. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg April 1996;83(4):528–531. [DOI] [PubMed] [Google Scholar]
- 10.Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg December 1996;62(6):1614–1616. [DOI] [PubMed] [Google Scholar]
- 11.Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol March 1 2008;26(7):1142–1147. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez BJ, Richards ML, Lohse CM, et al. Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg June 2012;36(6):1400–1405. [DOI] [PubMed] [Google Scholar]
- 13.Zerrweck C, Caiazzo R, Clerquin B, et al. Renal origin and size are independent predictors of survival after surgery for adrenal metastasis. Ann Surg Oncol October 2012;19(11):3621–3626. [DOI] [PubMed] [Google Scholar]
- 14.Howell GM, Carty SE, Armstrong MJ, et al. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol October 2013;20(11):3491–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table: Extra-adrenal metastases in patients AWD following resection


