Abstract
Background and objectives
Orthodontic relapse is a physiologic process that involves remodelling of the alveolar bone and principle periodontal ligament fibres. Raloxifene is an Food and Drug Administration (FDA)-approved selective oestrogen receptor modulator that inhibits systemic bone loss. In our study, we examined the effects of Raloxifene on alveolar bone modelling and orthodontic relapse in a rodent model.
Materials and methods
The efficacy of raloxifene was evaluated in 15-week-old male Wistar rats, 8 in each group (Control, Raloxifene, Raloxifene + 7-day relapse, Raloxifene + 14-day relapse) for a total of 42 days. All animals had 14 days of orthodontic tooth movement with a closed nickel–titanium coil spring tied from incisors to right first molar applying 5–8 gm of force. On the day of appliance removal, impression was taken with silicon material and the distance between first molar and second molar was filled with light-cured adhesive resin cement for retention phase. Raloxifene Retention, Raloxifene Retention + 7D, Raloxifene Retention + 14D groups received 14 daily doses of raloxifene (2.0 mg/kg/day) subcutaneously after orthodontic tooth movement during retention. After 14 days of retention, the retainer was removed and right first molar was allowed to relapse for a period of 14 days. Raloxifene injection continued for the Raloxifene + 14-day relapse group during relapse phase too. Control group received saline injections during retention. Animals were euthanized by CO2 inhalation. The outcome measure included percentage of relapse, bone volume fraction, tissue density, and histology analysis using tartrate-resistant acid phosphatase staining and determining receptor activator of nuclear factor-кB-ligand (RANKL) and osteoprotegerin expression.
Results
Raloxifene Retention + 14D group had significantly less (P < 0.05) orthodontic relapse when compared with other groups. There was a significant increase (P < 0.05) in bone volume fraction and tissue density in the Raloxifene Retention + 14D group when compared with other groups. Similarly, there was significant decrease in number of osteoclasts and RANKL expression in Raloxifene Retention + 14D group when compared with Raloxifene Retention + 7D group (P < 0.05).
Conclusion
Raloxifene could decrease post-orthodontic treatment relapse by decreasing bone resorption and indirectly enhancing bone formation.
Introduction
The retention strategy plays a critical role in the successful maintenance of position orthodontic moved teeth position. Removable or fixed retention is considered the standard long-term protocol for post-treatment stability. However, there has not been high-quality evidence to determine whether one retention strategy is effective over other and the duration of retention needed to achieve long-term stability (1–3). Regardless of the long-term retention strategy used, studies show that relapse can still occur (4). The reported frequency of relapse varies from approximately 14 to 70 per cent, based on different follow-up periods (1, 4, 5). Due to the complexity of the aetiology and the unpredictability of relapse, there is an unmet need for effective methods to prevent relapse. Biological interventions aimed to inhibit bone remodelling are some of the promising strategies to enhance post-orthodontic stability.
The primary factor regulating the process of alveolar bone remodelling is the ratio of receptor activator of nuclear factor (NF)-кB-ligand (RANKL), receptor activator of NF-кB (RANK), and osteoprotegerin (OPG) (6). Manipulation of RANK/RANKL/OPG pathway by biologics (small to large biomolecules) seems to be an effective approach for inhibiting relapse. The effects of administration of biologic/pharmacological agents, including bisphosphonates (7), osteoprotegerin (8–10), statins (11, 12), and relaxin (13) in animal models resulted in reduced relapse. However, in some of the studies, relapse was measured over a relatively short period of time, and in other studies, the biological mechanism of the biotherapeutics on the alveolar bone and preventing relapse was not clearly described (8). Additionally, the biologics used in previous studies had long-lasting effects on the bone modelling and remodelling, which is not the desirable clinical effect (7).
Sex hormones such as oestrogen, androgen, and progesterone play important role in bone modelling and remodelling (14, 15). Recently, selective oestrogen receptor modulators (SERMs) have been shown to reduce bone resorption systemically and preserve bone in various pre-clinical and clinical bone disorders (16, 17). SERMs are Food and Drug Administration (FDA)-approved biologics and have been used clinically to prevent bone loss in post-menopausal woman (18). Raloxifene is a second-generation SERM that imitates oestrogen action on bone, decreasing the number of osteoclasts by reducing interleukin-6 (IL-6) and IL-1β production and upregulating transforming growth factor (TGF-β3) (18, 19). Raloxifene is also known to increase the bone mineral density by shifting the RANKL/OPG ratio (18, 19). The bone protective effects of Raloxifene are not only limited to modulation of bone resorption, but Raloxifene also modulates bone formation through enhancing osteoblast proliferation (17). Raloxifene therapy in osteoporotic rats has shown an inhibition of osteoclastogenesis in alveolar bone healing and enhancement of peri-implant bone healing (20–23). Raloxifene acts through the endogenous oestrogen signalling pathway and treatment with this drug exhibits a very low incidence of adverse side effects (18). Given these advantages of Raloxifene, a pharmacological approach aimed at preventing the relapse after orthodontic tooth movement through known biological mechanism on alveolar bone may provide an effective and noncompliant method of preventing relapse. There is an unmet need for preventing relapse on orthodontically moved tooth/teeth and potential use of Raloxifene in preventing relapse has not been investigated.
In this research, we tested the hypothesis that the systemic administration of Raloxifene will prevent the relapse after the orthodontic tooth movement. Our objective with this study was to 1) determine the effects of Raloxifene administration on the prevention of relapse and 2) understand the effects of Raloxifene on alveolar bone modelling and remodelling. In our research, we used an established rat model to investigate the ability of systemic injection of Raloxifene to decrease relapse after orthodontic tooth movement (OTM), during the retention and relapse phases.
Material and methods
Ethical statement
The animals were housed in the animal facility, and all the experimental procedures involving Wistar rats were approved by the Institutional Care Committee of the University of Connecticut Health Center.
Overall study design
All experimental procedures were performed at the University of Connecticut Health under the guidelines of IACUC protocol #101652-0820. The efficacy of Raloxifene was evaluated in vivo using 15-week-old male Wistar rats (N = 32). An initial power analysis was used to determine the total sample size needed to detect an overall difference in retention across three treatment conditions [i.e. for a one-way analysis of variance (ANOVA)]. Based on a Cohen’s value f of 0.60 (ratio of population SD), an α-level of 0.05, and a desired power (1 − β) of 0.80, the total sample size estimated was n = 32 (or 8 rats in each group). Rats were randomly assigned to one of the four groups (Figure 1). Group 1: Control Group (n = 8) received saline injections during retention; Group 2: Raloxifene Group (n = 8) received Raloxifene during retention; Group 3: Raloxifene + 7-Day Relapse Group (n = 8) received Raloxifene during retention + 7 days of relapse and; Group 4: Raloxifene + 14-day Relapse Group (n = 8) received Raloxifene during the retention and relapse phases (Figure 1).
Figure 1.
Experimental design of the in vivo study depicting randomization of the 32 male Wistar rats into four groups.
Preparation of Raloxifene
Raloxifene (Sigma–Aldrich, Saint Lois, Missouri, USA) was dissolved using 20 per cent hydroxypropyl-β-cyclodextrin (Sigma–Aldrich) as diluent (24). Drug was kept at 4°C for up to 1 week prior to use.
Animal treatments
OTM was performed in all groups on the left side of the maxilla for a total of 14 days. The experimental procedures have been previously described (8). Animals were placed under general anaesthesia using a concoction of xylazine (13 mg/kg) and ketamine (87 mg/kg). To deliver a mesial orthodontic force to the left maxillary molar, a closed nickel–titanium coil spring (Ultimate Wireforms, Bristol, Connecticut, USA) delivering 5–8 gm of force was attached to the left maxillary first molar. As anchorage, the nickel–titanium coil spring was also attached to the two maxillary incisors using a 0.008-inch stainless steel wire on either side. The coils were secured with self-etching primer (Transbond Plus; 3M Unitek, Monrovia, California, USA) and light-cured adhesive resin cement (Transbond; 3M Unitek). To prevent appliance breakage and dislodging of the coils and springs, the mandibular incisors were trimmed twice a week. After appliance insertion, the rats were allowed to recover in the presence of an incandescent light for warmth and then returned to their cages.
During the retention phase (14 days following appliance insertion) animals received daily subcutaneously injections of saline or Raloxifene. Animals in the control (Group 1) were injected with saline, whereas animals in the experimental groups were injected with Raloxifene during retention (Group 2), Raloxifene Retention + 7D relapse (Group 3), and Raloxifene Retention + 14D relapse (Group 4) received Raloxifene (2.0 mg/kg/day) (25) (Figure 1). After 14 days of retention, the retainer was removed in all study groups and the first molar was allowed to relapse for another 7 days or 14 days, based on the assigned group (Figure 1). For the Raloxifene +14-day relapse (Group 4) injections of Raloxifene continued after retention, during the relapse phase (Figure 1). Animals in the Raloxifene +7-day relapse (Group 3) were euthanized after 7 days of relapse, and animals in the other groups were euthanized on day 14th of relapse (Figure 1). Animals were euthanized by inhalation of carbon dioxide followed by cervical dislocation. After euthanizing, the maxilla of each animal was then dissected and fixed at 4°C in 10 per cent formalin for 7 days.
Measurement of orthodontic tooth movement
To measure the OTM after 14 days and before the retention phase was initiated, the nickel–titanium coil spring was removed and an impression of the maxillary first and second molars were taken with polyvinyl siloxane. Impressions were poured with die stone, and casts of the maxillary arch were scanned using an iTero® Element 2 scanner (Align Technology, San Jose, California, USA). The three-dimensional scanned maxillary models were imported to Meshmixer software in STL file format. The amount of OTM was determined using the Meshmixer software, where measurements were made between the most convex points of the distal surface of first molar and the mesial surface of second molar (Figure 2). Measurements were conducted by one of the investigators who was blinded to the groups. All measurements were repeated after 2 weeks by the same investigator to assess the intra-examiner error. In all groups, the distance between the first and second molars was filled with a light-cured adhesive resin cement (Filtek; 3M Unitek) that served as a retainer for the 14-day retention phase.
Figure 2.
Measurement of the initial orthodontic tooth movement and the distance between the first and second molars after relapse. (A) Initial orthodontic tooth movement and (B) distance between the first and second molars after relapse. Histograms represent mean ± SD for n = 8 per group. #Statistically significant difference between groups Raloxifene Retention + 14D and Control and Raloxifene Retention. *Statistically significant difference between groups Raloxifene Retention + 7D and Control and Raloxifene Retention. # and *P < 0.05.
Micro-computed tomography analysis
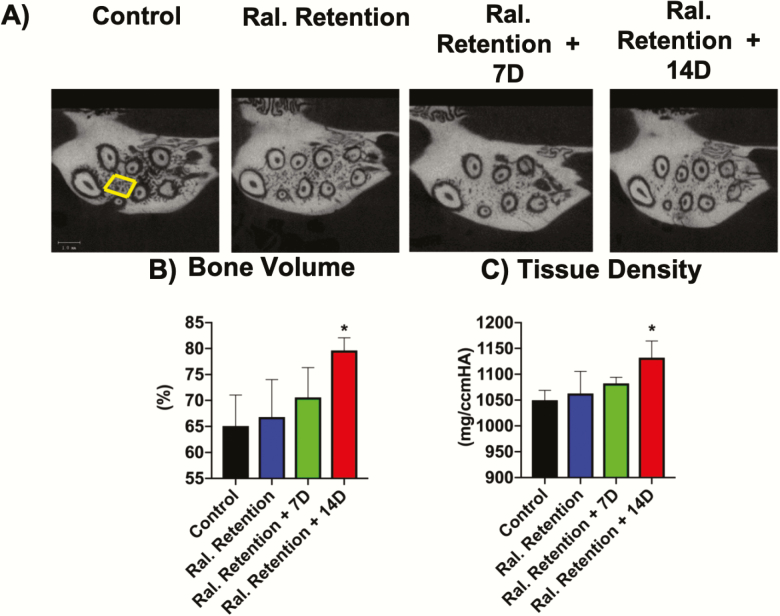
Micro-computed tomography (micro-CT) analysis was performed with a μCT40 instrument (Scanco Medical AG, Bruttisellen, Switzerland) using 55 kV, 145 mA, 1000 projections/rotation, and 300 ms. Bone volume fraction (BVF), tissue density (TD), and intermolar distance at the end of relapse phase were measured. Three-dimensional images were constructed using standard convolution and back projection algorithms with Shepp and Logan filtering, and were rendered within a 12.3-mm field of view at a discrete density of 578 704 voxel/mm3 (isometric 12-mm voxels). The serial images were used for quantitative analysis of alveolar bone changes (BVF and TD) in the region of interest (ROI) of the maxillary first molar (Figure 3A). The ROI was defined vertically as the most occlusal point of the furcation to the apex of the maxillary roots. Transversely, it formed a rectangular confirmation, which was bounded by the points of the most distal part of the distobuccal root, the distopalatal root, and the mesial side extending to the points of the most distal parts of the mesiobuccal and mesiopalatal roots (Figure 3A).
Figure 3.
Increased bone volume fraction and tissue density in Raloxifene Retention + 14D group. (A) Coronally reconstructed micro-CT images with depiction of the region of interest (ROI), in which bone volume (BVF) and tissue density (TD) were analysed. Histograms of (B) bone volume fraction and (C) tissue density in the control and experimental groups. Histograms (B and C) represent ±SD for n = 8 per group. *Statistically significant difference between Raloxifene Retention + 14D groups and other control and experimental groups. # and *P < 0.05. Scale bar = 1000 μm.
All images were analysed to determine the amount of relapse by measuring the distance between the maxillary first and second molars. The two points that were used for the measurement were the most distal point of the first molar (M1) and the most mesial point of the second molar (M2). The difference (M1 − M2 distance) was used to evaluate the distance which remained between the molars and was used to calculate the amount and percentage of relapse (Figure 2).
Histological analysis
Following micro-CT analysis, samples were decalcified using 14 per cent ethylene diamine tetra-acetic acid for 4 weeks. Samples were then dehydrated and embedded in paraffin. Sectioning at the sagittal view was performed obtaining 5-µm sections. Sections were collected when the distobuccal root of the first molar was fully sectioned. Histological sections were analysed by immunostaining, tartrate-resistant acid phosphatase (TRAP), and picrosirius red staining. Following heat antigen retrieval, the protocol for immunohistochemistry consisted of initially blocking the deparaffinized samples for 10 min at room temperature (Universal Blocking reagent, HK085-5k; BioGenex, Fremont, California, USA). Sections were incubated with rabbit polyclonal anti-RANKL antibody (AF1589; R&D Systems, Minneapolis, Minnesota, USA) or anti-osteoprotegerin (AB73400, Abcam, Cambridge, Massachusetts, USA) overnight at +4°C. Subsequently, the sections were washed with phosphate-buffered saline and incubated with Alexa Fluor 594 goat anti-rabbit IgG (A-11058; Life Technologies, Grand Island, New York, USA) for 1 hour at room temperature and mounted with a suspension of 50 per cent glycerol in phosphate-buffered saline containing nuclear stain (Hoechst H3570; Life Technologies, Carlsbad, California, USA).
TRAP staining was performed using a leukocyte acid phosphatase kit (386-1 KT; Sigma–Aldrich) according to the manufacturer’s instructions. TRAP-positive, multinucleated cells were counted on the alveolar bone surfaces on the mesial sides of the distobuccal roots. The area for quantification included a square with one side extending from the apex to the bifurcation and the other side extending 200 μm from the periodontal ligament (PDL) border inside the alveolar bone.
Deparaffinized sections were also stained with 1 per cent picrosirius red for 1 hour, washed with acidified water (0.5 per cent acetic acid water), and dehydrated with serial ethanol washes before mounting. The sections were examined and scanned with automated microscope using either fluorescent light or brightfield light on 10× magnification (Carl Zeiss, Thornwood, New York, USA).
Statistical analysis
We used descriptive analysis to summarize the data. We examined the outcome variables of OTM, relapse rate (in per cent), BVF, TD, osteoclast number, RANKL, and OPG expression. The relapse rate (in per cent) was the primary outcome. We used the D’Agostino and Pearson omnibus normality test to examine the normality of the data distribution. All the outcome measures were normally distributed. All statistical comparisons were made using one, two-way analysis of variance and post hoc Tukey’s honestly significant difference with a significance level of P value of <0.05. The intra-examiner reliability was measured through Cohen’s kappa, and it was 0.9 for the OTM measurement using Meshmixer. We based the selected sample size for each experiment on a power analysis. For an α value of 0.05, a β value of 0.1, and an SD of 10 per cent, we determined the sample size for in vivo studies to be 8 for a power of 0.8.
Results
Effects of Raloxifene on the rate and percentage of relapse
We found that there was no significant difference (P > 0.05) in OTM between the four different groups (Figure 2A). Measurement of the distance between the first and second molars (amount of OTM) in the 3D scanned models showed that in Group 1 (Control group), the OTM was 0.49 ± 0.08 mm; in Group 2 (Raloxifene Retention), the OTM was 0.44 ± 0.07 mm; in Group 3 (Raloxifene Retention + 7D relapse), the OTM was 0.41 ± 0.04 mm; and in Group 4 (Raloxifene Retention + 14D relapse), the OTM was 0.34 ± 0.05 mm (Figure 2A).
Following the removal of retainers on day 28, the next 14 days were considered the relapse phase. Micro-CT measurements of the distance between the first and second molars, after the relapse phase, showed that the first molar movement relapsed in all groups (Figure 2B and Table 1). However, there was significantly less (P < 0.05) relapse in Group 3 (Raloxifene Retention + 7D relapse group, relapse of 34 per cent) when compared with Group 4 (Raloxifene Retention + 14D relapse group, relapse of 54 per cent), Group 1 (Control group, relapse of 98 per cent), and Group 2 (Raloxifene Retention, Relapse of 82 per cent). Furthermore, there was significantly less (P < 0.05) relapse in Group 4 (Raloxifene Retention + 14D relapse) when compared with Group 1 (Control group) and Group 2 (Raloxifene Retention) (Figure 2B and Table 1). At the end of the study (day 42), the distance between the molars in the control (Group 1) was 0.007 ± 0.01 mm, whereas in the group receiving Raloxifene Retention (Group 2), the distance was 0.08 ± 0.12 mm; in the group receiving Raloxifene Retention + 7D relapse (Group 3), the distance was 0.27 ± 0.10 mm; and in the group receiving Raloxifene Retention + 14D relapse (Group 4), the distance was 0.15 ± 0.15 mm (Figure 2B).
Table 1.
Percentage of relapse in control and experimental groups
| Groups | % Relapse |
|---|---|
| Control | 98.48 |
| Raloxifene Retention | 81.56 |
| Raloxifene Retention + 7D | 33.93 |
| Raloxifene Retention + 14D | 54.14 |
Micro-CT analysis examining the effects of Raloxifene on bone parameters
Micro-CT analysis showed a significant increase (P < 0.05) in BVF for the Raloxifene Retention + 14D (Group 4) when compared with other groups (Figure 3A and 3B and Table 2). The BVF was 65 ± 5.9 per cent in the control (Group 1), 67 ± 7.2 per cent in the Raloxifene Retention (Group 2), 71 ± 5.8 per cent in the Raloxifene Retention + 7D (Group 3); and 80 ± 2.4 per cent in the Raloxifene Retention + 14D (Group 4).
Table 2.
Distribution of bone volume in the control and experimental groups
| Number of values | 8 | 8 | 8 | 8 |
| Minimum | 58.40 | 54.90 | 63.80 | 75.70 |
| 25% Percentile | 59.78 | 59.80 | 65.75 | 77.80 |
| Median | 63.80 | 68.50 | 68.50 | 79.75 |
| 75% Percentile | 71.13 | 72.78 | 76.45 | 81.88 |
| Maximum | 74.30 | 75.50 | 78.00 | 82.80 |
| Mean | 65.09 | 66.81 | 70.58 | 79.64 |
| SD | 5.948 | 7.228 | 5.751 | 2.413 |
| SEM | 2.103 | 2.556 | 2.572 | 0.8531 |
| Lower 95% CI | 60.12 | 60.77 | 63.44 | 77.62 |
| Upper 95% CI | 70.06 | 72.86 | 77.72 | 81.65 |
Similarly, the TD in the Raloxifene Retention + 14D (Group 4) was found to be significantly higher (P < 0.05) when compared with the other groups (Figure 3C and Table 3). The control (Group 1) had a TD of 1050 ± 19 mg/ccmHA, the Raloxifene Retention (Group 2) had a TD of 1063 ± 43 mg/ccmHA, the Raloxifene Retention + 7D (Group 3) had a TD of 1082 ± 12 mg/ccmHA, and the Raloxifene Retention + 14D (Group 4) had a TD of 1132 ± 33 mg/ccmHA (Figures 4 and 5B and Table 3).
Table 3.
Distribution of tissue density (mg/ccmHA) in the control and experimental groups
| Number of values | 8 | 8 | 8 | 8 |
| Minimum | 1012 | 967.0 | 1071 | 1082 |
| 25% Percentile | 1036 | 1049 | 1072 | 1112 |
| Median | 1055 | 1072 | 1082 | 1123 |
| 75% Percentile | 1063 | 1091 | 1094 | 1163 |
| Maximum | 1069 | 1103 | 1099 | 1177 |
| Mean | 1050 | 1063 | 1082 | 1132 |
| SD | 19.07 | 42.92 | 11.67 | 32.53 |
| SEM | 6.741 | 15.18 | 5.221 | 11.50 |
| Lower 95% CI | 1034 | 1027 | 1068 | 1105 |
| Upper 95% CI | 1066 | 1099 | 1097 | 1159 |
Figure 4.
(A) Histological staining and quantification showing a decrease in TRAP-positive multinucleated cells in the Raloxifene Retention + 14D group when compared with the Raloxifene Retention + 7D group. (B) Immunohistochemical staining and quantification showing decreased expression of RANKL Raloxifene Retention + 14D group when compared with the Raloxifene Retention + 7D group. (C) Immunohistochemical staining and quantification showing no difference in the expression of osteoprotegerin in between the control and experimental groups. Histograms represent ±SD for n = 6 per group. *Statistically significant difference between groups Raloxifene Retention + 7D group and Raloxifene Retention + 14D. *P < 0.05. Scale bar = 200 μm.
Figure 5.
Qualitative histological analysis of periodontal ligament collagen fibres by light microscopy. Animals (n = 5) were analysed in each group. Scale bar = 100 μm.
Histological analysis
We found a significant decrease (P < 0.05) in the number of TRAP-positive cells in the Raloxifene Retention + 14D group when compared with Raloxifene Retention + 7D group (Figure 4A). However, there was no difference between Raloxifene Retention + 14D group when compared with control and Raloxifene Retention group. Additionally, we found a significant decrease (P < 0.05) in RANKL expression in the Raloxifene Retention + 14D group when compared with Raloxifene Retention + 7D group (Figure 4B). However, there was no difference between Raloxifene Retention + 14D group when compared with control and Raloxifene Retention group (Figure 4B).
OPG labelling showed a moderate expression in all groups with no significant difference between four different groups (Figure 4C). Using light microscopy to analyse the collagen fibres of the PDL, following Sirius red staining, we found a more organized and thickened PDL (intense red staining) in the Raloxifene Retention + 7D (Group 3) and Raloxifene Retention + 14D (Group 4) when compared with the other groups (Figure 5).
Discussion
Preventing relapse after OTM is a clinical challenge. The alveolar bone continues to remodel following OTM, and a new compression zone appears in the direction opposite to the OTM. Our null hypothesis was rejected, and we observed that Raloxifene decreased relapse and enhanced post-orthodontic stability. In comparison to other pharmacological agents, Raloxifene has an advantage of concurrently simulating bone formation while inhibiting bone resorption. Furthermore, Raloxifene mimics the effects of oestrogen on bone tissue, has minimal to no side effects, and has not been correlated with medication-related osteonecrosis of the jaw (MRONJ) (26).
In this study, we found that Raloxifene significantly decreased the orthodontic relapse. The group of animals that received Raloxifene during the retention phase (Group 4) had the least relapse. This improved retention (less relapse) in the group receiving Raloxifene during retention and 14 days of relapse was accompanied by a significant increase in BVF and TD. Additionally, there was decrease in number of osteoclast and RANKL in Raloxifene Retention + 14D group when compared with Raloxifene Retention + 7D group.
Although the complete mechanism of action of Raloxifene has not been elucidated, studies have shown that Raloxifene increases OPG and decreases RANKL expression through the canonical Wnt/β-catenin signalling pathway. In our study, the group receiving Group 4 (Raloxifene Retention + 14D) showed moderate labelling of OPG and significantly decreased labelling for RANKL. Furthermore, it has been shown that Raloxifene modulates osteoclastic pathways mediated by tumor necrosis factor-α and TGF-β and decreases the proresorptive cytokines such as IL-1β and IL-6 (19). We also observed significant decrease in TRAP-positive osteoclasts in Group 4 (Raloxifene Retention + 14D) when compared with Group 3 (Raloxifene Retention + 7D).
Our findings were in agreement with the previously published animal studies on relapse after OTM (27, 28). Franzen et al. (27) has investigated the amount of relapse and the biological changes in the alveolar bone during relapse and they observed that 73 per cent of relapse occurred 1 day after appliance removal, whereas 93 per cent of relapse occurred 21 days after appliance removal. Similarly, we observed that the control group in our study had 98 per cent relapse 2 weeks after retention. The main difference in our study compared with other studies of relapse was the incorporation of a retention phase following active OTM and before the relapse phase. We believe that the retention phase included in our study was more representative of the clinical situation. We hypothesized that during the 14 days of retention, not only the alveolar bone and PDL fibres would regain their structural integrity, but also the administration of Raloxifene would lead to a decrease in the relapse magnitude by enhancing the quality of alveolar bone.
It has been shown that during OTM, the bone volume and the TD decrease, while there is an increase in the number of osteoclasts and the expression of bone resorption markers. It has been postulated that the newly formed bone behind the roots of moved teeth (tension side) may serve as an initial barrier to prevent relapse. However, without a long enough retention period, relapse will occur as bone is not adequately mineralized (8, 27). In our control group, the high relapse rate could be attributed to the lack of mature and mineralized bone. It is our understanding that immediately after OTM, the remodelled alveolar bone is not completely mineralized and could not resist to the relapse force caused by stretched transseptal fibres. Furthermore, it has been shown that bone mineralization markers (osteopontin and osteocalcin) are usually expressed in later stages of anabolic bone modelling (20). This observation could explain why 14 days of retention in our study did not decrease the percentage of relapse.
Comparing our Group 1 (control group) with the Group 2 (Raloxifene Retention), we did not find any significant differences in the relapse rate, bone volume, TD, osteoclast number, or in the expression of bone remodelling markers. Furthermore, by comparing the histological analysis and relapse rate between Group 2 (Raloxifene Retention) rats receiving Raloxifene during retention with Group 4 (Raloxifene Retention + 14D) rats receiving Raloxifene both during retention + 14 days of the relapse, we found that the transient manipulation of osteoclastogenesis and osteoblastogenesis solely during retention is not sufficient to manage relapse. Future studies examining the potential use of Raloxifene in the clinic for preventing orthodontic relapse should continue to administer the drug into the relapse phase.
A dose response was observed in our study; the animals receiving sustained injection of Raloxifene for the longest period of time, i.e. during retention + 14 days of the relapse phase (Group 4) had a 55 per cent reduction in relapse and they showed significantly more bone volume and TD. The short half-life of Raloxifene (24 hours) and physiological distal drift is a plausible explanation for these responses. Moreover, the short half-life of Raloxifene is beneficial in clinical applications since the drug would be only active during the therapeutic window without any long-term side effects. It has been shown that the expression of bone mineralization biomarkers was positively correlated with longer administration of Raloxifene (42 days) (26). Similarly, we observed that the group of rats receiving Raloxifene during both retention + 14 days of relapse (28 days total) had newly formed alveolar bone with higher bone quality. These characteristics provided the potential to resist the tendency for relapse.
To our knowledge, this is the first study to investigate the effects of FDA-approved Raloxifene in an animal model of orthodontic relapse. A growing number of animal studies have reported the effects of administering various biological agents including bisphosphonates (7), statins (11, 12, 29), osteoprotegerin (9), relaxin (13), and bone morphogenetic proteins (30) to modulate alveolar bone modelling with the objective of inhibiting orthodontic relapse. Although these pharmacological agents target bone and PDL remodelling, they have different half-lives and distinctly different mechanisms of action. It is challenging to compare these studies with our study as different investigators have utilized varied methodologies to measure relapse. The reduction in relapse reported in previous studies ranges between 8 and 50 per cent after 10–24 days of relapse (7–9, 12, 31). Similarly, we found approximately 13–44 per cent less relapse in the Raloxifene-treated groups.
Due to the limitations of rodent relapse models, including lack of secondary remodelling, translation of these results to the clinical setting needs further studies in higher animal models. Furthermore, in this study, Raloxifene was administered systemically. However, our future study will focus on local/controlled delivery of Raloxifene using microsphere.
Conclusion
1) Administration of Raloxifene during both the retention and relapse phases leads to decreased relapse, decreased expression of RANKL, and moderately increased expression of OPG.
2) There was increase in bone volume and TD when Raloxifene was administered during both the retention and the relapse phases.
Funding
American Association of Orthodontist Foundation; North Eastern Society of Orthodontist; National Institutes of Health (KO8DEO25914 to S.Y.).
Conflicts of interest
None to declare.
References
- 1. de Bernabé P.G., Montiel-Company J.M., Paredes-Gallardo V., Gandía-Franco J.L. and Bellot-Arcís C (2017) Orthodontic treatment stability predictors: a retrospective longitudinal study. The Angle Orthodontist, 87, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Littlewood S.J., Millett D.T., Doubleday B., Bearn D.R. and Worthington H.V (2016) Retention procedures for stabilising tooth position after treatment with orthodontic braces. Cochrane Database of Systematic Reviews, CD002283. doi: 10.1002/14651858.CD002283.pub4 [DOI] [PubMed] [Google Scholar]
- 3. Littlewood S.J., Millett D.T., Doubleday B., Bearn D.R. and Worthington H.V (2016) Retention procedures for stabilising tooth position after treatment with orthodontic braces. Cochrane Database of Systematic Reviews, CD002283. doi: 10.1002/14651858.CD002283.pub3 [DOI] [PubMed] [Google Scholar]
- 4. Steinnes J., Johnsen G. and Kerosuo H (2017) Stability of orthodontic treatment outcome in relation to retention status: an 8-year follow-up. American Journal of Orthodontics and Dentofacial Orthopedics, 151, 1027–1033. [DOI] [PubMed] [Google Scholar]
- 5. Little R.M., Riedel R.A. and Artun J (1988) An evaluation of changes in mandibular anterior alignment from 10 to 20 years postretention. American Journal of Orthodontics and Dentofacial Orthopedics, 93, 423–428. [DOI] [PubMed] [Google Scholar]
- 6. Huang H., Williams R.C. and Kyrkanides S (2014) Accelerated orthodontic tooth movement: molecular mechanisms. American Journal of Orthodontics and Dentofacial Orthopedics, 146, 620–632. [DOI] [PubMed] [Google Scholar]
- 7. Kim T.W., Yoshida Y., Yokoya K. and Sasaki T (1999) An ultrastructural study of the effects of bisphosphonate administration on osteoclastic bone resorption during relapse of experimentally moved rat molars. American Journal of Orthodontics and Dentofacial Orthopedics, 115, 645–653. [DOI] [PubMed] [Google Scholar]
- 8. Hudson J.B., Hatch N., Hayami T., Shin J.M., Stolina M., Kostenuik P.J. and Kapila S (2012) Local delivery of recombinant osteoprotegerin enhances postorthodontic tooth stability. Calcified Tissue International, 90, 330–342. [DOI] [PubMed] [Google Scholar]
- 9. Schneider D.A., Smith S.M., Campbell C., Hayami T., Kapila S. and Hatch N.E (2015) Locally limited inhibition of bone resorption and orthodontic relapse by recombinant osteoprotegerin protein. Orthodontics & Craniofacial Research, 18(Suppl 1), 187–195. [DOI] [PubMed] [Google Scholar]
- 10. Zhao N., Lin J., Kanzaki H., Ni J., Chen Z., Liang W. and Liu Y (2012) Local osteoprotegerin gene transfer inhibits relapse of orthodontic tooth movement. American Journal of Orthodontics and Dentofacial Orthopedics, 141, 30–40. [DOI] [PubMed] [Google Scholar]
- 11. Dolci G.S., Portela L.V., Onofre de Souza D. and Medeiros Fossati A.C (2017) Atorvastatin-induced osteoclast inhibition reduces orthodontic relapse. American Journal of Orthodontics and Dentofacial Orthopedics, 151, 528–538. [DOI] [PubMed] [Google Scholar]
- 12. AlSwafeeri H., ElKenany W., Mowafy M. and Karam S (2018) Effect of local administration of simvastatin on postorthodontic relapse in a rabbit model. American Journal of Orthodontics and Dentofacial Orthopedics, 153, 861–871. [DOI] [PubMed] [Google Scholar]
- 13. Hirate Y., Yamaguchi M. and Kasai K (2012) Effects of relaxin on relapse and periodontal tissue remodeling after experimental tooth movement in rats. Connective Tissue Research, 53, 207–219. [DOI] [PubMed] [Google Scholar]
- 14. Haruyama N., Igarashi K., Saeki S., Otsuka-Isoya M., Shinoda H. and Mitani H (2002) Estrous-cycle-dependent variation in orthodontic tooth movement. Journal of Dental Research, 81, 406–410. [DOI] [PubMed] [Google Scholar]
- 15. Zittermann A., Schwarz I., Scheld K., Sudhop T., Berthold H.K., von Bergmann K., van der Ven H. and Stehle P (2000) Physiologic fluctuations of serum estradiol levels influence biochemical markers of bone resorption in young women. The Journal of Clinical Endocrinology and Metabolism, 85, 95–101. [DOI] [PubMed] [Google Scholar]
- 16. Morita M., et al. (2016) Selective estrogen receptor modulators suppress hif1α protein accumulation in mouse osteoclasts. PLoS One, 11, e0165922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taranta A., Brama M., Teti A., De luca V., Scandurra R., Spera G., Agnusdei D., Termine J.D. and Migliaccio S (2002) The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone, 30, 368–376. [DOI] [PubMed] [Google Scholar]
- 18. Rey J.R., Cervino E.V., Rentero M.L., Crespo E.C., Alvaro A.O. and Casillas M (2009) Raloxifene: mechanism of action, effects on bone tissue, and applicability in clinical traumatology practice. The Open Orthopaedics Journal, 3, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viereck V., Gründker C., Blaschke S., Niederkleine B., Siggelkow H., Frosch K.H., Raddatz D., Emons G. and Hofbauer L.C (2003) Raloxifene concurrently stimulates osteoprotegerin and inhibits interleukin-6 production by human trabecular osteoblasts. The Journal of Clinical Endocrinology and Metabolism, 88, 4206–4213. [DOI] [PubMed] [Google Scholar]
- 20. Ramalho-Ferreira G., Faverani L.P., Momesso G.A.C., Luvizuto E.R., de Oliveira Puttini I. and Okamoto R (2017) Effect of antiresorptive drugs in the alveolar bone healing. A histometric and immunohistochemical study in ovariectomized rats. Clinical Oral Investigations, 21, 1485–1494. [DOI] [PubMed] [Google Scholar]
- 21. Luvizuto E.R., Dias S.S., Okamoto T., Dornelles R.C. and Okamoto R (2011) Raloxifene therapy inhibits osteoclastogenesis during the alveolar healing process in rats. Archives of Oral Biology, 56, 984–990. [DOI] [PubMed] [Google Scholar]
- 22. Ichimaru R., et al. (2018) Raloxifene reduces the risk of local alveolar bone destruction in a mouse model of periodontitis combined with systemic postmenopausal osteoporosis. Archives of Oral Biology, 85, 98–103. [DOI] [PubMed] [Google Scholar]
- 23. Ramalho-Ferreira G., Faverani L.P., Prado F.B., Garcia I.R. Jr and Okamoto R (2015) Raloxifene enhances peri-implant bone healing in osteoporotic rats. International Journal of Oral and Maxillofacial Surgery, 44, 798–805. [DOI] [PubMed] [Google Scholar]
- 24. Meixner C.N., Aref M.W., Gupta A., McNerny E.M.B., Brown D., Wallace J.M. and Allen M.R (2017) Raloxifene improves bone mechanical properties in mice previously treated with Zoledronate. Calcified Tissue International, 101, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamas A.Z., Caliman I.F., Dalpiaz P.L., de Melo A.F. Jr, Abreu G.R., Lemos E.M., Gouvea S.A. and Bissoli N.S (2015) Comparative effects of estrogen, raloxifene and tamoxifen on endothelial dysfunction, inflammatory markers and oxidative stress in ovariectomized rats. Life Sciences, 124, 101–109. [DOI] [PubMed] [Google Scholar]
- 26. Yogui F.C., Momesso G.A.C., Faverani L.P., Polo T.O.B., Ramalho-Ferreira G., Hassumi J.S., Rossi A.C., Freire A.R., Prado F.B. and Okamoto R (2018) A SERM increasing the expression of the osteoblastogenesis and mineralization-related proteins and improving quality of bone tissue in an experimental model of osteoporosis. Journal of Applied oral Science: Revista FOB, 26, e20170329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franzen T.J., Brudvik P. and Vandevska-Radunovic V (2013) Periodontal tissue reaction during orthodontic relapse in rat molars. European Journal of Orthodontics, 35, 152–159. [DOI] [PubMed] [Google Scholar]
- 28. Franzen T.J., Monjo M., Rubert M. and Vandevska-Radunovic V (2014) Expression of bone markers and micro-CT analysis of alveolar bone during orthodontic relapse. Orthodontics & Craniofacial Research, 17, 249–258. [DOI] [PubMed] [Google Scholar]
- 29. Han G., Chen Y., Hou J., Liu C., Chen C., Zhuang J. and Meng W (2010) Effects of simvastatin on relapse and remodeling of periodontal tissues after tooth movement in rats. American Journal of Orthodontics and Dentofacial Orthopedics, 138, 550.e1–550.e7; discussion 550. [DOI] [PubMed] [Google Scholar]
- 30. Hassan A.H., Al-Hubail A. and Al-Fraidi A.A (2010) Bone inductive proteins to enhance postorthodontic stability. The Angle Orthodontist, 80, 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y., Zhang T., Zhang C., Jin S.S., Yang R.L., Wang X.D., Jiang N., Gan Y.H., Kou X.X. and Zhou Y.H (2017) Aspirin blocks orthodontic relapse via inhibition of CD4+ T lymphocytes. Journal of Dental Research, 96, 586–594. [DOI] [PubMed] [Google Scholar]