Abstract
Objectives
To explore how alcohol affects the BMP-2 signaling pathway, which is known to play a critical role in bone and cartilage formation during fracture healing.
Methods
A rat model was used to demonstrate the detrimental effects of alcohol exposure on tibia fracture healing. Specific components of the BMP-2 pathway were analyzed in fracture callus at days 3, 7, 14, 21 post fracture via western immunoassays and ELISA.
Results
Alcohol exposure prior to tibia fracture demonstrated attenuation of downstream BMP-2 signaling. The BMP-2 antagonist, Chordin, may be the central component of the BMP-2 related changes demonstrated in this study. While alcohol affected BMP-related proteins at all time points, it appears day fourteen post fracture is a critical time point for alcohol-related modulation of callus formation in our model.
Conclusions;
This study may provide the scientific basis for further studies addressing whether the application of exogenous BMP-2 in patients with a history of alcohol abuse who sustain long bone fractures may or may not be of benefit.
Keywords: Bone Fracture, Fracture Nonunion, Alcohol, BMP-2, Tibia
INTRODUCTION
Alcohol abuse remains a significant health concern, with a reported 8.5% of US adults meeting the criteria for an alcohol use disorder (1). Episodic or binge alcohol consumption, which does not meet the criteria for an alcohol abuse disorder, is particularly prevalent amongst adolescent and young adults. Over 26% of the US population aged 18 or older reports binge drinking in the past month (2). Alcohol abuse has been associated with increased risk of osteoporosis and traumatic injury and thus is an important factor affecting many orthopaedic patients (3,4). Population studies have identified that binge drinking, is a risk factor for sustaining traumatic fractures (5). Alcohol abuse significantly increases the risk of developing fracture nonunion or delayed union (6,7, 8). A recent epidemiologic study by Zura et. al. found that diagnosed alcoholism increased the overall rate of fracture nonunion from 4.9 to 7.4 percent across all fracture types. (8). While nonunion rates are reported to be 4-10% across all fracture sites, the multifactorial nature of the problem can obscure the exact cause in individual patients (9,10). An understanding of the effect of alcohol consumption on the molecular and cellular signaling pathways which regulate fracture healing may offer insights into the etiology of impaired fracture healing.
Bone morphogenetic proteins (BMPs) have been shown to play an important role in bone formation and fracture healing (11, 12, 13, 14). BMP2 is used clinically as an adjunct to fracture healing (15, 16, 17). Recombinant human BMP2 (rhBMP2) is FDA-approved for clinical use, specifically, rhBMP2 has been approved for use in open tibia fractures fixed with intramedullary nails to reduce the nonunion rate of this injury (18), which can exceed 19% (40). While studies suggest that the use of BMP in the setting of an acute open fracture is safe and may aid in fracture repair, there is controversy concerning which fracture types (based on severity) would benefit most from the use of rhBMP2 (16, 19). An understanding of whether the pre-emptive use of rBMP2 may be beneficial in orthopaedic trauma patients with risk factors for poor fracture healing or nonunion would aid in the clinical management of these high-risk patients.
Prior studies have demonstrated a negative, dose-dependent relationship between alcohol exposure and fracture healing in rodents (4). Fracture healing involves a combination of intramembranous and endochondral bone formation (22). Local precursor cells from the periosteum contribute to intramembranous ossification and provide multipotent mesenchymal stem cells with chondro-osteo potential for endochondral-based bone repair. MSCs have been shown to express significant amounts of BMP2 when induced to undergo osteogenic differentiation (23, 24). BMP2 deficient mice are not able to heal fractures, even in the presence of osteogenic stimuli (25). Previous research from our laboratory has demonstrated that episodic alcohol exposure in mice prior to fracture injury resulted in both decreased fracture callus formation and callus biomechanical strength (21,25,39). Our laboratory has also demonstrated that BMP2 mRNA expression was decreased in intact bone from rats exposed to a chronic alcohol binge paradigm (20). Additionally, our laboratory has shown that alcohol exposure prior to fracture injury perturbs Canonical Wnt signaling activity during early fracture healing, which is critical for normal fracture repair (25, 27). Because of the importance of BMP2 signaling in fracture callus formation and because crosstalk occurs between the Canonical Wnt and BMP2 signaling pathways, investigating the relationship between alcohol exposure and BMP2 signaling was a logical step that has not been previously explored. The hypothesis of this study was that chronic binge alcohol exposure would attenuate BMP2 signaling and thus negatively impact fracture healing in a rat model of open tibia fracture injury.
MATERIALS AND METHODS
Chronic Binge Alcohol Model
This study was approved by the Loyola University Institutional Animal Care and Use Committee (IACUC). Fifty-six (56) Sprague Dawley rats (10 weeks of age) were obtained from Harlan Laboratories (Indianapolis, IN) and housed in an AALAC- approved facility. One week following their arrival, the 56 animals were randomly assigned to either the saline (28 animals) or alcohol treated group (28 animals), providing an N of 7 animals and time point studied in each group. Animals were weighed before the initiation of treatment and bi-weekly thereafter and allowed free access to both food and water throughout the study period. Alcohol administration was a once daily intra-peritoneal injection of a 20% (vol/vol) ethanol/saline solution at a dose of 3 g/kg, chosen to achieve blood alcohol levels (BAL) at the time of fracture injury of approximately 200 mg/dl (28), consistent with epidemiological data demonstrating that alcohol-intoxicated trauma patients arrive at the hospital with BAL’s averaging 150-mg/dl (29). Control animals were given an intra-peritoneal injection of an equal volume of sterile isotonic saline at the time of alcohol group injections. Injections were given for 3 consecutive days each week. No intra-peritoneal injections were given during the remaining 4 days of each week. This alcohol or saline treatment regimen was repeated for four consecutive weeks. Rats were then subjected to tibia fracture.
Stabilized Tibia Fracture Model
One hour after the administration of the final alcohol or saline injection, the animals were anesthetized with an induction dose of anesthesia (20mg/kg Ketamine, 2mg/kg xylazine) and prepped for surgery. A prophylactic dose of gentamicin (5mg/kg) was administered subcutaneously prior to the start of procedure. Animals were anesthetized completely by isoflurane inhalation. As depicted in Figure 1, under strict sterile technique a small incision was made to expose the patellar tendon and an entry hole was reamed with an 18-gauge needle through the proximal tibia at the anterior aspect of the tibial plateau to gain access into the medullary canal. An intramedullary pin (0.70mm) was then inserted into the tibia to provide stabilization. A hole was then created in the bone using a towel clip at the level of the mid-diaphysis to cause an area of weakness and a shaft fracture was then created manually using bone cutters. The intramedullary pin was cut flush and countersunk into the tibial plateau to avoid an intra-articular prominence. This was followed by closure of the wound with interrupted simple prolene suture. The animals received post-operative pain control in the form of an injectable sustained release narcotic (buprenorphine 1mg/kg), were placed in a clean cage, provided a heated environment during recovery, and allowed access to food and water. The animals were closely monitored until they regained normal activity levels.
Fig 1.
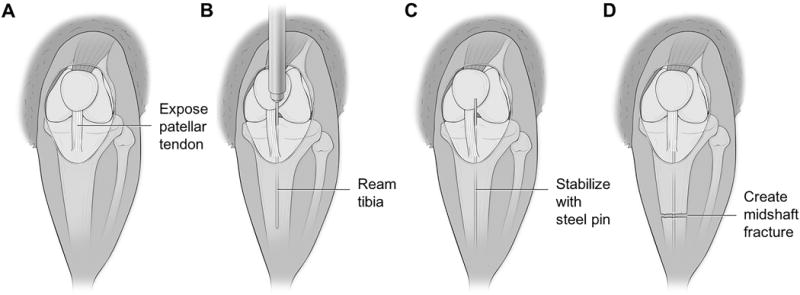
Anatomic illustrations depicting the surgical creation of the midshaft tibial fracture. (A) Illustration of right lower extremity of Sprague Dawley rat. A small midline incision was made through the skin distal to the knee joint. Skin was manually retracted towards the knee joint, exposing the patellar tendon. (B) Patellar tendon was displaced medially using forceps to expose tibial plateau. A 20gauge needle was used to ream through the tibial plateau and into the intramedullary canal. Needle was reamed distally through the canal for the entire length of the needle. (C) Reaming needle removed from canal and replaced with steel insect pin. The insect pin was inserted to the level where resistance was met. (D) Skin retracted distally to expose midshaft of tibia. Utilizing small bone cutters, a fracture was created through both cortices of the diaphyseal bone, however care was taken not to damage the intramedullary stabilizing pin. Skin again retracted proximally so the steel pin could be trimmed flush with the tibial plateau and clear from the knee joint.
Specimen Processing and Protein Isolation
Fracture tibiae from both experimental and control groups were harvested from humanely euthanized animals on post fracture days 3, 7, 14, and 21. Tibiae were flash frozen in liquid nitrogen and stored at -80° C. On a dry ice slab, the fracture callus was isolated with a cut proximal and distal to the fracture site using a Dremel tool (Dremel Inc., Racine, WI) The fracture callus was then pulverized in 3ml of lysis buffer (RIPA buffer, Halt phosphatase inhibitor cocktail (ThermoFisher Scientific, Waltham, MA), Complete mini protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO)) using a Freezer Mill 6770 (SPEX SamplePrep LLC, Metuchen, NJ). Samples were kept frozen in liquid nitrogen throughout the process. Total protein concentrations were measured using a Pierce BCA Protein Assay kit (ThermoFisher Scientific).
BMP Signaling Assays
Protein expressions of BMP2, BMPRII, BMPR1A, and the inhibitor Chordin were all assessed via the following commercially available ELISA kits: BMP2 (Boster Biological Technology, EKO312, Pleasanton, CA); BMPRII (Mybiosource, MBS701412, San Diego, CA); BMPRIA (Mybiosource, MBS720872); Chordin (Mybiosource, MBS2021328). Assays were run according to manufacturer specifications.
Immunoblots were used to assess protein concentrations of BMP2, Smad 4, and Phospho-Smad1/5/9. Fifteen (15) ug of total protein from each sample was resolved on 4-20% mini-protean TGX stain-free precast gels (Bio-Rad, Hercules, CA) After electrophoresis, gels were activated using Chemi doc, stain free gel protocol (Bio-Rad). Proteins were transferred to PVDF membranes, and total protein was determined via Chemi doc, stain free gel protocol. Probing of the blots was completed using I-bind Western Systems (ThermoFisher Scientific) with the following antibodies: BMP2 rb pAb (ab14933) (Abcam, Cambridge, MA), SMAD4 (#9515) (Cell Signaling, Danvers, MA), Phospho-Smad1/Smad5/Smad9 (#13820) (Cell Signaling) Visualization of bound antibodies was possible via secondary goat polyclonal antibody to rabbit IgG HRP (ab6721) (Abcam) and developed with SuperSignal West Pico chemiluminescent substrate (ThermoFisher Scientific). The blot was imaged with Chemi doc, Chemi-Hi sensitivity protocol. To ensure the equal loading of protein, blot signal volumes were normalized against total protein concentrations using Image lab software (Bio-Rad). Data was reported as a ratio of average signal volumes between the control and experimental groups.
Data Analysis
Statistical analysis was completed utilizing independent sample T-tests between control and experimental groups at individual time points. We did not examine statistical differences between the four time points. Confirmation w/Wilcoxon signed-rank test showed no difference amongst “P” values. Cut off for statistical significance was P <0.05 Values reported as mean ± SEM.
RESULTS
BMP2 Protein Analysis
Our study systematically analyzed the effects of episodic alcohol treatment on various components of the BMP signaling pathway, (Figure 2). The average BMP2 protein level in fracture callus from alcohol-treated rats was 257.13 pg/ml ± 12.71 (n=7), which was significantly higher than BMP2 levels in saline-treated rats (190.38 pg/ml ± 13.58 (n=5) day 14 post-fracture (p = 0.0055) (Figure 3B). No significant difference in fracture callus-associated BMP2 levels were observed between saline control and alcohol-treated animals at days 3, 7 or 21 post fracture. The significant increase in fracture callus-associated BMP2 protein levels observed in alcohol-treated rats at post-fracture day 14 was confirmed by Western Blot analysis. (N=7, p=.042) (Figure 3A).
Fig 2.
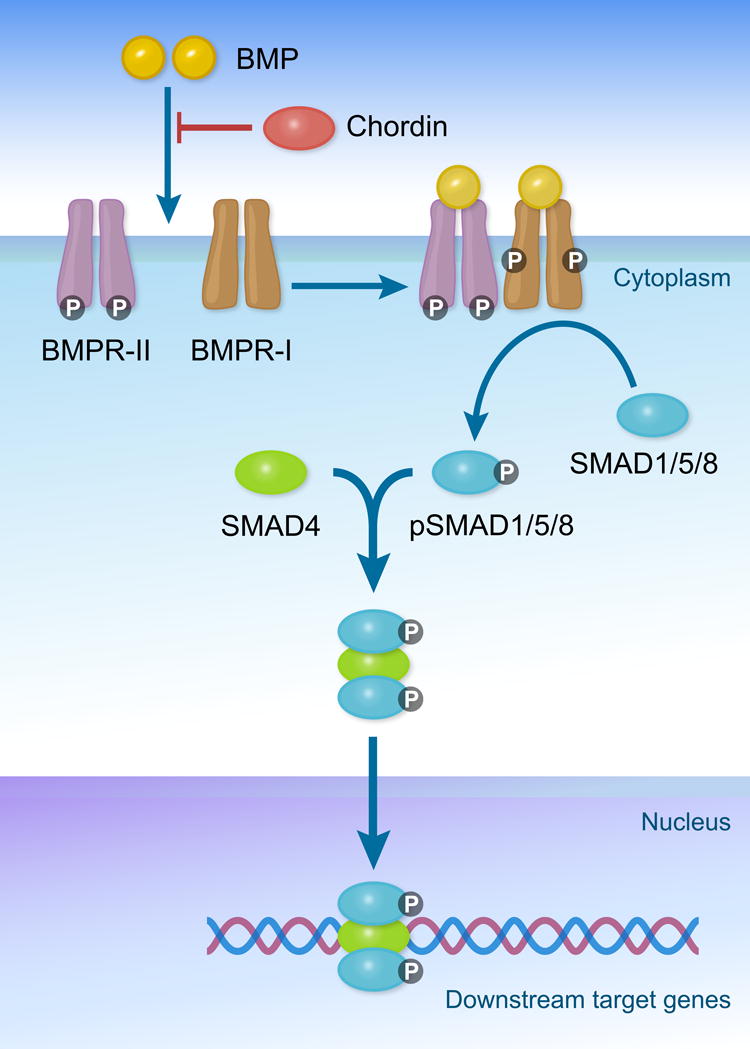
Illustration of selective components in the BMP signaling pathway, important for bone and cartilage formation and fracture healing. Bone morphogenetic proteins (BMP) induce intracellular signaling by binding the transmembrane receptors BMPR-II and BMPR-I. When these serine/threonine receptors are activated by BMPs, BMPR-I becomes phosphorylated. When both BMPR-II and BMPR-I are bound to BMPs and phosphorylated, downstream signaling is activated via the phosphorylation of transcription factors SMAD 1/5/8. Activated pSMAD 1/5/8 forms a complex with cofactor SMAD4, allowing for the regulated transcription of downstream target genes.
Fig 3.
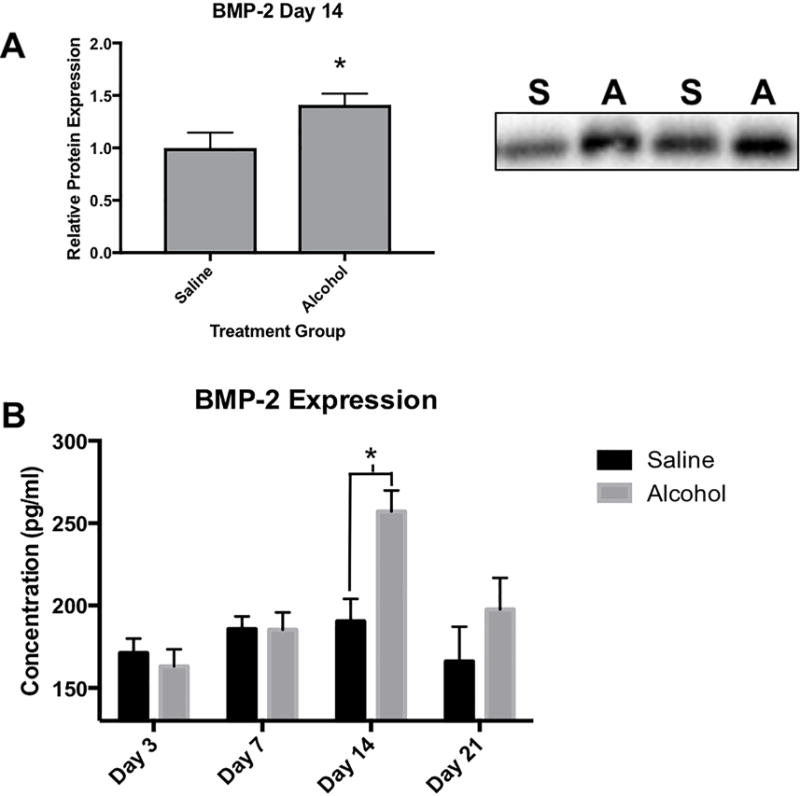
Effects of binge alcohol on BMP-2 protein expression in fracture callus. (A) Representative western blot for BMP-2 protein levels in fracture callus tissue homogenate at post fracture day 14. Saline samples labeled (S) and alcohol samples labeled (A). To ensure the equal loading of protein, blot signal volumes were normalized against total protein concentrations. Western data was reported as a ratio of average signal volumes between the control and experimental groups. Alcohol causes a significant increase in BMP-2 expression at post fracture day 14 as seen in the western blot data (p=.042), as well as in the ELISA immune assay (p=.0055) (B) Statistical significance was present when p <0.05 as determined by independent sample T-tests and displayed with (*).
BMP Receptor 2 and BMP Receptor 1A Analysis
BMP receptor 2 protein expression in callus samples was measured by ELISA. We found a statistically significant increase in in BMP receptor 2 expression in callus tissue from alcohol treated rats at day 14 post fracture (91.26 pg/ml ± 4.76, n=7) compared to expression in saline treated animals (50.81 pg/ml ± 7.30, n=5, p = 0.0007) (Figure 4A). No significant differences in BMP receptor 2 expression between saline control and alcohol-treated animals were seen at 3, 7 or 21-days post fracture. BMP receptor 1A protein expression was found to be significantly decreased in callus from alcohol treated rats when analyzed via ELISA, with an average of 6791.71 pg/ml ± 292.42 (n=7) compared to an average of 8425.30 pg/ml ± 436.38 (n=7) in callus from saline-treated rats at day 14 (p = 0.009) (Figure 4B). No significant differences in callus-associated BMP receptor 1A expression were observed between saline control and alcohol–treated animals at 3, 7 or 21 days post-fracture.
Fig 4.
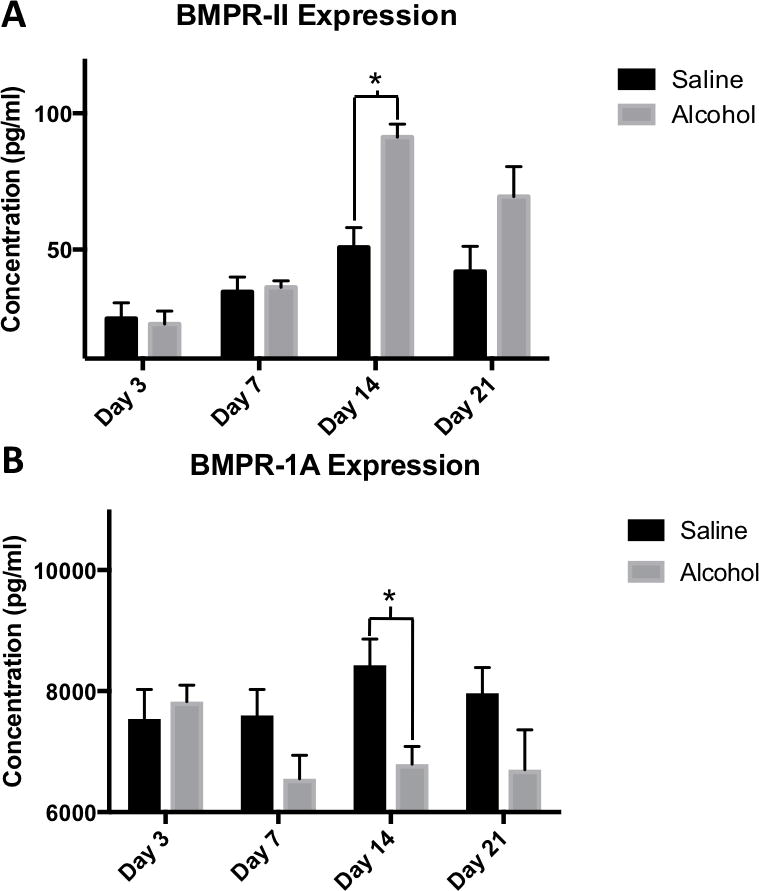
(A-B) Binge alcohol effects on BMPR-II and BMPR-1A protein expression in fracture callus. ELISA data of BMPR-II and BMPR-1A protein expression assessed at post fracture days 3, 7, 14 and 21. (A) Alcohol was shown to cause a significant increase in BMPR-II at day 14 (p<.001). (B) Alcohol was shown to cause a significant decrease in BMPR-1A at day 14 (p=.009). Statistical significance was present when p <0.05 as determined by independent sample T-tests and displayed with (*).
Chordin, SMAD4 and pSMAD 1,5,9 Analysis
Protein expression of the BMP2 antagonist Chordin in the fracture callus was determined by ELISA and found to be significantly increased at post-fracture days 3, 7, 14 and 21 in alcohol treated rats compared with saline-treated rats at the same time points post-fracture (Figure 5A). The largest difference in callus-associated chordin expression between alcohol and saline treated control rats was observed at post-fracture day 14 (Alcohol n=7 avg. 27413.63 pg/ml ± 1900.21, versus Saline n=7 avg. 9981.50 pg/ml ± 3455.46, p = 0.0008). Protein expression levels of callus-associated SMAD4, a downstream BMP2 signaling protein, were analyzed via western blot immunoassay, and found to be significantly increased at post-fracture day 14 in callus from the alcohol treated rats compared to callus from saline-treated control animal (n=7, p = 0.003) (Figure 5B). Protein expression of the BMP2 signaling protein, phosphorylated-SMAD 1,5,9, was determined via western blot immunoassay. A trend towards a decrease in pSMAD 1,5,9 in callus tissue from alcohol-treated rats was observed at post-fracture day 14 compared to callus from saline-treated rats at post-fracture day 14, however this difference did not reach statistical significance (p = 0.073) (Figure 5C).
Fig 5.
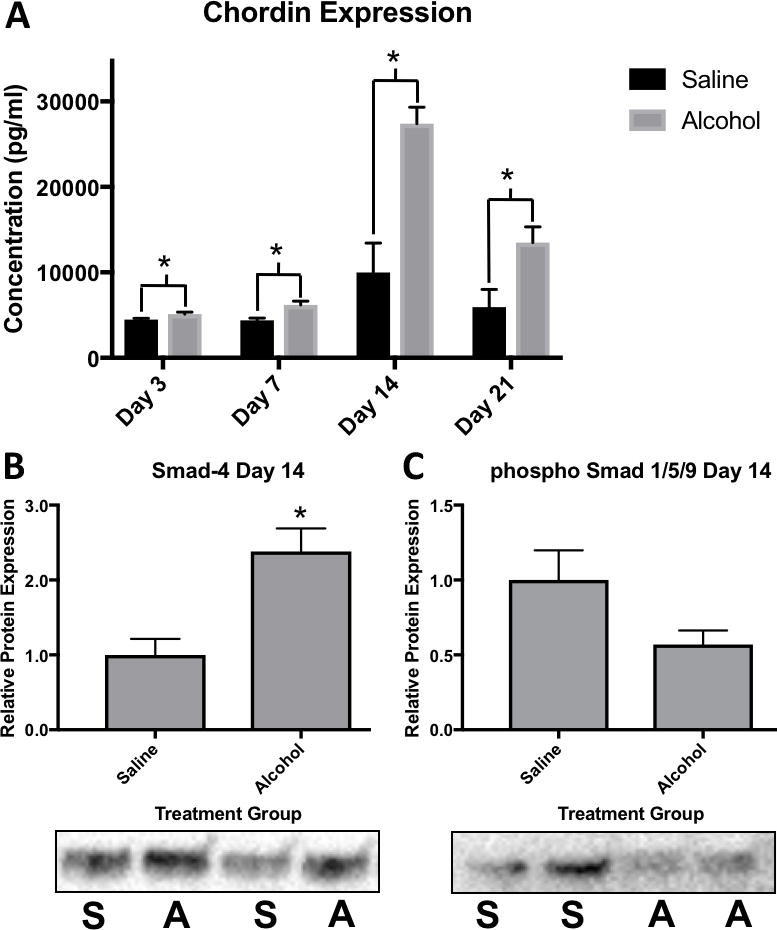
(A) Binge alcohol effects on Chordin protein expression in fracture callus. ELISA data of Chordin protein expression assessed at post fracture days 3, 7 14, and 21. Alcohol was shown to cause a significant increase in Chordin expression at all time points with the greatest difference occurring at post fracture day 14 (p<.001). (B-C) Effects of binge alcohol on SMAD4 and activated phospho Smad 1/5/9 protein expression in fracture callus. Representative western blot for SMAD4 and phospho Smad 1/5/9 protein levels in fracture callus tissue homogenate at post fracture day 14. Saline samples labeled (S) and alcohol samples labeled (A). To ensure the equal loading of protein, blot signal volumes were normalized against total protein concentrations. Western data was reported as a ratio of average signal volumes between the control and experimental groups. (B) Alcohol causes a significant increase in SMAD4 expression at post fracture day 14 as seen in the western blot data (p=.003). (C) Alcohol was shown to attenuate phospho Smad 1/5/9 expression at day 14, however this was not found to be significant (p=.08). Statistical significance was present when p <0.05 as determined by independent sample T-tests and displayed with (*).
DISCUSSION
Alcohol consumption is a significant contributing factor in over 40% of orthopaedic trauma cases and has been shown to increase the risk of fracture non-union (8, 30). As a result, the dangers associated with alcohol abuse and orthopaedic injuries are multifaceted; increasing the risk of injury and clinical complications, such as nonunion, which may necessitate multiple surgical procedures, increasing health-care costs, and negatively affecting the patient’s quality of life. The current options for treating fracture nonunion, including autogenous or allogenic bone graft, each have serious limitations. Thus, understanding the causes of nonunion as well as the molecular and cellular mechanisms which underlie the condition may lead to non-surgical or adjunct therapeutic strategies for treating non-healing fractures. As rBMP2 therapy is currently being utilized clinically as an adjunct to surgical intervention for types of fracture injuries with higher risks of delayed healing or nonunion, (such as open tibia fractures) the current study examined the effects of episodic alcohol treatment on fracture callus-associated BMP2 signaling during critical periods of fracture callus formation in a rat model of open tibia fracture injury. We found that episodic alcohol treatment perturbed callus-associated BMP2 signaling, suggesting that altered BMP2 signaling could underlie alcohol consumption-associated defects in fracture repair, and that exogenous rBMP2 therapy may have limited efficacy in alcohol abusing patients with orthopaedic injuries which are generally amenable to BMP2 treatment.
The effect of alcohol on both intact and fractured bone is well documented. The bone stiffness, strength, and energy required to sustain fracture is lower in alcohol treated animals compared to controls (31). Tibia cancellous bone mineral density as well as compressive strength are reduced with binge alcohol use (20). Both cortical and trabecular bone parameters are negatively affected in alcohol treated animals (41) and studies suggest that these bone changes are the result of alcohol-related effects on both osteoblastic and osteoclastic activity (32, 33, 34). As a result, fractures sustained with the same amount of impact energy may be more severe and comminuted in patients with alcohol use disorders.
As BMPs are known to play an important role in the formation of cartilage, bone, and during fracture healing (12, 13), their specific role in alcohol-related deficient fracture healing is of importance. Our lab previously has demonstrated that in alcohol treated animals, intact vertebrae had decreased BMP2 and BMP receptor II gene expression compared to control (20). In contrast, our current study found an unexpected increase in both BMP2 and the BMP receptor II protein levels in fracture callus samples from alcohol-treated rats at day 14 post-fracture. However, the finding that protein levels of the BMP2 inhibitor Chordin were significantly increased across all time points studied in callus samples from alcohol treated rats, suggests that the increases observed at post-fracture day 14 in both BMP2 and BMPR2 in alcohol-treated callus tissue may be a compensatory mechanism for an overall decrease in BMP2 signaling activity during fracture healing in alcohol-treated rats. A prior study by Kloen et al. demonstrated a general increase in BMP-inhibitors such as Chordin in tissue from nonunions compared to normal fracture callus (35), suggesting that perturbations in the balance of BMP and BMP-inhibitor expression may predispose a fracture towards nonunion and strongly suggests that the upregulation of Chordin expression we observed in alcohol-treated animals may be related to the deleterious effects of alcohol on the fracture healing process previously identified in rodent models (21,25,39) The effects of alcohol treatment on Chordin expression during the fracture repair process have not been previously reported and may represent a novel mechanistic underpinning for alcoholic nonunion.
We observed a significant decrease in BMP receptor 1A (BMPR1A) expression in the callus of alcohol treated mice at post-fracture day 14. BMPR1A is responsible for phosphorylation and activation of downstream SMAD proteins, suggesting that in addition to upregulating Chordin expression, alcohol may also attenuate BMP signaling during fracture healing through downregulation of SMAD activity. A prior study by Morgan et. al, demonstrated that pharmacological inhibition of BMPR1A signaling post-fracture resulted in impaired fracture healing, demonstrated by deficits in callus torsional strength and toughness (36), illustrating the important role of BMPR1A as a modulator of downstream BMP signaling and fracture healing. Since BMP2 downstream signaling is mediated through phosphorylation of SMAD 1,5,9 it was anticipated that we would see a significant decrease in SMAD phosphorylation. SMAD 1,5,9 was decreased in callus from alcohol treated rats at post fracture day 14, although this change was not found to be significantly different. A similar alcohol effect was observed in a study by Gerjevic et. al, who showed a decrease in phosphorylated SMAD1 and SMAD5 in hepatocytes of mice subjected to an alcoholic diet (37). Similar to our study, these results were not statistically significant but the trend suggests that overall BMP signaling in callus tissue may be decreased by alcohol.
Prior studies have also demonstrated the deleterious effects of alcohol on fracture healing in animal models. Sampson et al. looked specifically at gene expression during the inflammatory stages of fracture healing and found that numerous genes were either increased or decreased with the addition of alcohol (38). Interestingly, in their study SMAD4 gene expression was found to be decreased however their analysis was performed at 3 days’ post-fracture. In the current study, we found SMAD4 protein levels to be increased in the alcohol treated group at day 14 post-fracture. As noted above, cellular pathways often work on self-regulatory feedback, thus the increases observed in SMAD4, BMP2, and BMP receptor 2 levels in callus from the alcohol group at post fracture day 14 may be compensatory changes secondary to increased Chordin levels and an overall decrease in downstream BMP2 signaling activity. Our study also suggests that while BMP2 signaling-related proteins levels were affected by alcohol treatment across the time points, the fourteenth day after fracture appears to be a crucial time point for alcohol-related modulation of callus formation in our model.
The clinical significance of our study is it offers insights into both BMP2 signaling and effects of episodic alcohol consumption in the setting of a stabilized long bone fracture, providing a rationale for further studies addressing whether the exogenous application of BMP2 in patients with a history of alcohol abuse who sustain long bone fractures may or may not be of benefit. We may speculate that use of rhBMP2 in the alcoholic patient at risk of nonunion may be less effective than in a non-drinking patient, given the elevated levels of Chordin we observed in this study, and that alternative treatments regimens may be required in this patient population. Further studies investigating this topic are clearly warranted given the ubiquitous use of BMP2 as an adjunct to fracture healing.
Limitations to the current study include that it is a rodent-based study, and that we do not evaluate the effect of alcohol on crosstalk between BMP2 and other signaling pathways involved in fracture healing. Given the importance of BMP2 signaling in fracture healing, the alcohol-related changes we observe in BMP2 and downstream signaling in rats during fracture healing are likely also present in the fracture patient population that consumes excessive alcohol. Effects of alcohol on other cellular pathways which interact with BMP signaling and are important for fracture repair such as Canonical Wnt (25, 27) and FoxO (39) signaling are currently under investigation in our laboratory.
In summary, the current work adds new information on the molecular effects of alcohol on the fracture healing process, and provides a direction for future research on mechanisms responsible for alcohol abuse-related fracture nonunion.
Acknowledgments
This research was supported by funding from: NIH/NIAAA (R21AA021225) to JJC, and an Orthopaedic Trauma Association resident research grant to AB. We would like to thank Pegah Abbasnia, DVM, PhD, James Roemer, BS, and Loyola University Chicago Department of Orthopaedics for their assistance on various aspects of this project.
All listed authors contributed significantly to this study. A. Bratton received a resident research grant from the OTA, which partially funded the study and was involved in experimental design, animal care, surgeries and the writing of the manuscript. J. Eisenberg was involved in experimental design, animal surgeries, data collection, analysis and writing of the manuscript. A. Vuchkovska and P. Roper contributed to experimental design and animal surgeries. J. Callaci received NIH funding for the study and contributed to all aspects of the project. All authors have carefully read and approved the final written manuscript.
Footnotes
Presented in part at the Annual Meeting Orthopedic Research Society 2016.
References
- 1.Falk D, Yi H-y, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and drug use and disorders: findings from the national epidemiologic survey of alcohol and related conditions (NESARC) Alcohol Research & Health. 2008;31(2):100–110. 2008. [PMC free article] [PubMed] [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey on Drug Use and Health (NSDUH) 2015 Table 2.46B—Alcohol Use, Binge Alcohol Use, and Heavy Alcohol Use in Past Month among Persons Aged 12 or Older, by Demographic Characteristics: Percentages, 2014 and 2015. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-46b.
- 3.Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24:1693–1701. [PubMed] [Google Scholar]
- 4.Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077–2090. doi: 10.1097/01.alc.0000192039.21305.55. [DOI] [PubMed] [Google Scholar]
- 5.Marley W, Kelly G, Thompson N. Alcohol-related fracture admissions: a retrospective observational study. Ulster Med J. 2015;84(2):94–97. [PMC free article] [PubMed] [Google Scholar]
- 6.Duckworth AD, Bennet SJ, Aderinto J, et al. Fixation of intracapsular fractures of the femoral neck in young patients: risk factors for failure. J Bone Joint Surg Br. 2011;93:811–816. doi: 10.1302/0301-620X.93B6.26432. [DOI] [PubMed] [Google Scholar]
- 7.Williams G, Daly M, Proude EM, et al. The influence of alcohol and tobacco use in orthopaedic inpatients on complications of surgery. Drug & Alcohol Revs. 2008;27:55–64. doi: 10.1080/09595230701711108. [DOI] [PubMed] [Google Scholar]
- 8.Zura R, Xiong Z, Einhorn T, et al. Epidemiology of fracture nonunion in 18 human bones. AMA Surg. 2016;151(11):e162775. doi: 10.1001/jamasurg.2016.2775. [DOI] [PubMed] [Google Scholar]
- 9.Littenberg B, Weinstein LP, McCarren M, et al. Closed fractures of the tibial shaft: a meta-analysis of three methods of treatment. J Bone Joint Surg Am. 1998;80(2):174–83. doi: 10.2106/00004623-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83(Suppl 1 Pt 2):S151–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocrine reviews. 2003;24(2):218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 12.Urist MR, Silverman BF, DÜRING K, et al. 24 The Bone Induction Principle. Clin Orthop Relat Res. 1967;53:243–284. [PubMed] [Google Scholar]
- 13.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi A, Ishizuya T, Kintou N, et al. Effects of BMP-2, BMP-4, and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochem Biophys Res Commun. 1996;220(2):366–71. doi: 10.1006/bbrc.1996.0411. [DOI] [PubMed] [Google Scholar]
- 15.Einhorn TA, Majeska RJ, Mohaideen A, et al. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85-A:1425–35. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Garrison KR, Shemilt I, Donell S, et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;(6):CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures - A prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84(12):2123–34. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 18.http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm081154.htm
- 19.Nauth A, Ristiniemi J, McKee MD, et al. Bone morphogenetic proteins in open fractures: past, present, and future. Injury. 2009;40(Suppl 3):S27–S31. doi: 10.1016/S0020-1383(09)70008-7. [DOI] [PubMed] [Google Scholar]
- 20.Callaci JJ, Himes R, Lauing K, et al. Binge alcohol-induced bone damage is accompanied by differential expression of bone remodeling-related genes in rat vertebral bone. Calcif Tissue Int. 2009;84:474–484. doi: 10.1007/s00223-009-9240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obermeyer T, Yonick D, Lauing K, et al. Mesenchymal Stem Cells Facilitate Fracture Repair in an Alcohol-Induced Impaired Healing Model. J Orthop Trauma. 2012;26(12):712–718. doi: 10.1097/BOT.0b013e3182724298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi LX, Simmons DJ, Mainous E. Expression of BMP-2 by rat bone marrow stromal cells in culture. Calcif Tissue Int. 1999;64(1):63–68. doi: 10.1007/s002239900580. [DOI] [PubMed] [Google Scholar]
- 23.Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2(1):81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Karsenty G. Transcription factors in bone: developmental and pathological aspects. Trends Mol Med. 2002;8(7):340–5. doi: 10.1016/s1471-4914(02)02340-7. [DOI] [PubMed] [Google Scholar]
- 25.Lauing KL, Roper PM, Nauer RK, et al. Acute alcohol exposure impairs fracture healing and deregulates β‐catenin signaling in the fracture callus. Alcohol Clin Exp Res. 2012;36(12):2095–103. doi: 10.1111/j.1530-0277.2012.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sears BW, Volkmer D, Yong S, et al. Binge alcohol exposure modulates rodent expression of biomarkers of the immunoinflammatory response to orthopaedic trauma. J Bone Joint Surg Am. 2011;93(8):739–749. doi: 10.2106/JBJS.J.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauing KL, Sundaramurthy S, Nauer RK, Callaci JJ. Exogenous activation of Wnt/β-catenin signaling attenuates binge alcohol-induced deficient bone fracture healing. Alcohol Alcohol. 2014;49(4):399–408. doi: 10.1093/alcalc/agu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nation JR, Burkey RT, Grover CA. Lead/ethanol interactions. II: pharmacokinetics. Alcohol. 1993;10:363–367. doi: 10.1016/0741-8329(93)90021-f. [DOI] [PubMed] [Google Scholar]
- 29.Savola O, Niemela O, Hillbom M. Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol. 2005;40(4):269–73. doi: 10.1093/alcalc/agh159. [DOI] [PubMed] [Google Scholar]
- 30.Levy RS, Hebert CK, Munn BG, et al. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10:21–27. doi: 10.1097/00005131-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Sampson HW, Chaffin C, Lange J, et al. Alcohol Consumption by Young Actively Growing Rats: A Histomorphometric Study of Cancellous Bone. Alcohol Clin Exp Res. 1997;2:352–9. [PubMed] [Google Scholar]
- 32.Hogan HA, Sampson HW, Cashier E, et al. Alcohol Consumption by Young Actively Growing Rats: A Study of Cortical Bone Histomorphometry and Mechanical Properties. Alcohol Clin Exp Res. 1997;21(5):809–16. [PubMed] [Google Scholar]
- 33.Ronis MJJ, Mercer K, Chen JR. Effects of Nutrition and Alcohol Consumption on Bone Loss. Curr Osteoporos Rep. 2011;9(2):53–59. doi: 10.1007/s11914-011-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J-R, Lazarenko OP, Shankar K, et al. Inhibition of NADPH Oxidases Prevents Chronic Ethanol-Induced Bone Loss in Female Rats. J Pharmacol Exp Ther. 2011;336(3):734–742. doi: 10.1124/jpet.110.175091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kloen P, Lauzier D, Hamdy RC. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone. 2012;51(1):59–68. doi: 10.1016/j.bone.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Morgan EF, Pittman J, DeGiacomo A, et al. BMPR1A antagonist differentially affects cartilage and bone formation during fracture healing. J Orthop Res. 2016;34(12):2096–2105. doi: 10.1002/jor.23233. [DOI] [PubMed] [Google Scholar]
- 37.Gerjevic LN, Liu N, Lu S, et al. Alcohol activates TGF-beta but inhibits BMP receptor-mediated Smad signaling and Smad4 binding to hepcidin promoter in the liver. Int J Hepatol. 2011;2012:459278. doi: 10.1155/2012/459278. Epub 2011 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampson HW, Chaput CD, Brannen J, et al. Alcohol induced epigenetic perturbations during the inflammatory stage of fracture healing. Exp Biol Med. 2011;236(12):1389–1401. doi: 10.1258/ebm.2011.011207. [DOI] [PubMed] [Google Scholar]
- 39.Roper PM, Abbasnia P, Vuchkovska A, Natoli RM, Callaci JJ. Alcohol-related deficient fracture healing is associated with activation of FoxO transcription factors in mice. J Orthop Res. 2016;34(12):2106–2115. doi: 10.1002/jor.23235. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hak DJ. Management of aseptic tibial nonunion. The Journal of the American Academy of Orthopaedic Surgeons. 2011;19(9):563–73. doi: 10.5435/00124635-201109000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Alund AW, Mercer KE, Suva LJ, Pulliam CF, Chen JR, Badger TM, Van Remmen H, Ronis MJ. Reactive Oxygen Species Differentially Regulate Bone Turnover in an Age-Specific Manner in Catalase Transgenic Female Mice. J Pharmacol Exp Ther. 2016;358(1):50–60. doi: 10.1124/jpet.116.233213. [DOI] [PMC free article] [PubMed] [Google Scholar]


