SUMMARY
Checkpoint immunotherapy unleashes T cell control of tumors, but is undermined by immunosuppressive myeloid cells. TREM2 is a myeloid receptor that transmits intracellular signals that sustain microglial responses during Alzheimer’s disease. TREM2 is also expressed by tumor-infiltrating macrophages. Here, we found that Trem2−/− mice are more resistant to growth of various cancers than wild-type mice and are more responsive to anti-PD-1 immunotherapy. Furthermore, treatment with anti-TREM2 mAb curbed tumor growth and fostered regression when combined with anti-PD-1. scRNA-seq revealed that both TREM2 deletion and anti-TREM2 are associated with scant MRC1+ and CX3CR1+ macrophages in the tumor infiltrate, paralleled by expansion of myeloid subsets expressing immunostimulatory molecules that promote improved T cell responses. TREM2 was expressed in tumor macrophages in over 200 human cancer cases and inversely correlated with prolonged survival for two types of cancer. Thus, TREM2 might be targeted to modify tumor myeloid infiltrates and augment checkpoint immunotherapy.
In Brief
TREM2 is a pro-tumorigenic marker of tumor-infiltrating macrophages in mouse models and human tumors that can be targeted to curb tumor growth and improve the efficacy of checkpoint blockade therapy while remodeling the landscape of tumor-infiltrating macrophages.
Graphical Abstract

INTRODUCTION
The immune system plays an important protective function against tumor development and progression, effectively eliminating immunogenic cancer cells (Schreiber et al., 2011). To escape immunosurveillance, cancer cells muffle their immunogenic features and induce a microenvironment that actively suppresses immune responses. Suppressive mechanisms directly affect T cell responses by engaging immune checkpoints such as cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and programmed death-1 (PD-1) (Freeman et al., 2000; Leach et al., 1996). Tumors also coopt myeloid cells to actively suppress anti-tumor T cell responses. Myeloid cells constitute a significant cellular fraction of the microenvironment of many tumors and have been shown to inhibit T cell responses through multiple mechanisms (Mantovani et al., 2017). Although collectively considered suppressor cells, recent high-dimensional profiling studies have shown that tumor-infiltrating myeloid cells are considerably heterogeneous and may in fact include both immunostimulatory and immunosuppressive subsets (Broz and Krummel, 2015; Cassetta and Pollard, 2018; Gubin et al., 2018; Lavin et al., 2017). Immunostimulatory myeloid cells include type 1 dendritic cells (DC1s) and M1-like IFN-γ-induced macrophages; suppressive myeloid cells include M2-like macrophages, as well as a heterogeneous group of myeloid progenitor cells and immature myeloid cells collectively defined as myeloid-derived suppressor cells (MDSCs) (Veglia et al., 2018). Thus, depletion of suppressive myeloid cells from tumors, blockade of their functions, or induction of myeloid cells with immunostimulatory properties may constitute important approaches for improving immunotherapy strategies, perhaps in synergy with checkpoint blockade (Elinav et al., 2013).
Recently, attention has been focused on unique subsets of macrophages expressing the cell surface receptor, TREM2. TREM2 is an activating receptor of the Ig-superfamily that binds lipids and transmits intracellular signals through the adaptor DAP12 (Peng et al., 2010; Ulland et al., 2017). DAP12 recruits the protein tyrosine kinase Syk, which initiates a cascade of tyrosine phosphorylation events that activate downstream mediators such as PLCγ2, PI-3K, Vav, mTOR, and MAPK, ultimately leading to cell activation (Peng et al., 2010; Ulland et al., 2017). Although TREM2 is expressed on the cell surface, it is cleaved from the cell surface by metalloproteases and released as soluble TREM2 (sTREM2) (Ulland and Colonna, 2018). TREM2 cleavage may regulate cell activation; moreover, soluble TREM2 has been proposed to promote survival of neighboring cells (Zhong et al., 2017).
TREM2 has been extensively studied in microglia for its capacity to sustain microglial responses to neurodegenerative pathologies such as Alzheimer’s disease (Ulland and Colonna, 2018). However, TREM2 is also expressed in several peripheral macrophage populations involved in host defense and metabolism. During lung acute viral infection, TREM2+ macrophages release sTREM2, which inhibits apoptosis of macrophages, causing a feed-forward expansion of lung macrophages that converts acute infection into a chronic inflammatory disease (Wu et al., 2015). In the adipose tissue, TREM2 sustains the presence of a population of lipid-associated macrophages (LAMs) that prevent the dysmetabolism engendered by a high-fat diet (Jaitin et al., 2019). In atherosclerosis, TREM2+ macrophages are enriched in atherosclerotic lesions and specialize in lipid catabolism (Cochain et al., 2018). In the liver, a TREM2+CD9+ subset of macrophages that differentiate from circulating monocytes expands during liver cirrhosis and contributes to fibrosis (Ramachandran et al., 2019). In the skin, TREM2+ dermal macrophages secrete oncostatin M, which inhibits hair growth by maintaining hair follicle stem cells in a quiescent state (Wang et al., 2019). TREM2+ macrophages have also been reported in tumors (Lavin et al., 2017; Song et al., 2019), but the impact of TREM2 in tumor immune responses has not been addressed.
Here, we found that Trem2−/− mice are more resistant to tumor growth than wild-type (WT) mice using 3-methylcholanthrene (MCA)-induced sarcoma, colorectal cancer, and mammary tumor models. TREM2 deficiency was associated with alterations in macrophage subsets and an increase of intratumoral CD8+ T cells, some of which expressed PD-1. This observation prompted us to ask whether TREM2 blockade can enhance antitumor responses mediated by checkpoint immunotherapy. First, we showed that anti-PD-1 immunotherapy is more effective in TREM2-deficient than WT tumor-bearing mice. Moreover, administration of an Fc-mutated anti-TREM2 monoclonal antibody (mAb) to tumor-bearing mice blunted tumor growth and strongly enhanced the efficacy of anti-PD-1 immunotherapy. Analysis of the myeloid cell landscape by single-cell RNA sequencing (scRNA-seq) showed that both TREM2 deficiency and anti-TREM2 mAb treatment triggered marked changes in the macrophage populations infiltrating the tumor: CX3CR1+ and MRC1+ macrophage subsets declined, while novel subsets expressing potentially immunostimulatory molecules were induced. In parallel with the mouse data, we found that TREM2 is a marker of infiltrating macrophages in over 200 human tumors examined by immunohistochemistry (IHC). Moreover, TREM2 expression inversely correlated with greater overall survival and relapse-free survival in colorectal carcinoma (CRC) and triple-negative breast cancer (TNBC). We conclude that reshaping of tumor-associated macrophages by anti-TREM2 mAb is a promising avenue for complementing checkpoint immunotherapy.
RESULTS
TREM2 Deficiency Delays Growth of Transplanted Tumors and Modifies Tumor-Immune Infiltrate
To address the potential impact of TREM2 on immune responses to tumors, we chose mouse tumor models known to be associated with a microenvironment infiltrated by macrophages expressing TREM2. We first analyzed an MCA-induced sarcoma cell line (MCA/1956) in WT and Trem2−/− mice. MCA/1956 belongs to a panel of MCA-induced sarcomas known as progressors. Since these tumors were developed in immunocompetent WT mice, their immunogenic profiles were edited by the immune system, and hence, they grow unopposed when transplanted into naive syngeneic WT hosts (Alspach et al., 2019; Schreiber et al., 2011; Shankaran et al., 2001). These tumors have recently been shown to be infiltrated by a variety of macrophage subsets (Gubin et al., 2018), many of which express TREM2 (unpublished data).
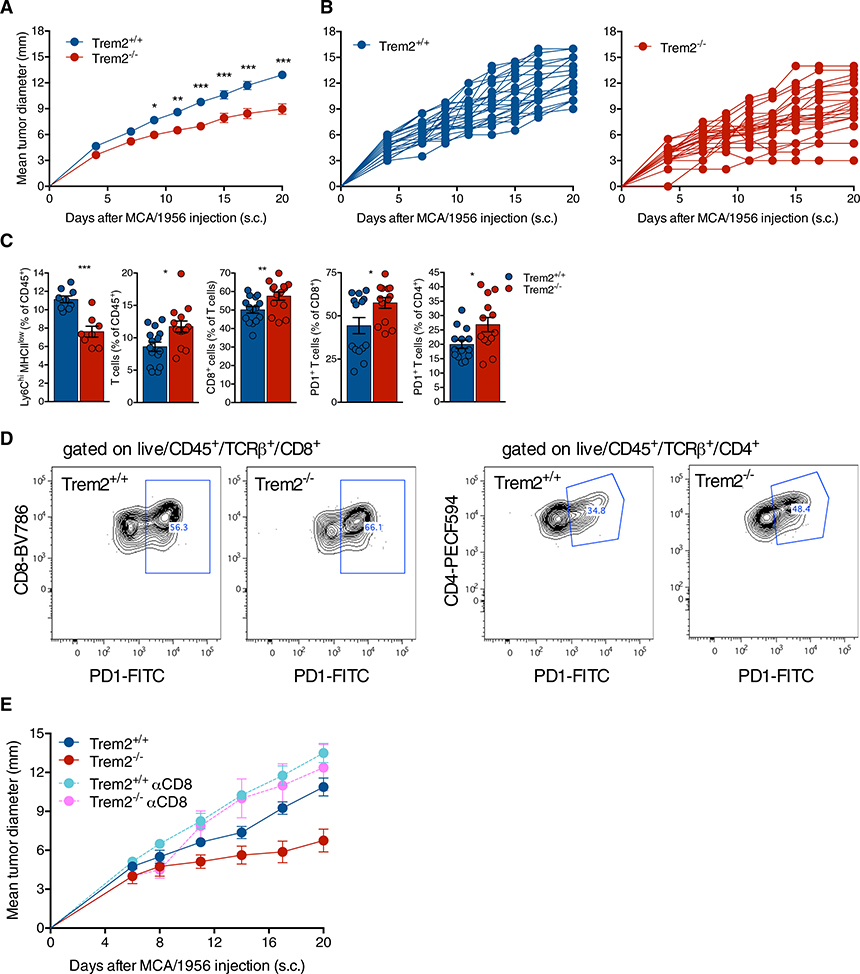
MCA/1956 grew progressively in WT mice, but was consistently attenuated in Trem2−/− mice (Figures 1A and 1B). We then examined the immune infiltrate of MCA/1956 tumors in WT and Trem2−/− mice 10 days after tumor injection by flow cytometry. Total CD11bhi myeloid cells were equally represented in WT and Trem2−/− infiltrates (Figure S1A); however, the relative proportion of a Ly6C+MHCIIlow/− subset was significantly reduced in the Trem2−/− infiltrates (Figure 1C), while the Ly6C+MHCII+ and Ly6C−MHCII+ subsets were present at similar frequencies (Figure S1A), suggesting that TREM2 deficiency may impact the myeloid infiltrate. Trem2−/− tumor infiltrates also contained more TCRβ+ T cells and CD8+ T cells than did WT infiltrates, in line with more robust tumor control in Trem2−/− mice (Figure 1C). In addition, significantly more CD8+ T cells and CD4+ T cells expressed PD-1 in the Trem2−/− infiltrates (Figures 1C and 1D), suggesting that TREM2 deficiency may promote increased T cell activation and potentially enhance responsiveness to anti-PD-1 checkpoint blockade. Lag3+ T cells, Tregs, and other lymphoid cells such as natural killer (NK) cells, γδ T cells, and B cells were equally represented in tumor infiltrates from WT and Trem2−/− mice (Figure S1B). To directly address the impact of T cells on the restrained tumor growth observed in Trem2−/− mice, we depleted CD8+ T cells and CD4+ T cells in tumor-bearing Trem2+/+ and Trem2−/− mice. Upon depletion of CD8+ T cells (Figure 1E), but not CD4+ T cells (Figure S1C), tumor growth was no longer attenuated, but in fact accelerated in Trem2−/− mice. Collectively, these data suggested that TREM2 deficiency modifies the MCA myeloid microenvironment in a manner that facilitates partial control of tumor growth by CD8+ T cells. Moreover, it appeared that CD8+ T cells generated in the TREM2-deficient environment may be more responsive to anti-PD-1.
Figure 1. TREM2 Deficiency Attenuates Growth of Transplantable Tumors.
(A and B) Tumor growth in Trem2+/+ and Trem2−/− mice injected subcutaneously (s.c.) with MCA/1956.
(C) Flow cytometry analysis of the immune infiltrate of MCA/1956 tumors 10 days after injection.
(D) Representative flow plots of PD-1 expression in CD8 (left panels) and CD4 (right panels) T cells.
(E) Tumor growth of MCA/1956 in Trem2+/+ and Trem2−/− after depletion of CD8 T cells.
Growth curves of groups (A and E) and single mice (B) are shown. **: Trem2+/+ versus Trem2−/−; *** Trem2−/− versus Trem2+/+ αCD8; *** Trem2−/− versus Trem2−/− αCD8; ns: Trem2+/+ versus Trem2+/+ αCD8; Trem2+/+ versus Trem2−/− αCD8; Trem2+/+ αCD8 versus Trem2−/− αCD8 (day 20). Data represent mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Two-way Anova with multiple comparisons (A and E) and Mann-Whitney test (C). See also Figure S1.
We extended our analysis to the growth of MC38 colorectal cancer, which has been shown to depend on the function of infiltrating macrophages (Rashidian et al., 2019). Meta-analyses of published RNA-seq data from the MC38 model (Arlauckas et al., 2018; Hoves et al., 2018) showed that macrophages express TREM2 (data not shown). Consistent with our results with the MCA/1956 model, MC38 tumor growth was more subdued in Trem2−/− mice than in WT mice (Figures S1D and S1E). Flow cytometric analysis of the tumor infiltrate of MC38 showed that TREM2 deficiency was associated with reduced representation of yet another subset of macrophages expressing CD206 (Figure S1F) that have been previously involved in immunosuppression (Allavena et al., 2010; Mantovani et al., 2017), whereas no obvious phenotypic changes were observed in the T cell compartment (data not shown). Finally, we assessed the expansion of PyMT mammary tumors, which are infiltrated by macrophages that have been shown to promote growth and metastatic potential (Franklin et al., 2014; Lin et al., 2001) and to express TREM2 (Ojalvo et al., 2009). Once again, tumor growth was more limited in Trem2−/− mice than in WT mice (Figures S1G and S1H). Cumulatively, these results demonstrated that TREM2 deficiency consistently restricts the growth of various tumors that are infiltrated by macrophages; moreover, flow cytometric analyses hinted that this restraint is associated with changes in infiltrating macrophages that may impact T cell responses.
Lack of TREM2 Impacts Tumor Myeloid and Lymphoid Landscape
To define the impact of TREM2 deficiency on the tumor-immune infiltrate at higher resolution, we analyzed immune cells in WT and Trem2−/− MCA/1956 tumors by scRNA-seq. We sorted live-CD45+ cells from MCA/1956 tumors 10 days after tumor injection (Figures S2A and S2B). We chose this time point to detect early changes in the myeloid compartment related to lack of TREM2, before these changes become entwined with altered T cell responses and modified pathology. We obtained data for an average of 19,758 cells across all conditions with a coverage of 13,545 UMI per cell. Unsupervised clustering by Uniform Manifold Approximation and Projection (UMAP) identified 14 clusters (Figures S2A–S2D). Expression of Ptprc (CD45) was evident in all clusters, with the exception of a few cells (cluster 12), which included contaminating non-immune cells. Cluster 9 expressed T cell markers (Cd3d, Cd4, and Cd8b1), while cluster 5 encompassed NK cells (Ncr1). Cluster 13 (Cd79a) and cluster 11 (S100a8) identified B cells and neutrophils, respectively. Cluster 8 corresponded to Mki67+ proliferating cells, predominantly CX3CR1+ macrophages. Cluster 10 included DC2-like cells based on the expression of Flt3 and Ccr7, while cluster 7 represented Xcr1+ DC1-like cells (Figure S2D).
Macrophage subsets were grouped in clusters 0, 1, 3, 4, 6, and 8 (Figures S2A–S2D). Some clusters were designated based on the expression of a characteristic marker such as CX3CR1-Macs-g, Mrc1-Macs-g, Nos2-Macs-g, and Cycling-Macs-g (Mki67+). Other clusters were simply identified as Macs-1–3-g, as they expressed a constellation of markers rather than an archetypal macrophage marker. All clusters were further labeled with a “g” to indicate that they were identified in a comparison between tumor-bearing mice with different genotypes (WT and Trem2−/−). We noticed that CX3CR1-Macs-g, Mrc1-Macs-g, Cycling-Macs-g, and Macs3-g were relatively meagerly represented in Trem2−/− tumors, whereas the Macs1-g cluster predominated (Figures S2B–S2D). Moreover, T cells (cluster 9) and NK cells (cluster 5) were enriched in Trem2−/− mice (Figures S2B and S2C). This initial evaluation established that lack of TREM2 impacts both myeloid and lymphoid tumor infiltrates. Moreover, TREM2 was expressed in all macrophage clusters, although at different levels, while it was absent in DCs and lymphoid cells (Figure S2E).
To delve more deeply into the influence of TREM2 on macrophage subsets, we further re-clustered macrophages into 8 subsets (Figures 2A–2D), all of which expressed TREM2 (Figures 2E and 2F). We confirmed that CX3CR1-Macs-g, Mrc1-Macs-g, Cycling-Macs-g, and Macs3-g were poorly represented in Trem2−/− MCA tumors. Genes that have been associated with immunosuppression in MCA, such as CX3CR1, Mrc1, Mertk, and CD81, were expressed by either CX3CR1- or Mrc1-Macs-g (Figures 2B–2D and 2G) (Gubin et al., 2018). Macs-3-g exhibited a profile reminiscent of the IFNα/β signature (Rsad2, Isg15, Ifit2, and Ifit3). Macs1-g and Macs2-g expressed monocyte markers such as Ccr2 and Il1r2, suggesting that they may encompass macrophages recently immigrated from blood. The Macs2-g subset, expressing C1qa and Cd72, was slightly diminished in Trem2−/− MCA tumors. On the other hand, the Macs1-g cluster, which expressed genes previously associated with IFN-γ imprinting and immunostimulation (Cxcl9 and Cd83) was much more predominant in Trem2−/−MCA tumors. Nos2-Macs-g (Nos2 and Arg1) and Macs4-g (Itga4) were not altered (Figures 2B–2D and 2G). Interestingly, TREM2 expression was particularly high in two of the clusters poorly represented in Trem2−/− MCA tumors, CX3CR1-Macs-g and Cycling-Macs-g, suggesting a correlation between the level of TREM2 expression and dependence on TREM2 for sustaining cell numbers. We also re-clustered lymphoid cells, revealing heightened expression of activation markers, such as IFNγ and PD-1 (Pdcd1), by T cells and NK cells in Trem2−/− MCA tumors (Figures S2F–S2I).
Figure 2. TREM2 Deficiency Remodels the Myeloid Compartment in MCA-Derived Sarcoma.
(A) UMAP plots of myeloid clusters from merged conditions.
(B) Cluster proportions in each condition: Trem2+/+ and Trem2−/−. Cluster identities were based on expression of key marker shown below.
(C) UMAP plots of selected cluster markers from merged conditions.
(D) UMAP plots of myeloid clusters in each condition.
(E) Violin plots of TREM2 expression level in each cluster.
(F) TREM2 expression in each condition.
(G) Heatmap showing normalized expression of selected genes in each myeloid cluster for each condition.
Tumors from Trem2+/+ and Trem2−/− male mice were analyzed 10 days after injection. See also Figure S2.
Finally, we compared the gene signatures of the 8 myeloid clusters identified within MCA tumors with those reported for the intratumoral macrophage compartment in a non-immunogenic MCA tumor related to ours, which, although on a 129 background, was treated with immune-checkpoint therapy (ICT) (Gubin et al., 2018). Mrc1 and CX3CR1 cluster reductions observed in Trem2−/− mice were consistent with similar reductions induced by ICT. In contrast, Nos2+ and Rsad2+ macrophages were only increased in ICT-treated but not in Trem2−/− mice (Figure 3A). We conclude that TREM2 deficiency drives a complex remodeling of macrophage infiltrate that promotes enrichment and activation of T cells and NK cells. Such reshaping resembles, only in part, that which was previously observed following ICT.
Figure 3. TREM2 Deficiency Drives a Complex Remodeling of Macrophage Infiltrate that Partly Resembles that Observed Following ICT.
Feature plots of selected genes in MCA/1956 sarcoma in Trem2+/+ and Trem2−/− mice (left panels) and in T3 sarcoma (Gubin et al., 2018) following ICT.
TREM2 Deficiency Enhances Checkpoint Blockade
The observed resistance of Trem2−/− mice to tumor growth prompted us to determine whether TREM2 deficiency can enhance antitumor responses unleashed by checkpoint blockade. Although the MCA/1956 sarcoma grows progressively in immunocompetent hosts, it retains sufficient immunogenicity to be effectively controlled by checkpoint immunotherapy with anti-PD-1 (Li et al., 2018). Indeed, when anti-PD-1 treatment was initiated early (on day 3 after injection of tumor cells), MCA/1956 was rejected in 100% mice (Figure 4A). To determine whether lack of TREM2 can enhance anti-PD-1 immunotherapy, we chose a sub-optimal scheme for PD-1 administration, starting treatment at a late time point (on day 8 after injection of tumor cells) (Li et al., 2018). Following this treatment protocol, only 40% of WT mice rejected the tumor, whereas 100% of Trem2−/− mice did (Figures 4B and 4C). Since MC38 is also responsive to checkpoint blockade (Harjunpää et al., 2018; Li et al., 2018; Woo et al., 2012), we examined whether lack of Trem2 could enhance suboptimal anti-PD-1 treatment of this tumor. Indeed, all Trem2−/− mice controlled tumor growth as opposed to only a few WT mice (Figures 4D and 4E).
Figure 4. TREM2 Deficiency Enhances Anti-PD-1 Immunotherapy.
(A) Experimental setup of the anti-PD-1 treatment. Optimal treatment started at day 3 (aPD-1-I); suboptimal treatment started at day 8 (aPD-1-II).
(B–E) Tumor growth of MCA/1956 (B and C) and MC38 (D and E) in Trem2+/+ and Trem2−/− mice treated with anti-PD-1. (B) ***: Trem2+/+ aPD-1 versus Trem2−/− aPD-1 (day 20). (D) ***: Trem2+/+ aPD-1 versus Trem2−/− aPD-1 (day 20).
(F) Flow cytometry analysis of lymphoid cells in Trem2+/+ and Trem2−/− mice treated with anti-PD-1 (day 14 after tumor injection).
Growth curves of groups (A, B, and D) and single mice (C and E) are shown. Data represent mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001; Mann-Whitney test.
We next analyzed the immune infiltrate 14 days after tumor injection to define T cell responses. Trem2−/− mice showed a trend in the increase of intratumoral αβ T cells, which was significant in anti-PD-1 treated mice (Figure 4F). CD8+ T cells were increased in Trem2−/− mice with or without anti-PD-1, as were PD-1+ CD8 T cells, corroborating our initial results (see Figure 1C). PD-1+ CD4 T cells were only increased in Trem2−/− mice treated with anti-PD-1 (Figure 4F). Treg, B, NK, and Lag3+ T cell proportions did not change in the absence of TREM2 (data not shown). Altogether, these results demonstrate that TREM2 deficiency augments the efficacy of ICT in two transplantable tumor models responsive to anti-PD-1. Enhancement of ICT was associated with augmented T cell infiltration and activation, at least in the MCA model.
Anti-TREM2 Treatment Is Protective in the Sarcoma Model
Since genetic deletion of Trem2 is associated with reduced tumor growth and enhanced response to checkpoint blockade, we sought to test the therapeutic potential of mAb modulation of TREM2. We previously showed that mAb 178 is specific for mouse TREM2 (Turnbull et al., 2006). This mAb was originally established using a standard hybridoma approach by immunizing rats with the recombinant ectodomain of TREM2. For tumor immunotherapy, we generated a recombinant form of mAb 178, in which the variable region of the heavy chain was grafted onto a mouse IgG2a constant region backbone that had been mutated in the Fc domain (LALAPG) to prevent recognition by Fc receptors and complement the consequent induction of antibody-dependent cellular cytotoxicity or antibody-dependent phagocytosis. Both native and Fc mutated anti-TREM2 antibodies specifically stained TREM2 transfected cells in a dose-dependent manner (Figure S3A). To see whether mAb 178 modulates TREM2, we tested the Ca2+-driven reporter cell line 2B4, stably transfected with TREM2 together with DAP12 (Figure S3B) (Wang et al., 2016). In these reporter cells, engagement of TREM2 promotes Ca2+ signals that lead to nuclear translocation of NFAT and NFAT-driven synthesis of EGFP, which is detected by flow cytometry. Lipidated HDL, a well-described TREM2 ligand (Song et al., 2017), induced EGFP in TREM2 reporter cells. Both native and Fc mutated mAb 178 inhibited HDL-induced EGFP expression, indicating that mAb 178 blocks ligand binding to TREM2 (Figure S3B). To exclude in vivo depleting activity of recombinant mAb 178, we assessed its impact on TREM2+ thioglycollate-induced peritoneal macrophages after intraperitoneal (i.p.) injection (Figure S3C). To evaluate TREM2+ macrophage numbers, we used an antibody recognizing a different epitope from that bound by mAb 178. The representation of TREM2+ macrophages was not affected by mAb 178 (Figure S3C), indicating no antibody-mediated depletion, consistent with lack of effector function in the mutated Fc fragment.
We then tested the recombinant anti-TREM2 in vivo in the MCA/1956 model with or without anti-PD-1. As control, we used an Fc mutated recombinant mAb specific for human ILT1, a receptor not encoded in mice. Administration of anti-TREM2 was initiated at day 2 after tumor injection and was repeated every 5 days until the end of the experiment. Anti-PD-1 was given following the suboptimal scheme described above (Figure 5A). Treatment with anti-TREM2 alone afforded significant but incomplete control of tumor growth (Figures 5A and 5B). However, the combination of anti-TREM2 and suboptimal anti-PD-1 conferred complete tumor control in all mice tested (Figures 5A and 5B). We conclude that anti-TREM2 mAb 178 has anti-tumor activity and augments anti-PD-1 checkpoint blockade. Interestingly, tumor growth was more attenuated in anti-TREM2-treated mice than in Trem2−/− mice (Figure 5C). Yet, treatment of Trem2−/− mice with the anti-TREM2 mAb did not further attenuate tumor growth, demonstrating that anti-TREM2 treatment has no off-target effects.
Figure 5. TREM2 Engagement Reduces MCA-Sarcoma Growth and Enhances Anti-PD-1-Induced Tumor Regression.
(A and B) Tumor growth in mice injected s.c. with the MCA/1956 cell line treated with anti-TREM2 and anti-PD-1 antibodies. (A) *: αPD-1 versus αTREM2+αPD-1 (day 24).
(C) Tumor growth of MCA/1956 tumors in Trem2+/+ and Trem2−/− mice treated with anti-TREM2. Anti-ILT1 was used as a control in both Trem2+/+ and Trem2−/− mice. **: CTRL versus CTRL Trem2−/−; ***: CTRL versus αTREM2; ns: CTRL Trem2−/− versus αTREM2 Trem2−/− (day 20).
(D) Flow cytometry analysis of the myeloid immune compartment 10 days after tumor injection in mice treated with anti-TREM2. Control groups, mice treated with anti-TREM2, anti-PD-1, and the combination of anti-TREM2 and anti-PD-1 are shown.
(E) IFNγ and TNFα production by CD8 and CD4 T cells stimulated ex vivo with PMA/I.
Data represent mean ± SEM. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001; Mann-Whitney test. Growth curves of groups (A and C) and single mice (B) are shown. See also Figure S3.
We next characterized the influence of anti-TREM2 on the tumor-immune infiltrate by flow cytometry. At an early time point (10 days after tumor injection), combined blockade of PD-1 and TREM2 was associated with diminished Ly6C+MHCII− and CD64+ subsets within the myeloid compartment. Neither anti-PD-1 nor anti-TREM2 treatment alone promoted significant changes (Figures 5D and S3D). At a later time point (24 days after tumor injection), in parallel with a considerable shrinkage of the tumor volume, anti-TREM2 alone showed a significant impact on the myeloid compartment: while the total number of macrophages was unchanged, the representation of Ly6ClowMHCII− cells, along with subsets expressing CD206, CD63, and CD9, was reduced (Figure S3E). Immunofluorescence analyses of tumor sections confirmed decreased CD206 expression within the tumor myeloid infiltrate (identified by staining for the myeloid marker Iba1) (Figure S3F). Mice treated with suboptimal anti-PD-1 evinced no obvious changes, likely due to the short duration of the treatment. Since mice treated with both anti-TREM2 and anti-PD-1 had entirely cleared the tumors, no infiltrate could be analyzed. Anti-TREM2 had no obvious impact on the representation of lymphoid subsets at early or late time points (data not shown). However, analysis of T cell cytokine production ex vivo indicated that anti-TREM2 treatment augments IFNγ and TNFα production by intra-tumoral CD8+ and CD4+ T cells, respectively (Figure 5E). Altogether, these data suggest that mAb-mediated modulation of TREM2 drives the remodeling of the intra-tumor myeloid compartment in a manner that promotes a protective T cell response and enhances anti-PD-1 immunotherapy.
Anti-TREM2 Treatment Reshapes the Intratumoral Macrophages Infiltrate
To define the impact of anti-TREM2 mAb on the immune populations at high resolution, we resorted to scRNA-seq. We assessed four treatment conditions: control antibody, anti-TREM2 plus control antibody, suboptimal anti-PD-1 plus control antibody, and anti-TREM2 plus suboptimal anti-PD-1. Two biological replicates for each condition were examined. scRNA-seq was performed on CD45+ cells sorted from MCA/1956 tumors and analysis was performed 10 days after injection of the tumor. We obtained data for an average of 2,726 cells per condition with a coverage of 14,622 UMI per cell. Unsupervised clustering by UMAP identified 16 clusters (Figures S3G–S3J). Ptprc (CD45) was expressed in all clusters, with the exception of few non-immune cells (cluster 14). Cluster 9 expressed T cell markers (Cd3d, Cd4, and Cd8b1), while cluster 2 encompassed NK cells (Ncr1). Clusters 11 and 13 represented two small clusters of γδ+ T cells (Trgv2+). Cluster 15 (Cd79a), cluster 8 (S100a8), and cluster 12 (SiglecH) identified B cells, neutrophils, and pDCs, respectively. Cluster 6 corresponded to Mki67+ proliferating cells, including both T cells and CX3CR1+ macrophages. Cluster 10 included DC2-like cells based on the expression of Flt3, Ccr7, Ccl22, and Ccl17, while cluster 7 represented Xcr1+ DC1-like cells (Figures S3G–S3J). Macrophage subsets were grouped in clusters 0, 1, 3, 4, and 5 (Figures S3G–S3J). While most clusters seemed similarly represented under all conditions, some of the macrophage clusters varied markedly, most notably, the exclusive presence of cluster 5 in the anti-TREM2-treated tumor (Figures S3G and S3J). No obvious changes were detected in the lymphoid compartment at this early time point.
We then separately re-clustered macrophages to obtain deeper profiling (Figures 6A and 6B). As in Figure 2, macrophage subsets were designated with either a gene name or a number, depending on whether they expressed a typical macrophage gene or a constellation of genes. Clusters were further designated with a “t” to indicate that they were distinguished following antibody treatments. We identified 8 subsets that broadly expressed TREM2 (Figures 6A–6E). CX3CR1-Macs-t resided primarily in cluster 2, as well in Cycling Macs-t (cluster 7). Most Mrc1-Macs-t were confined to cluster 4, with few Mrc1+ cells in clusters 2 and 7. Nos2-Macs-t were present in clusters 5 and 6 (Figures 6D and 6F), which were distinguished based on selective expression of Il1r2, Cd244, and Spp1 (cluster 5), as well as C3, CD38, and Ly6i (cluster 6) (Figures S4A and S4B). Cluster 3 exhibited a clear IFN signature, indicated by an enrichment for Isg15, Ifit3, and Rsad2 transcripts (Figures 6D and 6F). Macs1-t (cluster 0) and Macs2-t (cluster 1) encompassed cells expressing monocyte markers such as Ccr2 and IL1r2 and hence may be monocytes that recently immigrated into the tumor from blood. The distinction into two subsets was marked by Ly6i (Macs1-t) and Fgd2 (Macs2-t) (Figures 6D and 6F).
Figure 6. Anti-TREM2 Treatment Remodels the Myeloid Compartment in MCA-Derived Sarcoma.
(A) UMAP plots of myeloid clusters from merged conditions.
(B) Cluster proportions in each condition: controls (CTRL); mice treated with anti-PD-1 (αPD-1); mice treated with anti-TREM2 (αTREM2); mice treated with the combination of anti-PD-1 and anti-TREM2 (αTREM2 + αPD-1). Cluster identities were based on expression of key marker shown below.
(C) TREM2 expression in myeloid clusters from merged conditions.
(D) UMAP plots of myeloid clusters in each condition.
(E) UMAP plots of selected cluster markers from merged conditions.
(F) Heatmap showing normalized expression of selected genes in each myeloid cluster for each condition.
Tumors from control and treated female mice were analyzed 10 days after injection. See also Figures S3 and S4.
Some of the macrophage clusters were differentially represented after the distinct treatment regimens. Anti-TREM2 treatment, with or without anti-PD-1, induced de novo appearance of Nos2-Macs-t (cluster 5), which was virtually absent under other conditions (Figure 6E). The population of Mrc1-Macs-t contracted following treatment with anti-TREM2. One of the macrophage subsets that recently immigrated into the tumor, Macs1-t, also declined, while the other one—Macs2-t—expanded, perhaps reflecting changes in the intratumoral differentiation of blood-derived monocytes. Cycling-Macs-t (which were also CX3CR1+) were almost ablated by anti-TREM2 in combination with anti-PD-1 (Figures 6E and 6F). We conclude that anti-TREM2 treatment induces a complex remodeling of the myeloid compartment that drives the appearance of Nos2-Macs-t and a parallel decline of Mrc1-Macs-t, Cycling-Macs-t, and Macs1-t recent immigrants.
Given that previous analysis of ICT-treated MCA tumor showed an increase of Nos2- and Rsad2-expressing macrophages and a reduction of Mrc1- and CX3CR1-expressing macrophages (Figure 3) (Gubin et al., 2018), we examined the expression of the same genes in anti-TREM2-treated tumors (Figure S4C). Nos2+ cells were expanded and Mrc1+ cells were reduced in anti-TREM2 and anti-TREM2 plus anti-PD-1-treated MCA, in line with ICT-treated tumors. In contrast, Rsad2+ Macs were only increased following ICT and anti-TREM2 plus anti-PD-1, but not after anti-TREM2 treatment alone (Figure S4C). We further compared the gene signatures of myeloid clusters identified in Trem2+/+ and Trem2−/− tumor-bearing mice with those found in mice treated with blocking antibodies (Figure S4D). Results are represented in a heatmap showing the magnitude of difference/similarity of each cluster in the two experiments. CX3CR1 Macs_g, which declined in the absence of TREM2, overlapped with Mrc1 Macs_t, which comparably declined in anti-TREM2-treated mice, as well as with CX3CR1 Macs_t. Similarly, Nos2 Macs_g overlapped with the Nos2 Macs1_t and Nos2 Macs2_t clusters in anti-TREM2-treated mice. Finally, cycling Macs_g corresponded to cycling Macs_t, which were diminished in mice treated with combined anti-TREM2 and anti-PD-1. Altogether, TREM2 deficiency, and anti-TREM2 and anti-ICT treatments, induced some consistent changes in tumor-associated macrophages.
Trem2 Is Selectively Expressed in Human Tumor Macrophages
To address the significance of our findings in human tumors, we first analyzed TREM2 protein expression in macrophages from human normal and tumor specimens by IHC. TREM2 expression was not detected in a large majority of macrophages in peripheral tissues, with the exception of microglia, MITF+ placental macrophages, macrophages of the endometrium, and alveolar macrophages (Figure S5A). In contrast, TREM2+ macrophages were observed in 75% of carcinomas from various primary sites as assessed by screening of a multi-carcinoma tissue microarray containing 126 samples (data not shown). To circumvent potential tumor heterogeneity, we further extended this analysis to whole tissue sections obtained from 99 tumor samples, which included carcinomas from many sites. We confirmed the presence of TREM2+ macrophages in virtually all cases (representative examples are shown in Figure 7A). Morphology of TREM2+ tumor macrophages varied from small monocytoid to large foamy or multinucleated giant cells, with TREM2 expression mainly localized on cell membrane (Figures 7B and S5B). TREM2+ tumor macrophages co-expressed CD68, CD163, CSF1R, and nuclear MAFB; foamy TREM2+ tumor macrophages also co-expressed MITF, a transcription factor involved in lysosomal biogenesis that marks a regulatory myeloid subset of human placenta (Costa et al., 2017). No TREM2 expression was observed in BDCA2+ plasmacytoid DC, CD1c myeloid DC, or CD207 Langerhans cells (Figure S5B).
Figure 7. TREM2 Expression in Human Cancers and Association with Prognosis in CRC and TNBC Patients.
(A) IHC of tissue sections immunostained with anti-human TREM2 from primary carcinomas of skin (A), liver (B), lung (C), breast (D), bladder (E), colon (F), stomach (G), pancreas (H), and kidney (I), as well as from nodal lymphoma (J), cutaneous melanoma (K), and brain glioma (L). Sections are counterstained with hematoxylin. Magnification: 200×, scale bar: 100 μm.
(B) Morphology and phenotype of TREM2+ tumor macrophages. Sections are form primary carcinomas and melanomas and stained as labeled. TREM2 reactivity decorates the cell membrane as observed in high-power view (A). TREM2+ tumor macrophages co-stain for CD163 (B), CD68 (C), nuclear MAFB (D), CSF1R (E), and the MITF transcription factor (F). Sections are counterstained with hematoxylin. Magnification: 600× (A, scale bar: 33 μm) and 200× (B–F, scale bar: 100 μm).
(C and D) Kaplan-Meier survival curves generated for TREM2 expression. Patients were divided in high- and low- expressing groups based on 75% quantile of TREM2 expression. TCGA CRC (C) and TNBC (D) cohorts are shown.
See also Figures S5, S6, and S7.
TREM2+ macrophages were scant or absent in benign or low-grade neoplastic lesions, as observed for colon adenomas, low grade papillary urothelial carcinomas, and skin nevi (Figure S5C). Because of the clinical relevance of systemic dissemination of cancer cells, we tested TREM2 expression in macrophages from nodal (n = 33) and distant metastasis (n = 12). We found TREM2+ macrophages surrounding and infiltrating metastatic nests in tumor-draining lymph nodes, as well as in distant liver and lung metastasis (Figure S6). Altogether, TREM2 emerged as a marker of tumor-infiltrating macrophages in all cancers. One striking finding was the selective intratumor localization of TREM2+ macrophages as opposed to TREM2− macrophages, which were found in the surrounding stromal area. Since TREM2 expression is induced by CSF-1, GM-CSF, IL-4, and IL-13 (Cella et al., 2003), the expression of TREM2 may reflect intratumoral production of these cytokines.
TREM2 Correlates with Poor Disease Prognosis
We next explored The Cancer Genome Atlas (TCGA) database for correlations between TREM2 expression and clinical outcome in tumor types related to our experimental models. With a threshold of 75% quantile, TREM2 expression correlated with worse overall survival in the CRC cohort (Figure 7C). In the TNBC cohort, TREM2 expression correlated with worse overall and relapse-free survival using the 75% quantile as cutoff (Figure 7D). Similar results were obtained using the TREM2 median expression. Analysis of the TCGA database also revealed that TREM2 expression correlated with that of myeloid signature genes in both CRC and TNBC cohorts. In CRC patients, the top genes correlating with TREM2 included the scavenger receptor MSR1, the Fc receptor FCGR1A, the TREM2 adaptor protein TYROBP, the exhaustion marker HAVCR2, and several lipid metabolism genes (APOC1, APOE, and OLR1) previously associated with lipid-associated macrophage signature (Figure S7A) (Biswas and Mantovani, 2012; Cochain et al., 2018; Jaitin et al., 2019). Consistently, in TNBC patients, TREM2 highly correlated with MSR1, FCGR1C, TYROBP, monocyte-macrophage receptors (SIGLEC8 and LILRA2), genes associated with macrophage activation and phagocytosis (LY86, LY96, and RNASE6), macrophage metabolism (APOC1, ADORA3, TBXAS1, and FTL), and complement activation (C1QB and C1QC) (Figure S7B). Interestingly, in a multivariate survival analysis of TNBC, TREM2 had additional survival prognostic ability independent of other myeloid genes (Table S1), suggesting a direct contribution to pathogenesis. Other types of human tumors also showed a correlation between TREM2 and macrophage signature genes, but the association of TREM2 expression with patient overall or relapse-free survival was not significant (data not shown), suggesting that TREM2 impact on tumor-infiltrating macrophages and tumor immune responses might be context specific.
DISCUSSION
This study demonstrates that constitutive lack of TREM2 or anti-TREM2 treatment curbs tumor growth and leads to complete tumor regression when associated with suboptimal PD-1 immunotherapy. mAb inhibiting CTLA-4 or PD-1 have been extensively shown to unleash T cell effector functions to control tumors in both mice and some cancer patients (Sharma and Allison, 2015; Topalian et al., 2015). However, checkpoint blockade is incompletely effective for certain tumors, because they can escape using multiple mechanisms, one of which is the generation of a tumor microenvironment rich in myeloid cells with a strong immunosuppressive function. Thus, efforts are currently ongoing to complement checkpoint blockade with treatments targeting myeloid cells (Mantovani et al., 2017). Approaches have been developed to deplete myeloid cells from tumors, block their pro-tumoral functions, or restore their immunostimulatory properties. Among others, these strategies include inhibition of colony stimulating factor 1 receptor (CSF-1R) (Hume and MacDonald, 2012; Ries et al., 2014), CD24-Siglec10, CD47-SIRPα signaling (Barkal et al., 2019; Iribarren et al., 2018; Veillette and Chen, 2018; Willingham et al., 2012), and CXCR4-CXCL12 signaling (Hughes et al., 2015), as well as metabolic modulation, pharmacological modulation, and immunostimulation via an anti-CD40 agonistic antibody (Mantovani et al., 2017; Schoenberger et al., 1998; Wiehagen et al., 2017). The anti-TREM2 treatment presented here provides a novel therapeutic approach that broadens the armamentarium for myeloid cell targeting in tumors.
Analyses of the TCGA database suggested that anti-TREM2 treatment may be particularly promising in CRC and TNBC, because TREM2 expression inversely correlated with overall survival and relapse in these cancer patients. Beyond these correlations, our extensive pathology study of human tumors suggests that TREM2 may be a particularly attractive therapeutic target, as it was highly expressed in the vast majority of over 200 cases of primary and metastatic tumors that we examined by IHC, while it was poorly expressed in normal tissues. Therefore, TREM2 targeting may be widely applicable. We have previously shown that TREM2 is induced in human monocytes and mouse bone marrow cells upon exposure to GM-CSF and CSF-1 (Bouchon et al., 2001; Turnbull et al., 2006). Thus, we speculate that the high level of TREM2 expression in tumors reflects the production of these cytokines by several cell types in the tumor microenvironment, such as fibroblasts and myeloid cells. It is possible that the efficacy of anti-CSF-1 and anti-GM-CSF treatments previously observed in tumor models is partly due to the reduced expression of TREM2.
High-resolution analysis of the tumor cell infiltrate in the MCA model has revealed complex remodeling of the myeloid cell landscape in Trem2−/− and anti-TREM2 treated mice. This transformation is epitomized by a consistent decline in macrophage populations expressing CX3CR1 and MRC1, which is paralleled by the appearance of new macrophage clusters that express a set of activation markers in Trem2−/− mice, or iNOS in anti-TREM2-treated mice. These changes partially overlapped with those reported in a model of MCA-induced sarcoma treated with optimal anti-PD-1 (Gubin et al., 2018). It is worth noting that the changes induced by TREM2 targeting do not fit the M2/M1 paradigm. Diminished macrophage subsets expressed genes encoding molecules involved not only in immunosuppression, such as MRC1 and MERTK, but also in regulation of lipid metabolism, fibrosis, survival, and proliferation. The macrophages that expanded in Trem2−/− mice expressed CXCL9, which may reflect some exposure to IFN-γ, but they did not evince a clear IFN-γ-induced gene signature comparable to the classical M1 signature. Moreover, the macrophage subset inflated in anti-TREM2-treated mice expressed iNOS2, which has been associated with immunostimulation (Hibbs et al., 1987; Murray et al., 2014; Stuehr and Nathan, 1989), as well as immunosuppression (Shi et al., 2001). Even combining anti-TREM2 with anti-PD-1 failed to elicit a clear M1 signature, perhaps due to the suboptimal anti-PD-1 regimen used in our experiments, which was initiated late after tumor cell injection. The observed changes in tumor macrophage infiltrates enhanced T-cell-mediated control of tumor growth, although we noted some variability in the extent of T cell expansion and effector function when comparing Trem2−/−- and anti-TREM2-treated mice. It will be important to validate which key genes showcased in the transcriptional signatures of macrophages before and after TREM2 targeting stimulate or suppress T cell responses and how they operate.
The mechanisms by which TREM2 deficiency and anti-TREM2 treatment impact tumor macrophages remain unclear. We have previously shown that TREM2 cooperates with CSF-1 in sustaining macrophage proliferation and survival (Ulland et al., 2017). We noticed that two clusters diminished in Trem2−/− MCA express the highest levels of TREM2. Thus, it is possible that lack or blockade of TREM2 binding with endogenous ligands selectively affects the survival of certain macrophage subsets, allowing other subsets to expand. TREM2 modulation may also improve the antigen-presenting function of tumor macrophages, since Trem2−/− macrophages were shown to present antigens to T cells more effectively than WT macrophages in vitro (Ito and Hamerman, 2012). Interestingly, we noticed that anti-TREM2 treatment was more effective than TREM2 deficiency in controlling tumor size. While antibody treatment affects tumor-associated macrophages acutely, constitutive TREM2-deficiency might allow direct and/or indirect compensatory responses of myeloid cells that impact tumor growth. For example, constitutive lack of TREM2 may free up more DAP12 for other DAP12-associated receptors in macrophages, increasing their signaling and pro-survival capabilities. Additionally, anti-TREM2 may effectively interfere with some, but not all, TREM2-signaling pathways, which include not only proliferation and survival, but also metabolism, migration and chemokine production (Peng et al., 2010; Ulland et al., 2017). Finally, anti-TREM2 may actually over-activate rather than block TREM2-controlled pathways, leading to anergy and/or exhaustion. Future biochemical experiments will be important to characterize how anti-TREM2 interferes with ligand-binding as well as signaling pathways.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Marco Colonna (mcolonna@wustl.edu).
Materials Availability
Materials and reagents used in this study are listed in the Key Resources Table. Reagents generated in our laboratory in this study or previous studies are available upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| hTREM2 (rabbit, clone D8I4C) | Cell Signaling Technology | Cat #91068 |
| hCD163 (mouse, clone 10D6) | Thermo Scientific | Cat #MS-1103-S0 |
| hCD68 (mouse, clone PG-M1) | Dako | Cat #M0876 |
| hMITF (mouse, clone D5, 1:50) | Dako | Cat #M3621 |
| hCD1c (mouse, clone OTI2F4) | Abcam | Cat #ab156708 |
| hCD207 (mouse, clone 12D6) | Leica | Cat# NCL-L-LANGERIN |
| hMAFB (polyclonal rabbit) | Sigma-Aldrich | Cat #HPA005653 |
| hCSF-1R (rabbit, clone FER216) | Merck-Millipore | Cat #MABS1163 |
| mouse Iba1, (rabbit, clone E404W) | Cell Signalling Technology | Cat #17198S |
| mouse CD206-AlexaFluor488 (rat, clone C068C2) | Biolegend | Cat #141710 |
| anti-rabbit-AlexaFluor647 (donkey) | Invitrogen | Cat #A31573 |
| CD45-BV605 (clone 30-F11) | Biolegend | Cat #103139 |
| CD45-AlexaFluor700 (clone 30-F11) | Biolegend | Cat #103128 |
| CD11b-PerCPCy5.5 (clone M1/70) | Biolegend | Cat #101228 |
| CD11b-PECy7 (clone M1/70) | Biolegend | Cat #101216 |
| CD11b-BV421 (clone M1/70) | Biolegend | Cat #101235 |
| I-A/I-E-BV650 (clone M5/114.15.2) | Biolegend | Cat #107641 |
| Ly6C-BV421 (clone HK1.4) | Biolegend | Cat #128031 |
| Ly6C-APC (clone HK1.4) | Biolegend | Cat #128016 |
| Ly6C-PerCP (clone HK1.4) | Biolegend | Cat #128028 |
| Ly6C-PE (clone HK1.4) | eBioscience | Cat #12–5932-82 |
| Ly6G-AlexaFluor700 (clone 1A8) | Biolegend | Cat #127621 |
| Ly6G-APC (clone 1A8) | Biolegend | Cat #127614 |
| CD64-APC (clone X54–5/7.1) | Biolegend | Cat #139305 |
| CD64-BV605 (clone X54–5/7.1) | Biolegend | Cat #139323 |
| B220-BUV395 (clone RA3–6B2) | BD Biosciences | Cat #563793 |
| B220-PerCPCy5.5 (clone RA3–6B2) | BD Biosciences | Cat #552771 |
| CD206-PECy7 (clone C068C2) | Biolegend | Cat #141719 |
| iNOS/Nos2-PE (clone CXNFT) | Thermo Fisher | Cat #12–5920-80 |
| CD9-PE (clone MZ3) | Biolegend | Cat #124805 |
| CD63-PerCPCy5.5 (clone NVG-2) | eBioscience | Cat #46–0631-80 |
| PD-L2-PE/DazzleTM 594 (clone TY25) | Biolegend | Cat #107215 |
| TCRβ-PE (clone H57–597) | Biolegend | Cat #109208 |
| CD8a-BV785 (clone 53–6.7) | Biolegend | Cat #100749 |
| CD4-PE/DazzleTM 594 (clone RM4–5) | Biolegend | Cat #100566 |
| PD1-FITC (clone J43) | Invitrogen | Cat #11–9985-82 |
| CD19-PacificBlue (clone 6D5) | Biolegend | Cat #115523 |
| FOXP3-AlexaFluor647 (clone MF-14) | Biolegend | Cat #126408 |
| NK1.1-BV650 (clone PK136) | Biolegend | Cat #108736 |
| LAG-3-PECy7 (clone C9B7W) | Biolegend | Cat #125225 |
| TCRγδ-FITC (clone eBioGL3) | eBioscience | Cat #11–5711-85 |
| IFNγ-PE (clone XMG1.2) | Biolegend | Cat #505808 |
| TNFα-FITC (clone MP6-XT22) | Biolegend | Cat #506304 |
| TREM2-APC (clone 237920) | R&D | Cat #FAB17291A |
| Anti-human ILT1-Fc mutated (clone 135.5) | This paper | N/A |
| Anti-murine TREM2-Fc mutated (clone 178) | This paper | N/A |
| Anti-murine TREM2 (clone 178) | (Turnbull et al., 2006) | N/A |
| InVivoMab anti-mouse CD8a (53–6.7) | BioXCell | Cat #BE0004–1 |
| Anti-mouse CD4 (GK1.5) | In house | N/A |
| Rat IgG2a Isotype Control | Leinco Technologies, Inc. | Cat #I-1177 |
| InVivoMab rat IgG2b isotype control (LTF-2) | BioXCell | Cat #BE0090 |
| Bacterial and Virus Strains | ||
| 10-beta Comp. E. coli | New England Biolabs | Cat #C3019H |
| Biological Samples | ||
| Surgical samples of non-neoplastic tissues (human skin, lung, liver, brain, colon, stomach, uterus and placenta) and neoplastic tissues (primary carcinomas, tumor draining lymph nodes and distant metastasis). | Tissue bank of the Department of Pathology (ASST, Spedali Civili di Brescia, Brescia, Italy) | N/A |
| Multi-tumour tissue microarrays (TMA) | (Vermi et al., 2014) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Collagenase from Clostridium histolyticum | Sigma | Cat #C5138–5G |
| Aqua fluorescent reactive dye | Life technologies | Cat #L34966 |
| Novolink Polymer. | Leica Microsistem | Cat #RE7200-CE |
| Mach 4 MR-AP (Biocare Medical), | Biocare Medical | Cat #M4U536 |
| Diamminobenzidine | Leica Microsistem | Cat #AR9432 |
| Ferangie-blue | Biocare Medical | Cat #FB813 |
| Prolong Diamong antifade mountant | Thermofisher | Cat #P36961 |
| HiTrap® Protein A High Performance | GE Healthcare | Cat #17–0403-01 |
| XhoI | New England Biolabs | Cat #R0146S |
| BglII | New England Biolabs | Cat #R0144S |
| NheI-HF | New England Biolabs | Cat #R3131S |
| Expi293TM Expression Medium | Thermo Scientific | Cat #A1435101 |
| Lipoprotein, high density from human plasma | Sigma | Cat # L8039–10MG |
| Thioglycollate medium | Sigma | Cat #T9032 |
| Critical Commercial Assays | ||
| FOXP3/Transcription Factor Staining Buffer Set | Invitrogen (Thermo Fisher Scientific) | Cat #00–5523-00 |
| Endosafe LAL Cartridges | Charles River Laboratories, | Cat #PTS5505F |
| ExpiFectamineTM 293 Transfection Kit | Thermo Scientific | Cat #A14524 |
| EndoFree Plasmid Maxi Kit | QIAGEN | Cat #12362 |
| Gibson Assembly Master Mix | New England Biolabs | Cat #E2611S |
| ChromiumTM Single Cell 3’ GEM, Library & Gel Bead Kit v3, 16 rxns | 10× Genomics | Cat #PN-1000075 |
| ChromiumTM Chip B Single Cell Kit, 48 rxns | 10× Genomics | Cat #PN-1000073 |
| Chromium i7 Multiplex Kit | 10× Genomics | Cat #PN-120262 |
| dsDNA High Sensitivity Assay Kit | Invitrogen | Cat #Q32851 |
| Deposited Data | ||
| aTREM2 and TREM2 KO experiment with MCA sarcoma tumor, 10× single cell RNA-seq | this paper | GSE151710 |
| Experimental Models: Cell Lines | ||
| MCA/1956 | generated in house | N/A |
| MC38 | (Gilfillan et al., 2008) | N/A |
| PyMT | (Su et al., 2016) | N/A |
| Expi 293F | (Stadlbauer et al., 2019) | N/A |
| mTREM2-reporter cell line | (Wang et al., 2015) | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL6/J | Jackson | Cat #000664 |
| Trem2−/− and Trem2+/+ mice (C57BL6/J) | In house | N/A |
| Oligonucleotides | ||
| VH and CH1 gBlock (clone: 178) | Integrated DNA technologies | N/A |
| VL and CκgBlock (clone: 178) | Integrated DNA technologies | N/A |
| VH and CH1 gBlock (clone: 135.5) | Integrated DNA technologies | N/A |
| VL and CκgBlock (clone: 135.5) | Integrated DNA technologies | N/A |
| Recombinant DNA | ||
| Anti-murine Trem2 antibody plasmids (clone: 178) | generated in house | GENEWIZ |
| Fc-mutant mIgG2A control antibody plasmids (clone: 135.5) | generated in house | GENEWIZ |
| Software and Algorithms | ||
| FlowJo v9.3 | FlowJo | www.flowjo.com |
| Prism v6 | GraphPad | www.graphpad.com |
| Seurat | Butler et al, 2018 | N/A |
| Cellranger | https://support.10xgenomics.com/single-cell-gene-expression/software/downloads/latest | N/A |
| Phantasus | https://artyomovlab.wustl.edu/phantasus/ | N/A |
| ggplot2 | (Wickham, 2016) | N/A |
| R | R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. | N/A |
Data and Code Availability
The datasets generated in this study are available at GEO by GSE151710 ID.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Mice were of mixed sexes. Mice within experiments were age and sex matched. Mice were housed under specific pathogen free conditions. Mice from different genotypes were cohoused from birth and separated during the experiment (the day of tumor injection). Mice did not undergo any procedures prior to their stated use. Mice used in this study include WT C57BL/6J and Trem2−/− animals bred at Washington University School of Medicine animal facility. For experiments with wild-type groups only (e.g., experiments with anti-TREM2 treatment), wild-type mice were purchased from Jackson. All animals were backcrossed until at least > 98% C57BL/6J confirmed by genotype wide microsatellite typing. For tumor models, animals were injected at 8 weeks of age for MCA/1956 and MC38 cell lines. For the PyMT model, female mice were injected at 10 weeks of age. All studies performed on mice were done in accordance with the Institutional Animal Care and Use Committee at Washington University in St. Louis approved all protocols used in this study.
Tumor models
MCA/1956 or MC38 cells were washed and resuspended in PBS and injected subcutaneously (106 cells/mouse in 100μl PBS). Mice were previously shaved on the flank. 105 PyMT cells/mouse were suspended in 50μl of sterile PBS and Matrigel Matrix (Corning #354234) and injected into the mammary fat pad (MFP). Mice were monitored every day and tumors were measured by caliper every other day. Mice were sacrificed at day 10, at day 24 or when tumors reached 1.5cm of diameter.
In vivo treatments
Mice were treated intraperitoneally (i.p.) with anti-PD1 antibody (200μg/mouse) every 3 days, starting at day 3 or day 8 after tumor injection, as specified. Mice were treated i.p. with anti-TREM2 antibody (clone 178; 200μg/mouse) every 5 days, starting at day 2 after tumor injection. Anti-hILT1 (clone 135.5) was used as a control. Anti-CD4 and anti-CD8 treatments were started one day before tumor injection (200μg/mouse) and then administrated every 3 days for the entire duration of the experiment.
To isolate peritoneal macrophages, mice were treated with anti-TREM2 (clone 178) or control antibodies prior to 5% thioglycollate. We collected peritoneal cells after 72 h and stained them with the commercial antibody anti-TREM2 (clone 237920, R&D) in combination with antibodies specific for myeloid markers.
TREM2 blocking in vitro experiment
TREM2 reporter cell lines expressing GFP upon TREM2 engagement were produced in our laboratory and previously described (Wang et al., 2015). HDLs (100μg/mL; SIGMA) were immobilized on plates. Reporter cells were added and cultured overnight in the presence of antibodies (20μg/mL) in complete medium. Native (rat IgG2a) and recombinant Fc mutated (mouse IgG2a) anti-murine TREM2 antibodies (clone 178) were used. Recombinant Fc mutated (mouse IgG2a) anti-human ILT1 antibody (clone 135.5) was used as a control.
Human tissues
Formalin-fixed paraffin-embedded tissue blocks used for this study were retrieved from the tissue bank of the Department of Pathology (ASST, Spedali Civili di Brescia, Brescia, Italy). Non-neoplastic tissues included human skin, lung, liver, brain, colon, stomach, uterus, and placenta. Neoplastic tissues included multi-tumor TMA (Vermi et al., 2014), a set of primary carcinomas (n = 99), tumor-draining lymph nodes (n = 33) and distant metastasis (n = 12). Primary carcinomas from various sites are as follows: breast n = 14; ovary n = 10; skin = 5; lung n = 10; stomach n = 10; colon n = 10; pancreas n = 5; liver n = 4; kidney n = 7; bladder n = 10; gliomas n = 5; lymphomas n = 4 and melanomas n = 5.
METHOD DETAILS
Flow Cytometry
Single cell suspensions were prepared from tumors upon sacrifice. Tumors were minced and digested with Collagenase IV (Sigma) for 30 min at 37°C. Cells were filtered through 70-μm strainers, washed with PBS and stained for flow cytometry. The following antibodies were used: CD45-BV605 or –AlexaFluor700 (clone 30-F11), CD11b-PerCPCy5.5 or –PECy7 or –APC or -BV421 (clone M1/70), I-A/I-E-BV650 (clone M5/114.15.2), Ly6C-BV421 or –APC or –PerCPCy5.5 or –PE (clone HK1.4), Ly6G –AlexaFluor700 or –APC (clone 1A8); CD64 –APC or –BV605 (clone X54–5/7.1), B220-BUV395 or –PerCPCy5.5 (clone RA3–6B2); CD206 –PeCy7 (clone C068C2); iNOS/Nos2 –PE (clone CXNFT); CD9-PE (clone MZ3); CD63-PerCPcy5.5 (clone NVG-2); PD-L2-PECF594 (clone TY25); TCRβ-PE (clone H57–597); CD8-BV785 (clone 53–6.7); CD4-PECF594 (clone RM4–5); PD-1-FITC (clone J43); FOXP3-APC (clone MF-14); CD19-PacificBlue (clone 6D5); NK1.1-BV650 (clone PK136); Lag3-PECy7 (clone C9B7W); TCRγδ-FITC (clone eBioGL3); IFNγ-PE (clone XMG1.2); TNFα-FITC (clone MP6-XT22). TREM2 reporter cell line was stained with anti-TREM2 antibodies produced in our lab (clone 178). Anti-ratIgG-PE and anti-mouseIgG-PE (Southern Biotech) were used as secondary antibodies. Cells were incubated with Fc block prior to staining. Cell viability was determined by Aqua LIVE/Dead-405 nm staining (Invitrogen), negative cells were considered viable. Foxp3/Transcription Factor Staining Buffer Set (eBioscience) was used for intracellular staining. Cells were analyzed on BD X20 or BD FACSymphony (BD Bioscience). AriaII (BD Bioscience) was used for sorting. Data were analyzed with FlowJo software (Treestar).
Anti-TREM2 antibody production
Anti-mouse Trem2 monoclonal antibody (mAb) was cloned as previously described (Turnbull et al., 2006). For the Fc mutated antibody, the heavy chain variable (VH), CH1 and the light chain gene were sequenced from our 178 hybridoma (Syd Labs). To generate recombinant antibody, the heavy chain gBlocks (VH and CH1 mIgG2A; Integrated DNA technologies) was cloned into the pFUSEmIgG2A-Fc1 vector with L234A, L235A, P329G (LALA-PG) mutation (Lo et al., 2017), and the light chain gBlock (VL and Cκ; Integrated DNA technologies) was cloned into the mIgG2A-deficient pFUSE-mIgG2A-Fc1 vector. Then, the heavy chain and light chain plasmids were co-transfected into Expi293F cells (Thermo Scientific) for expression at mass ratio 1:2. Until cell viability reduces below 50% (5–7 days), the supernatant media was collected and antibody was purified with protein A agarose (GE Healthcare) (Stadlbauer et al., 2019). Following 3 days PBS dialysis, final preps were concentrated to about 10 mg/mL and freezed to store in −80°C. The heavy chain and light chain plasmids of the irrelevant Fc mutant mIgG2A control mAb (clone: 135.5) was co-transfected and purified similarly. After purification, all the antibody preps were confirmed with < 1EU/mL endotoxin levels (Charles River Endosafe cartridge technology).
T cell stimulation
Single cell suspensions were prepared from tumors upon sacrifice and leukocytes were enriched with a Percoll™ (GE Healthcare) gradient. Obtained cells were cultured in complete RPMI with or without PMA (10−7M, Sigma)-Ionomycin (500ng/mL, Sigma) and Protein Transport Inhibitor Cocktail (eBioscience, 500X) for 4 h. Cells were collected and stained for flow cytometry analysis.
Immunofluorescence staining and confocal imaging
Tumor masses were resected and fixed in 4% PFA at 4°C overnight. Fixed specimens were then dehydrated in 30% sucrose solution and cut into 50um-thick sections at the cryostat. Staining on free-floating sections was performed. Cryosections were blocked 4 h in 5%BSA solution and stained for 48 h at 4°C with rabbit anti-Iba1 (Cell Signaling Technology clone E404W, dilution 1:500) and rat anti-CD206-Alexafluor488 (Biolegend clone C068C2, dilution 1:200). Secondary staining was performed at room temperature for 2 h with fluorochrome-conjugated antibody (Life Technology Goat anti-rabbit Alexa-647, dilution 1:1000) and DAPI (Sigma, dilution 1:4000). Sections were then mounted on Superfrost glass slides (Fisher Scientific) and embedded in prolong diamond anti-fade mounting media (Thermofisher). Confocal imaging was carried out using a Zeiss LSM880 airyscan confocal microscope, with a 40X/1.4 oil-immersion objective. Each image was acquired in z stack/tile-scan mode to cover an area of 1mm2, and 10um of thickness. Percentage of staining covered area was calculated in ImageJ, after automated background thresholding.
Single cell RNaseq
Live CD45+ cells were sorted from processed tumors. Using the Chromium Single Cell 30 Reagent Kit v3 User Guide, single cell suspensions were partitioned into nanoliter droplets called Gel-bead-in-Emulsions (GEMs) to achieve single cell resolution. The cDNA generated within each individual GEMis tagged with a common 16nt 10× barcode and a 12nt unique molecular barcode during the RT reaction. Purified cDNA was amplified for 11 cycles before being purified using SPRIselect beads. Samples were then run on a Agilent Bioanalyzer to determine cDNA concentration. 10uL of purified cDNA was used to generate the Illumina library for sequencing. For sample preparation on the 10× Genomics platform, the Chromium Single Cell 30 GEM, Library & Gel Bead Kit v3 16 rxns (PN-1000075), Chromium Chip B Single Cell Kit, 48 rxns (PN-1000073) and Chromium i7 Multiplex Kit (PN-120262) were used. The single cell libraries were then sequenced on the Illumina NovaSeq 6000 S4 200 cycle flow cell generating 28×98 reads. A median sequencing depth of 50,000 reads/cell was targeted for each sample.
Immunohistochemistry
Four-micron thick tissue sections were used for immunohistochemical staining. Sections were incubated with anti-TREM2 antibody (clone D8I4C, 1:100, Cell Signaling Technology) and the reaction was revealed using Novolink Polymer (Leica Microsistem). For double staining, after completing the first immune reaction, the second was visualized using Mach 4 MR-AP (Biocare Medical), followed by Ferangi Blue. Finally, the slides were counterstained with Meyer’s Haematoxylin. For double stain, TREM-2 were coupled with anti-CD163 (clone 10D6, mouse, 1:50, Thermo Scientific), anti-CD68 (clone PG-M1, 1:200, Dako), anti-MITF (clone D5, 1:50, Dako), anti-CD1c (clone OTI2F4, 1:300, Abcam), anti-CD207 (clone 12D6, 1:150, Leica), anti-MAFB (polyclonal rabbit, 1:400, Sigma) and anti-CSF-1R (clone FER216, 1:1500, Millipore).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis
Data were shown as mean ± SEM Two-way ANOVA was used to model data generated from factorial design with the combination of 2 factors and two-way ANOVA for repeated-measures was used to model longitudinal tumor growth between treatments followed by post hoc comparisons on treatment difference at time points. Mann–Whitney U-test was used to compare two groups. Statistics were calculated with GraphPad Prism 6 (GraphPad Software).
Statistics and reproducibility
Figures 1A and 1B: n = 25 (Trem2+/+); n = 24 (Trem2−/−); four pooled experiments out of ten performed. Figure 1C: n = 8–15. Figure 1C (first panel): two pooled experiments. Figure 1C (T cells): three pooled experiments. Figure 1E: n = 4; one experiment performed; Figure 2: n = 2; one experiment performed. Figure 4A: n = 5 (CTRL); n = 5 (aPD1); n = 4 (aPD1II); one experiment performed. Figures 4B and 4C: n = 5; three experiments performed. Figures 4D and 4E: n = 4 (Trem2+/+ CTRL); n = 5 (Trem2−/− CTRL; Trem2+/+ aPD1; Trem2−/− aPD1); two experiments performed. Figure 4F: n = 7–8; pool of two experiments. Figures 5A and 5B: n = 5; three experiments performed. Figure 5C: n = 4 (CTRL; CTRL Trem2−/−; αTREM2 Trem2−/−); n = 5 (αTREM2); one experiment performed. Figures 5D and 5E: one experiment performed. Figure 6: n = 2; one experiment performed.
TCGA cohorts correlation analyses
The phenotype dataset (with survival outcomes) and the RNaseq dataset of each cancer cohort (the gene-level transcription estimates, as in log2(x+1) transformed RSEM normalized count) were downloaded from UCSC Xena (https://xenabrowser.net/) where genes are mapped onto the human genome coordinates using UCSC Xena HUGO probeMap in the RNaseq dataset. Spearman correlation coefficient was calculated between TREM2 and each of the genes in each cancer cohort, accompanied with sample size and p values. The individual scatterplot of each of the top genes with TREM2 (with fitted linear lines) was generated with Spearman correlation in the plot.
The Kaplan-Meier curve for overall survival (OS) and relapse free survival (RFS) was generated for TREM2 dichotomized by 75% quantile of TREM2 expression into high/low TREM2 expression group. The log-rank test was applied to test the survival difference between high/low TREM2 expression.
Single cell RNaseq analysis
Cellranger cell count was used to align samples to the reference mm10 genome and quantify reads. The Seurat package (Butler et al., 2018) in R was used for subsequent analysis. Cells with mitochondrial content greater than 12.5% and with less than 1100 genes were removed. Cells identified as doublets or mutliplets based on gene expression signatures, when more than one cell population-specific marker gene was highly expressed in one cell, were filtered out. Filtered data were normalized using a scaling factor of 10,000, nUMI was regressed with a negative binomial model, and data was log transformed. The highly variable genes were selected using the FindVariableFeatures. Principal component analysis was performed using the top 3000 variable genes. Clustering was performed using the FindClusters function. UMAP was used to project cells into two dimensions using 20 first principal components. For myeloid/lymphoid cells re-clustering we chose clusters that were identified as myeloid/lymphoid cells. For this cells we performed normalization, found variable genes and performed PCA, UMAP and clustering as described above. All visualization was done with ggplot2 R package (Wickham, 2016), heatmaps were done with Phantasus website (https://artyomovlab.wustl.edu/phantasus/).
For the heatmap showing the magnitude of difference/similarity of each cluster, we averaged gene expression across all clusters within all the conditions in the TREM2 knockout experiment. To form the signature of the anti-TREM2 treatment experiment, we determined differential expression for each cluster versus all to find the most differentially expressed genes and, for each cluster, we used the 25 most differentially expressed genes to form the signature. Then, we used averaged expression of these 25 genes to form a heatmap comparing clusters from the anti-Trem2 experiment with clusters from the TREM2 knockout experiment.
Supplementary Material
Highlights.
TREM2 is expressed by tumor-associated macrophages in different types of tumors
TREM2 deficiency and anti-TREM2mAb treatment both curb tumor growth in mice
Anti-PD-1 treatment is more efficacious when TREM2 is either absent or bound by a mAb
Modulation of TREM2 remodels the tumor macrophage landscape
ACKNOWLEDGMENTS
We thank the McDonnell Genome Institute at Washington University for sequencing, Ali Ellebedy and Aaron Schmitz for advising in the generation of the recombinant antibody, David Holtzman for supporting antibody purification, and Erica Lantelme and the Flow Cytometry Core at Washington University for sorting. We also thank Wandy Beatty, director of the Molecular Microbiology Imaging Facility at Washington University, for the valuable technical support. M.M. is a recipient of the Cancer Research Institute-Lloyd J. Old Memorial Fellowship in Tumor Immunology. W.V. is supported by AIRC IG23179. R.F. was supported by grants from the National Institutes of Health (R01 AR066551 and R01 CA235096). R.D.S. was supported by grants from the National Cancer Institute of the National Institutes of Health (RO1CA190700), the Parker Institute for Cancer Immunotherapy, the Cancer Research Institute, Janssen Pharmaceutical Company of Johnson and Johnson, the Prostate Cancer Foundation, and a Stand Up to Cancer-Lustgarten Foundation Pancreatic Cancer Foundation Convergence Dream Team translational research grant. Stand Up to Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. E.R.U. received support from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (AI114551 and DK058177).
DECLARATION OF INTERESTS
M. Colonna received research support from Alector, Amgen, Ono, and Pfizer for activities not related to the findings described in this publication. M. Colonna is a scientific advisory board member of Alector, Cell Signaling Technologies, and Bluefin, and has a patent to TREM2 pending. R.D.S. is a cofounder, scientific advisory board member, stockholder, and royalty recipient of Jounce Therapeutics and Neon Therapeutics and is a scientific advisory board member for A2 Biotherapeutics, BioLegend, Codiak Biosciences, Constellation Pharmaceuticals, NGM Biopharmaceuticals, and Sensei Biotherapeutics.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.07.013.
REFERENCES
- Allavena P, Chieppa M, Bianchi G, Solinas G, Fabbri M, Laskarin G, and Mantovani A (2010). Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin. Dev. Immunol. 2010, 547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, Meng W, Lichti CF, Esaulova E, Vomund AN, et al. (2019). MHCII neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlauckas SP, Garren SB, Garris CS, Kohler RH, Oh J, Pittet MJ, and Weissleder R (2018). Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics 8, 5842–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, Krishnan V, Hatakeyama J, Dorigo O, Barkal LJ, and Weissman IL (2019). CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SK, and Mantovani A (2012). Orchestration of metabolism by macrophages. Cell Metab. 15, 432–437. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Hernández-Munain C, Cella M, and Colonna M (2001). A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J. Exp. Med. 194, 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz ML, and Krummel MF (2015). The emerging understanding of myeloid cells as partners and targets in tumor rejection. Cancer Immunol. Res. 3, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L, and Pollard JW (2018). Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 17, 887–904. [DOI] [PubMed] [Google Scholar]
- Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, and Colonna M (2003). Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 198, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, Wolf D, Saliba AE, and Zernecke A (2018). Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 122, 1661–1674. [DOI] [PubMed] [Google Scholar]
- Costa ML, Robinette ML, Bugatti M, Longtine MS, Colvin BN, Lantelme E, Vermi W, Colonna M, Nelson DM, and Cella M (2017). Two Distinct Myeloid Subsets at the Term Human Fetal-Maternal Interface. Front. Immunol. 8, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, and Flavell RA (2013). Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13, 759–771. [DOI] [PubMed] [Google Scholar]
- Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, and Li MO (2014). The cellular and molecular origin of tumor-associated macrophages. Science 344, 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. (2000). Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, and Colonna M (2008). DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 205, 2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Esaulova E, Ward JP, Malkova ON, Runci D, Wong P, Noguchi T, Arthur CD, Meng W, Alspach E, et al. (2018). High-Dimensional Analysis Delineates Myeloid and Lymphoid Compartment Remodeling during Successful Immune-Checkpoint Cancer Therapy. Cell 175, 1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjunpää H, Blake SJ, Ahern E, Allen S, Liu J, Yan J, Lutzky V, Takeda K, Aguilera AR, Guillerey C, et al. (2018). Deficiency of host CD96 and PD-1 or TIGIT enhances tumor immunity without significantly compromising immune homeostasis. OncoImmunology 7, e1445949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs JB Jr., Taintor RR, and Vavrin Z (1987). Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235, 473–476. [DOI] [PubMed] [Google Scholar]
- Hoves S, Ooi CH, Wolter C, Sade H, Bissinger S, Schmittnaegel M, Ast O, Giusti AM, Wartha K, Runza V, et al. (2018). Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J. Exp. Med. 215, 859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M, et al. (2015). Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 75, 3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA, and MacDonald KP (2012). Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820. [DOI] [PubMed] [Google Scholar]
- Iribarren K, Buque A, Mondragon L, Xie W, Lévesque S, Pol J, Zitvogel L, Kepp O, and Kroemer G (2018). Anticancer effects of anti-CD47 immunotherapy in vivo. OncoImmunology 8, 1550619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, and Hamerman JA (2012). TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur. J. Immunol. 42, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, et al. (2019). Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 178, 686–698.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, Remark R, Sweeney R, Becker CD, Levine JH, et al. (2017). Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 169, 750–765.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, and Allison JP (1996). Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736. [DOI] [PubMed] [Google Scholar]
- Li XY, Das I, Lepletier A, Addala V, Bald T, Stannard K, Barkauskas D, Liu J, Aguilera AR, Takeda K, et al. (2018). CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J. Clin. Invest. 128, 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lin EY, Nguyen AV, Russell RG, and Pollard JW (2001). Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M, Kim HS, Tong RK, Bainbridge TW, Vernes JM, Zhang Y, Lin YL, Chung S, Dennis MS, Zuchero YJ, et al. (2017). Effector-attenuating Substitutions That Maintain Antibody Stability and Reduce Toxicity in Mice. J. Biol. Chem. 292, 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Marchesi F, Malesci A, Laghi L, and Allavena P (2017). Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojalvo LS, King W, Cox D, and Pollard JW (2009). High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am. J. Pathol. 174, 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, and Humphrey MB (2010). TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci. Signal. 3, ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, Portman JR, Matchett KP, Brice M, Marwick JA, et al. (2019). Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian M, LaFleur MW, Verschoor VL, Dongre A, Zhang Y, Nguyen TH, Kolifrath S, Aref AR, Lau CJ, Paweletz CP, et al. (2019). Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade. Proc. Natl. Acad. Sci. USA 116, 16971–16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. (2014). Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859. [DOI] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, and Melief CJ (1998). T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393, 480–483. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, and Smyth MJ (2011). Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, and Schreiber RD (2001). IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111. [DOI] [PubMed] [Google Scholar]
- Sharma P, and Allison JP (2015). The future of immune checkpoint therapy. Science 348, 56–61. [DOI] [PubMed] [Google Scholar]
- Shi FD, Flodström M, Kim SH, Pakala S, Cleary M, Ljunggren HG, and Sarvetnick N (2001). Control of the autoimmune response by type 2 nitric oxide synthase. J. Immunol. 167, 3000–3006. [DOI] [PubMed] [Google Scholar]
- Song W, Hooli B, Mullin K, Jin SC, Cella M, Ulland TK, Wang Y, Tanzi RE, and Colonna M (2017). Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimers Dement. 13, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Hawkins GA, Wudel L, Chou PC, Forbes E, Pullikuth AK, Liu L, Jin G, Craddock L, Topaloglu U, et al. (2019). Dissecting intratumoral myeloid cell plasticity by single cell RNA-seq. Cancer Med. 8, 3072–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer D, Zhu X, McMahon M, Turner JS, Wohlbold TJ, Schmitz AJ, Strohmeier S, Yu W, Nachbagauer R, Mudd PA, et al. (2019). Broadly protective human antibodies that target the active site of influenza virus neuraminidase. Science 366, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr DJ, and Nathan CF (1989). Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169, 1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Esser AK, Amend SR, Xiang J, Xu Y, Ross MH, Fox GC, Kobayashi T, Steri V, Roomp K, et al. (2016). Antagonizing Integrin β3 Increases Immunosuppression in Cancer. Cancer Res. 76, 3484–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, and Pardoll DM (2015). Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M, Piccio L, Hernandez M, and Colonna M (2006). Cutting edge: TREM-2 attenuates macrophage activation. J. Immunol. 177, 3520–3524. [DOI] [PubMed] [Google Scholar]
- Ulland TK, and Colonna M (2018). TREM2 - a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 14, 667–675. [DOI] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, et al. (2017). TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 170, 649–663.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, Perego M, and Gabrilovich D (2018). Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, and Chen J (2018). SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 39, 173–184. [DOI] [PubMed] [Google Scholar]
- Vermi W, Micheletti A, Lonardi S, Costantini C, Calzetti F, Nascimbeni R, Bugatti M, Codazzi M, Pinter PC, Schäkel K, et al. (2014). slanDCs selectively accumulate in carcinoma-draining lymph nodes and marginate metastatic cells. Nat. Commun. 5, 3029. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. (2015). TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, Yuan P, Mahan TE, Shi Y, Gilfillan S, et al. (2016). TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 213, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ECE, Dai Z, Ferrante AW, Drake CG, and Christiano AM (2019). A Subset of TREM2(+) Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell stem cell 24, 654–669.e6. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2-Elegant Graphics for Data Analysis (Springer; ). [Google Scholar]
- Wiehagen KR, Girgis NM, Yamada DH, Smith AA, Chan SR, Grewal IS, Quigley M, and Verona RI (2017). Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumor Immunity. Cancer Immunol. Res. 5, 1109–1121. [DOI] [PubMed] [Google Scholar]
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al. (2012). The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 109, 6662–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. (2012). Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Byers DE, Jin X, Agapov E, Alexander-Brett J, Patel AC, Cella M, Gilfilan S, Colonna M, Kober DL, et al. (2015). TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J. Exp. Med. 212, 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Chen XF, Wang T, Wang Z, Liao C, Wang Z, Huang R, Wang D, Li X, Wu L, et al. (2017). Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med. 214, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated in this study are available at GEO by GSE151710 ID.