Abstract
Leukocyte arrest on the endothelial cell surface during leukocyte extravasation is induced by rapid integrin activation by chemokines. We recently reported that fractalkine induces integrin activation without its receptor CX3CR1 through binding to the allosteric site (site 2) of integrins. Peptides from site 2 bound to fractalkine and suppressed integrin activation by fractalkine. We hypothesized that this is not limited to membrane-bound fractalkine. We studied if stromal cell-derived factor 1 (SDF1), another chemokine that plays a critical role in leukocyte arrest, activates integrins through binding to site 2. We describe here that (1) SDF1 activated soluble integrin αvβ3 in cell-free conditions, suggesting that SDF1 can activate αvβ3 without CXCR4; (2) site 2 peptide bound to SDF1, suggesting that SDF1 binds to site 2; (3) SDF1 activated integrins αvβ3, α4β1, and α5β1 on CHO cells (CXCR4-negative) and site 2 peptide suppressed the activation; (4) A CXCR4 antagonist AMD3100 did not affect the site 2-mediated integrin activation by SDF1; (5) Cell-surface integrins were fully activated in 1 min (much faster than activation of soluble αvβ3) and the activation lasted at least for 1 h. We propose that the binding of SDF1 to cell-surface proteoglycan facilitates the allosteric activation process; (6) Mutations in the predicted site 2-binding site in SDF1 suppressed integrin activation. These results suggest that SDF1 (e.g., presented on proteoglycans) can rapidly activate integrins in an allosteric manner by binding to site 2 in the absence of CXCR4. The allosteric integrin activation by SDF1 is a novel target for drug discovery.
Keywords: Integrin activation, Stromal cell-derived factor-1, allosteric ligand-binding site, chemokine
Integrins are a family of cell adhesion receptors that recognize ECM ligands, cell surface ligands, and soluble ligands (1). Integrins are transmembrane heterodimers, and at least 18 α and 8 β subunits are known (2). Leukocyte arrest on specific target vascular cells involves adhesive cascades mediated by sequential events, initiated by selectin-mediated adhesions (leukocyte rolling), followed by firm integrin-mediated arrest on immunoglobulin superfamily integrin ligands such as ICAM-1, VCAM-1 and MadCAM-1 (3). The leukocyte arrests involve an abrupt activation of leukocyte integrins by specialized chemokines displayed on the endothelial surface (arrest chemokines) (4–6). Leukocyte rolling is a critical step for leukocytes to encounter chemokines on the endothelial surface. Chemokines like CXCL12 (stromal cell-derived factor 1, SDF1), CCL21, CXCL1, CCL2 and CCL25 are the most potent physiological inducers of integrin-dependent leukocyte arrest, and subsequent leukocyte crawling over and diapedesis through vascular barriers. The mechanism of the abrupt activation of integrins by arrest chemokines, however, has not been established.
We previously reported that the chemokine domain of transmembrane chemokine fractalkine (FKN-CD) is an integrin ligand that binds to the classical ligand (e.g., RGD)-binding site of integrins (site 1) and induces integrin-FKN-CX3CR1 ternary complex (7). Also, the integrin-binding defective FKN mutant is an antagonist of FKN/CX3CR1 signaling, suggesting that integrins play a role in CX3CR1-dependent FKN signaling (7). It has been believed that integrin activation by chemokine is mediated by chemokine receptors and subsequently through signaling from inside the cells (inside-out signaling). Unexpectedly, we discovered that FKN-CD activates integrins in the absence of CX3CR1 by binding to another ligand-binding site (site 2) of integrins (8). Site 2 is located on the opposite side of site 1 in the integrin headpiece (8). Peptides from site 2 (e.g., residues 267–286 of β3) directly bound to FKN-CD and suppressed FKN-CD-induced integrin activation (8). Thus FKN-CD binding to site 2 induces activation of site 1 though conformational changes (in an allosteric mechanism). We also identified the secreted phospholipase A2-type IIA (sPLA2-IIA) as another integrin ligand that binds to site 2 (in addition to site 1) and activates integrins (9). FKN is a transmembrane-type chemokine, and thus it is possible that the direct integrin binding and the site-2-mediated integrin activation are unique to FKN. It is unclear if soluble chemokines can activate integrins through binding to site 2. One of the soluble chemokine SDF1 is a potent chemoattractant for leukocytes and is believed to regulate signaling events through two different receptors, CXCR4 and CXCR7 in leukocytes (10–13). Binding of CXCL12 to CXCR4 induces trimeric G protein signaling leading to activation of the Src, phosphoinositide-3 kinase (PI3K)/AKT, ERK, and JNK pathways, contributing to pro-inflammatory function such as protease production and cellular migration (14,15). Dysregulated expression of SDF1/CXCR4 were reported in rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis and inflammatory bowel disease (16–19). Indeed, both small molecule CXCR4 antagonists and CXCR4 knock-out mice exhibited reduced joint inflammation in both humans and a mouse model of arthritis, suggesting that SDF1/CXCR4 play a role in the recruitment of inflammatory cells to the joint (20–23). However, it is unclear that SDF1 acts through only CXCR4 and CXCR7. SDF1 may activate integrins in a receptor-independent manner as in the case of FKN or sPLA2-IIA.
In the present study, we determined if SDF1 also activates integrins in the absence of its receptors. We found that SDF1 activated integrins in a site-2 specific and CXCR4-independent manner. We propose that activation of integrins by binding to site 2 of integrins is a common mechanism of rapid integrin activation by arrest chemokines.
Materials and Methods
Materials-
GST-fusion proteins of fibronectin type III domains 8–11 (FN8–11) (7), and fibronectin H120 fragment (FN-H120) were described (24). Fibrinogen γ-chain C-terminal domain that lacks residues 400–411 (γC399tr) was synthesized as described (25).
Synthesis of SDF1-
The cDNA fragment of SDF1 was amplified using primers 5’-CGGGATCCAAGCCCGTCAGCCTGAGC-3’ and 5’-CGGAATTCTCACATCTTGAACCTCTTGTTTAAAGC-3’ with human SDF1 cDNA (Open Biosystems) as a template, and subcloned into the BamHI/EcoRI site of PET28a expression vector. The protein was synthesized in BL21 induced by isopropyl β-D-thiogalactoside as an insoluble protein. The protein was solubilized in 8 M urea, purified by Ni-NTA affinity chromatography, and refolded as previously described (26). The refolded protein was >90% homogeneous upon SDS-PAGE.
Activation of soluble αvβ3 by SDF1-
ELISA-type binding assays were performed as described previously (7). Briefly, wells of 96-well Immulon 2 microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated with 100 μl 0.1 M PBS containing γC399tr for 2 h at 37°C. Remaining protein binding sites were blocked by incubating with PBS/0.1% BSA for 30 min at room temperature. After washing with PBS, soluble recombinant αvβ3 (5 μg/ml) in the presence or absence of SDF1 was added to the wells and incubated in Hepes-Tyrodes buffer (10 mM HEPES, 150 mM NaCl, 12 mM NaHCO3, 0.4 mM NaH2PO4, 2.5 mM KCl, 0.1% glucose, 0.1% BSA) with 1 mM CaCl2 for 1 h at room temperature. After unbound αvβ3 was removed by rinsing the wells with binding buffer, bound αvβ3 was measured using anti-integrin β3 mAb (AV-10) followed by HRP-conjugated goat anti-mouse IgG and peroxidase substrates. For the time-course experiments, WT SDF1 and soluble αvβ3 were incubated for 1–60 min instead of 1 h.
Activation of integrins on the cell surface by SDF1-
Cells were cultured to nearly confluent in RPMI1640/10% FCS (K562 and U937 cells) or DMEM/10% FCS (CHO cells). Cells were resuspended with RPMI1640/0.02% BSA or DMEM/0.02% BSA and incubated for 30 min at room temperature to block protein binding sites. Cells were then incubated with WT SDF1 or mutants for 5 min at room temperature and then incubated with FITC-labeled integrin ligands (γC399tr, FN-H120, or FN8–11) for 15 min at room temperature. Cells were washed with PBS/0.02% BSA and analyzed by FACSCalibur (Becton Dickinson, Mountain View, CA). For blocking experiments, SDF1 was preincubated with Fc-β3 peptide for 30 min at room temperature. For blocking with AMD3100, cells were preincubated with AMD3100 (10 μM) for 30 min at room temperature. For time-course experiments, cells were incubated with WT SDF1 for 1–60 min at room temperature and then incubated with FITC-labeled integrin ligands for 5 min.
Fc site 2 peptide-
The Fc fragment of human IgG1 of the pFUSE-hIgG1-Fc1 vector was amplified and subcloned into the NdeI/NheI site of pET28a (designated pET28a-FcN). Site 2 peptide (QPNDGQSHVGSDNHYSASTTM, residues 267–287 of β3, C273 is changed to S) and a scrambled site 2 peptide (VHDSHYSGQGAMSDNTNSPQT) were synthesized as described (8) except that the BamHI/EcoRI fragment was subcloned into the BamHI/EcoRI site of pET28a-FcN. Proteins were synthesized in E. coli as insoluble proteins, purified by Ni-NTA affinity chromatography and refolded as described (7). The inserts were identified by DNA sequencing.
Binding of S2 peptide to SDF1-
ELISA-type binding assays were performed as described previously (7). Briefly, wells of 96-well Immulon 2 microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated with Fc-β3 peptide in 100 μl 0.1 M PBS for 2 h at 37°C. Remaining protein binding sites were blocked by incubating with PBS/0.1% BSA for 30 min at room temperature. After washing with PBS, SDF1 was added to the wells and incubated in PBS for 2 h at room temperature. After unbound SDF1 was removed by rinsing the wells with PBS, bound SDF1 was measured using anti-SDF1 antibody (R&D systems MAB350, mouse monoclonal antibody to Lys22-Lys89 of SDF1) and HRP-conjugated anti-mouse IgG.
Docking simulation-
Docking simulation of interaction between SDF1 and integrin αvβ3 (closed headpiece form, PDB code 1JV2) was performed using AutoDock3 as described (27). We used the headpiece (residues 1–438 of αv and residues 55–432 of β3) of αvβ3 (closed form, 1JV2.pdb). Cations were not present in integrins during docking simulation, as in the previous studies using αvβ3 (open form, 1L5G.pdb) (27,28). The ligand is presently compiled to a maximum size of 1024 atoms. Atomic solvation parameters and fractional volumes were assigned to the protein atoms by using the AddSol utility, and grid maps were calculated by using AutoGrid utility in AutoDock 3.05. A grid map with 127 × 127 × 127 points and a grid point spacing of 0.603 Å included the headpiece of αvβ3 (residue 1–438 of αv and residues 55–432 of β3). Kollman “united-atom” charges were used. AutoDock 3.05 uses a Lamarckian genetic algorithm (LGA) that couples a typical Darwinian genetic algorithm for global searching with the Solis and Wets algorithm for local searching. The LGA parameters were defined as follows: the initial population of random individuals had a size of 50 individuals; each docking was terminated with a maximum number of 1 × 106 energy evaluations or a maximum number of 27,000 generations, whichever came first; mutation and crossover rates were set at 0.02 and 0.80, respectively. An elitism value of 1 was applied, which ensured that the top ranked individual in the population always survived into the next generation. A maximum of 300 iterations per local search was used. The probability of performing a local search on an individual was 0.06, whereas the maximum number of consecutive successes or failures before doubling or halving the search step size was 4.
Other methods-
Treatment differences were tested using ANOVA and a Tukey multiple comparison test to control the global type I error using Prism 7 (Graphpad Software).
Results
SDF1 activates soluble integrin αvβ3 in cell-free conditions without CXCR4-
We previously reported that transmembrane chemokine FKN activates integrins in a CX3CR1-independent and site 2-dependent manner (8). This may be a mechanism of rapid integrin activation by FKN that leads to strong adhesion of leukocytes to the endothelial surface during leukocyte extravasation (4–6). It is unclear if this is limited to the transmembrane chemokine FKN. To address this question, we performed docking simulation of interaction between SDF1 (PDB code 1VMC) and integrin αvβ3 (PDB code 1JV2), which has been shown to have a closed headpiece (29). The simulation predicts that SDF1 binds to site 2 of αvβ3 (docking energy 18.5 kcal/mol) (Fig. 1a). The amino acid residues involved in the predicted model are shown in Table 1. To determine if SDF1 binds to site 2, we studied if site 2 peptide of β3 fused to Fc (Fc-β3) bind to SDF1. When SDF1 (His-tagged) is immobilized directly to wells of 96-well microtiter plate it did not bind to Fc-β3 (data not shown). So, we immobilized Fc-β3, incubated with soluble SDF1, and detected SDF1 using anti-SDF1 antibody (Fig. 1b). We found that SDF1 bound to Fc-β3 to the level much higher than that of parent Fc or scrambled β3 peptide (Fc-β3scr), suggesting that SDF1 binds to site 2. We studied if soluble chemokine SDF1 enhances the binding of soluble αvβ3 (extracellular domains) to its specific ligand γC399tr in cell-free conditions. To keep soluble αvβ3 in an inactive state, 1 mM Ca2+ was included in the assay medium. We found that SDF1 markedly enhanced the binding of soluble αvβ3 to γC399tr in a concentration- (Fig. 1c) and time-dependent manner (Fig. 1d). These findings suggest that SDF1 can activate soluble αvβ3 in cell-free conditions without CXCR4, while SDF1 at > 5 μg/ml was required to detect enhanced ligand binding to soluble αvβ3. We describe that SDF1 activated integrins on the cell surface at much lower SDF1 concentrations (see below).
Fig. 1. SDF1 binds to and allosterically activates αvβ3 without cognate receptor CXCR4.
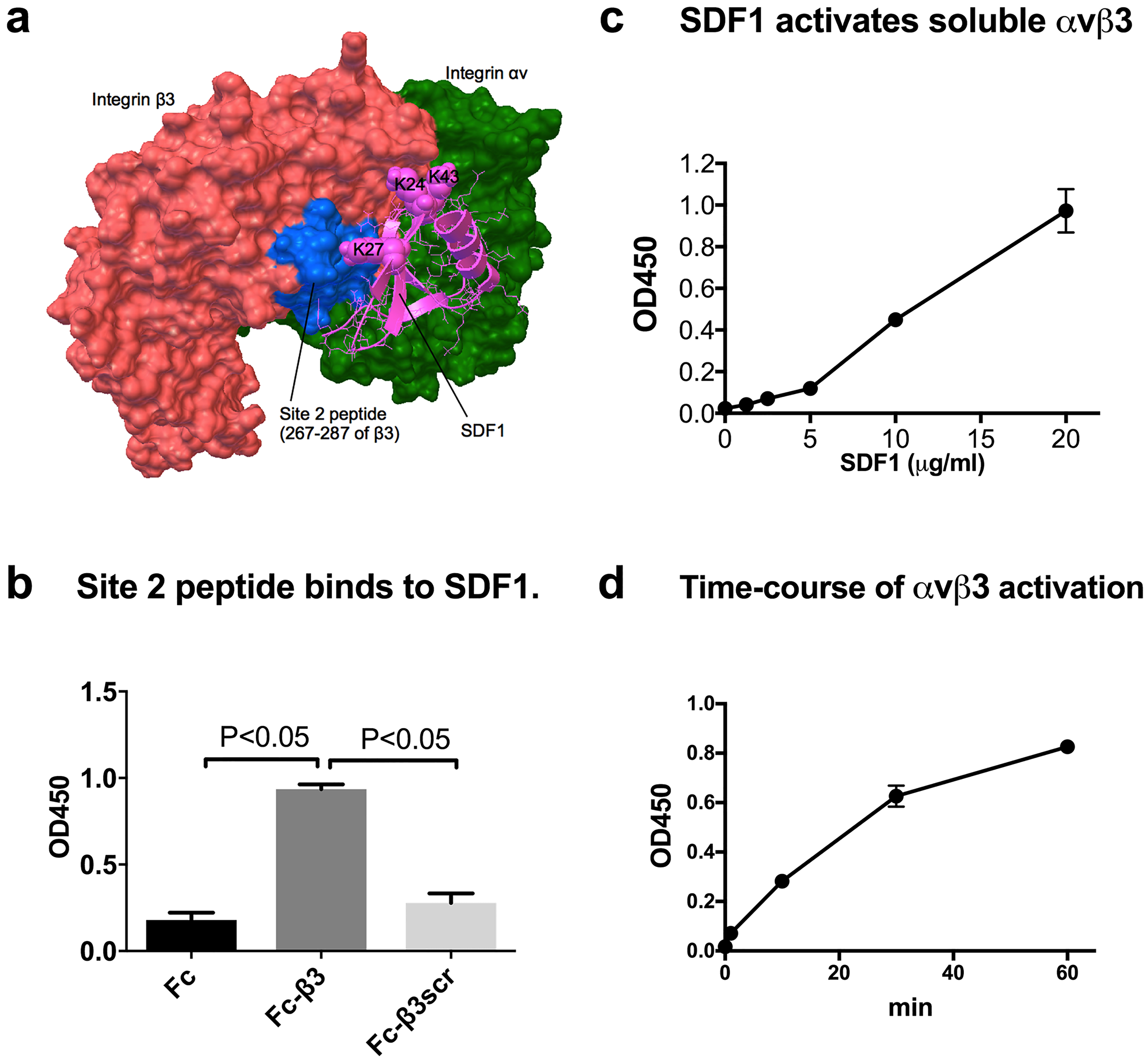
(a) Docking model of SDF1-αvβ3 (inactive) interaction. The position of site 2 peptide of β3 is shown in blue. The simulation predicted amino acid residues involved in integrin binding (Table 1), including Lys24, Lys27, and Lys43 of SDF1. (b) Peptide derived from site 2 (Fc-β3) binds to SDF1. SDF1 binding to immobilized Fc-β3 peptide, scrambled peptide (Fc-β3scr), or Fc was measured by using anti-SDF1 antibody. Data are shown as means +/− SEM of triplicate experiments. (c) SDF1 activates soluble αvβ3 in cell-free conditions. The binding of soluble αvβ3 to immobilized γC399tr was measured as described in the methods section. Data are shown as means +/− SEM of triplicate experiments. (d) Time course of SDF1-induced activation of soluble αvβ3. Data are shown as means +/− SEM of triplicate experiments.
Table 1.
Amino acid residues involved in the interaction between SDF1 and integrin αvβ3 (site 2).
| SDF1 | αv | β3 |
|---|---|---|
| Arg8, Arg12, Phe13, Glu15, His17, Val18, Ala19, asn22, Lys24, Lys27, Leu29, Arg41, Leu42, Lys43, Asn44, Asn45, Asn46, Arg47, Gln48 | Tyr18, Lys42, Asn44, Val51, Glu52, Phe88, Ser90, His91, Gln92, Trp93, Leu111, His113, Arg122 | Pro160, Val161, Ser162, Pro163, Met165, Tyr166, Ile167, Ser168, Glu171, Glu174, Asn175, Met187, Ala263, Gly264, Ile265, Gln267, Asp270, Gln272, Cys273, His274, Val275, Ser277, Asp278, His280, Tyr281, Ser282, Ala283, Ser284, Thr285, Thr286, Met287 |
Amino acid residues within 0.6 nm between SDF1 and αvβ3 were selected using pdb viewer (version 4.1).
Amino acid residues in β3 site 2 peptide (S2-β3) are shown in bold.
SDF1 activates cell-surface integrin αvβ3 in a CXCR4-independent manner-
It has been reported that CHO cells do not normally express CXCR4 or respond to SDF1 (30). We studied if SDF1 activates cell-surface integrin αvβ3 using CHO cells that express recombinant αvβ3 (β3-CHO). We used DMEM that includes high [Ca2+] to keep integrins inactive for integrin activation assays. We previously reported that site 2 peptides did not bind to γC399tr (8). We incubated the cells with FITC-labeled γC399tr in the presence of SDF1 and measured the bound FITC by flow cytometry. We found that SDF1 markedly enhanced binding of γC399tr to αvβ3 in a dose-dependent manner. One ng/ml (0.083 nM) SDF1 induced detectable αvβ3 binding to γC399tr in β3-CHO cells (Fig. 2a). The enhanced binding was inhibited by Fc-β3, but not by control Fc or Fc-β3scr (Fig. 2b). These findings suggest that SDF1 activates αvβ3 by binding to site 2. AMD3100, an inhibitor of CXCR4, did not affect SDF1-induced activation of αvβ3 (Fig. 2c). While CHO cells do not express CXCR4, these findings confirmed that CXCR4 is not involved in SDF1-induced activation of αvβ3 on CHO cells. We substituted Lys residues in the predicted SDF1-integrin interface at positions 24, 27, and 43 of SDF1 to Glu in combination. The SDF1 mutants did not induce integrin activation (Fig. 2d), suggesting that SDF1 binding to site 2 is required for integrin activation. This also suggests that these Lys residues are involved in site 2 binding (Fig. 4b–d), in consistent with the docking model. It has been reported that arrest chemokines very quickly activate integrins (4–6). We measured the time-course of SDF1-induced integrin activation in β3-CHO cells. We found that SDF1 were fully activated in 1 min (the shortest time point we can take in our assay) (Fig. 2e), which is much faster than SDF1-induced activation of soluble integrins in solution (Fig. 1a). We propose that SDF1 may be rapidly concentrated on the cell surface by binding to cell-surface proteoglycans and this process markedly facilitates the SDF1-induced integrin activation.
Fig. 2. SDF1 activates integrin αvβ3 on the cell surface by binding to site 2 in a CXCR4-independent manner.
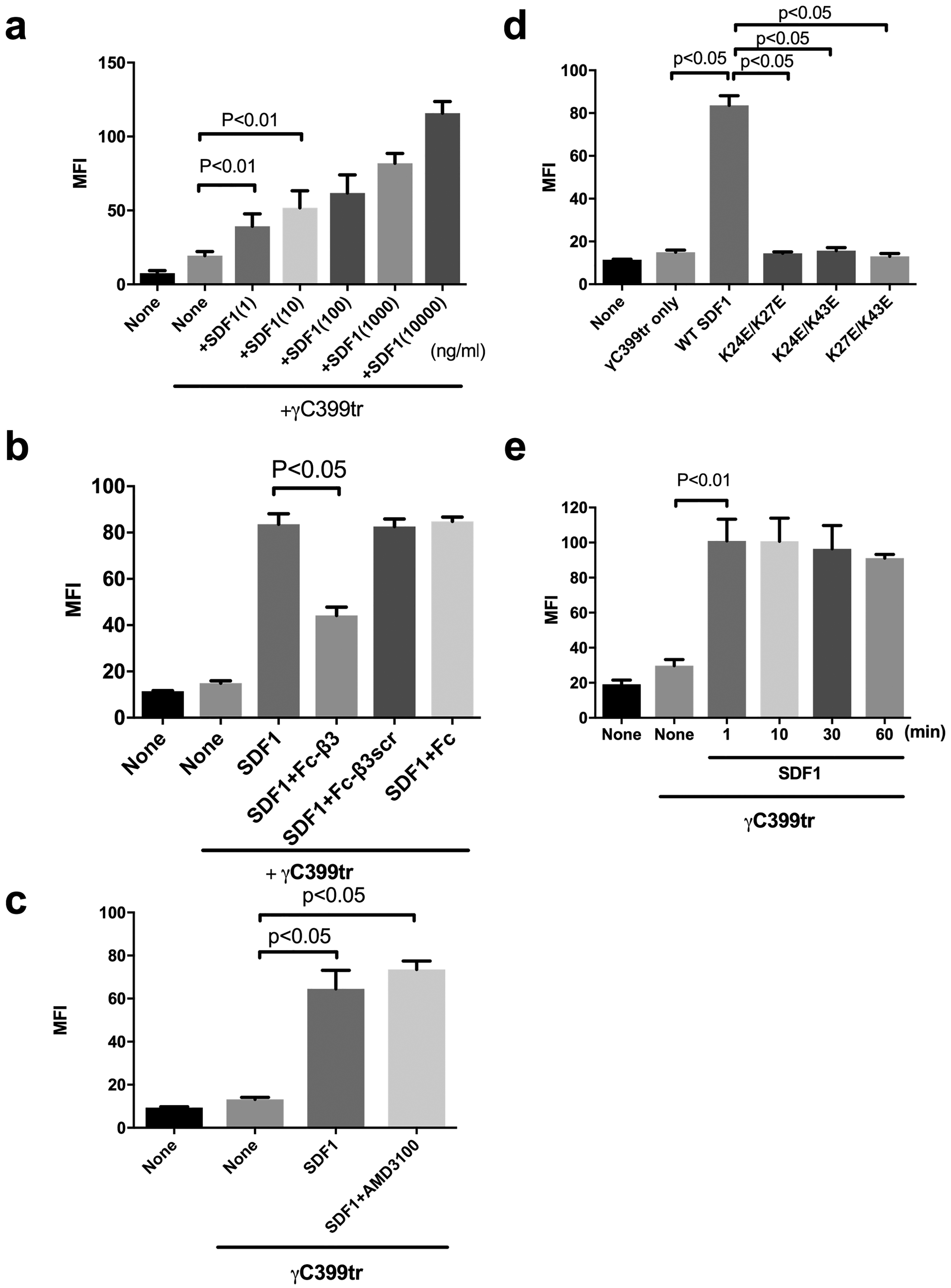
The binding of FITC-labeled γC399tr to the cell-surface αvβ3 on β3-CHO cells in the presence of SDF and Fc-β3 peptide was measured using flow cytometry as described in the methods section. MFI= median fluorescent intensity. Data are shown as means +/− SEM of triplicate experiments. (a) SDF1 activates integrin αvβ3 on β3-CHO cells in a dose-dependent manner (n=4). (b) Fc-β3 peptide, not Fc-β3scr or Fc, suppresses SDF1-mediated activation of integrin αvβ3. (c) AMD3100, a CXCR4 inhibitor, does not affect SDF1-mediated αvβ3 activation in an allosteric manner. (d) SDF1 mutations in the predicted site 2-binding interface of SDF1 are defective in αvβ3 activation. (e) SDF1 fully activates cell-surface αvβ3 in 1 min and the activation lasts for 1 h.
Fig. 4. SDF1 activates integrins α5β1 on the cell surface by binding to site 2 in a CXCR4-independent manner.
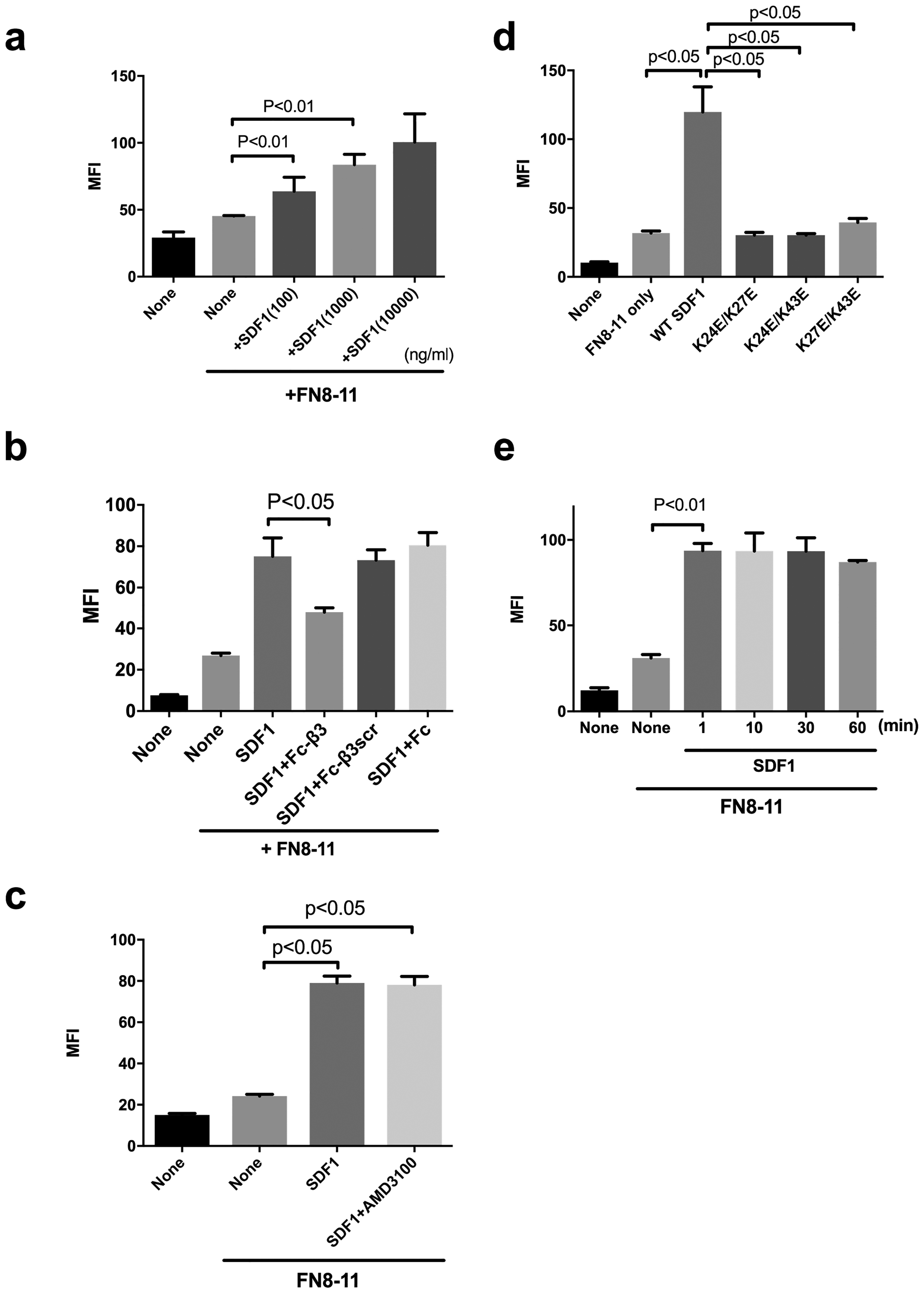
Parent CHO cells (α5β1+) were incubated with FITC-labeled ECM ligands (the fibronectin fragment FN8–11 specific to α5β1) in the presence of SDF1 (20 μg/ml) and/or Fc-β3 peptide (200 μg/ml) and the binding of the ligands was measured in flow cytometry. MFI= median fluorescent intensity. Data are shown as means +/− SEM of triplicate experiments. (a) SDF1 activates integrin α5β1 on CHO cells in a dose-dependent manner (n=4). (b) Fc-β3 peptide, not Fc-β3scr or Fc, suppresses SDF1-mediated activation of integrin α5β1. (c) AMD3100, a CXCR4 inhibitor, does not affect SDF1-mediated integrin α5β1 activation in an allosteric manner. (d) SDF1 mutations in the predicted site 2-binding interface of SDF1 are defective in α5β1 activation. (e) SDF1 fully activates cell-surface α5β1 in 1 min and the activation lasts for 1 h.
SDF1 activates α4β1 and α5β1 through site 2 in a CXCR4-independent manner-
αvβ3 is not a typical leukocyte integrin, but integrins α4β1 and α5β1 play a role in leukocyte functions (1,2). We studied if SDF1 activates α4β1 and α5β1 in CHO cells that express α4β1 (α4-CHO) or parent CHO cells (α5β1+) in DMEM that includes high [Ca2+] to keep integrins inactive. We measured the levels of binding of FITC-labeled ECM ligands [H120, a fibronectin fragment specific to α4β1 (24) and FN8–11, another fibronectin fragment that contains the cell-binding domain specific to α5β1 (14)] to cell surface integrins, and bound FITC was measured by flow cytometry. We obtained very similar results in α4β1 (Fig. 3) and α5β1 (Fig. 4) to that for αvβ3: One ng/ml SDF1 clearly enhanced integrin α4β1 binding to specific ligand H120, while 100 ng SDF1 was required to detect enhanced binding of integrin α5β1 to FN8–11. SDF1 markedly enhanced binding of ECM ligands to integrins α4β1 and α5β1 and the enhanced binding was inhibited by Fc-β3, but not by control Fc or Fc-β3scr. Also, AMD3100 did not affect the SDF1-induced activation of integrins α4β1 and α5β1. Importantly, SDF1 activated integrins in 1 min by binding to site 2 and the activation lasted at least for 1h. These findings suggest that SDF1-induced activation of integrins α4β1 and α5β1 in a site 2-dependent manner is not limited to αvβ3.
Fig. 3. SDF1 activates integrin α4β1 on the cell surface by binding to site 2 in a CXCR4-independent manner.
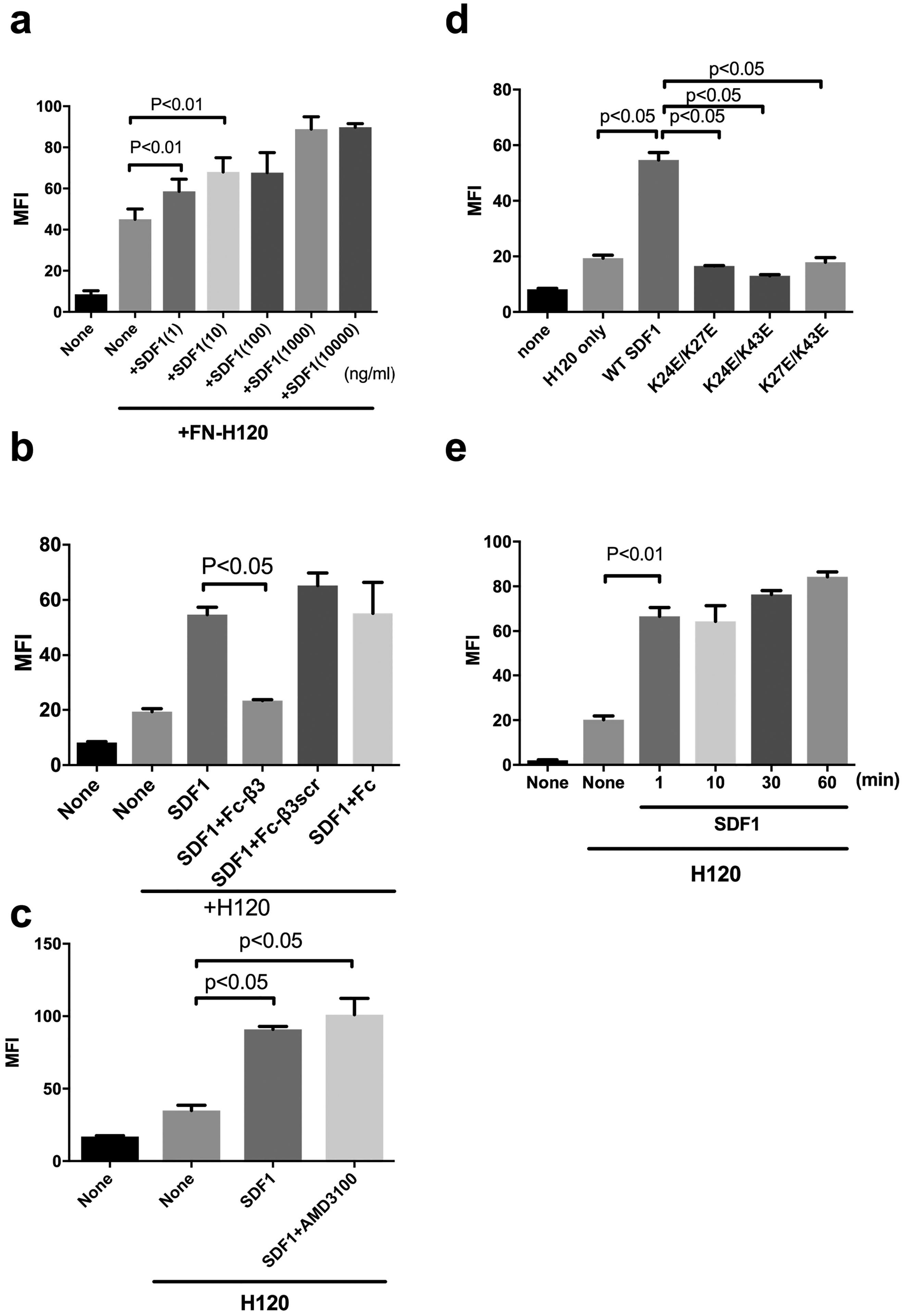
α4-CHO cells were incubated with FITC-labeled ECM ligands (the fibronectin fragment H120 specific to α4β1) in the presence of SDF1 (20 μg/ml) and/or Fc-β3 peptide (200 μg/ml) and the binding of the ligands was measured in flow cytometry. MFI= median fluorescent intensity. Data are shown as means +/− SEM of triplicate experiments. (a) SDF1 activates integrin α4β1 on α4-CHO cells in a dose-dependent manner (n=4). (b) Fc-β3 peptide, not Fc-β3scr or Fc, suppresses SDF1-mediated activation of integrin α4β1. (c) AMD3100, a CXCR4 inhibitor, does not affect SDF1-mediated integrin α4β1 activation in an allosteric manner. (d) SDF1 mutations in the predicted site 2-binding interface of SDF1 are defective in α4β1 activation. (e) SDF1 fully activates cell-surface α4β1 in 1 min and the activation lasts for 1 h.
Discussion
The present study establishes that a) soluble chemokine SDF1 directly binds to the allosteric binding site (site 2) in integrins. We further obtained evidence that b) SDF1 activated soluble αvβ3 in cell-free conditions, which does not involve signal transduction through CXCR4; c) Fc-β3 peptide, not control Fc-β3scr, bound to SDF1, suggesting that SDF1 binds to site 2; d) SDF1 activated integrins αvβ3, α5β1, and α4β1 on the cell surface and Fc-β3 peptide, but not control Fc-β3scr, suppressed SDF1-induced integrin activation; and e) CXCR4 antagonist AMD3100 did not suppress SDF1-mediated integrin activation on CHO cells, which suggests that SDF1 activates integrins by binding to site 2 in an allosteric manner, independent of CXCR4. f) Also, cell-surface integrins were fully activated within 1 min by SDF1, while it took over 1 h for soluble αvβ3 to be fully activated. We were not able to take time points shorter than 1 min. These findings suggest that cell-surface integrin activation by binding of SDF1 to site 2 is rapid and stable. Since SDF1 induced enhanced ligand binding to integrins occurs within or close to biological concentrations of SDF1 (1 or 100 ng/ml), suggesting that the SDF1-induced allosteric integrin activation is biologically relevant. It is unlikely that SDF1 at the biological concentrations (e.g., <10 ng/ml) detectably binds to integrins that has relatively low affinity to ligands (KD 10−6 to 10−7 M). SDF1 is known to bind to heparin and proteoglycans and thus expected to be highly concentrated on the cell surface proteoglycans (33,34). Therefore, we expect that SDF1 that has been presented and concentrated on the surface binds to site 2 of integrins and rapidly activates integrins independent of CXCR4 in physiological and pathological conditions. It is still unclear, however, if SDF1 binds to site 1. SDF1 and FKN are part of “arrest chemokines”, which are involved in tight binding of leukocytes to endothelial surface through rapid integrin activation (sub second) and subsequent extravasation (4). We propose that integrin activation by SDF1 and FKN (and perhaps other arrest chemokines) in a cognate GPCR-independent manner plays a role in rapid integrin activation (e.g., during leukocyte extravasation). The binding of chemokines to site 2 is a novel target for drug discovery.
Acknowledgement:
This work was partly supported by NIH R33CA196445 (to YT) and funding from The Kanae Foundation for the Promotion of Medical Science and from Mitsubishi Tanabe Pharma Corporation (to MF).
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2.Takada Y, Ye X, and Simon S (2007) The integrins. Genome Biol 8, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 [DOI] [PubMed] [Google Scholar]
- 4.Ley K (2014) Arrest chemokines. Front Immunol 5, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, and Butcher EC (1998) Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279, 381–384 [DOI] [PubMed] [Google Scholar]
- 6.Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, and Alon R (2005) Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nature immunology 6, 497–506 [DOI] [PubMed] [Google Scholar]
- 7.Fujita M, Takada YK, and Takada Y (2012) Integrins alphavbeta3 and alpha4beta1 Act as Coreceptors for Fractalkine, and the Integrin-Binding Defective Mutant of Fractalkine Is an Antagonist of CX3CR1. J Immunol 189, 5809–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita M, Takada YK, and Takada Y (2014) The Chemokine Fractalkine Can Activate Integrins without CX3CR1 through Direct Binding to a Ligand-Binding Site Distinct from the Classical RGD-Binding Site. PLoS One 9, e96372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita M, Zhu K, Fujita CK, Zhao M, Lam KS, Kurth MJ, Takada YK, and Takada Y (2015) Proinflammatory secreted phospholipase A2 type IIA (sPLA-IIA) induces integrin activation through direct binding to a newly identified binding site (site 2) in integrins alphavbeta3, alpha4beta1, and alpha5beta1. J Biol Chem 290, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, and Springer TA (1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382, 829–833 [DOI] [PubMed] [Google Scholar]
- 11.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, and Springer TA (1996) A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 184, 1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, and Bachelerie F (2005) The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 280, 35760–35766 [DOI] [PubMed] [Google Scholar]
- 13.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, and Schall TJ (2006) A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203, 2201–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busillo JM, and Benovic JL (2007) Regulation of CXCR4 signaling. Biochim Biophys Acta 1768, 952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, and Paya CV(2002) G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol 169, 5546–5554 [DOI] [PubMed] [Google Scholar]
- 16.Nanki T, Hayashida K, El-Gabalawy HS, Suson S, Shi K, Girschick HJ, Yavuz S, and Lipsky PE (2000) Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol 165, 6590–6598 [DOI] [PubMed] [Google Scholar]
- 17.Wang A, Guilpain P, Chong BF, Chouzenoux S, Guillevin L, Du Y, Zhou XJ, Lin F, Fairhurst AM, Boudreaux C, Roux C, Wakeland EK, Davis LS, Batteux F, and Mohan C (2010) Dysregulated expression of CXCR4/CXCL12 in subsets of patients with systemic lupus erythematosus. Arthritis and rheumatism 62, 3436–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, Newcombe J, Hohlfeld R, and Meinl E (2006) Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain: a journal of neurology 129, 200–211 [DOI] [PubMed] [Google Scholar]
- 19.Dotan I, Werner L, Vigodman S, Weiss S, Brazowski E, Maharshak N, Chen O, Tulchinsky H, Halpern Z, and Guzner-Gur H (2010) CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflammatory bowel diseases 16, 583–592 [DOI] [PubMed] [Google Scholar]
- 20.Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS, Perretti M, and Pitzalis C (2002) Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID Mice. Arthritis and rheumatism 46, 824–836 [DOI] [PubMed] [Google Scholar]
- 21.Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, Amara A, Curnow SJ, Lord JM, Scheel-Toellner D, and Salmon M (2000) Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol 165, 3423–3429 [DOI] [PubMed] [Google Scholar]
- 22.Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, and Fujii N (2004) Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS letters 569, 99–104 [DOI] [PubMed] [Google Scholar]
- 23.Chung SH, Seki K, Choi BI, Kimura KB, Ito A, Fujikado N, Saijo S, and Iwakura Y (2010) CXC chemokine receptor 4 expressed in T cells plays an important role in the development of collagen-induced arthritis. Arthritis Res Ther 12, R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M, Takada YK, and Takada Y (2015) The chemokine fractalkine can activate integrins without CX3CR1 through direct binding to a ligand-binding site distinct from the classical RGD-binding site. PloS one 9, e96372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama K, Zhang XP, Medved L, and Takada Y(1999) Specific binding of integrin alpha v beta 3 to the fibrinogen gamma and alpha E chain C-terminal domains. Biochemistry 38, 5872–5877 [DOI] [PubMed] [Google Scholar]
- 26.Saegusa J, Yamaji S, Ieguchi K, Wu CY, Lam KS, Liu FT, Takada YK, and Takada Y (2009) The direct binding of insulin-like growth factor-1 (IGF-1) to integrin alphavbeta3 is involved in IGF-1 signaling. J Biol Chem 284, 24106–24114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori S, Wu CY, Yamaji S, Saegusa J, Shi B, Ma Z, Kuwabara Y, Lam KS, Isseroff RR, Takada YK, and Takada Y (2008) Direct Binding of Integrin {alpha}v{beta}3 to FGF1 Plays a Role in FGF1 Signaling. J Biol Chem 283, 18066–18075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saegusa J, Akakura N, Wu CY, Hoogland C, Ma Z, Lam KS, Liu FT, Takada YK, and Takada Y (2008) Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins alphavbeta3 and alpha4beta1 and induces proliferation of monocytic cells in an integrin-dependent manner. J Biol Chem 283, 26107–26115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia W, and Springer TA (2014) Metal ion and ligand binding of integrin alpha5beta1. Proc Natl Acad Sci U S A 111, 17863–17868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott DH, Lopez J, Deng F, Liu Q, Ojode T, Chen H, Ulrick J, Kwatemaa N, Kelly C, Anaya-O'Brien S, Garofalo M, Marquesen M, Hilligoss D, DeCastro R, Malech HL, and Murphy PM (2011) AMD3100 is a potent antagonist at CXCR4(R334X) , a hyperfunctional mutant chemokine receptor and cause of WHIM syndrome. J Cell Mol Med 15, 2071–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz-Rodriguez F, Hidalgo A, and Teixido J (2001) Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood 97, 346–351 [DOI] [PubMed] [Google Scholar]
- 32.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, Zipori D, and Lapidot T (2000) The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95, 3289–3296 [PubMed] [Google Scholar]
- 33.Netelenbos T, van den Born J, Kessler FL, Zweegman S, Merle PA, van Oostveen JW, Zwaginga JJ, Huijgens PC, and Drager AM (2003) Proteoglycans on bone marrow endothelial cells bind and present SDF-1 towards hematopoietic progenitor cells. Leukemia 17, 175–184 [DOI] [PubMed] [Google Scholar]
- 34.Netelenbos T, Zuijderduijn S, Van Den Born J, Kessler FL, Zweegman S, Huijgens PC, and Drager AM (2002) Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J Leukoc Biol 72, 353–362 [PubMed] [Google Scholar]


