Abstract
Acute beer or alcohol ingestion reduces arterial stiffness, but the dose required to reduce arterial stiffness is unclear. Therefore, this study aimed to determine the acute effects of ingesting various amounts of beer on arterial stiffness in healthy men. Nine men (20–22 years) participated, in eight trials in random order on different days. The participants each consumed 25, 50, 100, or 200 mL of alcohol-free beer (AFB25, AFB50, AFB100, and AFB200) or regular beer (B25, B50, B100, and B200), and were monitored for 60 min thereafter. Arterial stiffness did not significantly change among all AFB and B25. However, B50, B100, and B200 caused a significant decrease in arterial stiffness for approximately 30–60 min: heart-brachial pulse wave velocity (B50: −4.5 ± 2.4%; B100: −3.4 ± 1.3%; B200: −8.1 ± 2.6%); brachial-ankle pulse wave velocity (B50: −0.6 ± 2.0%; B100: −3.3 ± 1.1%; B200: −9.3 ± 3.0%); heart-ankle pulse wave velocity (B50: −3.7 ± 0.3%; B100: −3.3 ± 0.9%; B200: −8.1 ± 2.7%); and cardio-ankle vascular index (B50: −4.6 ± 1.3%; B100: −5.6 ± 0.8%; B200: −10.3 ± 3.1%). Positive control alcoholic beverages reduced arterial stiffness, and these reductions did not significantly differ regardless of the type of beverage. Our data show that consuming about 50 mL of beer can start to reduce arterial stiffness, and that the reduced arterial stiffness is mainly attributable to the alcohol in beer.
Keywords: alcohol, arteriosclerosis, blood pressure, cardio-ankle vascular index, pulse wave velocity
Introduction
Arterial stiffening occurs with advancing age and impairs the ability of arteries to buffer BP pulsation and blood flow (Avolio et al., 1983; Tanaka and Safar, 2005). Thus, increased arterial stiffness has been identified as an independent risk factor for future cardiovascular diseases (Laurent and Boutouyrie, 2007; Vlachopoulos et al., 2010; Tanaka, 2019). Pulse wave velocity (PWV) and cardio-ankle vascular index (CAVI) are established indices of arterial stiffness (Vlachopoulos et al., 2010; Shirai et al., 2011). In particular, large elastic arteries progressively stiffen with advancing age, even in healthy individuals (Avolio et al., 1983; Gando et al., 2010). However, physical activity and dietary habits can alter the degree of arterial stiffening associated with age (Tanaka and Safar, 2005; Gando et al., 2016; Tanaka, 2019). Therefore, further scientific evidence of simple and effective methods for preventing arterial stiffening is needed.
Epidemiological studies have identified a J-shaped association between alcohol intake and arterial stiffness, and arterial stiffness is significantly lower in those who consume light-to-moderate amounts of alcohol than in those who consume none (Sierksma et al., 2004; Hougaku et al., 2005; Mattace-Raso et al., 2005; Di Castelnuovo et al., 2006; Costanzo et al., 2011; Poli et al., 2013; Panagiotakos et al., 2019). Beer is one of the most popular alcoholic beverages worldwide, and several studies have noted the vascular protective effects of beer or alcoholic beverages (Toda and Ayajiki, 2010; Arranz et al., 2012; de Gaetano et al., 2016; Zhou et al., 2016). Indeed, acute effects of beer or alcohol ingestion have been studied extensively; 500 mL red wine (0.8 g ethanol/kg body weight) (Mahmud and Feely, 2002), red wine and water, vodka and water, or beer (0.32 g ethanol/kg body weight and 7 mL/kg body weight of total volume) (Krnic et al., 2011), 400 mL beer and 400 mL water (total 800 mL), 800 mL dealcoholized beer, or 67 mL vodka and 733 mL water (total 800 mL) (approximately 0.21 g ethanol/kg body weight) (Karatzi et al., 2013), and 250 mL red wine (approximately 0.34 g ethanol/kg body weight) (Fantin et al., 2016). The findings of our recent study also showed that ingesting 200 mL of beer (0.14 g ethanol/kg body weight), corresponding to the lower daily limit of a mild-to-moderate drinker, reduces arterial stiffness in healthy young men (Nishiwaki et al., 2017a). That is, only a small amount of beer or alcohol may reduce or prevent arterial stiffening.
In contrast, many studies have indicated that moderate-to-excessive alcohol consumption increases arterial stiffness and the risk of cardiovascular diseases (Arranz et al., 2012; de Gaetano et al., 2016; Zhou et al., 2016; Wood et al., 2018). Low concentrations of alcohol increase endothelial function in human endothelial cells through nitric oxide (NO) production, whereas high concentrations induce endothelial dysfunction and apoptosis (Toda and Ayajiki, 2010; Zhou et al., 2016). Furthermore, the J-shaped association has not gained complete acceptance, especially from the viewpoint of alcohol intake and stroke, cancer, and liver diseases (Arranz et al., 2012; de Gaetano et al., 2016; Zhou et al., 2016; Wood et al., 2018). Several findings imply that moderate-to-excessive alcohol consumption confers negative effects on vascular health or longevity (Arranz et al., 2012; Goslawski et al., 2013; de Gaetano et al., 2016; Zhou et al., 2016; Wood et al., 2018). Accordingly, habitual consumption of small amounts of beer might be important to reduce or prevent arterial stiffening and positively affect health and longevity. However, as far as we can ascertain, little is known about the required dose of beer or alcohol required to reduce arterial stiffness.
Based on this background, the present study aimed to determine how much beer should be consumed to elicit reductions in arterial stiffness. Therefore, we investigated the acute effects of ingesting various amounts of beer on arterial stiffness in healthy young men. Considering our previous results (Nishiwaki et al., 2017a), we hypothesized the presence of a critical threshold of reductions in arterial stiffness when healthy young men ingest <200 mL of beer.
Materials and Methods
Participants
The mean age, height, body mass, body mass index (BMI), and body fat in nine healthy young males participated in this study were 21.1 ± 0.2 years, 171.0 ± 2.1 cm, 67.3 ± 3.8 kg, 22.9 ± 1.1 kg/m2, and 21.4 ± 2.0%, respectively. Because elastic properties of arteries fluctuate significantly with the phases of the menstrual cycle in young female (Hayashi et al., 2006), only male participants were recruited in this study. None of them had chronic diseases that could affect cardiovascular health, metabolism, or daily physical activity, a history of smoking, or were presently under medication. They all habitually consumed beverages containing alcohol, but none exceeded the recommended amount of alcohol consumption (40 g/day for men in Japan) beyond which the risk of developing lifestyle-related diseases is increased. The purpose, procedures, and risks of the study were explained to each participant. All participants provided written informed consent before participating in the study, which was reviewed and approved by the Human Ethics Committee at the Osaka Institute of Technology (approval numbers: 2016-5 and 2017-55) and implemented in accordance with the guidelines of the Declaration of Helsinki.
Sample Size and Experimental Procedures
We determined the sample size would be appropriate for the study by power calculations using G∗Power 3. In accordance with our previous findings (Nishiwaki et al., 2017a), we assumed that arterial stiffness would be transiently reduced by ∼10%. At least eight participants were needed to detect this difference at 80% power with a two-tailed α of 5%. We therefore planned to recruit more than eight participants in this study.
All experiments were conducted in a quiet, air-conditioned room at 22–24°C. To avoid potential diurnal variations, all experiments proceeded at the same time of day at least 4 h after a light meal. All participants abstained from beverages containing alcohol and caffeine, and avoided strenuous physical activity for 12 h before participating in experiments. In addition, the participants were advised to eat their habitual breakfast, lunch, and dinner on the day before each experiment.
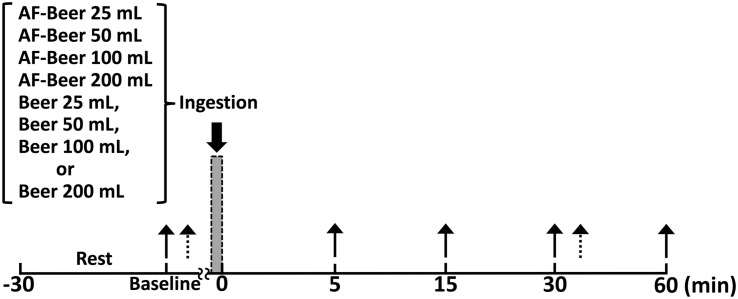
Figure 1 shows the time course of the study. All participants were assigned in random sequence to one trial per day for 8 days. The trials comprised the consumption of 25 (AF25), 50 (AF50), 100 (AF100), and 200 (AF200) mL of alcohol-free beer, and the same volumes of regular beer (B25, B50, B100, and B200, respectively). That is, the amounts (mL) of beer consumed by each participant relative to body mass (kg) were 0.38 ± 0.03 mL/kg (B25), 0.77 ± 0.05 mL/kg (B50), 1.53 ± 0.10 mL/kg (B100), and 3.06 ± 0.21 mL/kg (B200). Also, the amounts (g) of alcohol consumed by each participant relative to body mass (kg) were 0.017 ± 0.001 g/kg (B25), 0.034 ± 0.002 g/kg (B50), 0.067 ± 0.005 g/kg (B100), and 0.135 ± 0.009 g/kg (B200). The participants arrived at the laboratory every day for 8 days and rested for at least 30 min, then breath alcohol concentration (BAC), heart rate (HR), blood pressure (BP), and PWV were assessed to establish pre-ingestion baselines. Participants then consumed test drinks over a period of 3 min without food. The test beer (B) containing 5.5 vol% alcohol was The Premium Malt’s (Suntory Holdings, Osaka, Japan) and the control alcohol-free (AF) beer was All Free (Suntory Holdings, Osaka, Japan). All test drinks were poured into paper cups to blind the participants to which beer they consumed (single-blind study). After consuming a test drink, each participant rested on a comfortable chair for 60 min while the biometric measurements were repeated at 5, 15, 30, and 60 min post-ingestion.
FIGURE 1.
Time course of the experiment. Solid arrows: time points of measured breath alcohol level, heart-brachial pulse wave velocity, brachial-ankle pulse wave velocity, heart-ankle pulse wave velocity, cardio-ankle vascular index, and hemodynamics; broken arrows: time points of measured carotid-femoral pulse wave velocity; AF-Beer: alcohol-free beer.
Assessment of Each Parameter
The same investigators measured all parameters. BAC were measured in triplicate using an AL-1 breath alcohol detector (Daiji Industry, Osaka, Japan), and the mean values were analyzed (Nishiwaki et al., 2017a). The coefficients of variation (CV; a measure of reproducibility) of BAC measurement on two separate days were 9.4 ± 1.6%. According to a previous study (Nishiwaki et al., 2017a), circulating alcohol levels (%) were estimated using the formula: BAC (mg/L)/5. HR, BP, and PWV were measured using a semi-automated device (VS-1500AE/AN; Fukuda Denshi, Tokyo, Japan) with participants in the supine position (Nishiwaki et al., 2014, 2017b, 2019). Cuffs to measure BP and PWV were wrapped around both brachial upper arms and ankles, and then heart-brachial PWV (hbPWV), brachial-ankle PWV (baPWV), heart-ankle PWV (haPWV), and cardio-ankle vascular index (CAVI) were used as indexes of arterial stiffness. The carotid-femoral PWV (cfPWV), which is an index of central arterial stiffness, was measured at baseline (Before) and 30–45 min post-ingestion (After) using the same device (Nishiwaki et al., 2017a). Carotid and femoral arterial pressure waveforms were recorded by amorphous pulse wave sensors (TY-501A; Fukuda Denshi) attached to carotid and femoral arteries, and values were automatically calculated as the distance between the carotid and femoral artery sites divided by the transit time. The CVs of cfPWV, hbPWV, baPWV, haPWV, and CAVI measurements on two separate days were 7.5 ± 1.2, 4.2 ± 0.6, 2.7 ± 0.3, 2.6 ± 0.6, and 3.6 ± 0.6%, respectively (Nishiwaki et al., 2015, 2017a,2017b, 2019).
Supplementary Experiments
We conducted a positive control study to confirm whether alcohol in beer is the main source of changes in arterial stiffness (Supplementary Experiment 1). Spirytus 96° (96 vol% alcohol) vodka (Polmos Warszawa, Warszawa, Poland) was combined with alcohol-free beer to a final concentration as 5.5 vol% (AF + Alc) to coincide with the test beer. We then investigated the effects of ingesting this mixture on arterial stiffness in five healthy young males who participated in three trials in random order on separate days as follows: 50 mL of AF (AF50), 25 mL of AF + Alc (AF + Alc25), and 50 mL of AF + Alc (AF + Alc 50).
Supplementary Experiment 2 aimed to confirm whether ingesting different types of alcoholic beverages induce similar reductions in arterial stiffness. In random order on separate days, 10 healthy young males participated in five trials, and consumed the following on separate days: 100 mL each of pure water (PW), beer (B), sake (S), Japanese distilled spirits (D), and whisky (W). Reverse osmosis water was used for the PW. S of 13–14 vol% alcohol (Thuki, Gekkeikan Sake, Kyoto, Japan), D of 25 vol% alcohol (Kurokirishima, Kirishima Shuzo, Kagoshima, Japan), and W of 40 vol% alcohol (Suntory Whisky Kakubin, Suntory Holdings, Osaka, Japan) were combined with PW, then the mixture was adjusted to 5.5 vol% alcohol to match the test beer. All Supplementary Experiments proceeded in the same manner as the main study, and BAC, systemic PWVs, and CAVI were assessed at baseline and at 5, 15, 30, and 60 min post-ingestion.
Statistical Analysis
Results are presented as means ± SEM. Parameters at each baseline were compared using one-way repeated-measures ANOVA. Changes in each parameter were analyzed by two-way (trial × time) repeated-measures ANOVA. When the F-value was significant, the Bonferroni correction was applied for post hoc multiple comparisons. Relationships were assessed using Pearson’s correlation coefficient. All data were statistically analyzed using IBM SPSS Statistics 25J (IBM Japan, Tokyo, Japan) and Bell Curve for Excel Statistics 3.10 (Social Survey Research Information Co., Tokyo, Japan). To quantify the magnitude of the experimental effect between baseline and object value, effect size (ES) was calculated using G∗Power 3. Differences were considered significant at P < 0.05.
Results
Effects of Ingesting Beer on BAC and Arterial Stiffness
Two-way repeated-measures ANOVA revealed significant interaction in BAC (Figure 2A). Baseline BAC did not significantly differ across trials. The BAC in all AF trials did not significantly change throughout the experimental period, whereas that in all the beer trials changed significantly at 5 min and 15 min post-ingestion (P < 0.01). In particular, the BAC at B100 increased until 30 min (P < 0.01) and that of B200 increased throughout the study period (P < 0.01).
FIGURE 2.
Effects of ingesting different doses of beer on alcohol level (A) and CAVI (B). Broken lines: time point of beverage ingestion; Pre: baseline; AF-Beer: alcohol-free beer ingestion; CAVI: cardio-ankle vascular index; *p < 0.05 vs. each pre; data are expressed as mean ± SE.
Two-way repeated-measures ANOVA also revealed significant interactions in hbPWV, baPWV, haPWV, and CAVI (Table 1 and Figure 2B). Baseline PWV and CAVI did not differ significantly across trials as well as in all AF trials. Conversely, hbPWV (B100 15 min, ES = 0.88; B200 15 min, ES = 1.09), baPWV (B100 15 min, ES = 1.10; B200 15 min, ES = 1.06), haPWV (B100 15 min, ES = 1.25; B200 15 min, ES = 1.02), and CAVI (B100 15 min, ES = 2.04; B200 15 min, ES = 1.35) were decreased, particularly in the B100 and B200 trials at 30–60 min post-ingestion. In the B50 trial, hbPWV (15 min ES = 0.58; 30 min ES = 1.09), haPWV (15 min, ES = 4.18; 30 min, ES = 2.40), and CAVI (15 min, ES = 1.28; 30 min, ES = 3.00) were significantly reduced at about 30 min, but baPWV (15 min, ES = 0.06; 30 min, ES = 0.50) tended to decrease from each baseline level and baPWV responses were relatively blunt (small change). Neither PWV nor CAVI reduced significantly during post-ingestion of B25 (hbPWV 15 min, ES = 0.18; baPWV 15 min, ES = 0.04; haPWV 15 min, ES = 0.21; CAVI 15 min, ES = 0.00). The cfPWV did not change significantly in all AF trials. Conversely, cfPWV was significantly reduced in the B50 (ES = 0.42), B100 (ES = 0.50), and B200 (ES = 0.72) trials in a comparison of before and after values (each P < 0.05), but not in the B25 trial (ES = 0.39).
TABLE 1.
Effects of ingesting different doses of beer on PWVs.
| Variables | Baseline | 5 min | 15 min | 30 min | 60 min | Trial | Time | Interaction |
| Heart-brachial PWV, cm/s | ||||||||
| AF25 | 32411 | 3288 | 3299 | 33211 | 33710 | F = 0.500 | F = 11.521 | F = 3.443 |
| AF50 | 3209 | 3318* | 3369* | 34611* | 3399* | |||
| AF100 | 31711 | 32510* | 32911* | 32610* | 3357* | P = 0.831 | P < 0.001 | P < 0.001 |
| AF200 | 3218 | 33211* | 3269 | 3409* | 33612* | |||
| B25 | 32211 | 3217 | 32410 | 3339* | 34310* | |||
| B50 | 3269 | 3218 | 31212* | 31511* | 32811 | |||
| B100 | 32712 | 33316 | 31612* | 31314* | 33614 | |||
| B200 | 32510 | 31811* | 29913* | 30114* | 31712* | |||
| Brachial-ankle PWV, cm/s | ||||||||
| AF25 | 107329 | 106920 | 104223 | 103926 | 105037 | F = 0.172 | F = 6.616 | F = 2.066 |
| AF50 | 105333 | 105427 | 105939 | 105226 | 106626 | |||
| AF100 | 107932 | 106132 | 108240 | 107126 | 108234 | P = 0.990 | P < 0.001 | P = 0.002 |
| AF200 | 106926 | 106627 | 107033 | 106429 | 105731 | |||
| B25 | 108228 | 110341 | 108544 | 108140 | 106839 | |||
| B50 | 106225 | 105231 | 105838 | 104030 | 104034 | |||
| B100 | 107430 | 106632 | 103931* | 102933* | 105042* | |||
| B200 | 109436 | 108237 | 99452* | 100654* | 103954* | |||
| Heart-ankle PWV, cm/s | ||||||||
| AF25 | 60613 | 60511 | 59912 | 60315 | 61016 | F = 0.459 | F = 7.854 | F = 3.390 |
| AF50 | 6069 | 60313 | 60917 | 61615 | 61513 | |||
| AF100 | 59614 | 59615 | 60816 | 60311 | 61414 | P = 0.861 | P < 0.001 | P < 0.001 |
| AF200 | 59912 | 60713 | 60213 | 61414 | 60714 | |||
| B25 | 60218 | 60715 | 60719 | 60418 | 61017 | |||
| B50 | 60112 | 59812 | 57912* | 57914* | 59513 | |||
| B100 | 60415 | 60719 | 58417* | 57919* | 60420 | |||
| B200 | 60417 | 59615 | 55624* | 55824* | 58425* | |||
| Carotid-femoral PWV, cm/s | ||||||||
| AF25 | 61116 | − | − | 61318 | − | F = 0.188 | F = 0.772 | F = 1.395 |
| AF50 | 60618 | − | − | 60421 | − | |||
| AF100 | 62316 | − | − | 61816 | − | P = 0.987 | P = 0.383 | P = 0.223 |
| AF200 | 61321 | − | − | 61918 | − | |||
| B25 | 58917 | − | − | 61219* | − | |||
| B50 | 62222 | − | − | 59316* | − | |||
| B100 | 62019 | − | − | 60618* | − | |||
| B200 | 61010 | − | − | 59313* | − | |||
Data are expressed as mean ± SE. PWV, pulse wave velocity; AF25, AF50, AF100, and AF200, alcohol-free beer of 25, 50, 100, and 200 mL ingestion, respectively; B25, B50, B100, and B200, beer of 25, 50, 100, and 200 mL ingestion, respectively. * P < 0.05 vs. each baseline.
Effects of Ingesting Beer on Hemodynamic Parameters
Baseline HR and BP did not significantly differ. Although HR in B25, B50, and B100 tended to increase, the values did not reach statistical significance. HR in B200 significantly increased at 15 min post-ingestion (ES = 0.76), but not in any AF trial. Systolic BP, diastolic BP, and mean BP did not significantly change throughout the experimental period, but post-ingestion diastolic BP slightly decreased in the B50, B100, and B200 trials (Table 2).
TABLE 2.
Changes in hemodynamic parameters.
| Variables | Baseline | 5 min | 15 min | 30 min | 60 min | Trial | Time | Interaction |
| Heart rate, beats/min | ||||||||
| AF25 | 604 | 573 | 543 | 553 | 563 | F = 0.485 | F = 1.521 | F = 2.298 |
| AF50 | 573 | 563 | 583 | 593 | 573 | |||
| AF100 | 571 | 561 | 562 | 572 | 562 | P = 0.842 | P = 0.197 | P < 0.001 |
| AF200 | 613 | 593 | 603 | 573 | 573 | |||
| B25 | 582 | 572 | 562 | 552 | 593 | |||
| B50 | 593 | 594 | 635 | 624 | 583 | |||
| B100 | 583 | 573 | 613 | 613 | 583 | |||
| B200 | 563 | 634* | 675* | 645* | 614* | |||
| Systolic BP, mmHg | ||||||||
| AF25 | 1244 | 1213 | 1223 | 1223 | 1243 | F = 0.075 | F = 0.835 | F = 1.356 |
| AF50 | 1213 | 1213 | 1203 | 1253 | 1233 | |||
| AF100 | 1244 | 1254 | 1234 | 1243 | 1233 | P = 0.999 | P = 0.504 | P = 0.115 |
| AF200 | 1233 | 1212 | 1183 | 1254 | 1202 | |||
| B25 | 1223 | 1223 | 1244 | 1233 | 1222 | |||
| B50 | 1224 | 1213 | 1253 | 1234 | 1193 | |||
| B100 | 1245 | 1265 | 1243 | 1234 | 1225 | |||
| B200 | 1225 | 1224 | 1204 | 1224 | 1253 | |||
| Diastolic BP, mmHg | ||||||||
| AF25 | 692 | 702 | 682 | 682 | 692 | F = 0.590 | F = 2.271 | F = 0.788 |
| AF50 | 701 | 711 | 711 | 711 | 721 | |||
| AF100 | 701 | 701 | 721 | 701 | 691 | P = 0.762 | P = 0.062 | P = 0.771 |
| AF200 | 711 | 712 | 712 | 722 | 712 | |||
| B25 | 702 | 721 | 702 | 712 | 732 | |||
| B50 | 722 | 712 | 691 | 691 | 702 | |||
| B100 | 712 | 743 | 712 | 722 | 713 | |||
| B200 | 682 | 711 | 662 | 682 | 693 | |||
| Mean BP, mmHg | ||||||||
| AF25 | 892 | 902 | 882 | 872 | 912 | F = 0.097 | F = 1.648 | F = 0.915 |
| AF50 | 872 | 892 | 902 | 911 | 902 | |||
| AF100 | 893 | 892 | 913 | 892 | 892 | P = 0.998 | P = 0.163 | P = 0.583 |
| AF200 | 902 | 892 | 892 | 912 | 912 | |||
| B25 | 892 | 902 | 892 | 903 | 912 | |||
| B50 | 902 | 912 | 902 | 892 | 902 | |||
| B100 | 872 | 892 | 882 | 892 | 902 | |||
| B200 | 893 | 892 | 892 | 893 | 891 | |||
Data are expressed as mean ± SE. BP, blood pressure; AF25, AF50, AF100, and AF200, alcohol-free beer of 25, 50, 100, and 200 mL ingestion, respectively; B25, B50, B100, and B200, beer of 25, 50, 100, and 200 mL ingestion, respectively. * P < 0.05 vs. each baseline.
Relationships Between Alcohol Levels and Arterial Stiffness
In all pooled data of main experiments in beer trials from 15 min to 60 min, estimated circulating alcohol levels were significantly and negatively correlated with changes in hbPWV (r = −0.47, P < 0.001), baPWV (r = −0.31, P < 0.01), haPWV (r = −0.45, P < 0.001), and CAVI (r = −0.60, P < 0.001). Statistical correlations tended to be stronger between circulating alcohol and hbPWV, haPWV, and CAVI than baPWV. However, although the same volume of beer was consumed, changes in arterial stiffness did not correlate significantly with the volume of beer consumed per body mass (hbPWV 15 min, r = 0.33, P = 0.39; baPWV 15 min, r = −0.08, P = 0.84; haPWV 15 min, r = 0.04, P = 0.93; CAVI 15 min, r = 0.06, P = 0.88).
Supplementary Experimental Findings
Post-ingestion PWVs and CAVI in the AF50 trial (Supplementary Experiment 1) became significantly reduced when the participants consumed 50 mL of alcohol mixed with alcohol-free beer (AF + Alc50 trial; hbPWV 15 min, ES = 1.00; baPWV 15 min, ES = 0.74; haPWV 15 min, ES = 0.87; CAVI 15 min, ES = 0.73). However, PWV and CAVI did not change significantly in the AF + Alc25 trial (hbPWV 15 min, ES = 0.04; baPWV 15 min, ES = 0.64; haPWV 15 min, ES = 0.03; CAVI 15 min, ES = 0.00). These findings supported those of the B25 and B50 trials (Figures 3A–E).
FIGURE 3.
Supplementary experimental findings. Changes in breath alcohol levels (A), hbPWV (B), baPWV (C), haPWV (D), and CAVI (E) in Supplementary Experiment 1. Comparisons of the changes in hbPWV (F), baPWV (G), haPWV (H), and CAVI (I) in Supplementary Experiment 2. Broken lines, time points of beverage ingestion; Pre, baseline, AF-Beer, alcohol-free beer; AF-Beer + alcohol, alcohol mixed with alcohol-free beer as positive control; PW, pure water; B, beer; S, sake; D, Japanese distilled spirits; W, whisky; hbPWV, heart-brachial pulse wave velocity; baPWV, brachial-ankle pulse wave velocity; haPWV, heart-ankle pulse wave velocity; CAVI, cardio-ankle vascular index; *p < 0.05 vs. each pre; †p < 0.05 vs. PW. Data are expressed as mean ± SE.
Pulse wave velocity and CAVI were significantly reduced after ingesting 100 mL of B, S, D, and W but not PW (Supplementary Experiment 2). In addition, reductions in hbPWV, haPWV, and CAVI at 15 min post-ingestion were significantly greater for B (hbPWV, ES = 1.64; haPWV, ES = 1.19; CAVI, ES = 1.49), S (hbPWV, ES = 1.52; haPWV, ES = 1.43; CAVI, ES = 1.83), D (hbPWV, ES = 1.10; haPWV, ES = 1.30; CAVI, ES = 1.72), and W (hbPWV, ES = 2.01; haPWV, ES = 1.62; CAVI, ES = 1.66) than for PW. These degrees of reduction did not significantly differ among trials of alcohol, regardless of type. However, the reductions in baPWV were small, and did not significantly differ across trial (B vs. PW, ES = 0.20; S vs. PW, ES = 0.84; D vs. PW, ES = 0.23; W vs. PW, ES = 0.55) (Figures 3F–I).
Discussion
The salient findings are as follows. The CAVI and PWVs were significantly reduced in B50, B100, and B200 trials at 30–60 min post-ingestion, but not in either B25 or all AF trials. The findings of the supplementary experiments supported the results of main experiments, indicating that the alcohol in beer was the main contributor to acute reduction in PWV and CAVI. To the best of our knowledge, this is the first study to clarify the dose of beer required to induce reductions in arterial stiffness.
A significant reduction in arterial stiffness has been determined by ingesting ∼ 200 mL of beer, which corresponds to mild-to-moderate consumption (Nishiwaki et al., 2017a). However, to our knowledge, the amount of beer required to induce an acute reduction in arterial stiffness has remained unknown. The present results showed that CAVI and PWVs started to fall in the B50 trial and were reduced in the B100 and B200 trials, but not in the B25 and all AF trials. The positive control in supplementary experiment confirmed these findings of PWVs and CAVI. The gold-standard for assessing arterial stiffness is generally PWVs (Tanaka and Safar, 2005; Vlachopoulos et al., 2010; Ohkuma et al., 2017), and CAVI is also an index of arterial stiffness from the aorta to the ankle, after adjustment for BP, which is a major confounding factor (Shirai et al., 2011; Matsushita et al., 2019). In general, haPWV and CAVI are systemic indices of arterial stiffness from the aorta to the ankle (Tomiyama et al., 2003; Shirai et al., 2011). They comprise hbPWV that mainly reflects arterial stiffness of the upper limbs from the aorta to the brachial, which can serve as a marker of arterial stiffening of the proximal aorta (Sugawara et al., 2018, 2019), and baPWV, which mainly reflects arterial stiffness of central and lower limbs from the level of the brachial (thoracoabdominal) to the ankle (Yamashina et al., 2002). In addition, post-ingestion BP did not significantly change in either the B50, B100, or B200 trials. Although HR influences on PWV and HR significantly increased in the B200 trial (Callaghan et al., 1984; Lantelme et al., 2002), previous studies have indicates that fluctuations in HR between 60 and 70 beats/min do not obviously affect PWV values (Callaghan et al., 1984; Lantelme et al., 2002). Thus, our findings indicate that acute beer ingestion of about 50 mL (0.77 ± 0.05 mL of beer/kg body mass and 0.034 ± 0.002 g of alcohol/kg body mass) begins to reduce arterial stiffness.
The main constituents of beer are alcohol, antioxidant substances, water, and sugar (Arranz et al., 2012; Karatzi et al., 2013; de Gaetano et al., 2016). We previously showed that antioxidant substances and sugar do not affect beer-induced acute reduction in arterial stiffness (Nishiwaki et al., 2017a). The present study identified significant and negative correlations between estimated circulating levels of alcohol and changes in arterial stiffness. Furthermore, the findings of the two supplementary experiments supported the main results of present study, namely, that arterial stiffness was significantly reduced regardless of the type of alcohol consumed. Therefore, these findings show that the alcohol in beer is the main contributor to the acute reduction in arterial stiffness associated with ingesting a small amount of beer.
The physiological mechanism underlying vascular responses to alcohol ingestion have not yet been elucidated. However, changes in arterial stiffness are generally thought to result from structural changes in elastin and collagen content, functional changes in vasoconstrictor tone and endothelial functions, or a combination of both (Tanaka and Safar, 2005). Because the structure of the arterial walls is believed to change over weeks or years (Tanaka et al., 2000; Tanaka and Safar, 2005; Maeda et al., 2009; Sugawara et al., 2009), an acute change in arterial stiffness is probably mediated by functional changes (Sugawara et al., 2004; Maeda et al., 2009). Acute alcohol ingestion alters BP variability, which is index of sympathetic control of vasomotor tone (Buckman et al., 2015). Thus, one possible mechanism underlying acute effects of beer or alcohol ingestion on arterial function may be related to the change in sympathetic control of vasomotor tone. In addition, recent studies indicate that mental stress or comic movies-induced mirthful laughter affects arterial stiffness and/or vascular function (Vlachopoulos et al., 2006; Sugawara et al., 2010). Thus, ingesting a small amount of beer or alcohol might change mood states of participants, and thereby reducing vasomotor tone or arterial stiffness. Alternatively, studies in vitro have demonstrated that a low concentration of alcohol can promote NO release from endothelium by upregulation of NO synthase (Toda and Ayajiki, 2010; Zhou et al., 2016). NO is an important endogenous vasoactive substance that plays a key role in regulating blood pressure and protecting against pathological vascular damage. Therefore, ingesting alcohol might increase NO bioavailability in the vascular endothelium, thus reducing arterial stiffness (Karatzi et al., 2013). However, in this study, there is a lack of direct data to support our view and further studies are required.
Recent systematic reviews and meta-analyses found that an increase in PWV of 1 m/s corresponds to a >10% increase in risk for cardiovascular events or mortality (Vlachopoulos et al., 2010), and thus a reduction in arterial stiffness is considered of paramount importance. Many previous studies have demonstrated that both acute and chronic vascular responses to exercise are probably relevant (Kingwell et al., 1997; Kakiyama et al., 2005; Nishiwaki et al., 2015; Yamato et al., 2016). Thus, our results raise the possibility that repeated acute reductions in arterial stiffness and its accumulation would induce persistent reductions in arterial stiffness. Furthermore, according to our previous studies (Nishiwaki et al., 2014), we applied the linear regression between age and CAVI, and estimated “vascular age.” In the present study, alternations of vascular age corresponded to changes in 5–9 years between baseline and during beer ingesting trials. Therefore, our findings indicate that the magnitudes of reduction are not only statistically significant, but also clinically meaningful. However, as of now, whether regular ingestion of a small amount of beer or alcohol in daily life reduces arterial stiffness are unclear, and further intervention studies are thus needed.
Recent combined analysis by large-scale prospective studies have demonstrated that the threshold for lowest risk of total cardiovascular diseases and all-cause mortality is about 100 g of alcohol/week (Wood et al., 2018), which partially supports J-shaped relationships (Di Castelnuovo et al., 2006; Costanzo et al., 2011; Arranz et al., 2012; Poli et al., 2013; de Gaetano et al., 2016; Zhou et al., 2016; Panagiotakos et al., 2019). In addition, some meta-analyses have indicated that relative risks of vascular events can be reduced at 2–3 g/day of alcohol consumption and significantly reduced at approximately 5 g/day (Di Castelnuovo et al., 2006; Costanzo et al., 2011; Poli et al., 2013). In the present study, 50 and 100 mL of beer contained 2.2 and 4.4 g of alcohol, respectively. Therefore, our experimental findings can have high validity, because they are in line with these epidemiological results. However, the J-shaped association is not widely accepted, especially in terms of relationships between alcohol intake and stroke, cancer, and liver diseases (Arranz et al., 2012; Poli et al., 2013; de Gaetano et al., 2016; Zhou et al., 2016; Wood et al., 2018). Indeed, the American Heart Association states that those who consume excessive amounts of alcohol as well as those who abstain from alcohol, should not be encouraged to drink alcohol for health reasons (Arranz et al., 2012; Arnett et al., 2019). Thus, whether consuming a small amount (50–100 mL) of beer actually affects arterial stiffness, total health, and longevity remains unresolved and further investigations are required.
The strengths of our study include two supplementary experiments of positive control and ingesting different types of alcoholic beverages. However, this study has several important potential limitations. First, experimental studies have measured absolute amounts and body mass-adjusted relative amounts to determine alcohol intake (Krnic et al., 2011; Karatzi et al., 2013; Fantin et al., 2016; Nishiwaki et al., 2017a). Because recommended alcohol intake is defined by absolute amounts, and we intended that our study should be easily generalizable to the clinical settings, participants were given four different small absolute amounts of beer (25, 50, 100, and 200 mL). However, changes in arterial stiffness did not significantly correlate with relative amounts of alcohol per kilogram of body mass. Thus, the required dose for reduction in arterial stiffness was not profoundly affected by the minor differences in mass-based relative amounts ingested by each participant. Second, individual physiological responses to alcohol ingestion are affected by factors such as age, sex, amounts habitually consumed, and the arterial stiffness. Thus, our results are specific to young, healthy, male, mild-to-moderate consumers of alcohol, although it seems unlikely that changes in arterial stiffness can be profoundly affected by ingesting small amounts of beer. The number of participants was also very small although several supplementary experiments were performed. Therefore, statistical interpretation is limited and additional investigations using different protocols and study populations might uncover important new insights into the relationship between arterial stiffness and alcohol ingestion.
In conclusion, we found that about 50 mL of ingested beer (0.77 ± 0.05 mL of beer/kg body mass and 0.034 ± 0.002 g of alcohol/kg body mass) begins to reduce arterial stiffness, and that the reduction in arterial stiffness is mainly attributable to the alcohol in beer. These findings could thus offer new insights into the development of better methods for preventing arterial stiffening, regulating the circulation system, and vascular biology, especially from the viewpoint of blood distribution during physiological tasks or alcohol ingestion.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Ethics Committee at the Osaka Institute of Technology (approval numbers: 2016-5 and 2017-55). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MN, TY, and RN conceived, designed, and performed the study and analyzed the data. MN, TY, RN, and NM wrote the manuscript. MN and NM interpreted the data. All authors approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank the study participants for their cooperation.
Footnotes
Funding. This study was supported in part by a Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology (JSPS KAKENHI Grant Number 19K22830 to MN).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.01033/full#supplementary-material
References
- Arnett D. K., Blumenthal R. S., Albert M. A., Buroker A. B., Goldberger Z. D., Hahn E. J., et al. (2019). 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation 10.1161/CIR.0000000000000678 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz S., Chiva-Blanch G., Valderas-Martinez P., Medina-Remon A., Lamuela-Raventos R. M., Estruch R. (2012). Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 4 759–781. 10.3390/nu4070759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avolio A. P., Chen S. G., Wang R. P., Zhang C. L., Li M. F., O’rourke M. F. (1983). Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 68 50–58. 10.1161/01.cir.68.1.50 [DOI] [PubMed] [Google Scholar]
- Buckman J. F., Eddie D., Vaschillo E. G., Vaschillo B., Garcia A., Bates M. E. (2015). Immediate and complex cardiovascular adaptation to an acute alcohol dose. Alcohol. Clin. Exp. Res. 39 2334–2344. 10.1111/acer.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan F. J., Babbs C. F., Bourland J. D., Geddes L. A. (1984). The relationship between arterial pulse-wave velocity and pulse frequency at different pressures. J. Med. Eng. Technol. 8 15–18. 10.3109/03091908409032067 [DOI] [PubMed] [Google Scholar]
- Costanzo S., Di Castelnuovo A., Donati M. B., Iacoviello L., De Gaetano G. (2011). Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: a meta-analysis. Eur. J. Epidemiol. 26 833–850. 10.1007/s10654-011-9631-0 [DOI] [PubMed] [Google Scholar]
- de Gaetano G., Costanzo S., Di Castelnuovo A., Badimon L., Bejko D., Alkerwi A., et al. (2016). Effects of moderate beer consumption on health and disease: a consensus document. Nutr. Metab. Cardiovasc. Dis. 26 443–467. 10.1016/j.numecd.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Di Castelnuovo A., Costanzo S., Bagnardi V., Donati M. B., Iacoviello L., De Gaetano G. (2006). Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch. Intern. Med. 166 2437–2445. [DOI] [PubMed] [Google Scholar]
- Fantin F., Bulpitt C. J., Zamboni M., Cheek E., Rajkumar C. (2016). Arterial compliance may be reduced by ingestion of red wine. J. Hum. Hypertens. 30 68–72. 10.1038/jhh.2015.19 [DOI] [PubMed] [Google Scholar]
- Gando Y., Murakami H., Kawakami R., Yamamoto K., Kawano H., Tanaka N., et al. (2016). Cardiorespiratory fitness suppresses age-related arterial stiffening in healthy adults: a 2-year longitudinal observational study. J. Clin. Hypertens. 18 292–298. 10.1111/jch.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gando Y., Yamamoto K., Murakami H., Ohmori Y., Kawakami R., Sanada K., et al. (2010). Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension 56 540–546. 10.1161/hypertensionaha.110.156331 [DOI] [PubMed] [Google Scholar]
- Goslawski M., Piano M. R., Bian J. T., Church E. C., Szczurek M., Phillips S. A. (2013). Binge drinking impairs vascular function in young adults. J. Am. Coll. Cardiol. 62 201–207. 10.1016/j.jacc.2013.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Miyachi M., Seno N., Takahashi K., Yamazaki K., Sugawara J., et al. (2006). Variations in carotid arterial compliance during the menstrual cycle in young women. Exp. Physiol. 91 465–472. 10.1113/expphysiol.2005.032011 [DOI] [PubMed] [Google Scholar]
- Hougaku H., Fleg J. L., Lakatta E. G., Scuteri A., Earley C. J., Najjar S., et al. (2005). Effect of light-to-moderate alcohol consumption on age-associated arterial stiffening. Am. J. Cardiol. 95 1006–1010. 10.1016/j.amjcard.2004.12.051 [DOI] [PubMed] [Google Scholar]
- Kakiyama T., Sugawara J., Murakami H., Maeda S., Kuno S., Matsuda M. (2005). Effects of short-term endurance training on aortic distensibility in young males. Med. Sci. Sports Exerc. 37 267–271. 10.1249/01.mss.0000152733.12578.5a [DOI] [PubMed] [Google Scholar]
- Karatzi K., Rontoyanni V. G., Protogerou A. D., Georgoulia A., Xenos K., Chrysou J., et al. (2013). Acute effects of beer on endothelial function and hemodynamics: a single-blind, crossover study in healthy volunteers. Nutrition 29 1122–1126. 10.1016/j.nut.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell B. A., Berry K. L., Cameron J. D., Jennings G. L., Dart A. M. (1997). Arterial compliance increases after moderate-intensity cycling. Am. J. Physiol. 273 H2186–H2191. [DOI] [PubMed] [Google Scholar]
- Krnic M., Modun D., Budimir D., Gunjaca G., Jajic I., Vukovic J., et al. (2011). Comparison of acute effects of red wine, beer and vodka against hyperoxia-induced oxidative stress and increase in arterial stiffness in healthy humans. Atherosclerosis 218 530–535. 10.1016/j.atherosclerosis.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Lantelme P., Mestre C., Lievre M., Gressard A., Milon H. (2002). Heart rate: an important confounder of pulse wave velocity assessment. Hypertension 39 1083–1087. 10.1161/01.hyp.0000019132.41066.95 [DOI] [PubMed] [Google Scholar]
- Laurent S., Boutouyrie P. (2007). Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 49 1202–1206. 10.1161/hypertensionaha.106.076166 [DOI] [PubMed] [Google Scholar]
- Maeda S., Sugawara J., Yoshizawa M., Otsuki T., Shimojo N., Jesmin S., et al. (2009). Involvement of endothelin-1 in habitual exercise-induced increase in arterial compliance. Acta Physiol. 196 223–229. 10.1111/j.1748-1716.2008.01909.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud A., Feely J. (2002). Divergent effect of acute and chronic alcohol on arterial stiffness. Am. J. Hypertens. 15 240–243. 10.1016/s0895-7061(01)02315-9 [DOI] [PubMed] [Google Scholar]
- Matsushita K., Ding N., Kim E. D., Budoff M., Chirinos J. A., Fernhall B., et al. (2019). Cardio-ankle vascular index and cardiovascular disease: systematic review and meta-analysis of prospective and cross-sectional studies. J. Clin. Hypertens. 21 16–24. 10.1111/jch.13425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso F. U., Van Der Cammen T. J., Van Den Elzen A. P., Schalekamp M. A., Asmar R., Reneman R. S., et al. (2005). Moderate alcohol consumption is associated with reduced arterial stiffness in older adults: the Rotterdam study. J. Gerontol. A Biol. Sci. Med. Sci. 60 1479–1483. 10.1093/gerona/60.11.1479 [DOI] [PubMed] [Google Scholar]
- Nishiwaki M., Kora N., Matsumoto N. (2017a). Ingesting a small amount of beer reduces arterial stiffness in healthy humans. Physiol. Rep. 5:e13381. 10.14814/phy2.13381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki M., Takahara K., Matsumoto N. (2017b). Arterial stiffness in young adult swimmers. Eur. J. Appl. Physiol. 117 131–138. 10.1007/s00421-016-3505-9 [DOI] [PubMed] [Google Scholar]
- Nishiwaki M., Kurobe K., Kiuchi A., Nakamura T., Matsumoto N. (2014). Sex differences in flexibility-arterial stiffness relationship and its application for diagnosis of arterial stiffening: a cross-sectional observational study. PLoS One 9:e113646. 10.1371/journal.pone.0113646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki M., Nakano Y., Matsumoto N. (2019). Effects of regular high-cocoa chocolate intake on arterial stiffness and metabolic characteristics during exercise. Nutrition 60 53–58. 10.1016/j.nut.2018.09.021 [DOI] [PubMed] [Google Scholar]
- Nishiwaki M., Yonemura H., Kurobe K., Matsumoto N. (2015). Four weeks of regular static stretching reduces arterial stiffness in middle-aged men. Springerplus 4:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma T., Ninomiya T., Tomiyama H., Kario K., Hoshide S., Kita Y., et al. (2017). Brachial-Ankle Pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension 69 1045–1052. 10.1161/hypertensionaha.117.09097 [DOI] [PubMed] [Google Scholar]
- Panagiotakos D. B., Kouli G. M., Magriplis E., Kyrou I., Georgousopoulou E. N., Chrysohoou C., et al. (2019). Beer, wine consumption, and 10-year CVD incidence: the ATTICA study. Eur. J. Clin. Nutr. 73 1015–1023. 10.1038/s41430-018-0296-6 [DOI] [PubMed] [Google Scholar]
- Poli A., Marangoni F., Avogaro A., Barba G., Bellentani S., Bucci M., et al. (2013). Moderate alcohol use and health: a consensus document. Nutr. Metab. Cardiovasc. Dis. 23 487–504. [DOI] [PubMed] [Google Scholar]
- Shirai K., Hiruta N., Song M., Kurosu T., Suzuki J., Tomaru T., et al. (2011). Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J. Atheroscler. Thromb. 18 924–938. 10.5551/jat.7716 [DOI] [PubMed] [Google Scholar]
- Sierksma A., Lebrun C. E., Van Der Schouw Y. T., Grobbee D. E., Lamberts S. W., Hendriks H. F., et al. (2004). Alcohol consumption in relation to aortic stiffness and aortic wave reflections: a cross-sectional study in healthy postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 24 342–348. 10.1161/01.atv.0000110784.52412.8f [DOI] [PubMed] [Google Scholar]
- Sugawara J., Komine H., Hayashi K., Yoshizawa M., Otsuki T., Shimojo N., et al. (2009). Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int. J. Cardiol. 135 346–352. 10.1016/j.ijcard.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J., Maeda S., Otsuki T., Tanabe T., Ajisaka R., Matsuda M. (2004). Effects of nitric oxide synthase inhibitor on decrease in peripheral arterial stiffness with acute low-intensity aerobic exercise. Am. J. Physiol. Heart Circ. Physiol. 287 H2666–H2669. [DOI] [PubMed] [Google Scholar]
- Sugawara J., Tarumi T., Tanaka H. (2010). Effect of mirthful laughter on vascular function. Am. J. Cardiol. 106 856–859. 10.1016/j.amjcard.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Sugawara J., Tomoto T., Lin H. F., Chen C. H., Tanaka H. (2018). Aortic reservoir function of Japanese female pearl divers. J. Appl. Physiol. 125 1901–1905. 10.1152/japplphysiol.00466.2018 [DOI] [PubMed] [Google Scholar]
- Sugawara J., Tomoto T., Tanaka H. (2019). Heart-to-brachium pulse wave velocity as a measure of proximal aortic stiffness: MRI and longitudinal studies. Am. J. Hypertens. 32 146–154. 10.1093/ajh/hpy166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H. (2019). Antiaging effects of aerobic exercise on systemic arteries. Hypertension 74 237–243. 10.1161/hypertensionaha.119.13179 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Dinenno F. A., Monahan K. D., Clevenger C. M., Desouza C. A., Seals D. R. (2000). Aging, habitual exercise, and dynamic arterial compliance. Circulation 102 1270–1275. 10.1161/01.cir.102.11.1270 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Safar M. E. (2005). Influence of lifestyle modification on arterial stiffness and wave reflections. Am. J. Hypertens. 18 137–144. 10.1016/j.amjhyper.2004.07.008 [DOI] [PubMed] [Google Scholar]
- Toda N., Ayajiki K. (2010). Vascular actions of nitric oxide as affected by exposure to alcohol. Alcohol Alcohol. 45 347–355. 10.1093/alcalc/agq028 [DOI] [PubMed] [Google Scholar]
- Tomiyama H., Yamashina A., Arai T., Hirose K., Koji Y., Chikamori T., et al. (2003). Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement–a survey of 12517 subjects. Atherosclerosis 166 303–309. 10.1016/s0021-9150(02)00332-5 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Aznaouridis K., Stefanadis C. (2010). Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 55 1318–1327. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Kosmopoulou F., Alexopoulos N., Ioakeimidis N., Siasos G., Stefanadis C. (2006). Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom. Med. 68 231–237. 10.1097/01.psy.0000203171.33348.72 [DOI] [PubMed] [Google Scholar]
- Wood A. M., Kaptoge S., Butterworth A. S., Willeit P., Warnakula S., Bolton T., et al. (2018). Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 391 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina A., Tomiyama H., Takeda K., Tsuda H., Arai T., Hirose K., et al. (2002). Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 25 359–364. 10.1291/hypres.25.359 [DOI] [PubMed] [Google Scholar]
- Yamato Y., Hasegawa N., Sato K., Hamaoka T., Ogoh S., Iemitsu M. (2016). Acute effect of static stretching exercise on arterial stiffness in healthy young adults. Am. J. Phys. Med. Rehabil. 95 764–770. 10.1097/phm.0000000000000498 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zheng J., Li S., Zhou T., Zhang P., Li H. B. (2016). Alcoholic beverage consumption and chronic diseases. Int. J. Environ. Res. Public Health 13:522. 10.3390/ijerph13060522 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.