Abstract
Purpose
Delta‐like protein 3 (DLL3) is being developed as a predictive biomarker for DLL3‐targeting antibody‐drug conjugate and other therapies. Given the neuroendocrine features of Merkel cell carcinoma (MCC), we sought to evaluate DLL3 expression and its role in MCC.
Experimental Design
Formalin‐fixed and paraffin‐embedded MCC cases were consecutively selected. Immunohistochemistry was performed for DLL3 (SC16.65 antibody) and polyomavirus large T‐antigen (sc‐136172 antibody). Slides were read out for percentage of positive tumor cells. Cox proportional hazards model was applied to assess the association between DLL3 expression and overall survival (OS). A patient with a DLL3‐expressing MCC was treated with rovalpituzumab tesirine (Rova‐T) in the “other tumor” cohort of NCT02709889 and assessed for response.
Results
The median H‐score of DLL3 expression of 65 patients included was 60 (interquartile range, 30–100). Fifty‐eight cases (89%) had ≥1% tumor cells positive for DLL3 expression with any intensity, of which the median DLL3 expression was 50% (interquartile range, 25%–70%). Thirty‐four cases (52%) had ≥50% tumor cells positive for DLL3 expression with any intensity. Higher H‐score of DLL3 expression was associated with higher polyomavirus nuclear expression (p = .003) when it was dichotomized to negative versus positive. H‐score of DLL3 expression did not predict OS of patients with MCC (p = .4) after being adjusted for common clinicopathological factors. A patient treated with Rova‐T for refractory metastatic MCC achieved partial response.
Conclusions
DLL3 overexpression is very common in MCC by immunohistochemistry. The response to treatment suggests that DLL3 expression may have predictive relevance for DLL3‐targeting therapies in MCC.
Implications for Practice
Delta‐like protein 3 (DLL3) is being developed as a predictive biomarker to identify patients for treatment with DLL3‐targeting agents. Merkel cell carcinoma (MCC) is an aggressive neuroendocrine carcinoma of the skin. It was found that DLL3 overexpression is very common in MCC by immunohistochemistry and significantly associated with Merkel cell polyomavirus expression. Despite the lack of prognostic significance in this cohort, DLL3 expression may have predictive relevance for DLL3‐targeting therapies in MCC. The high levels of DLL3 expression in a subset of MCC may potentially be used to select patients to receive DLL3‐targeting therapies.
Keywords: Biomarker, DLL3, Merkel cell carcinoma, Rovalpituzumab tesirine
Short abstract
Delta‐like protein 3 (DLL3) immunohistochemistry is being developed as a predictive biomarker to identify patients with small cell lung cancer (SCLC) for treatment with the DLL3‐targeting antibody‐drug conjugate rovalpituzumab tesirine (Rova‐T). Considering the neuroendocrine features of SCLC and Merkel cell carcinoma (MCC), this article evaluates DLL3 expression, its association with common clinicopathological factors, and its prognostic role in MCC. In addition, response of a DLL3‐expressing MCC in a patient treated with Rova‐T is evaluated.
Introduction
Merkel cell carcinoma (MCC) is a rare but aggressive type of neuroendocrine carcinoma of the skin, which is associated with higher mortality compared with melanoma 1. The reported incidence of MCC tripled from 1986 to 2001, with current incidence of approximately 2,000 new cases per year in the U.S. 2. The initial management strategies of localized MCC include surgery and radiation; however, the recurrence rate is nearly 50% 3. Chemotherapy has limited clinical benefit for patients with metastatic MCC because more than half of patients have progression of disease by 3 months after initiation of chemotherapy 4. More recently, immune checkpoint inhibitors such as avelumab and pembrolizumab have rapidly become the new standard in MCC, which provided very durable responses with minimal toxicities in patients with chemotherapy naïve or treatment refractory disease 5, 6, 7, 8.
MCC is associated with risk factors including advanced age, chronic ultraviolet (UV) exposure, and immunosuppression 9. In fact, the carcinogenesis of MCC is strongly associated with immune mechanisms. Merkel cell polyomavirus (MCPyV) is also present in 80% of MCC in some series 10. The rest of MCPyV‐negative tumors are likely caused by chronic UV radiation and carry a high mutational burden with possible resultant neoantigens 11. Despite the reported tumor mutation burden, immune checkpoint inhibitors provided 56% overall response rate in patients with MCC as first‐line therapy 7 and 32% overall response rate as second and beyond lines of therapy 5. Thus, there is a clear unmet need for mechanism‐directed drug development in patients with advanced MCC who either cannot receive immunotherapy because of immunosuppression or autoimmune disease or have less than optimal response to immune checkpoint inhibitors.
Whole‐exome sequencing of MCC demonstrated not only frequent mutations in RB1 and TP53 but also additional cancer‐promoting mutations of genes involving chromatin modification, DNA‐damage, PI3K, and Notch pathways 11. Higher expression of jagged ligands JAG1 was associated with MCPyV‐negative MCC, and higher expression of Notch receptor NOTCH3 was associated with better overall survival 12. The Notch signaling pathway is important in regulating neuroendocrine versus epithelial cell fate in the developing lung 13. Dysregulated Notch pathway has implications in many malignancies 14. Delta‐like protein 3 (DLL3) is an inhibitory ligand of NOTCH receptors. DLL3 has minimal to no surface expression in normal tissue but is expressed in over 70% of small cell lung cancers (SCLCs) 15.
DLL3 immunohistochemistry (IHC) is being developed as a predictive biomarker to identify patients with SCLC for treatment with the DLL3‐targeting antibody‐drug conjugate rovalpituzumab tesirine (Rova‐T) 16. DLL3 is also under investigation as a target for other antibody constructs (AMG 757, NCT03319940). Given the neuroendocrine features of SCLC and MCC, and the dysregulated NOTCH expression in MCC, we sought to evaluate DLL3 expression, its association with common clinicopathological factors, and its prognostic role in MCC. In addition, we evaluated the response of a DLL3‐expressing MCC in a patient treated with Rova‐T.
Materials and Methods
An institutional biospecimen repository at the Mayo Clinic was searched to identify consecutive cases of formalin‐fixed and paraffin‐embedded (FFPE) MCC specimens. Only surgical resection specimens of the primary lesion were included in this study considering potential heterogeneity of DLL3 expression. All the cases were reviewed by a dermatopathologist to confirm the diagnosis. Representative tissue blocks were selected for IHC of DLL3 and MCPyV large T‐antigen. Patients’ demographic and clinical information were collected from the medical record retrospectively. Cases were staged according the American Joint Committee on Cancer 8th edition 17. This study was approved by Mayo Clinic Institutional Review Board.
Anti‐human DLL3 mouse antibody (clone SC16.65, immunoglobulin G2a [IgG2a]) was generated in house (Stemcentrx, Inc, South San Francisco, CA). The specificity of the antibody was confirmed by using engineered DLL3 overexpressing cell line, CRISPR knockdown cell line, and endogenously DLL3 expressing cell lines (supplemental online Figs. 1 and 2, supplemental online Table 1). IHC was performed on FFPE tissues sectioned at 5 μm mounted on positively charged glass slides. The whole process was done on an automated stainer, DAKO Link48, with DAKO Envision FLEX reagents (Agilent, Santa Clara, CA). Briefly, slides were incubated at 65°C for 60 minutes for baking, deparaffinization, and target retrieval in low pH 3‐in‐1 solution for 20 minutes at 97°C, followed by blocking with blocking solution for 5 minutes. After SC16.65 anti‐DLL3 antibody (final concentration at 3μg/mL) was incubated at room temperature for 60 minutes, slides were incubated with mouse linker for 20 minutes and horseradish peroxidase polymer for 20 minutes, followed by 3,3′‐Diaminobenzidine to visualize signal. After counterstain with hematoxylin, slides were dehydrated and cleared to coverslip. Stained slides were read under microscope for scoring at 10–20× magnification.
Slides were read out for percentage of positive tumor cells with 0 (negative), 1+ (weak), 2+ (moderate), and 3+ (strong) intensity side by side with an isotype control antibody (IgG2a). Any subcellular staining pattern (membranous, cytoplasmic, or punctate) was counted as overall positive. H‐score, combining the intensity of DLL3 staining and the percentage of DLL3‐positive cells was also calculated for each case. DLL3 expression was categorized as high (H‐score ≥ median) or low (H‐score < median). Polyomavirus status was determined by IHC using an antibody at 1 μg/mL that binds to MCPyV large T‐antigen (sc‐136172 at 1μg/mL; Santa Cruz Biotechnology, Inc., Dallas, TX). MCPyV nuclear expression was categorized as negative versus positive (Fig. 1).
Figure 1.

Merkel cell polyomavirus immunohistochemistry. (A): H&E section. (B): Tumor positive for Merkel cell polyomavirus (MCPyV). (C): H&E section. (D): Tumor negative for MCPyV. Original magnification 400× for all images.
Patients enrolled in an open‐label study of rovalpituzumab tesirine in subjects with DLL3‐expressing advanced solid tumors (NCT02709889) received rovalpituzumab tesirine at 0.2–0.4 mg/kg intravenously on day 1 of each 6‐week cycle. DLL3 expression was based on immunohistochemistry of baseline tumor tissue. Staining in ≥1% of tumor cells was considered positive for this trial. Responses were evaluated using RECIST 1.1 every 6 weeks until 6 months, then every 12 weeks until disease progression. One patient with MCC was enrolled into this clinical trial and was treated at 0.2 mg/kg.
Continuous variables were summarized as medians and interquartile ranges (IQRs) and compared with the Wilcoxon rank‐sum test. Categorical variables were summarized as counts and percentages, and compared between groups with Fisher's exact tests. Overall survival (OS) was calculated from the date of tumor diagnosis to the date of death or censored at the time of last follow‐up. Cox proportional hazards models were used to compare OS between high versus low DLL3 expression groups. Hazard ratios (HRs), 95% confidence intervals (CIs), and log‐rank test p‐values were reported for survival analyses. All statistical tests were two‐sided. A p value <.05 was considered statistically significant for these exploratory analyses.
Results
A total of 65 patients with MCC were included for DLL3 expression evaluation in this study. Patients’ baseline demographic and clinical characteristics in the entire cohort, low DLL3 group, and high DLL3 group were summarized in Table 1. More female patients had high levels of DLL3 expression than male patients (67% vs. 36%, p = .03). The median follow‐up time of the entire cohort was 23 months (IQR, 9–47 months). The majority of the patients (97%) with MCC were diagnosed with localized disease initially. Among 64 patients with available information on primary therapy, 62 patients (95%) had surgical resection. Only one (2%) patient had a positive surgical margin. Twenty‐five patients (38%) were observed after surgical resection; 33 patients (52%) received adjuvant therapy. For those who received treatment, 5 (8%) patients received chemotherapy alone in the form of cisplatin or carboplatin plus etoposide, 27 patients (42%) received radiation therapy alone, and 5 (8%) patients received chemotherapy in the form of cisplatin or carboplatin plus etoposide with radiation.
Table 1.
Baseline demographic and clinical characteristics
| Variables | Total (n = 65) | Low DLL3 (n = 35) | High DLL3 (n = 30) | p value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 21 (32.3) | 7 (33.3) | 14 (66.7) | .03 |
| Male | 44 (67.7) | 28 (63.6) | 16 (36.4) | |
| Median age (IQR), yr | 73.0 (66.0–83.0) | 76.0 (69.0–82.5) | 70.0 (62.0–83.0) | .2 |
| T stage, n (%) | ||||
| 0 | 3 (4.6) | 1 (33.3) | 2 (66.7) | .8 |
| 1 | 37 (56.9) | 20 (54.1) | 17 (45.9) | |
| 2 | 12 (18.5) | 5 (41.7) | 7 (58.3) | |
| 3 | 4 (6.2) | 3 (75.0) | 1 (25.0) | |
| 4 | 2 (3.1) | 1 (50.0) | 1 (50.0) | |
| N stage, n (%) | ||||
| 0 | 36 (55.4) | 20 (55.6) | 16 (44.4) | .9 |
| 1a | 3 (4.6) | 1 (33.3) | 2 (66.7) | |
| 1b | 23 (35.4) | 12 (52.2) | 11 (47.8) | |
| 2 | 2 (3.1) | 1 (50.0) | 1 (50.0) | |
| M stage, n (%) | ||||
| 0 | 62 (95.4) | 33 (53.2) | 29 (46.8) | 1 |
| 1 | 2 (3.1) | 1 (50.0) | 1 (50.0) | |
| AJCC stage, n (%) | ||||
| IA | 18 (27.7) | 12 (66.7) | 6 (33.3) | .7 |
| IB | 7 (10.8) | 2 (28.6) | 5 (71.4) | |
| IIA | 8 (12.3) | 4 (50.0) | 4 (50.0) | |
| IIC | 2 (3.1) | 1 (50.0) | 1 (50.0) | |
| IIIA | 3 (4.6) | 1 (33.3) | 2 (66.7) | |
| IIIB | 23 (35.4) | 12 (52.2) | 11 (47.8) | |
| IV | 2 (3.1) | 1 (50.0) | 1 (50.0) | |
| AJCC stage group, n (%) | ||||
| I/II | 35 (53.8) | 19 (54.3) | 16 (45.7) | .8 |
| III/IV | 28 (43.1) | 14 (50.0) | 14 (50.0) | |
| Median tumor size (IQR), cm | 1.7 (1.3–2.4) | 1.8 (0.9–2.4) | 1.6 (1.6–2.2) | .9 |
| Median positive LN (IQR) | 0 (0–1.8) | 0 (0–1) | 0 (0–2) | .6 |
| Median follow‐up (IQR), mo | 23.0 (9.0–47.0) | 23.0 (9.0–48.0) | 21.0 (9.5–44.8) | 1 |
| Treatment, n (%) | ||||
| Observation | 25 (38.5) | 16 (64.0) | 9 (36.0) | .6 |
| Radiation | 27 (41.5) | 13 (48.1) | 14 (51.9) | |
| Chemotherapya | 5 (7.7) | 2 (40.0) | 3 (60.0) | |
| Chemotherapy and radiation | 5 (7.7) | 2 (40.0) | 3 (60.0) | |
| Recurrence, n (%) | ||||
| No | 29 (44.6) | 13 (44.8) | 16 (55.2) | .2 |
| Yes | 28 (43.1) | 17 (60.7) | 11 (39.3) | |
| Vial status, n (%) | ||||
| Alive | 31 (47.7) | 12 (38.7) | 19 (61.3) | .03 |
| Dead | 34 (52.3) | 23 (67.6) | 11 (32.4) | |
| Median percentage of DLL3‐positive cells, any intensity (IQR) | 50 (25–70) | 25 (8.0–40.5) | 72.5 (65.0–83.8) | <.001 |
| Median DLL3 H‐score (IQR) | 60 (30–100) | 30.0 (42.0) | 100.0 (57.5) | <.001 |
| MCPyV nuclear expression, n (%) | ||||
| Negative | 26 (40.0) | 20 (76.9) | 6 (23.1) | .003 |
| Positive | 39 (60.0) | 15 (38.5) | 24 (61.5) |
Chemotherapy includes cisplatin + etoposide or carboplatin + etoposide.
Abbreviations: AJCC, American Joint Committee on Cancer; DLL3, Delta‐like protein 3; IQR, interquartile range; LN, lymph node; MCPyV, Merkel cell polyomavirus.
The median percentage of DLL3 positive cells at any intensity was 50 (IQR, 25–70) for all cases. As shown in Figure 2A, 34 of 65 (52%) patients had ≥50% DLL3‐positive cells; 58 of 65 (89%) patients had ≥1% DLL3‐positive cells. The median DLL3 H‐score was 60 (IQR, 30–100). In the entire cohort, 39 (60%) patients had positive MCPyV nuclear expression, which was significantly associated with a higher DLL3 score (p = .003), as showed in Figure 2B.
Figure 2.
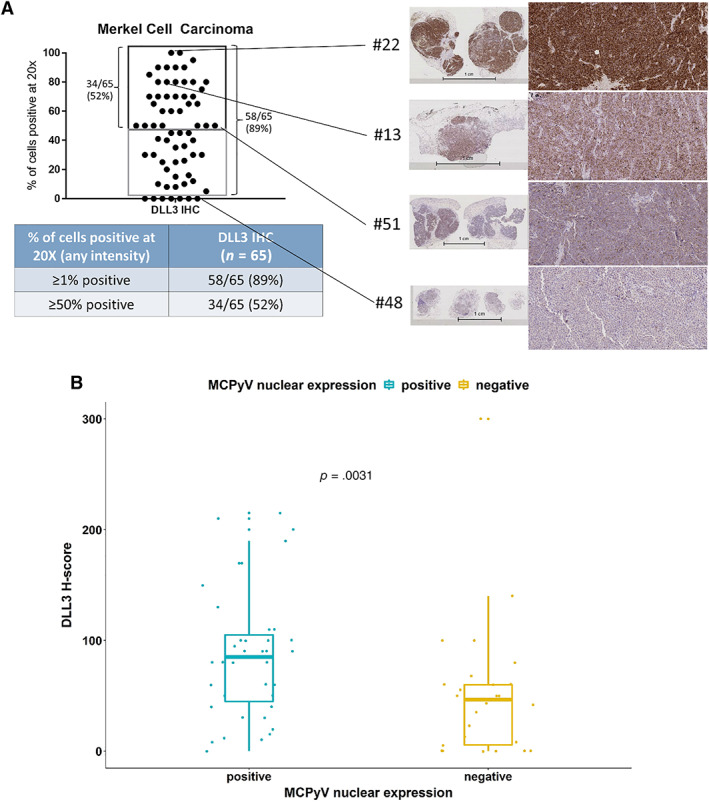
DLL3 prevalence in Merkel cell carcinoma. (A): DLL3 expression by immunohistochemisty with magnification 20× and its prevalence in Merkel cell carcinoma (MCC). (B): Higher DLL3 expression is associated with positive MCPyV expression in MCC.
Abbreviations: DLL3, Delta‐like protein 3; MCPyV, Merkel cell polyomavirus.
DLL3 expression in patients with MCC was not associated with OS (p = .15; Fig. 3). Patients with MCC in the high DLL3 expression group had median OS of 78 months (95% CI, 26 months to not reached). Patients with MCC in the low DLL3 expression group had median OS of 28 months (95% CI, 19 months to not reached). As summarized in Table 2, univariate survival analysis identified older age, larger tumor size, higher number of positive lymph nodes, and negative MCPyV nuclear expression as poor prognostic factors. Multivariable survival analysis showed that after adjusting for other common clinical covariates, higher number of positive lymph nodes (HR, 1.5; p = .002), presence of distant metastases (HR, 31; p = .02), and negative MCPyV nuclear expression (HR, 3.7; p = .02) were independent poor prognostic factors for OS. DLL3 H‐score was not a significant prognostic factor for OS of patients with MCC. Of note, there was no interaction between MCPyV nuclear expression and DLL3 expression group (p = .6) in multivariable Cox proportional hazards model.
Figure 3.
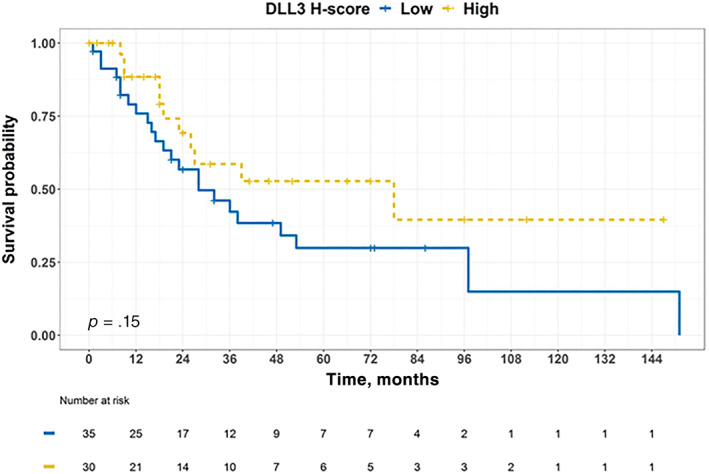
The Kaplan‐Meier curve for overall survival stratified by DLL3 expression in patients with Merkel cell carcinoma. Patients were censored at the time of last follow‐up if they were alive.
Abbreviation: DLL3, Delta‐like protein 3.
Table 2.
Univariate and multivariable survival analysis for overall survival
| Variables | HR (95% CI) (univariate) | HR (95% CI) (multivariable) |
|---|---|---|
| Gender (male vs. female) | 2.6 (0.99–6.6) p = .05 | 1.7 (0.4–7.3) p = .5 |
| Age (per 1 yr increase) | 1.04 (1.01–1.07) p = .02 | 1.05 (1.00–1.1) p = .06 |
| AJCC stage (III/IV vs. I/II) | 1.5 (0.8–3.0) p = .2 | |
| Tumor size (per 1 cm increase) | 1.4 (1.05–1.9) p = .02 | 1.4 (0.9–1.9) p = .1 |
| Positive LN (per 1 more positive LN) | 1.3 (1.1–1.4) p = .001 | 1.5 (1.2–2.0) p = .002 |
| Distant metastasis (yes vs. no) | 2.5 (0.3–19) p = .4 | 31 (1.8–524) p = .02 |
| MCPyV nuclear expression (negative vs. positive) | 3.3 (1.6–6.8) p = .001 | 3.7 (1.3–11) p = .02 |
| DLL3 H‐score (high vs. low) | 0.6 (0.3–1.2) p = .2 | 1.6 (0.5–5.1) p = .4 |
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; DLL3, Delta‐like protein 3; HR, hazard ratio; LN, lymph node; MCPyV, Merkel cell polyomavirus.
A 67‐year‐old patient with metastatic Merkel cell carcinoma whose disease progressed after standard chemotherapy (four cycles of cisplatin and etoposide) and immune checkpoint inhibitor (five cycles of pembrolizumab) was enrolled into an open‐label study of Rova‐T in subjects with DLL3‐expressing advanced solid tumors (NCT02709889). Thirty percent of this patient's tumor cells expressed DLL3 in the available tissue specimen. This patient received three doses of Rova‐T at 0.2 mg/kg. This patient's target lesions decreased 57% after the first two cycles of Rova‐T, consistent with radiographic partial response by RECIST 1.1 (Fig. 4).
Figure 4.

A patient with Delta‐like protein 3 (DLL3)–positive metastatic Merkel cell carcinoma achieved radiographic partial response to rovalpituzumab tesirine based on RECIST criteria. The changes of three largest target lesions were shown in computed tomography scans prior to cycle 1, cycle 2, and cycle 3 rovalpituzumab tesirine, respectively.
Discussion
In this study, we evaluated DLL3 expression in a representative cohort of patients with MCC. The common poor prognostic factors, including older age, higher stage, and negative MCPyV nuclear expression, identified from this patient cohort were consistent with previous reports 18. Although higher DLL3 expression was found to be associated with positive MCPyV nuclear expression, DLL3 expression was not associated with overall survival. This was likely because of our patient cohort, which primarily consisted of patients with localized MCC whose resected tumor specimens were used to profile DLL3 expression. Because of the lack of sufficient paired primary and metastatic tumor specimens, we could not evaluate differential DLL3 expression between primary and metastatic pairs.
The lack of prognostic significance of DLL3 expression in MCC is also similar to what has been observed in SCLC 19. This findings suggest that DLL3 may not be the driver protein in the pathogenesis and progression of MCC; rather, DLL3 expression might be ancillary in the disease process. This finding is supported by the seemingly multifaceted roles of Notch signaling pathway previously studied in MCC. For example, rare NOTCH mutations involving NOTCH 1–4 were almost exclusively present in MCPyV‐negative MCC 20. Higher expression of JAG1 was associated with MCPyV‐negative MCC, and higher expression of Notch receptor NOTCH3 was associated with better OS 12. In contrast, MCPyV‐positive MCC had the lowest number of somatic mutations among all malignancies 11, but MCPyV‐positive MCC had higher DLL3 expression according to our findings. Despite the lack of prognostic significance of DLL3 expression in this cohort of patients with MCC who underwent surgical resection, the partial response to treatment with Rova‐T observed in the only patient with MCC treated on NCT02709889 suggests that DLL3 expression may have predictive relevance with this therapy. Thus, DLL3 expression may represent a relevant therapeutic target for the majority of patients with MCC.
In conclusion, our study demonstrated that DLL3 expression was prevalent in MCC across different stages. DLL3 is the therapeutic target for the antibody drug conjugate Rova‐T. However, larger studies will be needed to confirm whether DLL3 expression might be a predictive biomarker of DLL3 targeting in patients with MCC. DLL3 is also a therapeutic target for other agents in development, such as a bispecific anti‐DLL3/CD3 antibody (AMG 757, NCT03319940) and chimeric antigen receptor T cells targeting DLL3 (AMG 119, NCT03392064). Overall, our study suggests that DLL3 is frequently upregulated in MCC and that DLL3‐targeting therapies warrant further exploration for this indication.
Author Contributions
Conception and design: Hao Xie, Laura R. Saunders, Aaron S. Mansfield
Acquisition of data: Hao Xie, Frederic J. Kaye, Kumiko Isse, Yan Sun, Johanna Ramoth, Dorothy M. French, Thomas J. Flotte, Yan Luo, Laura R. Saunders, Aaron S. Mansfield
Analysis and interpretation of data: Hao Xie, Kumiko Isse, Yan Sun, Johanna Ramoth, Dorothy M. French, Yan Luo, Laura R. Saunders, Aaron S. Mansfield
Writing, review, and/or revision of the manuscript: Hao Xie, Frederic J. Kaye, Kumiko Isse, Yan Sun, Johanna Ramoth, Dorothy M. French, Thomas J. Flotte, Yan Luo, Laura R. Saunders, Aaron S. Mansfield
Study supervision: Laura R. Saunders, Aaron S. Mansfield
Disclosures
Kumiko Isse: AbbVie (E); Yan Sun: AbbVie (E); Johanna Ramoth: AbbVie (E); Dorothy M. French: AbbVie (E); Yan Luo: AbbVie (E); Laura R. Saunders: AbbVie (E); Aaron S. Mansfield: Bristol‐Myers Squibb, Novartis, Verily (RF), AbbVie, AstraZeneca, Bristol‐Myers Squibb, Genentech Inc. (SAB‐Honorary to institution), Mesothelioma Applied Research Foundation Board of Directors (other‐nonremunerated member). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information
Acknowledgments
We appreciate the help from Elena A. Nelson and Chrystal Bailey with coordinating patient data and the help from Bobbi‐Ann Jebens with administrative assistance.
F.J.K. was supported by the Gatorade Trust, Department of Medicine, University of Florida. A.S.M. was supported by CA015083 and K12CA90628.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: Implications for immunotherapy of a polyomavirus‐associated cancer. Curr Oncol Rep 2011;13:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freeman MB, Holman DM, Qin J et al. Merkel cell carcinoma incidence, trends, and survival rates among adults aged ≥50 years from United States Cancer Statistics. J Am Acad Dermatol 2019;80:1154–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen PJ, Bowne WB, Jaques DP et al. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J Clin Oncol 2005;23:2300–2309. [DOI] [PubMed] [Google Scholar]
- 4. Iyer JG, Blom A, Doumani R et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med 2016;5:2294–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufman HL, Russell J, Hamid O et al. Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: A multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol 2016;17:1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nghiem P, Bhatia S, Lipson EJ et al. Durable tumor regression and overall survival in patients with advanced merkel cell carcinoma receiving pembrolizumab as first‐line therapy. J Clin Oncol 2019;37:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nghiem PT, Bhatia S, Lipson EJ et al. PD‐1 blockade with pembrolizumab in advanced merkel‐cell carcinoma. N Engl J Med 2016;374:2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Angelo SP, Russell J, Lebbé C et al. Efficacy and safety of first‐line avelumab treatment in patients with stage IV metastatic merkel cell carcinoma: A preplanned interim analysis of a clinical trial. JAMA Oncol 2018;4:e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harms PW, Harms KL, Moore PS et al; International Workshop on Merkel Cell Carcinoma Research (IWMCC) Working Group. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat Rev Clin Oncol 2018;15:763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng H, Shuda M, Chang Y et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goh G, Walradt T, Markarov V et al. Mutational landscape of MCPyV‐positive and MCPyV‐negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 2016;7:3403–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wardhani LO, Matsushita M, Kuwamoto S et al. Expression of Notch 3 and Jagged 1 is associated with merkel cell polyomavirus status and prognosis in merkel cell carcinoma. Anticancer Res 2019;39:319–329. [DOI] [PubMed] [Google Scholar]
- 13. Morimoto M, Nishinakamura R, Saga Y et al. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012;139:4365–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brzozowa‐Zasada M, Piecuch A, Michalski M et al. Notch and its oncogenic activity in human malignancies. Eur Surg 2017;49:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saunders LR, Bankovich AJ, Anderson WC et al. A DLL3‐targeted antibody‐drug conjugate eradicates high‐grade pulmonary neuroendocrine tumor‐initiating cells in vivo. Sci Transl Med 2015;7:302ra136. [DOI] [PMC free article] [PubMed]
- 16. Rudin CM, Pietanza MC, Bauer TM et al. Rovalpituzumab tesirine, a DLL3‐targeted antibody‐drug conjugate, in recurrent small‐cell lung cancer: A first‐in‐human, first‐in‐class, open‐label, phase 1 study. Lancet Oncol 2017;18:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amin MB, ed. AJCC Cancer Staging Manual, 8th ed. Chicago IL: American Joint Committee on Cancer Springer, 2017. [Google Scholar]
- 18. Moshiri AS, Doumani R, Yelistratova L et al. Polyomavirus‐negative merkel cell carcinoma: A more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol 2016;137:819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka K, Isse K, Fujihira T et al. Prevalence of Delta‐like protein 3 expression in patients with small cell lung cancer. Lung Cancer 2018;115:116–120. [DOI] [PubMed] [Google Scholar]
- 20. Harms PW, Vats P, Verhaegen ME et al. The distinctive mutational spectra of polyomavirus‐negative merkel cell carcinoma. Cancer Res 2015;75:3720–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supporting Information


