Abstract
Background
Cervical cancer (CC) is a global problem; it is among the five leading causes of cancer death in women. Several studies have examined the association between age and disease prognosis; however, controversy still exists. The objective of the present study is to determine if age at diagnosis has an impact on overall survival (OS) and disease‐free survival (DFS).
Materials and Methods
Retrospective cohort of 2,982 patients with CC treated at the National Cancer Institute of Mexico from 2005 to 2015. We collected demographic, clinical, and treatment data, as well as current status, of 2 groups: women under and over 40 years of age. We calculated OS and DFS rates with Kaplan‐Meier estimates. Cox proportional hazards modeling was used to determine risks.
Results
The median follow‐up time was 26.5 months (percentile [P]25–P75, 11–60.23). When comparing DFS, OS, stage, and histologic subtype between young patients <40 and adult patients >40, we did not observe any difference. We found that in both groups, locally advanced and advanced stage, neuroendocrine subtype, hydronephrosis, and positive inguinal lymph nodes increased the risks of death and recurrence. Having been pregnant was identified as protective factor in DFS (hazard ratio, 0.54; 95% confidence interval, 0.04–0.71).
Conclusion
We corroborated that age at diagnosis is not a prognostic factor for decreased or increased OS or DFS, and in both groups, the stage, histologic subtype, hydronephrosis, and node involvement were identified as factors adverse to OS and DFS, and pregnancy history was a protective factor in DFS.
Implications for Practice
The present study directly affects everyday clinical practice because it allows us to focus on the most relevant prognostic factors in patients with cervical cancer. When planning treatment and follow‐up, clinicians should focus on stage at diagnosis, histologic subtype, hydronephrosis, and distant metastasis instead of patients’ age. They should also be aware of any previous pregnancies and poor response, or nonresponse, to treatment, which results in disease progression and persistence. Paying attention to these factors affecting overall survival and disease‐free survival will help treat patients better and increase their chances of survival and improve their quality of life.
Keywords: Cervical cancer, Young women, Worst prognosis
Short abstract
Cervical cancer is among the five leading causes of cancer morbimortality in women. This article focuses on age at diagnosis and the related effect on overall and disease‐free survival in this patient population.
Introduction
For decades, cervical cancer (CC) has been recognized as a global health problem 1, 2. In 2018, CC ranked fifth in incidence and mortality in women worldwide (rates: 13.1 per 100,000 and 6.9 per 100,000, respectively) 3. It is estimated that the 5‐year prevalence of this disease is 1,474,265 3, with incidence peaks among women aged 30 to 44 years 4, 5.
In Mexico, in the last two decades, CC mortality rates decreased, from 9.0 in 2000 to 6.21 in 2013 per 100,000 women 6. Even so, the CC occupies the second place of mortality by cancer in Mexican women. Regarding the incidence rate, CC ranked third in 2018 (11.0 per 100,000 women), behind breast and thyroid cancer. The mortality rate was 5.8 per 100,000 women, and the 5‐year prevalence is 22,000 3.
Some studies have highlighted the role of age in prognosis 7 and reported that younger patients could have a worse prognosis 8, 9. This is perhaps associated with substance abuse, which was significantly more common among younger patients. Patients who smoked or consumed drugs or alcohol had a significantly lower survival rate in comparison with nonsmokers and nonconsumers 10. Other reports showed only very young women (≤35) had poorer survival or that an interaction existed between age, positive lymph nodes, and stage 11, 12, 13, 14.
In contrast, other reports examined whether treatment was a prognostic factor for survival and compared groups of young and adult women. Their results revealed that age had no prognostic value for overall survival and that there is no difference between young and adult women regardless of treatment or stage at diagnosis 15, 16.
The available reports do not agree on the definition of “young” and “adult” women; they have short follow‐up times and small sample sizes, which could make difficult to assess the effect of age on survival in these groups of patients.
The objective of our analysis is to determine the overall survival (OS) and disease‐free survival (DFS) in a retrospective cohort study of patients with CC treated at the National Cancer Institute of Mexico (INCan) and identify if there is a difference between young (≤40) and adult women (>40) while accounting for the presence of other known prognostic factors (e.g., stage, histologic type, treatment, demographic characteristics) to establish a connection between age and prognosis in patients with CC.
Materials and Methods
We conducted a retrospective cohort study. The Research Ethics Committee in INCan approved the study (Rev/31/16). It included 2,982 women with invasive CC who were diagnosed and treated from 2005 to 2015 in the INCan. Patients who arrived at the hospital with previous treatment (including inadvertent cancer operated on or with radiotherapy and/or chemotherapy), as well as patients with fertility‐sparing treatments and disease in situ, were excluded from this analysis.
Data were collected from medical records with the aid of data entry clerks previously trained. A group of oncologists specialized in CC standardized the data, which included demographic and clinicopathological characteristics, such as stage at diagnosis, treatment, comorbidities (diabetes, hypertension, and overweight or obesity), and current status (alive or dead). Patients were classified as early stage (International Federation of Gynecology and Obstetrics [FIGO] stage IA1–IB1), locally advanced (FIGO stage IB2‐IVA), or advanced (FIGO stage IVB); FIGO 2009 classification was used 17. The given treatments for early stage were surgery and adjuvant treatment. Adjuvant treatment was given according to 2 of 3 Sedlis's criteria for intermediate risk of recurrence. Patients were given tele‐radiotherapy and brachytherapy, and with a high‐risk criterion they were given concomitant chemo‐radiotherapy 18. Those with locally advanced stage (IB2‐IVA) were treated with concomitant chemo‐radiotherapy and brachytherapy, and patients with advanced stage (IVB) were treated with systemic chemotherapy, except for stages IVB (for para‐aortic nodes) that were treated with chemo‐radiotherapy concomitant and extended field radiation to para‐aortics.
We define current state as the following: live without disease, live with disease, dead without disease, dead with disease.
Data were validated by an independent epidemiologist and an oncologist.
Statistical Analysis
Clinicopathological characteristics are displayed as median and SD for continuous variables and as frequencies and percentages for categorical variables. When applicable, we performed a chi‐squared test, Student's t test, or Fisher's exact test to compare groups.
We calculated OS by considering the time from diagnosis to the last date of medical appointment or death. Death was considered as the last event, and patients alive were censored. DFS was determined based on the last date of treatment until recurrence, death, or last medical appointment. Medians and interquartile range are reported.
Using the Kaplan‐Meier method and plots, we conducted the survival analysis. The statistical differences between survival and clinical characteristics (stage at diagnosis and histologic type) were calculated using a log‐rank or Wilcoxon test. We used a proportional hazards model to identify the clinical variables that could predict survival in the study population. The model diagnosis was made by testing the proportionality assumption and with an analysis of residuals. We established a p value of ≤.05 to be statistically significant. All analyses were done with Stata, version 14.2 (StataCorp, College Station, TX).
Results
The study included 2,982 women with CC treated at INCan from 2005 to 2015, of which 596 (19.99%) were younger than 40 years, and 2,386 (80.01%) were older than 40 years. The median follow‐up time for all patients was 26.5 months (25th to 75th percentile [P25–P75], 11–60.23), with a range of 0.03–137.46 months; the median for the younger patients was 25.57 months (P25–P75, 11.9–63.13), and the median for the older patients was 26.6 months (P25–P75, 10.62–60). The distribution of demographic and clinical characteristics is summarized in Table 1. Most patients had locally advanced disease (IB2‐IVA, FIGO 2009): 73.10% of the younger patients and 78.10% of the older ones. However, younger patients had more early‐stage disease (1A‐1B1, FIGO 2009) when compared with older patients, at 19.31% versus 14.97%, respectively; with respect to advanced disease, the proportion in young patients was 7.58% compared with older patients, at 6.93% (p = .031). There was no difference in histologic subtypes between both groups (p = .879). Tumor staging was not possible in 42 (7.05%) younger patients and 235 (9.85%) older patients because they had already undergone surgery or even started treatment at the moment of admission. These patients were excluded from the subsequent analyses or were controlled (in the case of the multivariate analysis).
Table 1.
Demographic and clinical characteristics of women with cervical cancer, INCan 2005–2015
| Characteristic | Total (n = 2,982) | Age < 40, (n = 596), mean (SD) | Age > 40 (n = 2,386), mean (SD) | p value |
|---|---|---|---|---|
| Number of pregnancies | 2,720 | 3.05 (±2.00) | 5.15 (±3.56) | >.001 |
| Lymph node clinical involvement | ||||
| None | 569 (96.44) | 2,282 (96.33) | .662 | |
| Neck | 6 (1.02) | 17 (0.72) | ||
| Inguinal | 15 (2.54) | 70 (2.95) | ||
| Total | 2,959 | 590 (100) | 2,369 (100) | |
| Hydronephrosis | 2,982 | 77 (12.92) | 267 (11.19) | .214 |
| Stage FIGO | ||||
| IA1 | 32 (5.37) | 65 (2.72) | .001 | |
| IA2 | 7 (1.17) | 19 (0.80) | ||
| IB1 | 68 (11.41) | 238 (9.97) | ||
| IB2 | 45 (7.55) | 107 (4.48) | ||
| IIA1 | 10 (1.68) | 55 (2.31) | ||
| IIA2 | 3 (0.50) | 28 (1.17) | ||
| IIB | 188 (31.54) | 778 (32.61) | ||
| IIIA | 74 (12.42) | 327 (13.10) | ||
| IIIB | 50 (8.39) | 210 (8.80) | ||
| IVA | 35 (5.87) | 175 (7.33) | ||
| IVB | 42 (7.05) | 149 (6.24) | ||
| Could not be determined | 42 (7.05) | 235 (9.85) | ||
| Total | 2,982 | 596 (100) | 2,386 (100) | |
| Histologic type | ||||
| Epidermoid | 492 (83.25) | 1944 (81.99) | .879 | |
| Adenocarcinoma | 72 (12.18) | 310 (13.07) | ||
| Adenosquamous | 12 (2.03) | 61 (2.57) | ||
| Neuroendocrine | 10 (1.69) | 34 (1.43) | ||
| Other | 5 (0.85) | 22 (0.939 | ||
| Total | 2,962 | 591 (100) | 2,371 (100) | |
| Recurrence | 2,834 | 104 (18.09) | 435 (19.26) | .524 |
| Persistence | 2,830 | 137 (23.70) | 494 (21.94) | .363 |
| Progression | 2,824 | 198 (34.43) | 668 (29.70) | .028 |
| Type of recurrence | ||||
| Local | 21 (20.39) | 85 (19.81) | .900 | |
| Locoregional | 16 (15.53) | 60 (13.99) | ||
| Systemic | 66 (64.08) | 284 (66.20) | ||
| Total | 532 | 103 (100) | 429 (100) |
Abbreviation: FIGO; International Federation of Gynecology and Obstetrics.
Despite younger patients having early‐stage disease, the proportion of disease progression was more common in young than older patients (34.43% vs. 29.70%; p = .028). Younger patients (23.04%) underwent radical or simple hysterectomy, whereas older patients (15.68%) mainly had radical hysterectomy. We found differences in the types of surgery and treatment between younger and older patients; radical hysterectomy was more frequently in older patients (62.22% vs. 85.87%), and adjuvant treatment was also more common in older patients (9.75% vs. 14.64%).
The 10‐year OS and DFS rates were, respectively, 60.5% and 62.4% for younger patients and 69.46% and 66.572% for older patients. The Wilcoxon test did not reveal significant differences in OS or DFS (Fig. 1A, B).
Figure 1.

Overall survival and disease‐free survival in patients with cervical cancer according to age. (A): Overall survival and (B) disease‐free survival (all patients).
Abbreviations: DFS, disease‐free survival; INCan, National Cancer Institute of Mexico.
When comparing both groups following the 2009 FIGO staging system (early, locally advanced, and advanced), we did not find statistically significant differences regarding OS and DFS after 2, 5, and 10 years (Fig. 2; Table 2). In both groups, histologic subtypes did not have any effect on OS or DFS after 2, 5, and 10 years (Fig. 3; Table 2).
Figure 2.
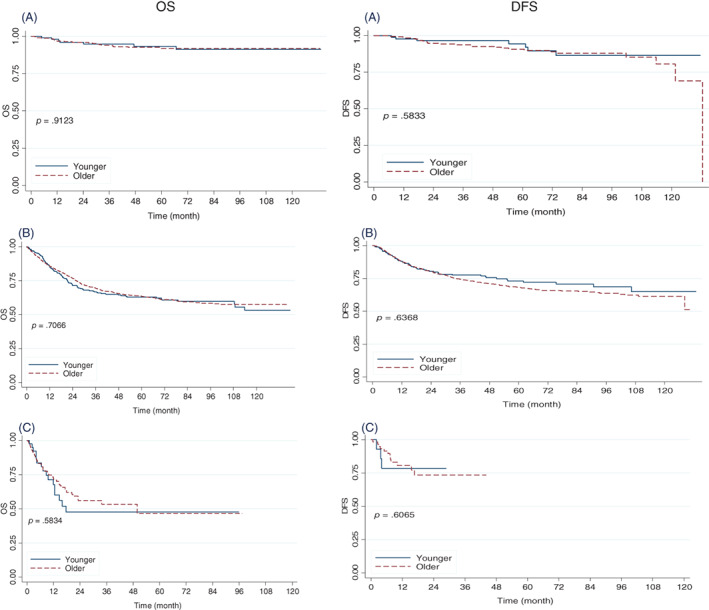
Overall survival and disease‐free survival according to age (younger vs. older patients with cervical cancer according to stage) National Cancer Institute of Mexico. 2005–2015. (A): Early. (B): Locally advanced. (C): Advanced.
Abbreviations: DFS, disease‐free survival; OS, overall survival.
Table 2.
OS and DFS of younger and older patients according to stage and histologic subtype
| Characteristics | 2‐yr survival | 5‐yr survival | 10‐yr survival | ||||
|---|---|---|---|---|---|---|---|
| <40 yr of age, % (95% CI) | >40 yr of age, % (95% CI) | <40 yr of age, % (95% CI) | >40 yr of age, % (95% CI) | <40 yr of age, % (95% CI) | >40 yr of age, % (95% CI) | p value | |
| OS | |||||||
| Early (IA1‐IB1) | 96.0 (89.8–98.5) | 96.0 (93.0–97.7) | 93.4 (85.6–97.0) | 92.0 (87.8–94.7) | 91.2 (81.7–95.9) | 92.0 (87.8–94.7) | .9123 |
| Locally advanced (IB2‐IVA) | 71.4 (66.2–76.0) | 76.7 (74.4–78.8) | 62.9 (57.0–68.2) | 63.4 (60.4–66.2) | 53.1 (43.8–61.6) | 57.4 (53.9–60.8) | .7066 |
| Advanced (IVB) | 47.5 (28.3–64.5) | 56.1 (45.5–65.3) | 47.528.3–65.5) | 46.6 (30.7–61.0) | .5834 | ||
| DFS | |||||||
| Early (IA1‐IB1) | 96.61 (89.83–98.89) | 94.72 (91.07–96.90) | 94.46 (85.19–97.99) | 90.78 (85.94–94.01) | 86.53 (72.32–93.75) | 80.63 (66.40–89.30) | .5833 |
| Locally advanced (IB2‐IVA) | 80.50 (74.86–85.01) | 80.52 (77.88–82.89) | 73.06 (66.21–78.75) | 68.23 (64.76–71.43) | 65.00 (53.90–74.06) | 61.46 (56.73–65.83) | .6368 |
| Advanced (IVB) | 78.57 (47.25–92.54) | 73.54 (56.91–84.57) | .6065 | ||||
| OS histologic type | |||||||
| Epidermoid | 73.77 (69.16–77.81) | 78.65 (76.26–80.58) | 66.78 (61.57–71.46) | 67.25 (64.63–69.73) | 58.09 (49.60–65.65) | 62.79 (59.62–65.78) | .5960 |
| Adenocarcinoma | 83.41 (71.95–90.48) | 77.24 (71.73–81.81) | 77.46 (64.59–86.14) | 68.09 (61.62–73.71) | 73.78 (59.03–83.91) | 65.21 (58.16–71.37) | .1754 |
| Adenosquamous | 74.07 (39.07–90.86) | 77.68 (63.89–86.72) | 74.07 (39.07–90.86) | 67.09 (51.09–78.89) | .7972 | ||
| Neuroendocrine | 68.57 (30.46–88.71) | 47.62 (28.78–64.28) | 68.57 (30.46–88.71) | 22.68 (5.40–47.03) | .3835 | ||
| DFS histologic type | |||||||
| Epidermoid | 82.69 (77.83–86.58) | 83.50 (81.21–85.54) | 76.80 (70.84–81.70) | 73.74 (70.71–76.50) | 70.57 (60.86–78.30) | 67.27 (62.64–71.45) | .9133 |
| Adenocarcinoma | 86.2 (74.26–92.89) | 81.74 (75.43–86.58) | 77.81 (62.55–87.44) | 73.52 (66.01–79.63) | 65.97 (46.97–79.54) | 66.59 (57.11–74.44) | .6395 |
| Adenosquamous | 85.71 (33.41–97.86) | 81.88 (63.74–91.51) | 85.71 (33.41–97.86) | 70.89 (47.52–85.30) | .7214 | ||
| Neuroendocrine | 66.67 (5.41–94.52) | 66.18 (36.37–84.49) | 66.67 (5.41–94.52) | 39.71 (13–96) | .5071 | ||
Abbreviations: CI, confidence interval; DFS, disease‐free survival; OS, overall survival.
Figure 3.

Overall survival and disease‐free survival by age (younger vs. older patients with cervical cancer according to histologic type. National Cancer Institute of Mexico. 2005–2015. (A): Epidermoid. (B): Adenocarcinoma. (C): Adenosquamous. (D): Neuroendocrine.
Abbreviations: DFS, disease‐free survival; OS, overall survival.
Adjusting for other known prognostic factors, we performed a multivariate analysis with a Cox proportional hazards model to evaluate OS and DFS. We did not find significant differences in survival between younger (<40) and older (>40) patients.
The statistically significant factors in OS were as follows: locally advanced stage (hazard ratio [HR], 3.34; 95% confidence ratio [CI], 2.12–5.26; p < .001), advanced stage (HR, 3.86; 95% CI, 2.28–6.55; p < .001), neuroendocrine histologic type (HR, 1.81; 95% CI, 1.14–2.89; p < .001), hydronephrosis (HR, 1.61; 95% CI, 1.29–1.99; p < .001), inguinal lymph nodes involvement (HR, 2.10; 95% CI, 1.37–3.40; p < .001), pregnancies (HR, 1.74; 95% CI, 1.27–2.39; p < .001), persistence (HR, 2.67; 95% CI, 2.12–3.37; p < .001), and progression (HR, 3.84; 95% CI, 2.99–4.94; p < .001; Table 3). The prognostic factors for DFS were locally advanced stage (HR, 1.92; 95% CI, 1.29–2.86; p < .001), persistence (HR, 0.32; 95% CI, 0.25–0.45; p < .001), and progression (HR, 14.50; 95% CI, 11.38–18.48; p < .001; Table 4). Unlike the factors involved in OS, hydronephrosis did not affect DFS. Having been pregnant at any point in life was a protective factor in DFS and reduced the risk of recurrence by 46% (95% CI, 0.40–0.71; p < .001) in comparison with women who had never been pregnant.
Table 3.
Cox proportional hazards model
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | ||||
| Under 40 | 1.00 | 1.00 | ||
| Over 40 | 1.01 (0.85–1.21) | .870 | 0.97 (0.80–1.19) | .786 |
| Stage | ||||
| Early | 1.00 | 1.00 | ||
| Locally advanced | 6.07 (4.16–8.88) | >.001 | 3.34 (2.12–5.26) | >.001 |
| Advanced | 12.37 (7.94–19.29) | >.001 | 3.86 (2.28–6.55) | >.001 |
| Histologic type | ||||
| Epidermoid | 1.00 | 1.00 | ||
| Adenocarcinoma | 0.92 (0.75–1.14) | .462 | 0.97 (0.75–1.26) | .841 |
| Adenosquamous | 1.04 (0.67–1.62) | .867 | 0.83 (0.46–1.52) | .553 |
| Neuroendocrine | 2.65 (1.73–4.06) | >.001 | 1.81 (1.14–2.89) | .013 |
| Other | 1.06 (0.48–2.38) | .881 | 1.27 (0.52–3.10) | .599 |
| Hydronephrosis | ||||
| No | 1.00 | 1.00 | ||
| Yes | 3.20 (2.68–3.81) | >.001 | 1.61 (1.29–1.99) | >.001 |
| Not tested | 0.62 (0.51–0.76) | >.001 | 0.85 (0.66–1.09) | .198 |
| Lymph node involvement | ||||
| None | 1.00 | 1.00 | ||
| Cervix | 3.05 (1.58–5.90) | .001 | 1.64 (0.79–3.39) | .186 |
| Inguinal | 3.59 (2.60–4.96) | >.001 | 2.10 (1.37–3.40) | .001 |
| Pregnancies | ||||
| No | 1.00 | 1.00 | ||
| Yes | 2.04 (1.55–2.69) | >.001 | 1.74 (1.27–2.39) | .001 |
| Recurrence | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.80 (1.54–2.44) | >.001 | 1.10 (0.88–1.38) | .394 |
| Persistence | ||||
| No | 1.00 | 1.00 | ||
| Yes | 7.19 (6.16–8.40) | >.001 | 2.67 (2.12–3.379) | >.001 |
| Progression | ||||
| No | 1.00 | 1.00 | ||
| Yes | 8.49 (7.22–10.00) | >.001 | 3.84 (2.99–4.94) | >.001 |
No. observations: 2240, no. events: 589, LR chi2 (20): 804.43, log probability: −3916.95, Prob >0.0000; Harrell's C = 0.8043, Somers’ D = 0.6087. Overall survival according to age group, INCan 2005–2015.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 4.
Cox proportional hazards model
| Characteristic | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | ||||
| Under 40 | 1.00 | 1.00 | ||
| Over 40 | 1.04 (0.83–1.29) | .756 | 1.01 (0.77–1.31) | .939 |
| Stage | ||||
| Early | 1.00 | 1.00 | ||
| Locally advanced | 3.40 (2.40–4.81) | >.001 | 1.92 (1.29–2.86) | .001 |
| Advanced | 3.68 (2.03–6.69) | >.001 | 1.76 (0.92–3.38) | .089 |
| Histologic type | ||||
| Epidermoid | 1.00 | 1.00 | ||
| Adenocarcinoma | 1.11 (0.86–1.42) | .428 | 0.96 (0.71–1.29) | .780 |
| Adenosquamous | 0.82 (0.44–1.54) | .544 | 0.98 (0.50–1.91) | .953 |
| Neuroendocrine | 2.31 (1.24–4.34) | .009 | 0.98 (0.48–2.00) | .953 |
| Other | 0.69 (0.17–2.76) | .596 | 1.41 (0.06–3.14) | .393 |
| Hydronephrosis | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.81 (1.37–2.41) | >.001 | 1.28 (0.88–1.85) | .200 |
| Not tested | 0.56 (0.44–0.71) | >.001 | 0.65 (0.47–0.90) | .009 |
| Lymph node involvement | ||||
| None | 1.00 | 1.00 | ||
| Neck | 2.26 (0.73–7.05) | .158 | 0.49 (0.07–3.51) | .474 |
| Inguinal | 2.56 (1.50–4.36) | .001 | 0.82 (0.38–1.78) | .620 |
| Pregnancies | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.69 (0.55–0.87) | .002 | 0.54 (0.40–0.71) | >.001 |
| Persistence | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.58 (1.22–2.04) | .001 | 0.32 (0.25–0.45) | >.001 |
| Progression | ||||
| No | 1.00 | 1.00 | ||
| Yes | 9.74 (8.01–11.85) | >.001 | 14.50 (11.38–18.48) | >.001 |
Disease‐free survival according to age group, INCan 2005–2015. No. observations, 1,849, no. events: 370; LR chi2 (18): 551.57, log probability: −23.09.80, Prob >0.0000; Harrell's C = 0.7955, Somers’ D = 0.5909.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Discussion
Previous studies have reported that younger patients have worse survival rates 8, 9, 10, 11, 12, 13, 14; however, the results have been contradictory. Recent findings suggest that other prognostic factors, such as histologic type, stage, or type of treatment, could mediate survival.
Our study evaluated the OS and DFS in women with CC in terms of age at diagnosis. We retrospectively analyzed 2,982 women divided into two groups: younger (<40) and older (>40) patients who were treated at INCan, the main oncology reference center in Mexico, from 2005 to 2015.
Our results show that age does not affect OS or DFS in women with CC, even when stage and histologic type were controlled. Our findings agree with several reports stating that age at diagnosis is not a prognostic factor 15, 16.
Seamon et al. 19 reported opposite results: they argued that OS decreased by 3% for every increase of one year in age. They retrospectively studied a cohort divided into three groups (<40, 40–50, and > 50) from 2001 to 2010, but the HR was 1.03 (95% CI, 1.02–1.04), which is a similar result to the one obtained for DFS. Comorbidities and stage at diagnosis, rather than age, affect OS and DFS 19.
When Quinn et al. retrospectively studied a 1973–2015 SEER cohort divided into age groups (20–49, 50–69, and > 70), they found that women older than 50 years showed poorer OS and were diagnosed in late stages. They also found that despite treatments being less aggressive than the ones administered to younger patients, older patients had poorer overall survival (50–69 years; HR, 1.46; >70 HR, 2.87) 20.
These differences could be explained, first, by the year in which the studies were conducted. Before 1999, patients with locally advanced cancer only received radiotherapy. After 1999, concomitant radiotherapy and chemotherapy became the standard treatment. Some studies report the positive effects chemotherapy had on survival 21, 22, 23, 24, 25.
Based on our study, it is worth noting that the population treated at INCan is similar to the rest of Mexico and Latin‐American countries. We treat patients in late stages (locally advanced and advanced: 73.10% and 7.58%, respectively, of patients under 40 years, and 78% and 6.93% in the case of patients over 40 years), unlike developed countries where early stages prevail, which might distort the assessment of OS and DFS.
In our sample, even though younger patients were diagnosed in early stages, they showed higher progression rates than older patients. It could be explained by lower susceptibility to conventional treatments or more aggressive cancers. Nevertheless, he interaction between these factors did not show a effect in survival.
We also identified other factors predicting OS and DFS. We found that stage IVB due to inguinal lymph node involvement (HR, 2.10; 95% CI, 1.37–3.40; p < .001) was a statistically significant factor in OS. In this sense, FIGO 2018 classification 26 The recently modified staging system in the lymph node involvement section was modified taking into account the prognostic relevance of lymph node metastasis. The changes were as follows: stage IIIC1 was assigned if there are metastases to pelvic lymph nodes, and IIIC2 is assigned in case of para‐aortic lymph node metastasis; in the previous FIGO 2009 classification, paraaortic metastasis was a stage IVB and currently is only considered stage IVB due to distant visceral metastases or when metastatic lymph nodes are outside the two previous sites, including the inguinal ones. According to our analysis, stage IVB caused by inguinal node metastases has a worse prognosis.
Also, in both groups we found that neuroendocrine carcinoma and hydronephrosis were negative predictors of OS. According to our study, locally advanced stage was the highest risk factor for OS, which indicates that advanced stages at diagnosis are still the main factor related to CC mortality. Early detection and, on a larger scale, prevention seem to be the best strategy to reduce the deaths caused by this neoplasia.
This is the first study focused on evaluating survival in a large cohort of patients with long follow‐up times in Mexico. Our study showed that we could expect a 10‐year survival rate of 60% irrespective of age at diagnosis.
However, our analysis was retrospective and not randomized, so bias could exist. More specific studies are needed to examine age‐specific prognosis, particularly in the case of older patients and type of treatment. Other limitations of our study include the following: (a) data were collected in one care center, but the data from patients who did not receive initial treatment in our center were not associated with OS or DFS; (b) the difference in the number of patients in each age group, particularly in the <40 group, which limited the analysis of separated subgroups; (c) the time to receive treatment was not always appropriate; (d) the cause of death is unknown in some cases, so were ignored if age affected disease‐specific survival and relative survival.
The strength of our study lies in its sample size: it is the largest series on CC and with the longest follow‐up times published in Latin‐America. The limitations are its retrospective nature and that cases were taken from only one institution.
Conclusion
As we corroborated the results of our study, which are in agreement with other reports, we concluded that age at diagnosis of CC is not a prognostic factor for OS and DFS. In the analysis of both groups, the stage, histologic subtype, hydronephrosis, and stage IVB due to inguinal lymph node involvement were identified as factors adverse to OS and DFS, and pregnancy history was a protective factor in DFS.
Author Contributions
Conception/design: David Isla‐Ortiz, Elizabeth Palomares‐Castillo, Nancy Reynoso‐Noverón
Provision of study material or patients: David Isla‐Ortiz, Nora Ramírez‐Calderón, Nancy Reynoso‐Noverón
Collection and/or assembly of data: David Isla‐Ortiz, Elizabeth Palomares‐Castillo, Nora Ramírez‐Calderón, Nancy Reynoso‐Noverón
Data analysis and interpretation: David Isla‐Ortiz, Nancy Reynoso‐Noverón
Manuscript writing: David Isla‐Ortiz, Elizabeth Palomares‐Castillo, Nancy Reynoso‐Noverón
Final approval of manuscript: David Isla‐Ortiz, Elizabeth Palomares‐Castillo, José Emilio Mille‐Loera, Nora Ramírez‐Calderón, Alejandro Mohar‐Betancourt, Abelardo A. Meneses‐García, Nancy Reynoso‐Noverón
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Arbyn M, Autier P, Ferlay J. Burden of cervical cancer in the 27 members states of the European Union: Estimates for 2004. Ann Oncol 2007;18:1423–1425. [DOI] [PubMed] [Google Scholar]
- 2. Arbyn M, Castellsagué X, de Sanjosé S et al. Worldwide burden of cervical cancer in 2008. Ann Oncol 2011;22:2675–2686. [DOI] [PubMed] [Google Scholar]
- 3. Bary F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4. Bosetti C, Rodríguez T, Chatenoud L et al. Trends in cancer mortality in Mexico, 1981‐2007. Eur J Cancer Prev 2011;20:355–363. [DOI] [PubMed] [Google Scholar]
- 5. Gómez‐Dantés H, Lamadrid‐Figeroa H, Cahuana‐Hurtado L et al. The burden of cancer in Mexico, 1990‐2013. Salud Publica Mex 2016;58:118–131. [DOI] [PubMed] [Google Scholar]
- 6. Mohar‐Betancourt A, Reynoso‐Noverón N, Armas‐Texta D et al. Cancer trends in Mexico: Essential data for creation and follow‐up of public policies. J Glob Oncol 2017; 3:740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kapp D, Fischer D, Gutierrez E et al. Pretreatment prognostic factors in carcinoma of the uterine cervix: A multivariable analysis of the effects of age, stage, histology and blood counts on survival. Int J Radiat Oncol Biol Phys 1983;9:445–455. [DOI] [PubMed] [Google Scholar]
- 8. Murrell D, Helm C, Bourne H. Carcinoma of cervix in women up to 35 year of age. Clin Cncol (R Coll Radiol) 1990;2:260–263. [DOI] [PubMed] [Google Scholar]
- 9. Lau H, Juang C, Chen Y et al. Aggressive characteristics of cervical cancer in young women in Taiwan. Int J Gynaecol Obstet 2009;107:220–223. [DOI] [PubMed] [Google Scholar]
- 10. Serur E, Fruchter R, Maiman M, et al. Age, substance abuse, and survival of patients with cervical carcinoma. Cancer 1995;75:2530–2538. [DOI] [PubMed] [Google Scholar]
- 11. Rutledge F, Mitchell M, Munsell M et al. Youth as a prognostic factor in carcinoma of cervix: a matched analysis. Gynecol Oncol 1992;44:123–130. [DOI] [PubMed] [Google Scholar]
- 12. Pelkofski E, Stine J, Wages N et al. Cervical cancer in women aged 35 years and younger. Clin Ther 2016;38:459–466. [DOI] [PubMed] [Google Scholar]
- 13. Prempree T, Patanaphan V, Sewchand W et al. The influence of patient's age and tumor grade on the prognosis of carcinoma of the cervix. Cancer 1983;51:1764–1771. [DOI] [PubMed] [Google Scholar]
- 14. Lybeert M, Meerwaldt J, van Putten W. Age as a prognostic factor in carcinoma of the cervix. Radiother Oncol 1987;9:147–151. [DOI] [PubMed] [Google Scholar]
- 15. de Rijke J, van der Putten H, Lutgens L et al. Age‐specific differences in treatment and survival of patients with cervical cancer in the southeast of The Netherlands, 1986‐1996. Eur J Cancer 2002;38:2041–2047. [DOI] [PubMed] [Google Scholar]
- 16. Póká R, Juhász B, Lampé L. Cervical cancer in young women: A poorer porgnosis? Int J Gynaecol Obstet 1994;46:33–37. [DOI] [PubMed] [Google Scholar]
- 17. Kim HS, Song YS. International Federation of Gynecology and Obstetrics (FIGO) staging system revised: What should be considered critically for gynecologic cancer? J Gynecol Oncol 2009;20:135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sedlis A, Bundy BN, Rotman MZ et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–183. [DOI] [PubMed] [Google Scholar]
- 19. Seamon LG, Tarrant RL, Fleming ST et al. Cervical cancer survival for patients referred to a tertiary care center in Kentucky. Gynecol Oncol 2011;123:565–570. [DOI] [PubMed] [Google Scholar]
- 20. Quinn BA, Deng X, Colton A et al. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachyterapy 2019;18:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keys HM, Bundy BN, Stehman FB et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154–1161. [DOI] [PubMed] [Google Scholar]
- 22. Rose PG, Bundy BN, Watkins EB et al. Concurrent cisplatin‐based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–1153. [DOI] [PubMed] [Google Scholar]
- 23. Whitney CW, Sause W, Bundy BN et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea in stage IIB/IVA in carcinoma of the cervix. J Clin Oncol 1999;17:1339–1348. [DOI] [PubMed] [Google Scholar]
- 24. Peters WA 3rd, Liu PY, Barrett RJ 2nd et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high‐risk early‐stage cancer of the cervix. J Clin Oncol 2000;18:1606–1613. [DOI] [PubMed] [Google Scholar]
- 25. Morris M, Eifel PJ, Lu J et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para‐aortic radiation for high‐risk cervical cancer. N Engl J Med 1999;340:1137–1143. [DOI] [PubMed] [Google Scholar]
- 26. Bhatla N, Aoki D, Sharma DN et al. Cancer of the cervix uteri. Int J Gynecol Obstet 2018;143(suppl 2):22–36. [DOI] [PubMed] [Google Scholar]


