Abstract
Background
Taxanes usually follow anthracyclines in breast cancer neo/adjuvant treatment, likely because of their later introduction into clinical practice. However, there is no biological rationale that justifies this current standard of care. We compared a taxane followed by an anthracycline‐based regimen with the reverse sequence in the neoadjuvant setting.
Patients and Methods
In a randomized, open‐label, single‐center phase II trial, women with inoperable, locally advanced, HER2‐negative breast cancer were stratified by hormone receptor status and randomized to three cycles of docetaxel (T) followed by three cycles of fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus three cycles of FAC followed by three cycles of docetaxel. Surgery, radiotherapy, and adjuvant hormonal therapy were administered as per local guidelines. The primary endpoint was pathological complete response (pCR), and secondary endpoints included toxicity, event‐free survival (EFS), and overall survival (OS).
Results
Treatment sequence did not improve pCR, which was 7% with T‐FAC and 3% with FAC‐T. However, after a median follow‐up of 79 months, the 5‐year EFS rate was 75.7% (95% confidence interval [CI], 65.4%–87.7%) with T‐FAC and 48.2% (95% CI, 37.0%–62.7%) with FAC‐T (hazard ratio [HR], 0.46; 95% CI, 0.26–0.81; log‐rank p = .0054), and the 5‐year OS rate was 89.7% (95% CI, 82.2%–97.8%) with T‐FAC and 64.7% (95% CI, 53.6%–78.1%) with FAC‐T (HR, 0.41; 95% CI, 0.22–0.78; p = .0052). There were no unexpected toxicities.
Conclusion
We showed for the first time an improvement in EFS and OS with taxane‐first compared with anthracycline‐first sequencing chemotherapy in HER2‐negative, locally advanced breast cancer. Confirmation of these results may have implications for clinical practice.
This trial was registered with Clinicatrials.gov identifier NCT01270373.
Implications for Practice
The NeoSAMBA trial showed a benefit for taxane‐first sequencing chemotherapy consistent with the systematic review of the literature as well as the larger Neo‐tAnGo study. Many recent and current ongoing clinical trials have already followed this treatment strategy. As a taxane‐before‐anthracycline sequence carries neither an incremental cost nor an increased toxicity, and given the available literature on this issue, reinforced that taxane‐first regimen can be easily incorporated into daily clinical practice while awaiting confirmation of these findings from larger trials.
Keywords: Breast neoplasms, Doxorubicin, Taxoids
Short abstract
Despite remarkable achievements in breast cancer research, little attention has been paid to the sequence of chemotherapy agents in breast cancer treatment. This article compares an anthracycline‐based followed by a taxane neoadjuvant chemotherapy regimen with the reverse sequence in patients with locally advanced, HER2‐negative invasive breast cancer.
Introduction
It has been more than 40 years since a pivotal trial showed that 12 months of postoperative chemotherapy consisting of cyclophosphamide, methotrexate, and fluorouracil (CMF) decreased the risk of recurrence of breast cancer in women with positive axillary lymph nodes [1]. Breast cancer adjuvant chemotherapy evolved over the years to incorporate anthracyclines, taxane‐containing regimens, and a combination of chemotherapy with targeted therapy for the HER2‐positive subset [2, 3]. We have also learned that administering neoadjuvant chemotherapy does not compromise distant recurrence, breast cancer survival, or overall survival (OS) rates [4]. Dose intensification with chemotherapy drugs given at a shorter treatment interval provided further improvement [5]. Taking all these advances over the last decades, breast cancer adjuvant chemotherapy reduced mortality by almost half when compared with no adjuvant chemotherapy. Recent efforts have focused on tailoring adjuvant chemotherapy to the women most likely to benefit from it, potentially sparing toxicity and cost through gene‐expression assays [6, 7].
Despite these remarkable achievements, little attention has been paid to the sequence of chemotherapy agents. The delivery of doxorubicin followed by CMF significantly reduced the risk of disease relapse and death when compared with an alternating regimen of doxorubicin and CMF, despite the fact that both regimens included the same drugs, at the same doses, with identical treatment duration and dose intensity [8]. Currently, anthracyclines and taxanes represent the main chemotherapy drugs for breast cancer adjuvant treatment. Taxanes usually follow anthracyclines in breast cancer neo/adjuvant treatment, likely because of their later introduction into clinical practice. However, there is no biological rationale that justifies this current standard of care. A systematic review of the published data descriptively summarized the results from 15 mainly nonrandomized studies (eight in the neoadjuvant setting and seven in the adjuvant setting) totaling almost 5,000 patients with no disadvantages in terms of efficacy or toxicity for sequences in which the taxane was administered first [9]. A recent meta‐analysis showed that there was considerable variation in the level of evidence supporting equivalent outcomes for the order in which taxanes are delivered in the neoadjuvant setting [10].
We prospectively compared an anthracycline‐based regimen followed by a taxane neoadjuvant chemotherapy with the reverse sequence in patients with locally advanced, HER2‐negative invasive breast cancer.
Materials and Methods
Study Design and Treatment
This randomized, parallel, open‐label, phase II trial was performed at a single center, the Instituto Nacional de Câncer, in Brazil. The NeoSAMBA trial (Neoadjuvant: Does the Sequence of Anthracycline and Taxane Matter: Before or After?) was approved by the local ethics committee on July 21, 2010. This trial was registered with Clinicatrials.gov identifier NCT01270373.
We enrolled women aged older than 18 years with a histological diagnosis of invasive HER2‐negative breast cancer (defined according to the American Society of Clinical Oncology/College of American Pathologists guidelines) and a clinical tumor size of more than 50 mm (T3) or a clinically N2 axillary involvement [11]. Other eligibility criteria were adequate cardiac, bone marrow, hepatic, and renal function and appropriate Eastern Cooperative Oncology Group performance status (0–2). No previous exposure to chemotherapy, radiotherapy, or endocrine therapy and no uncontrolled diabetes or peripheral neuropathy were allowed. All patients provided written informed consent.
After stratification by hormone receptor status, women were randomly assigned via a computerized system on a 1:1 ratio to the two treatment groups. Hormone receptor was considered as positive when >1% of tumor cell nuclei were immunoreactive. Neither patients nor investigators were masked to treatment allocation.
Baseline studies included tumor biopsy, routine blood tests, chest x‐ray, abdominal ultrasound, bone scintigraphy, electrocardiogram, and echocardiogram. Tumor and blood samples were collected for translational analyses at three time points and will be reported elsewhere. All patients were scheduled to receive six cycles of a 21‐day regimen of neoadjuvant intravenous chemotherapy. The experimental arm consisted of three cycles of docetaxel 100 mg/m2 (T) followed by three cycles of fluorouracil 500 mg/m2, doxorubicin 50 mg/m2, and cyclophosphamide 500 mg/m2 (T‐FAC), and the control arm consisted of the identical doses of FAC followed by docetaxel (FAC‐T). Primary prophylaxis with granulocyte colony‐stimulating factor was not provided. Surgery was performed no later than 10 weeks after the end of the last chemotherapy cycle. Radiotherapy and adjuvant hormonal therapy were administered as per local guidelines.
Patients were followed up every 21 days during the chemotherapy period with physical examination and blood tests and on average every 6 months postsurgery, according to the local clinical practice.
Outcomes
The primary endpoint was pathological complete response (pCR), defined as absence of residual invasive disease in the breast and axillary lymph nodes. Secondary endpoints included toxicity, relative dose intensity, event‐free survival (EFS), and OS. Event‐free survival was defined as the time from randomization to disease progression, recurrence (either local or distant), or death from any cause. Overall survival was the time elapsed between the date of randomization to death from any cause. We assessed adverse events after each chemotherapy cycle using Common Terminology Criteria for Adverse Events, version 3.0. We also recorded use of growth factor support and red blood cell transfusion.
Statistical Analysis
A pathological response rate of less than 10% was expected as anticipated in patients treated with neoadjuvant chemotherapy locally. Considering a type I error of 0.1 and a type II error of 0.1, a total of 56 evaluable patients per arm should be included. A 5% dropout rate was awaited.
R‐project packages “survival” and “survminer” were used for analyses [12, 13, 14]. Two‐sided p values less than .05 were considered statistically significant.
All patients who received any treatment were included in the safety, primary endpoint, EFS, and OS analyses, which were performed on an intent‐to‐treat basis.
Patients without an EFS or OS event were censored at the last known follow‐up time for the corresponding analysis. Fisher's exact test was used to compare dose reductions and pCR, and the Mann‐Whitney U test was used for treatment interval comparison between arms. We constructed Kaplan‐Meier curves, used log‐rank tests to compare them, and used Cox proportional‐hazards models to assess hazard ratios (HRs) and adjust for other prognostic factors. We report the long‐term results of EFS and OS.
The institutional data monitoring committee oversaw the trial. The study was developed and conducted in accordance with the national regulations and the principles of Good Clinical Practice.
Results
Patient Characteristics and Treatment Exposure
From August 10, 2010, through November 29, 2012, we enrolled 120 patients. Two patients were found to be ineligible after randomization, leaving 118 patients for the analyses of secondary endpoints (Fig. 1).
Figure 1.
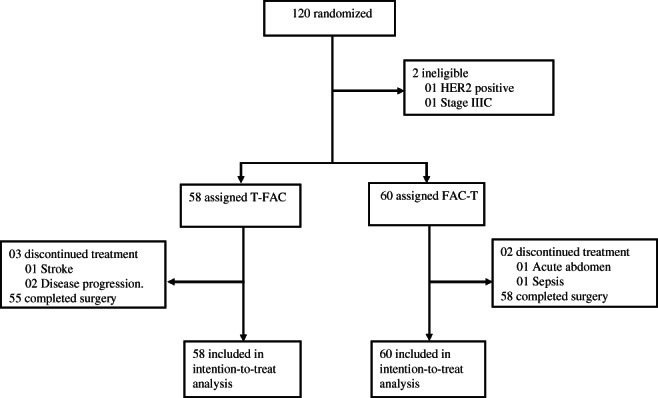
Trial profile.
Patients and tumor baseline characteristics are depicted in Table 1 and were relatively well balanced. Ninety‐four patients (80%) had stage III disease, and 31 (26%) had triple‐negative breast cancer (TNBC). No notable difference of the relative dose intensity existed between the two arms in the T or FAC components. There was a numerically higher dose reduction rate in the FAC‐T arm (18%) when compared with the reverse sequence (5%) related to the T component (p = .04). Dose interruptions, blood transfusions, and growth factor use were similar between the randomized treatment groups (Table 2). The median time interval between the end of chemotherapy and surgery was 38 days (interquartile range [IQR], 35–51) and 42 days (IQR, 33–55) in the T‐FAC and FAC‐T arms (p = .66), respectively. Radiation therapy followed surgery at a median time of 49 days (IQR, 41–61) in the T‐FAC arm and 49 days (IQR, 41–57) in the FAC‐T arm (p = .70). Five patients in each arm had radiation prior to surgery because of inoperable tumors.
Table 1.
Baseline characteristics of the intention‐to‐treat population
| Characteristics | T‐FAC (n = 58), n (%) | FAC‐T (n = 60), n (%) |
|---|---|---|
| Age groups, years | ||
| <40 | 10 (17) | 18 (30) |
| 41–64 | 44 (76) | 33 (55) |
| >64 | 4 (7) | 9 (15) |
| Age, median (IQR), years | 50 (44–59) | 50 (38–60) |
| ECOG performance status a | ||
| 0 | 43 (75) | 42 (71) |
| 1 | 14 (25) | 17 (29) |
| Histology | ||
| IDC | 51 (88) | 54 (90) |
| ILC | 5 (9) | 3 (5) |
| Other | 2 (3) | 3 (5) |
| Stage | ||
| IIB | 14 (24) | 10 (17) |
| IIIA | 19 (33) | 21 (35) |
| IIIB | 25 (43) | 29 (48) |
| Subtype | ||
| Hormone receptor positive | 42 (72) | 45 (75) |
| Triple negative | 16 (18) | 15 (25) |
| Grade a | ||
| 1 | 4 (10) | 3 (7) |
| 2 | 29 (71) | 28 (65) |
| 3 | 8 (20) | 12 (28) |
Data not available for all randomized patients.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FAC, fluorouracil, doxorubicin, and cyclophosphamide; IDC, infiltrating ductal carcinoma; IQR, interquartile range; ILC, infiltrating lobular carcinoma; T, docetaxel.
Table 2.
Treatment delivered
| Treatment features | T‐FAC (n = 58) | FAC‐T (n = 60) |
|---|---|---|
| RDI FAC, median (IQR) | 98.2 (93.6–100.6) | 99.0 (95.8–100.6) |
| RDI T, median (IQR) | 98.5 (96.7–100.4) | 97.7 (91.9–100.2) |
| Dose reduction, n (%) | 3 (5) | 11 (18) |
| Dose interruption, n (%) | 4 (7) | 4 (7) |
| Blood transfusion, n (%) | 1 (2) | 2 (3) |
| GCSF use, n (%) | 12 (21) | 14 (23) |
Abbreviations: FAC, fluorouracil, doxorubicin, and cyclophosphamide; GSCF, granulocyte colony‐stimulating factor; IQR, interquartile range; RDI, relative dose intensity; T, docetaxel.
Efficacy Results
Only six patients (5%) had a pCR, and a post hoc analysis showed that 12 patients (10%) had a residual cancer burden score of 0 or 1 [15]. The treatment sequence did not improve pCR, which was seen in four (7%) patients in the T‐FAC and two (3%) in the FAC‐T arms, respectively (p = .43). Only patients with triple‐negative disease had a pCR (p < .001 for the comparison with hormone receptor–positive disease; Table 3).
Table 3.
Rates of pathologic complete response and residual cancer burden according to breast cancer subtype and treatment sequence
| Subtype/Treatment | (n = 118), n | pCR (n = 6), n (%) | RCB 0–I (n = 12), n (%) |
|---|---|---|---|
| Subtype | |||
| Triple negative | 31 | 6 (19.4) | 9 (29.0) |
| Hormone receptor positive | 87 | 0 (0) | 3 (3.4) |
| Treatment sequence | |||
| T‐FAC | 58 | 4 (6.9) | 6 (10.3) |
| FAC‐T | 60 | 2 (3.3) | 6 (10) |
Abbreviations: FAC, fluorouracil, doxorubicin, and cyclophosphamide; pCR, pathologic complete response; RCB, residual cancer burden; T, docetaxel.
The median follow‐up was 79 months (95% confidence interval [CI], 76–84 months). At the time of analysis, 18 patients (31%) and 31 patients (52%) had a recurrence: 2 patients (3%) and 6 patients (10%) had a local recurrence as a first event, whereas 16 (28%) and 26 (43%) had a distant recurrence, in the T‐FAC and FAC‐T arms, respectively. One patient (2%) in the FAC‐T arm had a simultaneous local and distant recurrence (Table 4).
Table 4.
Site of first invasive recurrence
| Site | T‐FAC (n = 58), n (%) | FAC‐T (n = 60), n (%) |
|---|---|---|
| Total | 18 (31) | 31 (52) a |
| Local | 2 (3) | 6 (10) |
| Regional | 0 | 0 |
| Distant | 16 (28) | 26 (43) |
One patient had simultaneous local and distant recurrence.
Abbreviations: FAC, fluorouracil, doxorubicin, and cyclophosphamide; T, docetaxel.
The 5‐year EFS rate was 75.7% (95% CI, 65.4%–87.7%) with T‐FAC and 48.2% (95% CI, 37.0%–62.7%) with FAC‐T (HR, 0.46; 95% CI, 0.26–0.81; log‐rank p = .0054). The 5‐year OS rate was 89.7% (95% CI, 82.2%–97.8%) with T‐FAC and 64.7% (95% CI, 53.6%–78.1%) with FAC‐T (HR, 0.41; 95% CI, 0.22–0.78; p = .0052), respectively (Fig. 2). After excluding the patients who had dose reductions, the HR for EFS remained statistically significant (HR, 0.427; 95% CI, 0.23–0.79). In a multiple Cox proportional‐hazards regression, only treatment sequence had statistically significant impact on EFS (adjusted HR, 0.46; 95% CI, 0.26–0.81; p = .007) and OS (adjusted HR, 0.43; 95% CI, 0.23–0.82; p = .009), after adjusting for stage and hormone receptor.
Figure 2.
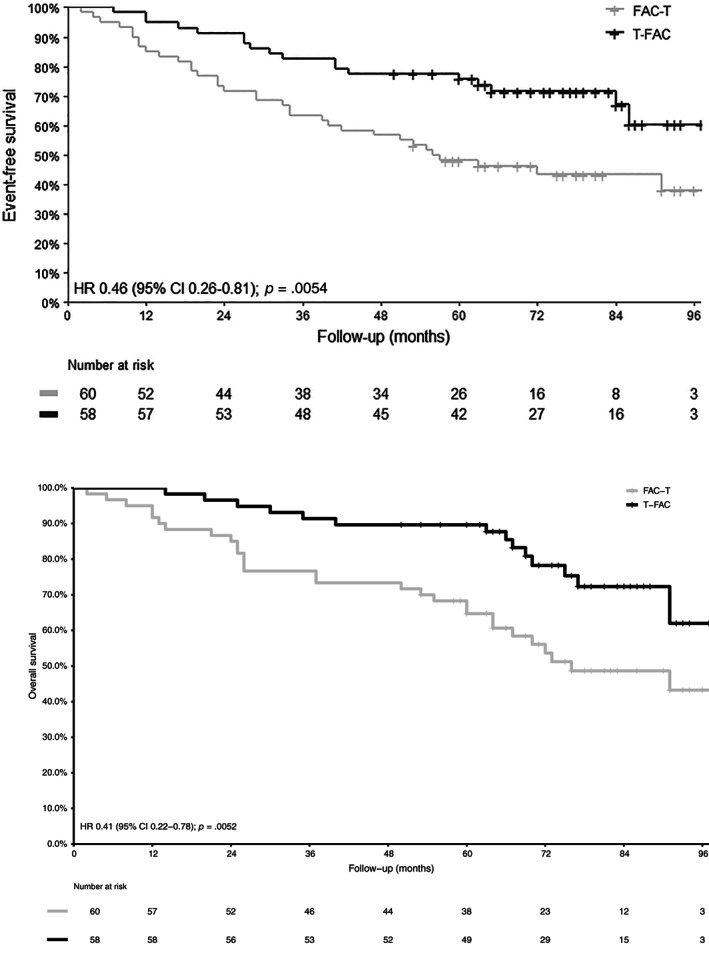
Event‐free survival and overall survival.
Abbreviations: CI, confidence interval; HR, hazard ratio; FAC‐T, fluorouracil, doxorubicin, and cyclophosphamide followed by docetaxel; T‐FAC, docetaxel followed by fluorouracil, doxorubicin, and cyclophosphamide.
Safety Results
Overall, the rates of grade 3 or higher adverse events were as expected, and there was no notable difference between the two treatment groups other than hypertension (10%) and muscle or joint pain (8%), which were both higher in the anthracycline‐first arm than in the taxane‐first arm (Table 5). Other adverse events included neutropenia and febrile neutropenia, acute hypersensitivity, hyperglycemia, and infection. Two patients died during the protocol treatment period, both in the FAC‐T group. One patient died after an acute abdomen following the first cycle of docetaxel, and the second one died of sepsis 46 days after the end of chemotherapy.
Table 5.
Summary of grade 3 or higher adverse events >5%
| Adverse events | T‐FAC (n = 58), n (%) | FAC‐T (n = 60), n (%) |
|---|---|---|
| Hematological | ||
| Neutropenia | 6 (10) | 8 (13) |
| Febrile neutropenia | 12 (21) | 13 (22) |
| Nonhematological | ||
| Acute hypersensitivity | 4 (7) | 4 (7) |
| Hyperglycemia | 2 (3) | 3 (5) |
| Hypertension | 0 (0) | 6 (10) |
| Infection | 2 (3) | 4 (7) |
| Muscle or joint pain | 0 (0) | 5 (8) |
Abbreviations: FAC, fluorouracil, doxorubicin, and cyclophosphamide; T, docetaxel.
Discussion
In this single‐center, randomized phase II trial we showed no difference in pCR with taxane‐first when compared with anthracycline‐first sequencing neoadjuvant chemotherapy in inoperable, HER2‐negative, locally advanced breast cancer. On the other hand, there was an improvement in EFS and OS with a taxane‐first chemotherapy. Treatment delivery and toxicity were similar between both arms.
In the NeoSAMBA trial, despite a higher pCR rate for the triple‐negative cohort, there was a low pCR for the overall patient population comprising mainly of hormone receptor–positive disease. How can the low pCR and the benefit in time‐to‐event outcome be reconciled? Although these results could be due to chance, these findings were described by large meta‐analyses of breast cancer neoadjuvant studies [16]. As expected, the frequency of pCR in patients with hormone receptor–positive tumors is low when compared with triple‐negative and HER2‐positive tumors. Although pCR has been the most commonly used endpoint in neoadjuvant trials, its role as a surrogate endpoint for EFS and OS has been debated. The weakest association between pCR and long‐term outcomes has been described for hormone receptor–positive and low‐grade tumors; moreover, pCR has not been confirmed as a prognostic marker in patients with luminal A or luminal B breast cancer [16]. The GeparSepto trial compared nab‐paclitaxel with solvent‐based paclitaxel (sb‐paclitaxel) in addition to anthracycline‐based neoadjuvant chemotherapy. The recently published update showed a higher pCR in TNBC and at the same time revealed the benefit of nab‐paclitaxel in long‐term invasive disease–free survival (iDFS) irrespective of hormone receptor status. Interestingly, patients with a Ki‐67 of less than 20% who were treated with nab‐paclitaxel did not show a higher pCR rate but had a better iDFS compared with those treated with sb‐paclitaxel [17]. Other potential explanations for these findings include the fact that adjuvant endocrine treatment for hormone receptor–positive breast cancer can improve the outcome of residual disease and that treatment sensitivity of the primary tumor and micrometastatic disease may differ. These combined results suggest an uncoupling of pCR and long‐term outcomes in HER2‐negative hormone receptor–positive breast cancer.
With regard to sequencing, the study Z1041 compared sequential epirubicin‐based treatment followed by paclitaxel and trastuzumab versus concurrent treatment with paclitaxel and trastuzumab followed by epirubicin and trastuzumab neoadjuvant chemotherapy for patients with HER2‐positive breast cancer and showed no significant difference in pCR rates, disease‐free survival, or OS [18]. Active anti‐HER2 targeted treatment may overcome the impact related to the order of chemotherapy agents in HER2‐positive breast cancer. The Neo‐tAnGo study, the largest neoadjuvant trial to compare chemotherapy treatment sequences, without a selection for breast cancer subtypes, showed an absolute 5% pCR improvement in the taxane‐before‐anthracycline arm. At the time of publication, with a median follow‐up of 47 months, there was no difference in disease‐free survival or OS according to treatment sequence, and long‐term results of this large trial are awaited [19]. In NeoSAMBA, HER2‐positive disease was excluded, minimizing the potential sequence and treatment interaction with anti‐HER2 agents. At the same time, 80% of the patients had stage III disease (as opposed to 80% of the patients included in Neo‐tAnGo with T2 disease), representing a population at very high risk of disease recurrence, in which the absolute magnitude of the benefit of a treatment is likely higher than in early‐stage cancers. Of note, the NeoSAMBA trial used doxorubicin and docetaxel, whereas the Neo‐tAnGo trial included epirubicin and paclitaxel as the anthracycline and taxane agent, respectively.
The study limitations included being a relatively small phase II single‐center trial that had pCR and not EFS and OS as its primary objectives. Also, patients enrolled in the study had a large disease burden, and they may have been understaged with the diagnostic imaging studies used. The National Comprehensive Cancer Network guidelines suggest chest and abdominal computed tomography (CT) and even positron‐emission tomography/CT in locally advanced breast cancer [20]. Moreover, chemotherapy regimens have changed over the years when the NeoSAMBA trial was ongoing. Although the optimal treatment duration is not currently known, six cycles of neoadjuvant chemotherapy for locally advanced disease may not be ideal. More recently, increasing the dose density of adjuvant chemotherapy by shortening intervals between treatment courses and the addition of adjuvant capecitabine to those without a pCR after standard anthracycline and taxane neoadjuvant chemotherapy further improved the outcomes [5, 21]. It is still to be determined if the observed impact of taxane‐first chemotherapy will be maintained with these more recent adjuvant regimens.
Underlying biological mechanisms may help to explain the benefits of taxane‐first sequencing. in vitro studies have shown differences in the ability of the drugs to induce cross‐resistance to each other. Cells selected for resistance to paclitaxel showed little cross‐resistance (fourfold) to doxorubicin. In contrast, cells selected for resistance to doxorubicin exhibited a dramatic 4,700‐fold and 14,600‐fold cross‐resistance to paclitaxel and docetaxel, respectively [22]. This study suggested that doxorubicin‐resistant cells exhibited higher P‐glycoprotein and breast cancer resistance protein as well as procaspase‐9 downregulation as possible explanations for this differential induction of cross‐resistance. Acquired resistance to doxorubicin via NF‐κB activation but not its upstream receptor TLR4 may also explain differential drug resistance, whereas taxane can induce drug resistance via the TLR4–NF‐κB pathway [23]. Therapies that increase vessel maturity and the density of perfused vessels might be optimal for alleviating hypoxia. Reengineering the tumor microenvironment to eliminate hypoxia and promote normoxia could lead to improved treatment outcomes. Taxanes may decrease interstitial fluid pressure and increase vessel maturity, and the density of perfused vessels which might be optimal for alleviating hypoxia, whereas doxorubicin had no such significant effect [24]. Such findings raise the hypothesis that tumors undergoing such changes could ultimately have a better overall response because of the improved penetration of the second drug. Another potential mechanism relates to senescence. The strongest induction of the senescent phenotype was seen with the DNA‐interactive agents doxorubicin and cisplatin, whereas the lowest was seen with the microtubule‐targeting drugs docetaxel and vincristine. The induction of senescence may lead to a persistent cytostatic state that may render tumor cells more resistant to subsequent therapies [25, 26]. At the same time, taxanes and anthracyclines have been reported to interfere with tumor microenvironment immune components, and we do not know yet the impact of different chemotherapeutic agents sequence in modeling such tumor milieu [27]. The acquisition of a phenotype marked by an increased abundance of CD44 (CD44Hi) by breast cancer cells as a tolerance response to taxanes, activating a metabolic switch that confers tolerance against unrelated standard‐of‐care chemotherapeutic agents, such as anthracyclines, merits further investigation [28].
NeoSAMBA collected tissue and blood samples before treatment initiation, at the time of chemotherapy switch, and at surgery. Correlative studies including immune mediators are ongoing, and a prospective phase III trial is planned.
Conclusion
In summary, the NeoSAMBA trial showed a benefit for taxane‐first sequencing chemotherapy consistent with the systematic review of the literature as well as the larger Neo‐tAnGo study. Many recent and current ongoing clinical trials have already followed this treatment strategy. As a taxane‐before‐anthracycline sequence carries neither incremental cost nor increased toxicity, and given the available literature on this issue, taxane‐first regimen can be easily incorporated into daily clinical practice while we wait for confirmation of these findings from larger trials.
Author Contributions
Conception/design: José Bines, Fabiola Kestelman, Eduardo Millen, Martin Bonamino
Provision of study material or patients: José Bines, Fabiola Kestelman, Fabiana Resende Rodrigues, Lilian Faroni, Aline Gonçalves, Erika Ebecken, Pedro Maroun, Eduardo Millen
Collection and/or assembly of data: Isabele A Small, Roberta Sarmento, Silvania Silva
Data analysis and interpretation: José Bines, Isabele A Small, Martin Bonamino
Manuscript writing: José Bines
Final approval of manuscript: José Bines, Isabele A Small, Roberta Sarmento, Fabiola Kestelman, Silvania Silva, Fabiana Resende Rodrigues, Lilian Faroni, Aline Gonçalves, Erika Ebecken, Pedro Maroun, Eduardo Millen, Martin Bonamino
Disclosures
The authors indicated no financial relationships.
Acknowledgments
Our sincere gratitude to Everardo D. Saad for his scientific input. This work was supported by Fundação do Câncer, Brazil. An additional unrestricted educational grant was received from Sanofi‐Aventis, Brazil, which provided free Taxotere (docetaxel).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Bonadonna G, Brusamolino E, Valagussa P et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976;294:405–410. [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists’ Collaborative Group . Comparisons between different polychemotherapy regimens for early breast cancer: Meta‐analyses of long‐term outcome among 100 000 women in 123 randomized trials. Lancet 2012;379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 2005;353:1673–1684. [DOI] [PubMed] [Google Scholar]
- 4. Early Breast Cancer Trialists’ Collaborative Group . Long‐term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta‐analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Early Breast Cancer Trialists’ Collaborative Group . Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient‐level meta‐analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet Oncol 2019;393:1440–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardoso F, van't Veer LJ, Bogaerts J et al. 70‐gene signature as an aid to treatment decisions in early‐stage breast cancer. N Engl J Med 2016;375:717–729. [DOI] [PubMed] [Google Scholar]
- 7. Sparano JA, Gray RJ, Makower DF et al. Adjuvant chemotherapy guided by a 21‐gene expression assay in breast cancer. N Engl J Med 2018;379:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonadonna G, Zambetti M, Moliterni A et al. Clinical relevance of different sequencing of doxorubicin and cyclophosphamide, methotrexate, and fluorouracil in operable breast cancer. J Clin Oncol 2004;22:1614–1620. [DOI] [PubMed] [Google Scholar]
- 9. Bines J, Earl H, Buzaid AC et al. Anthracyclines and taxanes in the neo/adjuvant treatment of breast cancer: Does the sequence matter? Ann Oncol 2014;25:1079–1085. [DOI] [PubMed] [Google Scholar]
- 10. Zaheed M, Wilcken N, Willson M et al. Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer. Cochrane Database Syst Rev 2019(2)CD012873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolff AC, Hammond ME, Schwartz JN et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–145. [DOI] [PubMed] [Google Scholar]
- 12. R: The R Project for Statistical Computing . Available at https://www.r-project.org/. Accessed August 10, 2019.
- 13. Therneau TM. Survival Analysis [R package survival version 2.42‐6]. Available at https://CRAN.R-project.org/package=survival. Accessed August 10, 2019.
- 14. Kassambara A, Kosinski M, Biecek P et al. survminer: Drawing Survival Curves using “ggplot2.” 2018. Available at https://CRAN.R-project.org/package=survminer. Accessed August 10, 2019.
- 15. Symmans WF, Peintinger F, Hatzis C et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 16. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 17. Untch M, Jackisch C, Schneeweiss A et al. NAB‐paclitaxel improves disease‐free survival in early breast cancer: GBG 69–GeparSepto. J Clin Oncol 2019;37:2226–2234. [DOI] [PubMed] [Google Scholar]
- 18. Buzdar AU, Suman VJ, Meric‐Bernstam F et al. Disease‐free and overall survival among patients with operable HER2‐positive breast cancer treated with sequential vs concurrent chemotherapy: The ACOSOG Z1041 (Alliance) randomized clinical trial. JAMA Oncol 2019;5:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Earl HM, Vallier AL, Hiller L et al. Effects of the addition of gemcitabine, and paclitaxel‐first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high‐risk early breast cancer (Neo‐tAnGo): An open‐label, 2 × 2 factorial randomised phase 3 trial. Lancet Oncol 2014;15:201–212. [DOI] [PubMed] [Google Scholar]
- 20. NCCN Clinical Practice Guideline in Oncology . Breast Cancer. 3.2019. Accessed September 6, 2019.
- 21. Masuda N, Lee SJ, Ohtani S et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017;376:2147–2159. [DOI] [PubMed] [Google Scholar]
- 22. Guo B, Villeneuve DJ, Hembruff SL et al. Cross‐resistance studies of isogenic drug‐resistant breast tumor cell lines support recent clinical evidence suggesting that sensitivity to paclitaxel may be strongly compromised by prior doxorubicin exposure. Breast Cancer Res Treat 2004;85:31–51. [DOI] [PubMed] [Google Scholar]
- 23. Xu F, Wang F, Yang T et al. Differential drug resistance acquisition to doxorubicin and paclitaxel in breast cancer cells. Cancer Cell Int 2014;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taghian AG, Abi‐Raad R, Assaad SI et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: Clinical implications. J Clin Oncol 2005;23:1951–1961. [DOI] [PubMed] [Google Scholar]
- 25. te Poele RH, Okorokov AL, Jardine L et al. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 2002;62:1876–1883 [PubMed] [Google Scholar]
- 26. Ewald JA, Desotelle JA, Wilding G et al. Therapy‐induced senescence in cancer. J Natl Cancer Inst 2010;102:1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kersten K, Salvagno C, de Visser KE. Exploiting the immunomodulatory properties of chemotherapeutic drugs to improve the success of cancer immunotherapy. Front Immunol 2015;6:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldman A, Khiste S, Freinkman E et al. Targeting tumor phenotypic plasticity and metabolic remodeling in adaptive cross‐drug tolerance. Sci Signal 2019:12:eaas8779. [DOI] [PMC free article] [PubMed] [Google Scholar]


