Abstract
Background
Epstein‐Barr virus (EBV)‐positive gastric cancers (GCs) have been recently identified as a molecular subgroup showing excellent outcomes after surgery for early‐stage disease and responsiveness to immune checkpoint inhibitors (ICIs) for metastatic stage. No data are available on the prevalence, clinical characteristics, and prognosis of this subgroup of GCs in the metastatic setting.
Materials and Methods
In this cohort study, we assessed the impact of EBV status in patients with metastatic GC treated with chemotherapy at two Italian institutions.
Results
Among the 175 cases analyzed, only 7 (4%) were EBV positive and all showed long‐lasting and even complete responses to first‐line chemotherapy with fluorouracil and platinum and a significantly better survival compared with EBV‐negative patients (3‐year overall survival: 80% vs. 20.1%; hazard ratio: 0.12).
Conclusion
If confirmed in larger data sets, our results may give a strong rationale for investigating the addition of ICIs to chemotherapy, in order to maximize the chance of achieving durable and complete responses in this uncommon subtype of GC.
Implications for Practice
To date, no data are available on the prevalence and clinical characteristics of patients with Epstein‐Barr virus (EBV)‐positive metastatic gastric cancer (GC), a specific subtype of GC showing excellent outcomes after radical surgery in early‐stage disease and responsiveness to immune checkpoint inhibitors (ICIs). This cohort study showed that patients with EBV‐positive GC who did not receive ICIs had exceptional, long‐lasting, and even complete responses to first‐line chemotherapy with fluorouracil and platinum and a significantly better survival compared with EBV‐negative patients. If confirmed in larger series, these results may give a strong rationale for investigating the combination of chemotherapy and ICIs to achieve durable and potentially complete response in this uncommon subtype of GC.
Keywords: Gastric cancer, Epstein‐Barr virus infection, Molecular classification
Short abstract
This article assesses the effect of Epstein‐Barr virus status in patients with metastatic gastric cancer.
Introduction
In The Cancer Genome Atlas (TCGA), four gastric cancer (GC) subtypes were identified based on molecular profiling of primary tumors: Epstein‐Barr virus (EBV) positive, which represent less than 10% of cases and are characterized by extensive DNA hypermethylation, PIK3CA mutations, CD274 and PDCD1LG2 amplifications (encoding for programmed death‐ligand 1 [PD‐L1] and PD‐L2, respectively), and activation of immune signaling pathways; microsatellite instability (MSI) tumors, which are detected in 7%–20% of GCs, with hypermutation and high immune cell infiltration; and genomically stable (GS) and chromosomal instability (CIN), reported in 20% and 50%, respectively 1. Because TCGA subtypes are assigned by means of complex (epi)genomic and transcriptomic profiling techniques, their clinical translation is challenging. However, MSI and EBV can be easily assessed as individual and reproducible biomarkers to identify the two corresponding molecular subgroups in the clinical practice.
In resectable disease, the subgroup of patients with MSI‐high GC show a significantly better survival when compared with the microsatellite stable (MSS) one, with lack of benefit from neoadjuvant/adjuvant chemotherapy 2, 3, 4, 5. Similarly, EBV‐positive GC is characterized by the best outcomes after radical surgery as compared with other molecular subtypes 6, but the relatively lower prevalence has hampered the investigation of its role as a predictive marker of the efficacy of multimodality treatments. The excellent outcomes of the MSI and EBV subgroups in radically resected early‐stage disease are thought to be related to the host immune response, which is driven by hypermutation and elevated neoantigen load in the MSI subtype and by the persistency of the viral infection in the EBV one.
In the metastatic setting, the prevalence of MSI is extremely low (about 4% of cases) 7, as is also shown for patients with metastatic colorectal cancer. The prognostic impact of MSI in metastatic GC (mGC) in the preimmunotherapy era has been already explored, even if the available data led to nonconclusive results. However, the same studies are not available for the EBV subtype so far. The aim of this report was to investigate the prevalence, clinical‐pathological‐molecular characteristics, chemotherapy response, and survival of patients with EBV‐positive mGC.
Materials and Methods
Patients
We included patients with metastatic gastric or gastroesophageal junction (GEJ) cancers who were treated with chemotherapy at two Italian oncology departments: Fondazione IRCCS Istituto Nazionale dei Tumori of Milan and University Hospital of Modena. Patients treated with immune checkpoint inhibitors (ICIs) were excluded. Tumor formalin‐fixed paraffin‐embedded (FFPE) samples were collected from primary tumor or from metastases obtained before any systemic therapy.
Medical records were reviewed to collect the following characteristics: age, gender, primary tumor location (GEJ vs. gastric body or antrum), Lauren histotype (intestinal vs. diffuse vs. mixed), human epidermal growth factor receptor 2 (HER2) status, microsatellite instability (MSI‐high vs. MSI‐low/microsatellite stable), primary tumor resection, time to metastases (synchronous vs. metachronous), number of metastatic sites, specific metastatic sites, treatments received and survival outcomes.
Molecular Analyses
FFPE tumor tissue blocks obtained prior to the start of first‐line therapy were used for molecular analyses. EBV infection was evaluated by EBV‐encoded RNA (EBER) in situ hybridization (ISH). ISH was performed using a BenchMark Ultra Platform (Ventana Medical Systems, Tucson, AZ), according to the manufacturer's instructions, using a cocktail of oligonucleotide probes labeled with fluorescein. EBV positivity was defined as presence of blue nuclear staining in tumor cells.
Microsatellite instability was assessed by means of multiplex polymerase chain reaction using five quasi‐monomorphic mononucleotide markers: BAT‐25, BAT‐26, NR‐21, NR‐24, and MONO‐27 (MSI Analysis System, version 1.2; Promega, Madison, WI). Cases with instability at two or more of the five markers were classified as MSI‐high.
Statistical Analyses
The association between clinical, pathological, or molecular characteristics and EBV status was analyzed using chi‐square test, Fisher's exact test, or Mann‐Whitney U test, as appropriate. Overall survival (OS) was defined as the time from diagnosis of metastatic disease to death or last follow‐up for alive patients. Survival analyses were performed using the Kaplan‐Meier method and Cox proportional hazards regression models. Variables resulting significantly associated with OS at the univariable analysis were fitted in a multivariable model. A p value of less than .05 was considered statistically significant. All statistical analyses were conducted using the R software (version 3.5.0).
Results
We included 290 patients with mGC treated from January 2011 to June 2019, of whom 35 were excluded because of insufficient follow‐up/clinical data and 80 because of insufficient tumor content in the centrally collected tumor blocks. The remaining 175 patient were evaluable for this analysis (Fig. 1).
Figure 1.
CONSORT diagram of patients evaluated in our study.
Among the overall population of 175 cases, EBV‐positive disease was found in 7 cases (4%). Table 1 shows patients' and disease baseline in the overall population and according to EBV status. No clinicopathological characteristics differences were observed between patients with EBV‐positive and EBV‐negative metastatic gastric cancers. However, all EBV‐positive cases were MSS, intestinal‐type, and HER2 negative. In the six patients with EBV‐positive mGC who received first‐line therapy with platinum and fluoropyrimidine combined chemotherapy, response rate was 100%, with three partial and three complete responses (Table 2).
Table 1.
Patients and disease baseline characteristics in the overall population and according to EBV status
| Characteristics | Overall (n = 175), n (%) | EBV positive (n = 7), n (%) | EBV negative (n = 168), n (%) | p valuea |
|---|---|---|---|---|
| Age, years | .066 | |||
| Median | 63 | 69 | 62 | |
| IQR | 51–70 | 66–72 | 50–69 | |
| Gender | .257 | |||
| Female | 66 (37.7) | 1 (14.3) | 65 (38.7) | |
| Male | 109 (62.3) | 6 (85.7) | 103 (61.3) | |
| Site | .439 | |||
| Gastric body or antrum | 121 (69.1) | 6 (85.7) | 115 (68.5) | |
| Gastroesophageal junction | 54 (30.9) | 1 (14.3) | 53 (31.5) | |
| Histotype | .203 | |||
| Intestinal | 109 (62.3) | 7 (100) | 102 (60.7) | |
| Diffuse | 53 (30.3) | 0 | 53 (31.6) | |
| Mixed | 13 (7.4) | 0 | 13 (7.7) | |
| HER2 status | .348 | |||
| Positiveb | 37 (21.1) | 0 | 37 (22) | |
| Negative | 138 (78.9) | 7 (100) | 131 (78) | |
| Time to metastases | .999 | |||
| Metachronous | 32 (18.3) | 1 (14.3) | 31 (18.5) | |
| Synchronous | 143 (81.7) | 6 (85.7) | 137 (81.5) | |
| Primary tumor resected | .440 | |||
| Yes | 70 (40) | 4 (57.1) | 66 (39.3) | |
| No | 105 (60) | 3 (42.9) | 102 (60.7) | |
| Number of metastatic sites | .999 | |||
| 1 | 95 (54.3) | 4 (57.1) | 91 (54.2) | |
| >1 | 80 (45.7) | 3 (42.9) | 77 (45.8) | |
| Liver metastases | .675 | |||
| No | 127 (72.6) | 6 (85.7) | 121 (72.1) | |
| Yes | 48 (27.4) | 1 (14.3) | 47 (27.9) | |
| Peritoneal metastases | .712 | |||
| No | 91 (52) | 3 (42.9) | 88 (52.4) | |
| Yes | 84 (48) | 4 (57.1) | 80 (47.6) | |
| Lymph node metastases | .999 | |||
| No | 76 (43.4) | 3 (42.9) | 73 (43.5) | |
| Yes | 99 (56.6) | 4 (57.1) | 95 (56.5) | |
| MSI status | .999 | |||
| MSI‐low/MSS | 160 (91.4) | 7 (100) | 153 (91.1) | |
| MSI‐high | 15 (8.6) | 0 | 15 (8.9) |
Chi‐square test, Fisher's exact test, or Mann‐Whitney U test, as appropriate.
HER2‐positive cases were defined as those with a score of 3+ at immunohistochemical staining (IHC) or IHC 2+ and amplified at the fluorescence in situ hybridization test.
Abbreviations: EBV, Epstein‐Barr virus; HER2, human epidermal growth receptor 2; IQR, interquartile range; MSI, microsatellite instability; MSS, microsatellite stable.
Table 2.
Patient characteristics, treatments received, and treatment‐related outcomes in the subgroup of patients with EBV‐positive disease
| Patient | Gender | Age | No. of treatment lines | Treatment regimen | Best responsea | No. of treatment cycles | Individual patient PFS, months | Individual patient OS, months |
|---|---|---|---|---|---|---|---|---|
| 013 | M | 65 | 1 | Docetaxel + oxaliplatin | CR | 6 | 51.8 | 52.8b |
| 031 | M | 81 | 0 | NAc | NA | NA | NA | 3.7b |
| 032 | M | 59 | 1 | FOLFOX | CR | 5 | 2.6d | 5.6b |
| 035 | F | 69 | 1 | CAPOX | CR | 8 | 93.3 | 95.0b |
| 068 | M | 67 | 1 | FOLFOX | PR | 10 | 56.8 | 66.9b |
| 156 | M | 69 | 2 | FOLFOX (first line) | PR | 12 | 12.0 | 42.1 |
| Paclitaxel + ramucirumab (second line) | SD | 6 | 6.4 | |||||
| 172 | M | 74 | 2 | FOLFOX (first line) | PR | 11 | 8.2 | 25.2 |
| Paclitaxel + ramucirumab (second line) | SD | 8 | 3.3 |
Response were evaluated according to RECIST 1.1.
Patient alive and progression‐free at time of data cutoff (November 15, 2019).
This patient received only best supportive care.
Patient still on treatment at time of data cutoff (November 15, 2019).
Abbreviations: CR, complete response; F, female; M, male; NA, not applicable; PR, partial response; SD, stable disease.
At a median follow‐up of 24.2 months, the median OS was not reached in patients with EBV‐positive mGC and 20.1 months (95% confidence interval [CI], 17.1–24.1) in patients with EBV‐negative mGC, with a 3‐year survival rate of 80% (95% CI, 51.6–100) and 26.5% (95% CI, 18.7–37.6), respectively (hazard ratio [HR], 0.12; 95% CI, 0.02–0.86; p = .035; Fig. 2). In the multivariable model including the other characteristics significantly associated with OS at the univariate analysis (i.e., primary tumor resection and number of metastatic sites), EBV positivity confirmed an independent prognostic role (adjusted HR: 0.12; 95% CI, 0.02–0.89; p = .038) as shown in Table 3, whereas MSI status didn't show any significant impact on survival (adjusted HR: 1.25; 95% CI, 0.57–2.70; p = .577).
Figure 2.
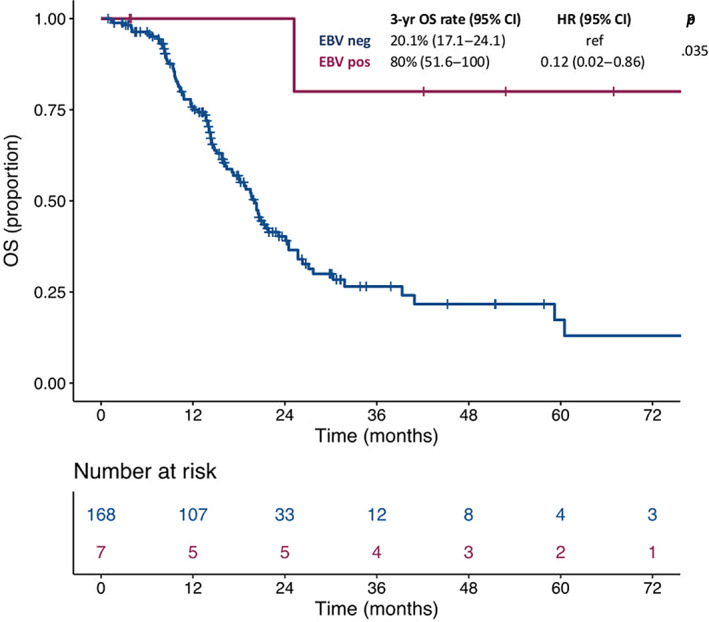
Kaplan‐Maier survival curves according to molecular subgroups.Abbreviations: CI, confidence interval; EBV, Epstein‐Barr virus; HR, hazard ratio; OS, overall survival.
Table 3.
Cox proportional hazards regression models for OS
| Characteristic | Univariate analysis | Multivariable model | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Agea | .404 | — | ||
| 51 | Ref | — | ||
| 70 | 0.88 (0.65–1.19) | |||
| Gender | .483 | — | ||
| Female | Ref | — | ||
| Male | 0.17 (0.75–1.81) | |||
| Site | .754 | — | ||
| Gastric body or antrum | Ref | — | ||
| Gastroesophageal junction | 1.07 (0.70–1.65) | |||
| Histotype | .200 | — | ||
| Intestinal | Ref | — | ||
| Diffuse | 1.45 (0.92–2.30) | |||
| Mixed |
1.50 (0.68–3.32) |
|||
| HER2 status | .866 | — | ||
| Positiveb | Ref | — | ||
| Negative | 1.04 (0.64–1.69) | |||
| Time to metastases | .084 | — | ||
| Metachronous | Ref | — | ||
| Synchronous | 1.71 (0.93–3.15) | |||
| Primary tumor resected | .002 | .004 | ||
| Yes | Ref | Ref | ||
| No | 2.05 (1.31–3.19) | 1.97 (1.24–3.11) | ||
| Number of metastatic sites | .033 | .181 | ||
| 1 | Ref | Ref | ||
| >1 | 1.56 (1.04–2.36) | 1.34 (0.87–2.04) | ||
| Liver metastases | .662 | — | ||
| No | Ref | — | ||
| Yes | 1.10 (0.71–1.72) | |||
| Peritoneal metastases | .089 | — | ||
| No | Ref | — | ||
| Yes | 1.43 (0.95–2.16) | |||
| MSI status | .610 | — | ||
| MSI‐low/MSS | Ref | — | ||
| MSI‐high | 1.22 (0.56–2.65) | |||
| EBV status | .035 | .038 | ||
| Negative | Ref | Ref | ||
| Positive | 0.12 (0.02–0.86) | 0.12 (0.02–0.89) | ||
The values represent the first and third quartile, respectively, of the variable distribution.
HER2‐positive cases were defined as those with a score of 3+ at immunohistochemical staining (IHC) or IHC 2+ and amplified at the fluorescence in situ hybridization test.
Abbreviations: —, not applicable; CI, confidence interval; EBV, Epstein‐Barr virus; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; MSI, microsatellite instability; MSS, microsatellite stable.
Discussion
In this study we used a simple, fast, and widespread assay to assess EBV status in patients with mGC and we evaluated the impact of EBV positivity on treatment outcomes and prognosis with specific regard to the metastatic setting.
Our analysis shows that patients with EBV‐positive mGC have an extraordinarily good prognosis compared with EBV‐negative ones. This observation is in line with the data obtained in patients with resectable early‐stage disease 6, 8, 9 and is potentially related to the dramatic efficacy of cytotoxic chemotherapy and the chance of achieving treatment‐induced complete and/or durable response. We should acknowledge that the relationship between the natural history of EBV‐positive mGC, the host immune response, and the immunogenic cell death/antigen release induced by chemotherapy cannot be easily established. Moreover, there has been no systematic or preclinical investigation on the effect of EBV infection on the chemosensitivity of EBV‐associated tumors. Finally, we also acknowledge that patients with EBV‐negative disease had relatively good survival in our series, probably owing to a combination of several factors, such as the continuous improvement of second‐ and further‐line treatments and the management at a tertiary cancer center.
In our series, the prevalence of MSI‐high status was relatively higher than the 4%–5% reported in clinical trials 7, 10, which may be due to the usual limitations of cohort studies and to potential differences between trial‐eligible and real‐world patients with cancer. Regarding the prognostic role of MSI, we did not show any significant OS difference between patients with MSI‐high versus MSS mGC. Even if all available evidences are limited by the retrospective nature and the extremely small sample size, a previous series suggested a worse outcome of the patients' subgroup with MSI‐high mGC after standard chemotherapy 11, but survival after relapse was not significantly different according to MSI status in a recent meta‐analysis of four trials 2. However, several series in colorectal cancer showed that MSI‐high status turns from a positive prognostic biomarker to a negative one when focusing on early versus metastatic disease. Such aggressiveness of MSI‐high metastatic disease may be related to the elevated burden of immune suppression typical of advanced disease 12, and it is also due to chemo‐ and anti‐epidermal growth factor receptor resistance 13, 14, 15.
Our data have clinical implications for the management of patients with mGC and should be interpreted in light of the growing evidence and strong biological rationale for the dramatic efficacy of ICIs in the EBV‐positive molecular subgroup 16, 17. Although first‐line pembrolizumab and combination of chemotherapy plus pembrolizumab provided a significantly higher benefit compared with chemotherapy alone in the MSI‐high subgroup 17, the specific benefit of adding chemotherapy to programmed cell death protein 1 (PD‐1) blockade in this patient population is yet to be determined. On the contrary, even if specific evidences from immunotherapy trials in the EBV‐positive subgroup are lacking, the use of immunotherapy is biologically supported by the elevated T‐cell infiltrate and immune checkpoint expression in EBV‐related cancers and clinically confirmed by proof‐of‐concept studies 16. Our results highlight the importance of implementing EBV testing in the future clinical practice and the strong rationale for the design of molecularly stratified clinical trials. Even if the comparison of less toxic PD‐1 blockade with chemotherapy in patients with EBV‐positive mGC is warranted, large‐scale randomized trials in this small subgroup are rather unfeasible. We strongly encourage the design of proof‐of‐concept trials with the combination of first‐line chemotherapy and immunotherapy, with the aim to maximize the chance of achieving durable responses and potential disease cure in this uncommon subtype at risk of being neglected.
Conclusion
Even in need of further confirmation in larger data sets, the extraordinarily good prognosis showed by patients with EBV‐positive mGC in our analysis may give a strong rationale for investigating the addition of ICIs to chemotherapy in this uncommon subtype of GC.
Author Contributions
Conception/design: Salvatore Corallo, Federica Morano, Filippo de Braud, Filippo Pietrantonio, Maria Di Bartolomeo
Provision of study material or patients: Salvatore Corallo, Federica Morano, Massimo Salati, Andrea Spallanzani, Maria Antista, Michele Prisciandaro, Alessandra Raimondi, Carlo Sposito, Vincenzo Mazzaferro, Filippo Pietrantonio, Maria Di Bartolomeo
Collection and/or assembly of data: Salvatore Corallo, Giovanni Fucà, Massimo Salati, Riccardo Lobefaro, Vincenzo Guarini, Maria Antista
Data analysis and interpretation: Salvatore Corallo, Giovanni Fucà, Annunziata Gloghini, Chiara Costanza Volpi, Desirè Viola Trupia, Riccardo Lobefaro, Massimo Milione, Laura Cattaneo, Filippo Pietrantonio, Maria Di Bartolomeo
Manuscript writing: Salvatore Corallo, Giovanni Fucà, Annunziata Gloghini, Chiara Costanza Volpi, Filippo Pietrantonio, Maria Di Bartolomeo
Final approval of manuscript: Salvatore Corallo, Giovanni Fucà, Federica Morano, Massimo Salati, Andrea Spallanzani, Annunziata Gloghini, Chiara Costanza Volpi, Desirè Viola Trupia, Riccardo Lobefaro, Vincenzo Guarini, Massimo Milione, Laura Cattaneo, Maria Antista, Michele Prisciandaro, Alessandra Raimondi, Carlo Sposito, Vincenzo Mazzaferro, Filippo de Braud, Filippo Pietrantonio, Maria Di Bartolomeo
Disclosures
Federica Morano: Servier (H); Filippo Pietrantonio: Amgen, Roche, Merck‐Serono, Eli Lilly and Company, Sanofi, Bayer, Servier (C/A), Bristol‐Myers Squibb (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The study was approved by the Institutional Review Board of Fondazione IRCCS Istituto Nazionale dei Tumori of Milan (INT 117/15). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study. This work was supported by Italian Association of Cancer Research (AIRC), IG 23624 (F.P.).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Wen‐Liang Fang, Kuo‐Hung Huang, Shi‐Ching Chang et al. Comparison of the Clinicopathological Characteristics and Genetic Alterations Between Patients with Gastric Cancer with or Without Helicobacter pylori Infection. The Oncologist 2019;24:e845–e853.
Implications for Practice: Patients with gastric cancer with Helicobacter pylori (HP) infection had fewer PI3K/AKT pathway genetic mutations, less tumor recurrence, and better survival than those without HP infection, especially for Epstein‐Barr virus (EBV)‐negative and intestinal‐type gastric cancer. HP infection is an independent prognostic factor regarding overall survival and disease‐free survival. Future in vivo and in vitro studies of the correlation among HP infection, PI3K/AKT pathway, and EBV infection in gastric cancer are required.
References
- 1. The Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pietrantonio F, Miceli R, Raimondi A et al. Individual patient data meta‐analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol 2019;37:3392–3400. [DOI] [PubMed] [Google Scholar]
- 3. Smyth EC, Wotherspoon A, Peckitt C et al. Mismatch repair deficiency, microsatellite instability, and survival: An exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol 2017;3:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi YY, Kim H, Shin SJ et al. Microsatellite instability and programmed cell death‐ligand 1 expression in stage II/III gastric cancer: Post hoc analysis of the CLASSIC randomized controlled study. Ann Surg 2019;270:309–316. [DOI] [PubMed] [Google Scholar]
- 5. Di Bartolomeo M, Morano F, Raimondi A et al. Prognostic and predictive value of microsatellite instability, inflammatory reaction and PD‐L1 in gastric cancer patients treated with either adjuvant 5‐FU/LV or sequential FOLFIRI followed by cisplatin and docetaxel: A translational analysis from the ITACA‐S Trial. The Oncologist 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sohn BH, Hwang JE, Jang HJ et al. Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas Project. Clin Cancer Res 2017;23:4441–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchs CS, Doi T, Jang RW et al. Safety and efficacy of pebrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE‐059 trial. JAMA Oncol 2018;4:e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kohlruss M, Grosser B, Krenauer M et al. Prognostic implication of molecular subtypes and response to neoadjuvant chemotherapy in 760 gastric carcinomas: Role of Epstein–Barr virus infection and high‐ and low‐microsatellite instability. J Pathol Clin Res 2019;5:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roh CK, Choi YY, Choi S et al. Single patient classifier assay, microsatellite instability, and Epstein‐Barr virus status predict clinical outcomes in stage II/III gastric cancer: Results from CLASSIC Trial. Yonsei Med J 2019;60:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shitara K, Özgüroğlu M, Bang YJ et al. Pembroluzumab versus paclitaxel for previously treated, advanced gastric or gastro‐oesophageal junction cancer (KEYNOTE‐061): A randomised, open‐label, controlled, phase 3 trial. Lancet 2018;392:123–133. [DOI] [PubMed] [Google Scholar]
- 11. Janjigian YY, Sanchez‐Vega F, Jonsson P et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 2018;8:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corso S, Isella C, Bellomo SE et al. A comprehensive PDX gastric cancer collection captures cancer cell‐intrinsic transcriptional MSI traits. Cancer Res 2019;79:5884–5896. [DOI] [PubMed] [Google Scholar]
- 13. Goldstein J, Tran B, Ensor J et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high‐level microsatellite instability (MSI‐H). Ann Oncol 2014;25:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Venderbosch S, Nagtegaal ID, Maughan TS et al Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morano F, Corallo S, Lonardi S et al. Negative hyperselection of patients with RAS and BRAF wild‐type metastatic colorectal cancer who received panitumumab‐based maintenance therapy J Clin Oncol 2019;37:3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim ST, Cristescu R, Bass AJ et al. Comprehensive molecular characterization of clinical responses to PD‐1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449–1458. [DOI] [PubMed] [Google Scholar]
- 17. Shitara K, Van Cutsem E, Bang Y et al. Pembrolizumab with or without chemotherapy vs chemotherapy in patients with advanced G/GEJ cancer including outcomes according to microsatellite instability‐high status in KEYNOTE‐062. Ann Oncol 2019;30(suppl 5):v851–v934. [Google Scholar]


