Abstract
Lessons Learned
Administration of lapatinib with food significantly increased its plasma concentration in Chinese patients with metastatic breast cancer.
There were no serious adverse events during the study and no significant differences in lapatinib‐related adverse events between the fasted and fed states.
Background
Lapatinib, a small molecular reversible dual tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR) and human epidermal growth receptor 2 (HER2), was approved for use in combination with capecitabine to treat metastatic HER2‐positive breast cancer. Administration of lapatinib in the fasted state was recommended; however, our preliminary phase II trial data showed that administration of lapatinib with food increased its concentration.
Methods
This study was a single‐center, open‐label, and prospective self‐controlled clinical study. Ten Chinese patients with metastatic breast cancer were enrolled from June 2017 to April 2018. They were required to receive lapatinib plus physician's choice of chemotherapy. Patients were required to take lapatinib orally on an empty stomach continually for 10 days, and then take lapatinib with food continually for the next 10 days. Plasma concentration was measured by liquid chromatography on the 9th and 10th day of each state.
Results
Area under the concentration‐time curve (AUC) of the fasted state and the fed state was 21.23 ± 8.91 mg*h/L (coefficient of variation (CV)% 42%) and 60.60 ± 16.64 mg*h/L (CV% 27%), respectively. The mean plasma concentration in the fasted state was 0.88 ± 0.39 mg/L (CV% 45%), and that in the fed state was 2.53 ± 0.77 mg/L (CV% 30%). Compared with taking lapatinib on an empty stomach, receiving lapatinib with food significantly increased the plasma concentration of lapatinib (Wilcoxon match‐paired test, p = .005). In addition, there were no serious adverse events during the study or significant difference in lapatinib‐related adverse events between the two states.
Conclusion
Our study shows that receiving lapatinib with food can increase its plasma concentration with no significantly increased drug‐related toxicity. We suggest that a larger‐sample‐size clinical trial is needed to fully understand the effect of administration of lapatinib with food.
Discussion
Lapatinib (TYKERB, Tyverb/Tykerb, GlaxoSmithKline, Bentford, United Kingdom) is a small molecular reversible dual tyrosine kinase inhibitor of EGFR and HER2. The current recommended method of administering lapatinib is 1 hour before or 2 hours after a meal. Several previous clinical trials indicated that food could increase the plasma concentration of lapatinib. However, there have been no investigations of the effect of food on the bioavailability of lapatinib in Chinese patients with metastatic breast cancer so far.
This was a single‐center, open‐label, and prospective phase II study. Thirteen patients were enrolled from June 2017 to April 2018 in Sun Yat‐sen University Cancer Center, Guangzhou, China. In this study, we found that administration of lapatinib with food significantly increased the plasma concentration of lapatinib in Chinese patients with metastatic breast cancer. AUC of the fasted state and the fed state was 21.23 ± 8.91 mg*h/L (Coefficient of variation (CV)% 42%) and 60.60 ± 16.64 mg*h/L (CV% 27%), respectively (Fig. 1A). The mean plasma concentration in the fasted state was 0.88 ± 0.39 mg/L (CV% 45%), and that in the fed state was 2.53 ± 0.77 mg/L (CV% 30%; Fig. 1B, 1C). In addition, there were no serious adverse events during the study or significant difference in lapatinib‐related adverse events between the two states.
Figure 1.
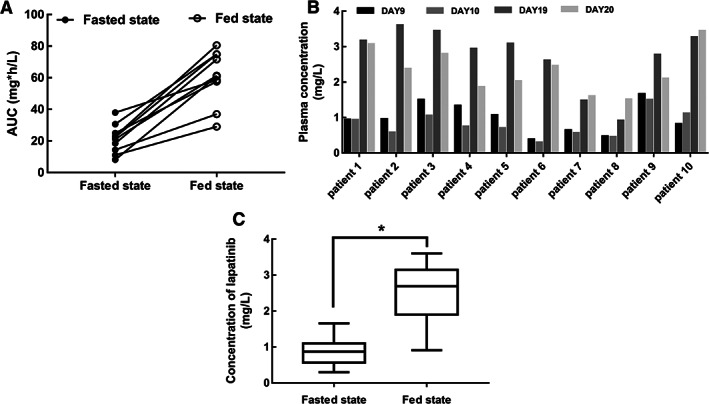
Administration of lapatinib with food increases its plasma concentration. (A): Interindividual changes in lapatinib AUC in each state. (B): Plasma concentration (μg/mL) of lapatinib of 10 patients on the 9th, 10th, 19th, and 20th day after dosing. (C): Plasma concentration of lapatinib of the two states; the results were presented as mean ± SD (Wilcoxon match‐paired test, *p < .05).Abbreviation: AUC, area under the concentration‐time curve.
In conclusion, this study indicated that administering lapatinib with food increased its plasma concentration in Chinese patients with metastatic breast cancer, and lapatinib was well tolerated in the fed state. We suggest that a larger‐sample‐size clinical trial should be needed to confirm the effect of administration of lapatinib with food.
Trial Information
| Disease | Breast cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study – 1 | Phase II |
| Type of Study – 2 | Single arm |
| Primary Endpoint | Correlative endpoint |
| Secondary Endpoint | Safety |
| Additional Details of Endpoints or Study Design | |
| This was a single‐center, open‐label, and prospective phase II study (NCT03075995). The study involved two clinical states; all patients were required to receive lapatinib plus physician's choice chemotherapy. In the fasted state, all participants took lapatinib (750–1,250 mg a day according to investigator's direction) on an empty stomach for 10 days. In the fed state, all patients were assigned to take lapatinib with breakfast for the next 10 days. The breakfast consisted of one egg, 250 mL whole milk, and 100 g bread or fried rice noodle. Patients were required to write down daily drug intake, food intake, and relative adverse reactions in a diary. Blood samples were collected on days 9, 10, 19, and 20. | |
| Patients with HER2‐positive metastatic breast cancer who planned to take lapatinib for at least 1 month were eligible. All patients were provided a full explanation of the study by the study investigator and signed an informed consent before study procedures. | |
| The primary endpoint was to quantify the impacts of food on the steady‐state plasma concentration of lapatinib in patients with metastatic breast cancer. The secondary endpoint was to identify any safety distinctions between the different modes of lapatinib administration. No patients were allowed to take drugs known to inhibit CYP3A4 enzyme activity during the study. | |
| Toxicities were assessed and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. A sample size of 10 patients provided 90% power for the test on the account of the data from the 27‐patient phase I study mentioned above, using one‐side alpha level of 0.025. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Drug 1 | |
| Generic/Working Name | Lapatinib |
| Trade Name | Tykerb |
| Company Name | GlaxoSmithKline |
| Drug Type | Small molecule |
| Drug Class | HER2/Neu |
| Dose | 750–1,250 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | 750–1,250 mg once daily on an empty stomach for 10 days, and then take the same dose with food continually for the next 10 days |
Patient Characteristics
| Number of Patients, Male | 0 | |
| Number of Patients, Female | 10 | |
| Stage | Stage IV | |
| Age | Median (range): 46 (31–72) years | |
| Number of Prior Systemic Therapies | Median (range): 2.5 (1–6) | |
| Performance Status: ECOG |
0 — 10 1 — 0 2 — 0 3 — 0 Unknown — 0 |
|
| Other | Prior surgery, n (%) | 10 (100) |
| Prior chemotherapy, n (%) | ||
| 1 | 3 (30) | |
| 2 | 3 (30) | |
| 3 | 2 (20) | |
| >3 | 2 (20) | |
| Prior hormonal therapy, n (%) | 3 (30) | |
| Prior targeted therapy, n (%) | 9 (90) | |
| Baseline treatments, n (%) | ||
| NL | 1 (10) | |
| NHL | 4 (40) | |
| GHL | 1 (10) | |
| XHL | 4 (40) | |
| Cancer Types or Histologic Subtypes | Breast cancer, 10 |
Abbreviations: G, gemcitabine; H, trastuzumab; L, lapatinib; N, Navelbine; X, capecitabine.
Primary Assessment Method
| Title | New assessment |
| Number of Patients Screened | 13 |
| Number of Patients Enrolled | 10 |
| Number of Patients Evaluable for Toxicity | 10 |
| Number of Patients Evaluated for Efficacy | 10 |
| Evaluation Method (OutcomeNotes) | Other (The primary endpoint was to quantify the impact of food on the steady‐state plasma concentration of lapatinib in patients with metastatic breast cancer.) |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Diarrhea | 80% | 0% | 20% | 0% | 0% | 0% | 20% |
| Vomiting | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
| Fatigue | 90% | 10% | 0% | 0% | 0% | 0% | 10% |
There was no significant difference between the two states.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Pharmacokinetics/Pharmacodynamics of Lapatinib in Each State (n = 10)
| Parameters | Fasted state geometric mean (CV%) | Fed state geometric mean (CV%) |
|---|---|---|
| AUC, mg*h/L | 21.23 (42%) | 60.60 (27%) |
| Concentration, mg/L | 0.88 (45%) | 2.53 (30%) |
Compared with taking lapatinib on an empty stomach, receiving lapatinib with food significantly increased the plasma concentration of lapatinib (Wilcoxon match‐paired test, p = .005).
Abbreviations: AUC, area under the concentration‐time curve; CV, coefficient of variation.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Lapatinib was proved to inhibit the phosphorylation of human epidermal growth receptor 2 (HER2) and its downstream proteins 1, 2. Previous studies indicated that different methods of administration could influence the plasma concentration of lapatinib in patients with solid metastatic cancers; however, data for administration of lapatinib with food in Chinese patients with metastatic breast cancer are still limited.
Here, we aimed to investigate the effect of food on the bioavailability of lapatinib in order to increase the cost‐effectiveness of lapatinib in Chinese patients with metastatic breast cancer. To our knowledge, our study is the first study to investigate the effect of administration of lapatinib with food in Chinese patients with metastatic breast cancer. In addition, we compared blood samples collected on multiple time points during the steady concentration state in order to analyze the plasma concentration more accurately.
This was a single‐center, open‐label, and prospective phase II study. Thirteen patients were enrolled from June 2017 to April 2018 in Sun Yat‐sen University Cancer Center, Guangzhou, China. The flow chart of the study is shown in Figure 2. In this study, we found that administration of lapatinib with food significantly increased the plasma concentration of lapatinib in Chinese patients with metastatic breast cancer. Our results showed that area under the concentration‐time curve (AUC; Fig. 1A) and the plasma concentration of the fed state was significantly higher than that of the fasted state (Fig. 1B, 1C).
Multiple clinical trials have demonstrated the safety and efficacy of lapatinib. It was approved by the Food and Drug Administration for using in combination with capecitabine to treat metastatic breast cancer on account of results in a phase III trial (NCT078572) 3. Safety, tolerability, and pharmacokinetic parameters of lapatinib were assessed in several phase I trials. The first phase I study (EGF10003) indicated that multiple oral doses of lapatinib up to 175 mg were well tolerated in healthy subjects. Additionally, the steady‐state plasma concentrations were achieved on day 7 and the effective half‐life of lapatinib was 24 hours 4. A phase I study (EGF10004) conducted in patients with HER2‐overexpressing advanced or metastatic cancers showed that the maximum tolerated dose of lapatinib was 1,600 mg once daily and clinical activity was observed at a dose 500–1,600 mg 5. Clinical activity and tolerability of lapatinib as first‐line monotherapy was evaluated in HER2‐positive metastatic breast cancer (phase II study, EGF20009) 6 and metastatic gastric cancer 6, 7. The recommended dose of lapatinib was 1,250 mg daily in combination with capecitabine, and administration of lapatinib in the fasted state was recommended. More recently, dual HER2 blockade (lapatinib in combination with trastuzumab) plus aromatase inhibitors (AIs) showed superior outcome versus trastuzumab plus AI in postmenopausal women with HER2‐positive/hormone receptor–positive metastatic breast cancer 8.
The current recommended method of administering lapatinib is 1 hour before or 2 hours after a meal. Several previous clinical trials indicated that food could increase the plasma concentration of lapatinib 9, 10, 11, 12. It was first evaluated in 19 healthy subjects in a phase I study (EGF10003) 4. The results showed that the maximum concentration (Cmax) of lapatinib was 1.60‐fold higher in the fed state compared with the fasted state. A subsequent larger study was conducted in 27 patients with advanced solid tumors 11, which demonstrated that lapatinib exposure AUC was 2.67‐fold higher in low‐fat‐diet state and 4.25‐fold higher in high‐fat‐diet state compared with fasted state. Furthermore, the Cmax of lapatinib was 2.42‐fold higher in the low‐fat‐diet group and 3.03‐fold higher in the high‐fat‐diet group. Another phase I study assessed plasma concentration of 1,250 mg lapatinib in fed and fasted state 10 and demonstrated that the Cmax of lapatinib was 3.20‐fold higher in fed state compared with that in fasted state. Recently, a phase I study that included 25 patients with advanced solid tumors indicated that administration of lapatinib after low‐fat meals could increase the AUC of lapatinib by 1.8‐fold, whereas high‐fat meals could increase it by 2.61‐fold 9. Moreover, Cmax of lapatinib was 1.90‐fold and 2.66‐fold higher in the low‐fat‐diet and high‐fat‐diet groups, respectively, than in the fasted group. However, there have been no investigations of the effect of food on the bioavailability of lapatinib in Chinese patients with metastatic breast cancer so far.
Food can slow the gastric emptying, increase the secretion of bile, and prolong the intestinal transit time 13. Delayed gastric emptying could offer more time for complete dissolution of lapatinib, bile salt could increase the permeability of the drug, and prolonged transit time of small bowels could facilitate the uptake of lapatinib to the circulation. Moreover, fatty acids could increase the permeability of the epithelial cell membrane. Thus, taking lapatinib with food could increase the plasma concentration as a result of the reasons mentioned above. However, a phase I study indicated that these played only a small part in increasing the plasma concentration of lapatinib 11, because the dissolution of lapatinib was so fast (6‐minute half‐life in vitro) that delayed gastric emptying might not notably impact the bioavailability of lapatinib. It was also proposed that food could enhance the autoinhibition of CYP3A4 with lapatinib so that receiving lapatinib with food could increase its plasma concentration.
In our study, patients were required to take lapatinib with breakfast—in the fed state. The breakfasts consisted of one egg, 250 mL whole milk, and 100 g bread or fried rice noodle. Our result showed that the plasma concentrations of lapatinib were higher when taking lapatinib with food containing fat. It is consistent with previous studies.
Safety and tolerance of lapatinib were estimated in previous studies 9, 10, 11, 12. A phase I study (EGF10004) showed that the most common adverse events (AEs) were diarrhea (42%) and skin rash (31%). Generally speaking, lapatinib was well tolerated in patients with solid metastatic tumors. In this study, no serious adverse events occurred. The most common drug‐related adverse event was diarrhea (2/10, Common Terminology Criteria for Adverse Events [CTCAE] grade 1–2), and other drug‐related AEs were nausea, vomiting, and fatigue (1/10, CTCAE grade 1–2). This was consistent with previous studies. More importantly, administering lapatinib with food did not enhance the toxicity of lapatinib compared with fasted administration of lapatinib.
There are several limitations to this study. The correlation between increased plasma concentration of lapatinib and clinical efficacy is still unclear. Some previous studies indicated that clinical efficacy could be observed at the dose of 900–1,200 mg daily 5, but the concentration‐efficacy data were limited. In our study, we did not investigate the clinical efficacy of receiving lapatinib with food; the mechanisms and clinical efficacy of increased plasma concentration of lapatinib still need further investigation. In addition, we did not assess the long‐term adverse events in this study.
In conclusion, this study preliminarily indicated that administering lapatinib with food increased its plasma concentration in Chinese patients with metastatic breast cancer, and lapatinib was well tolerated in the fed state. Changing the method of administration of lapatinib may increase the cost‐effectiveness of lapatinib (decrease the dose of lapatinib or enhance the clinical efficacy of lapatinib). Nevertheless, the clinical efficacy and the long‐term adverse events data were limited in our study. It is hard to change the recommended method of taking lapatinib (1 hour before or 2 hours after a meal) only based on this study. Nevertheless, this study was a one‐arm study with 10 patients. We suggest that a larger‐sample‐size clinical trial is needed to understand in detail the effect of administration of lapatinib with food.
Disclosures
The authors indicated no financial relationships.
Figure
Figure 2.
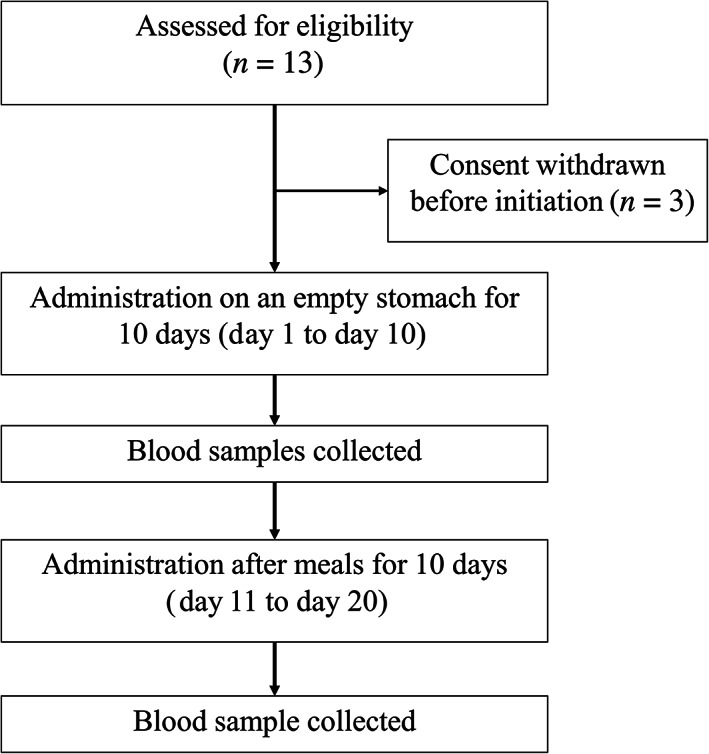
Study design.
Acknowledgments
This research was funded by the China Medical Foundation (NO.316.2204), National Natural Science Foundation of China (U1601224), and 5010 program of Sun Yat‐Sen University (2017011).
Footnotes
- ClinicalTrials.gov Identifier: NCT03075995
- Sponsor: China Medical Foundation
- Principal Investigator: Shusen Wang
- IRB Approved: Yes
References
- 1. Spector NL, Xia W, Burris H 3rd et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 2005;23:2502–2512. [DOI] [PubMed] [Google Scholar]
- 2. Konecny GE, Pegram MD, Venkatesan N et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER‐2‐overexpressing and trastuzumab‐treated breast cancer cells. Cancer Res 2006;66:1630–1639. [DOI] [PubMed] [Google Scholar]
- 3. Geyer CE, Forster J, Lindquist D et al. Lapatinib plus capecitabine for HER2‐positive advanced breast cancer. N Engl J Med 2006;355:2733–2743. [DOI] [PubMed] [Google Scholar]
- 4. Bence AK, Anderson EB, Halepota MA et al. Phase I pharmacokinetic studies evaluating single and multiple doses of oral GW572016, a dual EGFR‐ErbB2 inhibitor, in healthy subjects. Invest New Drugs 2005;23:39–49. [DOI] [PubMed] [Google Scholar]
- 5. Burris HA 3rd, Hurwitz HI, Dees EC et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 2005;23:5305–5313. [DOI] [PubMed] [Google Scholar]
- 6. Gomez HL, Doval DC, Chavez MA et al. Efficacy and safety of lapatinib as first‐line therapy for ErbB2‐amplified locally advanced or metastatic breast cancer. J Clin Oncol 2008;26:2999–3005. [DOI] [PubMed] [Google Scholar]
- 7. Iqbal S, Goldman B, Fenoglio‐Preiser CM et al. Southwest Oncology Group study S0413: A phase II trial of lapatinib (GW572016) as first‐line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol 2011;22:2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnston SRD, Hegg R, Im SA et al. Phase IIi, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2‐positive, hormone receptor‐positive metastatic breast cancer: ALTERNATIVE. J Clin Oncol 2018;36:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Devriese LA, Koch KM, Mergui‐Roelvink M et al. Effects of low‐fat and high‐fat meals on steady‐state pharmacokinetics of lapatinib in patients with advanced solid tumours. Invest New Drugs 2014;32:481–488. [DOI] [PubMed] [Google Scholar]
- 10. Burris HA 3rd, Taylor CW, Jones SF et al. A phase I and pharmacokinetic study of oral lapatinib administered once or twice daily in patients with solid malignancies. Clin Cancer Res 2009;15:6702–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith D, Koch K, Lee D et al. The effect of food on the pharmacokinetics of GW572016. EJC Suppl 2003;1:S169–S169. [Google Scholar]
- 12. Koch KM, Reddy NJ, Cohen RB et al. Effects of food on the relative bioavailability of lapatinib in cancer patients. J Clin Oncol 2009;27:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: Underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet 2004;43:1127–1156. [DOI] [PubMed] [Google Scholar]


