Abstract
On June 28, 2018, the Committee for Medicinal Products for Human Use adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Vyxeos, intended for the treatment of acute myeloid leukemia (AML). Vyxeos was designated as an orphan medicinal product on January 11, 2012. The applicant for this medicinal product was Jazz Pharmaceuticals Ireland Limited. Vyxeos is a liposomal formulation of a fixed combination of daunorubicin and cytarabine, antineoplastic agents that inhibit topoisomerase II activity and also cause DNA damage. The strength of Vyxeos is 5 units/mL, where 1 unit equals 1.0 mg cytarabine plus 0.44 mg daunorubicin. The marketing authorization holder Jazz Pharmaceuticals had found that this was an optimal ratio for the efficacy of the product. Study CLTR0310‐301, a phase III, multicenter, randomized, trial of Vyxeos (daunorubicin‐cytarabine) liposome injection versus standard 3+7 daunorubicin and cytarabine in patients aged 60–75 years with untreated high‐risk (secondary) AML, showed a statistically significant difference between the two groups in overall survival (OS) with a median OS of 9.56 months in the daunorubicin‐cytarabine arm compared with 5.95 months for standard chemotherapy (hazard ratio, 0.69; 95% confidence interval, 0.52–0.90; one‐sided p = .003).
The most common side effects were hypersensitivity including rash, febrile neutropenia, edema, diarrhea/colitis, mucositis, fatigue, musculoskeletal pain, abdominal pain, decreased appetite, cough, headache, chills, arrhythmia, pyrexia, sleep disorders, and hypotension.
Implications for Practice
Vyxeos has demonstrated a clinically significant improvement in overall survival compared with the standard of care 7+3 in the proposed population of patients with newly diagnosed acute myeloid leukemia (AML) with myelodysplasia‐related changes and therapy‐related AML. This is remarkable given the very poor prognosis of these patients and their unmet medical need. Secondary endpoints support the primary outcome, in particular an increased rate of hematopoietic stem cell transplantation, which is potentially the only curative treatment in AML.
Keywords: Vyxeos (cytarabine and daunorubicin), Therapy‐related acute myeloid leukemia, Acute myeloid leukemia with myelodysplasia‐related changes, European Medicines Agency
Short abstract
This article addresses development and authorization of Vyxeos for the treatment of acute myeloid leukemia, summarizing the scientific review of the application leading to regulatory approval in the European Union.
Background
Acute myeloid leukemia (AML) is a clonal disorder of hematopoietic progenitor cells, whereby a population of leukemic stem cells is thought to give rise to the proliferation of abnormal myeloid precursor cells (blasts), which fail to differentiate 1, 2. AML is the most frequent form of leukemia, accounting for approximately 25% of all leukemias in adults in the Western world. The incidence of AML increases sharply with age, ranging from 1.8 cases per 100,000 people aged less than 65 years to 13.7 cases per 100,000 people aged more than 65 years. More than half of the patients with newly diagnosed AML in developed countries are aged more than 65 years, with a median age at diagnosis of 67, and AML is more common in men than in women. The estimated prevalence of AML in the European Union is 1.1 per 10,000 3.
AML is a biologically heterogeneous disease that can be classified into three distinct categories based on clinical ontogeny. De novo AML arises in the absence of an identified exposure or prodromal stem cell disorder (like in myelodysplastic syndrome [MDS]). Therapy‐related AML (t‐AML) develops as a late complication in patients with prior exposure to leukemogenictherapies. Secondary AML is a subset of AML meeting World Health Organization (WHO) AML criteria; patients have a history of prior chemotherapy (treatment‐related AML) or AML arising from an antecedent hematologic disorder such as MDS, myeloproliferative neoplasm, combined myelodysplasia and myeloproliferative disease, or chronic myelomonocytic leukemia. This is an important subgroup of accounting for 10%–30% of all patients with AML who have poorer response rates, shorter duration of remission, and shorter overall survival when compared with patients with de novo AML 4.
At the time of the marketing authorization of Vyxeos (CPX‐351) in the European Union, approved agents included decitabine, which is authorized for the treatment of adult patients with newly diagnosed de novo or secondary AML, according to the WHO classification, who are not candidates for standard induction chemotherapy. Azacitidine is also authorized for the treatment of adult patients who are not eligible for hematopoietic stem cell transplantation (HSCT) with AML with 20%–30% blasts and multilineage dysplasia, according to WHO classification, and AML with >30% marrow blasts according to the WHO classification. In addition, histamine dihydrochloride is authorized for adult patients with AML in first remission concomitantly treated with interleukin‐2. Midostaurin is authorized in combination with standard daunorubicin and cytarabine induction and high‐dose cytarabine consolidation chemotherapy followed by midostaurin single‐agent maintenance therapy for adult patients with newly diagnosed AML who are FLT3 mutation positive. Finally, Gemtuzumab ozogamicin is authorized in combination therapy with daunorubicin and cytarabine for the treatment of patients aged at least 15 years with previously untreated, de novo CD33‐positive AML, except acute promyelocytic leukemia.
Vyxeos is a liposomal formulation of a fixed combination of daunorubicin and cytarabine in a 1:5 molar ratio. The 1:5 molar ratio has been shown in vitro and in vivo to maximize synergistic antitumor activity in AML.
Daunorubicin has antimitotic and cytotoxic activity, which is achieved by forming complexes with DNA, inhibiting topoisomerase II activity, inhibiting DNA polymerase activity, affecting regulation of gene expression, and producing DNA‐damaging free radicals.
Cytarabine is a cell cycle phase–specific antineoplastic agent, affecting cells only during the S‐phase of cell division. Intracellularly, cytarabine is converted into cytarabine‐5‐triphosphate (ara‐CTP), which is the active metabolite. The mechanism of action is not completely understood, but it appears that ara‐CTP acts primarily through inhibition of DNA synthesis. Incorporation into DNA and RNA may also contribute to cytarabine cytotoxicity. Cytarabine is cytotoxic to proliferating mammalian cells in culture.
Nonclinical Aspects
The pharmacokinetics (PK) studies were performed in mice, rats, and dogs and involved investigations of single‐ and repeat‐dose PK, tissue distribution, metabolism, and excretion of Vyxeos following intravenous (IV) administration. Daunorubicin‐cytarabine molar ratios in animal plasma were maintained near the desired 1:5 molar ratio for at least 24 hours after IV administration. This was attributed to high retention of the drug cargo within the liposomes. in vivo release from Vyxeos liposomes is deduced to be very slow because only a very small fraction (<1%) of the total drug concentration in the circulation exists as free drug.
Upon repeat‐dose administration of Vyxeos in animals, there were no major deviations from dose proportionality or time linearity for daunorubicin or cytarabine. Very good allometric relationships indicated that excretion, metabolism, and distribution data in animals were likely to translate directly to humans.
Tissue distribution data for daunorubicin and cytarabine suggested that Vyxeos liposomes are slowly taken up by the mononuclear phagocyte system in several tissues, particularly spleen. Intracellular accumulation of Vyxeos is higher within leukemic than normal bone marrow cells (2.2‐fold higher for daunorubicin; 9.5‐fold higher for cytarabine), indicating selective targeting. After internalization by leukemia cells, Vyxeos liposomes undergo degradation, releasing the drug cargo within the intracellular environment and enabling the drugs to exert their antineoplastic activity. There were different patterns of tissue distribution for 14C‐daunorubicin and 14C‐cytarabine associated with Vyxeos versus the nonliposomal formulations. No unexpected or novel toxicities were noted for Vyxeos beyond those reported for daunorubicin or cytarabine.
Clinical Pharmacokinetics
The PK of daunorubicin and cytarabine administered as Vyxeos was investigated by measuring total plasma concentration (i.e., encapsulated and unencapsulated) of each medicinal product in adult patients following dosing administration of daunorubicin 44 mg/m2 and cytarabine 100 mg/m2 as a 90‐minute intravenous infusion on days 1, 3, and 5. The mean (coefficient of variation) maximum plasma concentration values observed at day 5 were 26.0 (32.7%) μg/mL and 62.2 (33.7%) μg/mL for daunorubicin and cytarabine, respectively. The mean (coefficient of variation) area under the curve values were 637 (38.4%) μg·h/mL and 1,900 (44.3%) μg·h/mL for daunorubicin and cytarabine, respectively. When daunorubicin and cytarabine are administered as components of Vyxeos, the liposomes appear to govern their tissue distribution and rates of elimination; therefore, whereas the nonliposomal medicinal products have markedly different clearance, volume of distribution, and terminal half‐life, Vyxeos causes these PK parameters to converge. There was no evidence of time dependence, and dose proportionality was observed for each medicinal product over the range of 1.3 mg/3 mg per square meter to 59 mg/134 mg per square meter.
Age, sex, race, body weight, body mass index, and white blood cell count do not have a clinically important effect on the exposure of total daunorubicin or cytarabine after adjusting dose by body surface area.
The PK of total daunorubicin and cytarabine was not altered in patients with bilirubin ≤50 μmol/L; however, the PK in patients with bilirubin >50 μmol/L is unknown. Given that daunorubicin is metabolized by the liver, changes in hepatic function induced by concomitant therapies may affect metabolism, PK, therapeutic efficacy, and/or the toxicity of Vyxeos.
Main Study
Study CLTR0310‐301 (also referred as Study 301; Fig. 1) was a phase III, multicenter, open‐label, randomized, trial of Vyxeos (daunorubicin‐cytarabine) liposomal formulation injection versus standard 3+7 daunorubicin and cytarabine in patients aged 60–75 years with untreated high‐risk (secondary) AML 5. The primary objective was to confirm the efficacy of Vyxeos compared with 7+3 as first‐line therapy in this group of patients.
Figure 1.
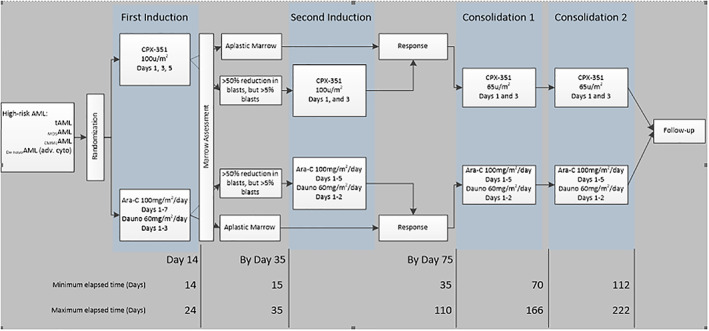
Study design (Study CLTR0310‐301).Abbreviations: Ara‐C, cytarabine; CMMoLAML, AML arising from chronic myelomonocytic leukemia; Dauno, daunorubicin; de novoAML (adv. cyto), de novo acute myeloid leukemia; MDSAML, AML arising from myelodysplastic syndrome; tAML, therapy‐related AML.
The secondary objectives were to confirm the safety of Vyxeos and improvement in the rate of leukemia‐free state, postinduction response, remission duration, event‐free survival (EFS), and overall best response rate. The baseline demographics and disease characteristics are presented in Table 1.
Table 1.
Baseline demographics and disease characteristics (intention‐to‐treat population, Study CLTR0310‐301)
| CPX‐351 (n = 153) | 7+3 (n = 156) | |
|---|---|---|
| Age | ||
| Median (range), years | 68 (60–75) | 68 (60–75) |
| 60–70, n (%) | 96 (63) | 102 (65) |
| 70–75, n (%) | 57 (37) | 54 (35) |
| Sex, n (%) | ||
| Male | 94 (61) | 96 (62) |
| Female | 59 (39) | 60 (38) |
| Race, n (%) | ||
| American Indian or Alaska Native | 1 (1) | 0 |
| Asian | 6 (4) | 2 (1) |
| Black | 7 (5) | 6 (4) |
| White | 128 (84) | 139 (89) |
| Other | 11 (7) | 8 (5) |
| Multiple | 0 | 1 (1) |
| ECOG performance group, n (%) | ||
| PS 0 | 37 (24) | 45 (29) |
| PS 1 | 101 (66) | 89 (57) |
| PS 2 | 15 (10) | 22 (14) |
| Extramedullary disease, n (%) | 5 (3) | 5 (3) |
| AML subtype, n (%) | ||
| CMMol to AML | 11 (7) | 12 (8) |
| De novo AML | 41 (27) | 37 (24) |
| MDS with HMA to AML | 50 (33) | 55 (35) |
| MDS without HMA to AML | 21 (14) | 19 (12) |
| t‐AML | 30 (20) | 33 (21) |
| Genetic mutations, n (%) | ||
| FLT3 positive mutation | 22 (14) | 21 (14) |
| NPM1 positive mutation | 13 (9) | 12 (8) |
| CEBPA mutated | 12 (8) | 5 (3) |
| Cytogenetic risk assessment, n (%) | ||
| Better risk | 7 (5) | 5 (3) |
| Intermediate risk | 64 (45) | 58 (40) |
| Poor risk | 72 (50) | 83 (57) |
Abbreviations: AML, acute myeloid leukemia; CMMoL, chronic myelomonocytic leukemia; ECOG, Eastern Cooperative Oncology Group; HMA, hypomethylating agents; MDS, myelodysplastic syndrome; PS, performance status; t‐AML, therapy‐related AML.
The study comprised two phases: a treatment phase and a follow‐up phase.
For the treatment phase, patients were to receive up to two inductions and up to two consolidations with either CPX‐351 or cytarabine and daunorubicin given as a 7+3. The number of inductions and consolidations received depended on response confirmed by bone marrow assessment.
The initial induction course began within 24 hours of randomization.
Each unit of CPX‐351 contained 1 mg cytarabine and 0.44 mg daunorubicin base in liposomes.
First induction:
CPX‐351: 100 units/m2 by 90‐minute IV infusion on days 1, 3, and 5
7+3: cytarabine 100 mg/m2/day on days 1 through 7 by continuous infusion; daunorubicin 60 mg/m2/day on days 1, 2, and 3
Second induction:
CPX‐351 100 units/m2 by 90‐minute IV infusion on days 1 and 3
5+2: cytarabine 100 mg/m2/day on days 1 through 5 by continuous infusion; daunorubicin 60 mg/m2/day on days 1 and 2
Consolidation:
CPX‐351 65 units/m2 by 90‐minute IV infusion on days 1 and 3
5+2: cytarabine 100 mg/m2/day on days 1 through 5 by continuous infusion; daunorubicin 60 mg/m2/day on days 1 and 2.
The regimen used for the control group is in line with the European guidelines for patients above the age of 60 years 6, 7.
Efficacy Data and Additional Analyses
Both arms in the pivotal study were balanced with regard to baseline demography and disease characteristics. A significant improvement of 3.6 months in median overall survival (OS) with Vyxeos compared with the 7+3 standard treatment has been demonstrated in the pivotal study intention‐to‐treat (ITT) population (median OS 9.56 months vs. 5.95 months; hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.52–0.90; one‐sided p = .003; Fig. 2). This is a clinically relevant effect, as the 7+3 treatment has been the standard treatment for over 4 decades and no other therapy has demonstrated superior efficacy over it. The OS benefit was seen across all randomization strata (except for the AML with myelodysplasia‐related changes with prior hypomethylating agent therapy) and in those with the adverse risk FLT3 mutation.
Figure 2.

Kaplan‐Meier curve for overall survival (intention‐to‐treat population, Study CLTR0310‐301).Abbreviation: CI, confidence interval.
A significantly greater proportion of patients in the Vyxeos group achieved complete remission (CR) or CR plus complete remission with incomplete platelet or neutrophil recovery (CRi) compared with the 7+3 group (CR, 37.3% vs. 25.6%; CR + CRi, 47.7% vs. 33.3%). No clinical differences were seen in remission duration between Vyxeos versus 7+3 (median 6.93 months vs. 6.11 months). Favorable results for Vyxeos versus 7+3 treatment were also documented for EFS (median 2.53 months vs. 1.31 months, HR, 0.74; one‐sided p = .011), achieving a morphologic leukemia‐free state (69.0% vs. 55.5%) and the rate of bridging to transplant (52 [34.0%] vs. 39 [25.0%]; Table 2).
Table 2.
Proportion of patients receiving stem cell transplant (intention‐to‐treat population, Study CLTR0310‐301)
| Endpoint (n CPX‐351, n 7+3) | CPX‐351, n (%) | 7+3, n (%) | Odds ratio (95% CI) |
|---|---|---|---|
| HSCT (153, 156) | 52 (34.0) | 39 (25.0) |
1.54 (0.92–2.56) One‐sided p = .049 |
| Age, years | |||
| 60–69 (96, 102) | 36 (37.5) | 33 (32.4) | 1.25 (0.70–2.25) |
| 70–75 (57, 54) | 16 (28.1) | 6 (11.1) | 3.12 (1.12–8.72) |
| AML subtype | |||
| CMMoLAML | 3 (27.3) | 0 | Not evaluable |
| de novoAML | 17 (41.5) | 11 (29.7) | 1.67 (0.65–4.28) |
| MDSAML with prior HMA | 14 (28.0) | 14 (25.5) | 1.14 (0.48–2.71) |
| MDSAML without prior HMA | 7 (33.3) | 5 (26.3) | 1.40 (0.36–5.49) |
| t‐AML | 11 (36.7) | 9 (27.3) | 1.54 (0.53–4.49) |
Abbreviations: AML, acute myeloid leukemia; CI, confidence interval; CMMoLAML, AML arising from chronic myelomonocytic leukemia; de novoAML, de novo AML; HMA, hypomethylating agents; HSCT, hematopoietic stem cell transplantation; MDSAML, AML arising from myelodysplastic syndrome; t‐AML, therapy‐related AML.
More patients in the ITT population in the Vyxeos treatment group received HSCT in CR or CRi compared with patients in CR or CRi for the 7+3 regimen (76.9% vs. 61.5%).
Supportive Studies: Study 205
Study 205 was a phase II, multicenter, randomized, open‐label, parallel‐arm, active controlled study in patients between the ages of 18 and 65 years with AML in first relapse. The primary objective was to estimate the efficacy of CPX‐351 (liposomal formulation of daunorubicin and cytarabine) compared with intensive salvage therapy in patients with AML in first relapse 8. A total of 126 patients were randomized to CPX‐351 (81 patients) and salvage therapy (45 patients). Most patients (82%) were aged between 18 and 60 years and had de novo AML (89.6%).
The proportion of patients surviving at 1 year, the primary endpoint, was higher in the CPX‐351 arm (35.8%, 29/81 patients) than in the salvage therapy arm (27.3%, 12/44 patients), but the difference was not statistically significant (p = .43; Fisher's exact test). The median survival was 259 days (8.51 months) in the CPX‐351 and 191 days (6.28 months) in the salvage arm (p = .19, based on log‐rank test; HR of survival by 1 year was 0.75; 95% CI, 0.5–1.2).
Safety Data
In Study 301, similar percentages of patients in the Vyxeos group (92%) and the 7+3 group (91%) reported adverse events (AEs) of grade ≥ 3. The most frequently reported in both groups were febrile neutropenia (68% and 71%, in Vyxeos and 7+3, respectively), pneumonia (20% and 15%), and hypoxia (13% and 15%). The most frequently reported hypersensitivity was rash, and the majority of these were not serious (38.9%). The incidence of all hypersensitivity events was 66.9%, mainly nonserious. Severe myelosuppression (including fatal infections and hemorrhagic events) has been reported in patients after administration of a therapeutic dose of Vyxeos. Therefore, consideration should be given to the prophylactic use of anti‐infective therapy, colony‐stimulating factors, and transfusions. Blood counts should be regularly monitored until recovery.
During the treatment period, cardiac AEs were more frequent in the Vyxeos group than in all controls, but the difference is driven by grade 1 AEs, whereas similar incidences were reported for grade 3–5 AEs. The most frequently reported cardiac AEs (CPX‐351 vs. all controls) were tachycardia (15.2% vs. 12.7%, respectively), followed by chest pain (7.2% vs. 5.9%) and atrial fibrillation (6.9% vs. 8.9%), and there were small imbalances in pericardial effusion (5.1% vs. 2.5%) and cardiac murmur (4.3% vs. 0.8%). Grade 5 AEs were reported for two patients in CPX‐351 and included cardiorespiratory arrest (n = 1) and sudden cardiac death (n = 1). The most frequently reported serious cardiac AEs was ejection fraction decreased (2.4% for CPX‐351 vs. 3.4% all controls).
Benefit‐Risk Assessment and Discussion
This application was supported by a phase III clinical study (Study 301), and the overall design was considered adequate. The patient population was adequately selected and reflects those patients with high‐risk AML with documented prior hematological disorder as defined in the proposed indication. The pivotal study included only patients without prior anti‐AML treatment and who were aged more than 60 years. The comparator was the standard 7+3 regimen, which was appropriate and allows a clear comparison of the liposomal formulation combination of cytarabine and daunorubicin versus the two components administered separately. The choice of endpoints with OS as primary was satisfactory considering the poor prognosis in this type of AML population. All other secondary endpoints are also considered adequate.
AML is the common acute leukemia in adults, accounting for approximately 90% of all acute leukemias in those aged more than 18 years. Overall survival remains poor, with <50% 5‐year survival in patients under 45 years and < 5% in those over 65 years 9. Among all AML types, high‐risk AML as defined by therapy‐related AML or AML with myelodysplasia‐related changes carries a very poor prognosis and represents an unmet medical need.
Vyxeos has demonstrated a clinically significant improvement in overall survival compared with the standard of care 7+3 in the proposed population of patients with newly diagnosed AML with myelodysplasia‐related changes and therapy‐related AML. Secondary endpoints support the primary outcome, in particular an increased rate of HSCT (due to the CR + Cri rates in patients aged more than 70 years), which is potentially the only curative treatment in AML.
Improved median OS by 3.6 months (ITT population median OS 9.56 months Vyxeos vs. 5.95 months 7+3; HR, 0.69; 95% CI, 0.52–0.90; one‐sided p = .003).
Improved response rates (ITT population: CR + CRi 47.7% Vyxeos vs. 33.3% 7+3; odds ratio [OR], 1.77; 95% CI, 1.11–2.81; two‐sided p = .016).
Improved median EFS by 1.2 months (ITT population: median EFS 2.53 months Vyxeos vs. 2.31 months 7+3; HR, 0.74; 95% CI, 0.58–0.96; two‐sided p = .021).
Improved morphologic leukemia‐free state (ITT population: 69% Vyxeos vs. 55.5% 7+3; OR, 1.78; 95% CI, 1.05–3.03; one‐sided p = .017).
Improved rate of HSCT (ITT population: 34% Vyxeos vs. 25% 7+3; OR, 1.54; 95% CI, 0.92–2.56; two‐sided p = .097).
Easier dosing schedule with Vyxeos of three (or two) 90‐minute infusions every other day compared with 7‐day (or 5‐day) continuous infusion of cytarabine along with subsequent administration of daunorubicin at induction.
Due to the neutropenia experienced with Vyxeos, infections of various types were very common adverse reactions (ADRs). Pneumonia, sepsis, and bacteremia were the most frequently seen serious infection ADRs in the clinical studies population. The incidence of infection events was 78.1%; the incidence of nonserious events of infections was 73.1%; the incidence of serious events of infections was 28.5%; the incidence of infections that led to discontinuation was 0.5%.
The safety profile appears similar to the known profile of 7+3 except for a more prolonged myelosuppression due to the liposomal formulation and an increase in serious AE, in particular infections. Vyxeos is associated with a more prolonged myelosuppression than 7+3 due to its PK properties of releasing a “full” cargo of daunorubicin and cytarabine to the bone marrow. Subsequently, it is not surprising that the recovery of neutropenia and thrombocytopenia takes longer for CPX‐351, around 7–10 more days. However, infections were reported with similar incidence in both arms (around 92%–93%), but the difference between CPX‐351 and 7 + 3 was seen in serious infections (32% vs. 21%), especially bacteremia. The risk of serious infections appears to be managed with supportive care, as it did not translate into a higher discontinuation of study treatment or overall AE‐related mortality. According to the pivotal study protocol, prophylactic use of antibiotics was recommended during periods of neutropenia, and the use of growth factors was allowed according to local practice. There is no evidence of increased cardiac toxicity, and in fact, despite longer exposure to treatment, the cumulative doses of daunorubicin are lower than with 7+3.
In view of the effect in terms of OS and the known ADRs for such a combination (cytarabine and daunorubicin), the benefit‐risk balance in the proposed indication was considered positive. The Committee for Orphan Medicinal Products found that a clinically relevant advantage linked to the significant benefit based on efficacy was associated not only with the delivery system but also with the optimal ratio between the two products in the liposomal formulation. The two components offered a clinically relevant advantage to the free association of daunorubicin and cytarabine in the treatment of these patients.
Conclusion
The pivotal study showed similar outcome across age subgroups (60–69 years and 70–75 years), although it excluded patients aged less than 60 years. Most patients that receive the standard treatment need to be fit for intensive chemotherapy, and therefore, patients’ performance status and their suitability to receive the treatment is based on clinical judgement and the physician's decision. For patients aged less than 60 years with high‐risk AML scheduled to receive the standard 7+3, Vyxeos seems an optimized formulation that is likely to be superior to 7 + 3 given the results in patients aged more than 60 years from the pivotal study. It appears there are no differences in disease biology for treatment‐related AML or AML with myelodysplasia‐related changes between adult age subgroups. Prognosis is very poor, and these subtypes of AML represent an unmet medical need irrespective of age. The exposure response analysis conducted in adult patients included few patients below the age of 60 years, and it indicated the proposed dose is the most appropriate.
Author Contributions
Conception/design: Francesco Pignatti
Collection and/or assembly of data: Kyriaki Tzogani, Karri Penttilä, Tuomo Lapveteläinen, Robert Hemmings, Janet Koenig, João Freire, Silva Márcia, Susan Cole, Paola Coppola, Beatriz Flores, Yolanda Barbachano, Silvia Domingo Roige, Francesco Pignatti
Data analysis and interpretation: Karri Penttilä, Tuomo Lapveteläinen, Robert Hemmings, Janet Koenig, João Freire, Silva Márcia, Susan Cole, Paola Coppola, Beatriz Flores, Yolanda Barbachano
Manuscript writing: Francesco Pignatti
Final approval of manuscript: Kyriaki Tzogani, Karri Penttilä, Tuomo Lapveteläinen, Robert Hemmings, Janet Koenig, João Freire, Silva Márcia, Susan Cole, Paola Coppola, Beatriz Flores, Yolanda Barbachano, Silvia Domingo Roige, Francesco Pignatti
Disclosures
The authors indicated no financial relationships.
Acknowledgments
The scientific assessment summarized in this report is based on important contributions from the rapporteur and co‐rapporteur assessment teams, members of the Committee for Medicinal Products for Human Use (CHMP), and additional experts following the application for a marketing authorization from the company. This publication is a summary of the European Public Assessment Report (EPAR), CHMP assessment. The EPAR is published on the European Medicines Agency (EMA) Web site (www.ema.europa.eu). For the most current information on this marketing authorization, please refer to the EMA Web site. The authors of this paper remain solely responsible for the opinions expressed in this publication.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Estey E, Döhner H. Acute myeloid leukaemia. Lancet 2006;368:1894–1907. [DOI] [PubMed] [Google Scholar]
- 2. Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther 2009;31:2349–2370. [DOI] [PubMed] [Google Scholar]
- 3. Visser O, Trama A, Maynadié M et al.; RARECARE Working Group . Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer 2012;48:3257–3266. [DOI] [PubMed] [Google Scholar]
- 4. Leone G, Mele L, Pulsoni A et al. The incidence of secondary leukemias. Haematologica 1999;84:937–945. [PubMed] [Google Scholar]
- 5. Lancet JE, Uy GL, Cortes JE et al. CPX‐351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol 2018;36:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone RM, Berg DT, George SL et al. Postremission therapy in older patients with de novo acute myeloid leukemia: A randomized trial comparing mitoxantrone and intermediate‐dose cytarabine with standard‐dose cytarabine. Blood 2001;98:548–553. [DOI] [PubMed] [Google Scholar]
- 7. Döhner H, Estey E, Grimwade D et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cortes JE, Goldberg SL, Feldman EJ et al. Phase II, multicenter, randomized trial of CPX‐351 (cytarabine:daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer 2015;121:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thein MS, Jemal A, Baer MR et al. Improved survival rates among acute myeloid leukemia (AML) patients 65 to 74 years: Population‐based estimates over three decades. Blood 2011;118:3591a.21821704 [Google Scholar]


