Abstract
Lessons Learned
Bintrafusp alfa had a manageable safety profile and demonstrated preliminary clinical activity in heavily pretreated patients with solid tumors (including hepatocellular carcinoma) with no or limited treatment options.
Findings from this study suggest bintrafusp alfa may be a novel therapeutic approach for patients with advanced solid tumors.
Additional trials are needed to further explore safety and efficacy of bintrafusp alfa in specific tumor types.
Background
Bintrafusp alfa is a first‐in‐class bifunctional fusion protein composed of the extracellular domain of transforming growth factor‐β (TGF‐β) RII receptor (a TGF‐β “trap”) fused to a human immunoglobulin (Ig) G1 antibody blocking programmed death‐ligand 1 (PD‐L1). Bintrafusp alfa is designed to neutralize TGF‐β signaling by “trapping” and sequestering all TGF‐β isoforms, and this trap function is physically linked to PD‐L1 blockade in the tumor microenvironment.
Methods
NCT02699515 was a phase I, open‐label, dose‐escalation study of bintrafusp alfa (3, 10, and 20 mg/kg every 2 weeks) in Asian patients with advanced solid tumors, including a hepatocellular carcinoma (HCC) safety‐assessment cohort. The primary objective was safety and tolerability; the secondary objective is best overall response.
Results
As of August 24, 2018, 23 patients (including 9 in the HCC cohort) received bintrafusp alfa. Eight patients experienced treatment‐related adverse events (TRAEs). Three patients had grade 3 TRAEs (13.0%; hypoacusis, hyponatremia, hypopituitarism, increased blood creatine phosphokinase, and intracranial tumor hemorrhage); one had grade 4 hyponatremia (4.3%). No treatment‐related deaths occurred. In the dose‐escalation cohort, two patients had a confirmed partial response, and 3 had stable disease (SD), for an overall response rate of 14.3% and a disease control rate (DCR) of 35.7%. In the HCC cohort, one patient had SD (DCR, 11.1%). A dose‐proportional pharmacokinetics profile was observed at doses of >3 mg/kg.
Conclusion
Bintrafusp alfa had a manageable safety profile and preliminary efficacy in heavily pretreated patients with advanced solid tumors, including HCC.
Discussion
TGF‐β performs multiple, highly diverse cellular functions, which, in the context of a tumor microenvironment, can lead to stimulation of multiple relevant tumorigenic processes, including epithelial‐mesenchymal transition, fibrosis, and angiogenesis 1, 2, 3. Antibodies targeting the PD‐(L)1 pathway have shown antitumor activity in a variety of indications 4, 5. However, a majority of patients do not respond to anti–PD‐(L)1 monotherapy, potentially because of insufficient generation or inadequate function of effector T cells 6. Because their mechanisms of action are nonredundant and complementary, the simultaneous inhibition of the TGF‐β and PD‐(L)1 pathways may provide a novel treatment approach with the potential for increased activity.
Bintrafusp alfa (M7824) is a first‐in‐class bifunctional fusion protein composed of the extracellular domain of TGF‐βRII receptor (a TGF‐β “trap”) fused to a human IgG1 antibody blocking PD‐L1. Here we report results from an ongoing phase I study (NCT02699515) designed to evaluate the safety and tolerability of bintrafusp alfa in 23 Asian patients with heavily pretreated metastatic or locally advanced solid tumors (including HCC) unselected for tumor PD‐L1 expression.
Bintrafusp alfa at 3–20 mg/kg every 2 weeks (Q2W) was associated with an overall manageable safety profile. Grade 3–4 TRAEs occurred in three patients. One immune‐related adverse event (grade 1 hyperthyroidism) occurred. Two patients (8.7%) experienced infusion‐related reactions, which were mild and manageable. Two patients (8.7%) discontinued the study because of TRAEs. The maximum tolerated dose was not reached at the highest dose level tested in this study (20 mg/kg), and no treatment‐related deaths occurred. Finally, no TGF‐β–related skin adverse events were reported in the dose‐escalation and HCC cohorts of this study.
The clinical activity in the 3‐mg/kg and 20‐mg/kg dose‐escalation cohorts was consistent with antitumor activity reported in mouse models and findings of a global phase I study (NCT02517398) in which clinical activity was observed across all evaluated doses 7, 8. Two patients in the dose‐escalation component had confirmed partial response, and three had SD as their best overall response per RECIST 1.1, as assessed by the investigator, for an overall response rate of 14.3% and DCR of 35.7% (Fig. 1). Median progression‐free survival and overall survival in the dose‐escalation cohort were 1.4 months (95% confidence interval [CI], 0.9–6.7) and 4.8 months (95% CI, 2.1–18.6), respectively. The pharmacokinetic profile was similar to the global study 8, 9.
Figure 1.

Change from baseline in the sum of longest diameters according to RECIST 1.1. (A): Dose‐escalation cohort (n = 14) and (B): HCC cohort (n = 9).Abbreviations: HCC, hepatocellular carcinoma; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
The ability of bintrafusp alfa to simultaneously block TGF‐β and PD‐L1 may contribute to the increased clinical benefit observed in heavily pretreated patients compared with historical data of other anti–PD‐(L)1 agents. Although the small sample size precludes any meaningful conclusions from being drawn, the overall findings from this phase I study in Asian patients with advanced solid tumors are encouraging, and further evaluation of bintrafusp alfa in larger patient groups is warranted.
Trial Information
| Disease | Hepatocellular carcinoma |
| Disease | Advanced cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of Study | Phase I, dose escalation and safety cohorts |
| Primary Endpoint | Safety |
| Secondary Endpoints | Best overall response, pharmacokinetics |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Drug 1 | |
| Generic/Working Name | Bintrafusp alfa |
| Company Name | Merck KGaA, Darmstadt, Germany, and GlaxoSmithKline |
| Drug Type | Bifunctional fusion protein |
| Drug Class | TGF‐β and PD‐L1 |
| Dose | 3–20 mg/kg |
| Route | IV |
| Schedule of Administration | Every 2 weeks (Q2W) |
Dose Escalation Table
| Dose Level | Dose of drug: bintrafusp alfa | Number enrolled | Number evaluable for toxicity |
|---|---|---|---|
| 1 | 3 mg/kg Q2W | 4 | 4 |
| 2 | 10 mg/kg Q2W | 3 | 3 |
| 3 | 20 mg/kg Q2W | 7 | 7 |
Patient Characteristics
| Number of Patients, Male | 11 (47.8) |
| Number of Patients, Female | 12 (52.2) |
| Age | Median (range): 55 (38–75) |
| Performance Status: ECOG |
0 — 17 1 — 6 2 — 3 — Unknown — |
Primary Assessment Method
| Title | Efficacy |
| Number of Patients Screened | 26 |
| Number of Patients Enrolled | 23 |
| Number of Patients Evaluable for Toxicity | 23 |
| Number of Patients Evaluated for Efficacy | 23 |
| Evaluation Method | RECIST 1.1 |
Adverse Events
| All Dose Levels, All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All Grades |
| Aspartate aminotransferase increased | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Blood creatine phosphokinase increased | 91% | 4% | 0% | 4% | 0% | 0% | 9% |
| Anorexia | 91% | 9% | 0% | 0% | 0% | 0% | 9% |
| Fatigue | 91% | 0% | 9% | 0% | 0% | 0% | 9% |
| Hyponatremia | 91% | 0% | 0% | 4% | 4% | 0% | 9% |
| Nausea | 91% | 4% | 4% | 0% | 0% | 0% | 9% |
| Fever | 91% | 4% | 4% | 0% | 0% | 0% | 9% |
| Hearing impaired | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Intracranial hemorrhage | 96% | 0% | 0% | 4% | 0% | 0% | 4% |
| Hypopituitarism | 96% | 0% | 0% | 4% | 0% | 0% | 0% |
Adverse events reported were TRAEs in at least two patients and/or were grade ≥3. One patient may have experienced multiple TRAEs.
Abbreviation: NC/NA, no change from baseline, no adverse event reported.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Hypoacusis | 3 | Probable |
| Hypopituitarism | 3 | Probable |
| Ileus | 3 | Unrelated |
| Nausea | 2 | Probable |
| Hyperbilirubinemia | 3 | Unrelated |
| Decreased appetite | 3 | Unrelated |
| Hyponatremia | 4 | Probable |
| Blood creatine phosphokinase increased | 3 | Probable |
| Cancer pain | 2 | Unrelated |
| Intracranial tumor hemorrhage | 3 | Probable |
| Malignant ascites | 2 | Unrelated |
| Pulmonary hemorrhage | 1 | Unrelated |
| Respiratory failure | 5 | Unrelated |
| Upper gastrointestinal hemorrhage | 3 | Unrelated |
| Pyrexia | 2 | Unrelated |
| Paraneoplastic syndrome | 3 | Unrelated |
| Dyspnea | 3 | Unrelated |
| Ascites | 2 | Unrelated |
| Disease progression | 5 | Unrelated |
Dose‐Limiting Toxicities
| Dose level | Number enrolled | Number evaluable for toxicity | Number with a dose‐limiting toxicity | Dose‐limiting toxicity information |
|---|---|---|---|---|
| 1 | 3 mg/kg Q2W | 4 | 0 | N/A |
| 2 | 10 mg/kg Q2W | 3 | 0 | N/A |
| 3 | 20 mg/kg Q2W | 7 | 1 | Intracranial tumor hemorrhage |
Abbreviation: N/A, not applicable.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Bintrafusp alfa (M7824) is a first‐in‐class bifunctional fusion protein composed of the extracellular domain of the human transforming growth factor‐β (TGF)‐βRII (a TGF‐β “trap”) fused via a flexible linker to the C terminus of each heavy chain of an immunoglobulin G1 antibody blocking programmed death‐ligand 1 (PD‐L1; anti–PD‐L1) 7, 8, 10. Preclinical data suggest that improved clinical efficacy may be observed through targeting both TGF‐β and PD‐(L)1 pathways compared with either pathway alone. Furthermore, as a bifunctional fusion protein, bintrafusp alfa may have improved efficacy based on the hypothesis that bintrafusp alfa binding to PD‐L1 in the tumor microenvironment may facilitate local TGF‐β trapping 7. Additionally, a manageable safety profile and encouraging early signs of clinical activity have been reported with bintrafusp alfa across all doses tested (0.3–20 mg/kg) in a first‐in‐human global phase I, 3 + 3 dose‐escalation study (NCT02517398), which enrolled patients with heavily pretreated advanced solid tumors 8. This promising antitumor activity and manageable tolerability profile have also been demonstrated in multiple expansion cohorts of the same trial 11, 12, 13, 14.
We report results of NCT02699515, an ongoing phase I, open‐label, dose‐escalation and dose‐expansion trial of bintrafusp alfa in Asian patients with metastatic or locally advanced solid tumors. For the dose‐escalation phase of this study, patients received bintrafusp alfa at 3, 10, or 20 mg/kg via 1‐hour intravenous infusion every two weeks (Q2W) until confirmed progressive disease (PD), unacceptable toxicity, or trial withdrawal. Patients in the hepatocellular carcinoma (HCC) safety‐assessment cohort received bintrafusp alfa 3 or 10 mg/kg Q2W intravenously until confirmed PD, unacceptable toxicity, or trial withdrawal.
The primary endpoints for the trial were the occurrence of dose‐limiting toxicities during the first 3 weeks of treatment in the dose‐escalation part and the number, severity, and duration of treatment‐related adverse events (TRAEs) according to National Cancer Institute‐CTCAE v4.03. Secondary endpoints included best overall response according to RECIST 1.1 and the pharmacokinetics profile of bintrafusp alfa. Additional secondary endpoints for the HCC safety‐assessment cohort were duration of response, disease control rate (DCR), progression‐free survival (PFS), and overall survival (OS). Efficacy and safety were analyzed in all patients who received at least one dose of bintrafusp alfa.
Between March 17, 2016, and August 24, 2018, 26 Asian patients with metastatic or locally advanced solid tumors, including 10 with HCC, were screened; 23 patients, including 9 with HCC, were enrolled (Fig. 2) and received bintrafusp alfa Q2W at 3 mg/kg (4 and 3 patients in the dose‐escalation and HCC cohort, respectively), 10 mg/kg (3 and 6 patients in the dose‐escalation and HCC cohort, respectively), or 20 mg/kg (7 patients in the dose‐escalation cohort).
Figure 2.
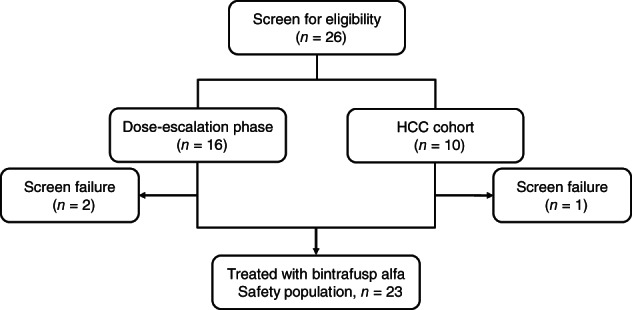
Study CONSORT of the dose‐escalation phase and HCC cohort of study NCT02699515.Abbreviation: HCC, hepatocellular carcinoma.
This was a heavily pretreated population, with 64.3% of patients having received at least four prior lines of therapy (Table 1). As of the data cutoff, median duration of treatment was 5.9 weeks (range, 2–122) in the dose‐escalation cohort, with one patient in the dose‐escalation cohort still receiving treatment. The most common reason for discontinuation was PD (17 patients [73.9%]).
Table 1.
Patient baseline and disease characteristics
| Characteristic | DE cohort (n = 14) | HCC cohort (n = 9) | Total (n = 23) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 4 (28.6) | 7 (77.8) | 11 (47.8) |
| Female | 10 (71.4) | 2 (22.2) | 12 (52.2) |
| Age, median (range), yr | 53 (38‐75) | 63 (39‐71) | 55 (38‐75) |
| No. of prior anticancer therapies, n (%) | |||
| 0 | 0 | 0 | 0 |
| 1 | 0 | 6 (66.7) | 6 (26.1) |
| 2 | 2 (14.3) | 1 (11.1) | 3 (13.0) |
| 3 | 3 (21.4) | 1 (11.1) | 4 (17.4) |
| ≥4 | 9 (64.3) | 1 (11.1) | 10 (43.5) |
| ECOG performance status, n (%) | |||
| 0 | 11 (78.6) | 6 (66.7) | 17 (73.9) |
| 1 | 3 (21.4) | 3 (33.3) | 6 (26.1) |
| Hepatitis viral infection, n (%) | |||
| Hepatitis B | 0 | 1 (11.1) | 1 (4.3) |
| Hepatitis C | 0 | 6 (66.7) | 6 (26.1) |
| Primary disease, n (%) | |||
| Adenoid cystic carcinoma of the tongue | 1 (7.1) | 0 | 1 (4.3) |
| Colorectal cancer | 1 (7.1) | 0 | 1 (4.3) |
| Descending colon cancer | 1 (7.1) | 0 | 1 (4.3) |
| Gastric/GEJ cancer | 3 (21.4) | 0 | 3 (21.4) |
| Hepatocellular carcinoma | 0 | 9 (100.0) | 9 (39.1) |
| Ovarian cancer | 2 (14.3) | 0 | 2 (14.3) |
| Pancreatic cancer | 1 (7.1) | 0 | 1 (4.3) |
| Parotid gland cancer | 1 (7.1) | 0 | 1 (4.3) |
| Renal pelvis cancer | 1 (7.1) | 0 | 1 (4.3) |
| Sigmoid colon cancer | 1 (7.1) | 0 | 1 (4.3) |
| Stomach cancer | 1 (7.1) | 0 | 1 (4.3) |
| Vulvar cancer | 1 (7.1) | 0 | 1 (4.3) |
Abbreviations: DE, dose‐escalation; ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; HCC, hepatocellular carcinoma.
Among the 23 evaluable patients, 8 (34.8%) experienced TRAEs. Grade ≥3 TRAEs were observed in three patients (13.0%; grade 4 hyponatremia and grade 3 hypopituitarism; grade 3 intracranial tumor hemorrhage; and grade 3 increased blood creatine phosphokinase level, hyponatremia, and hypoacusis). The patient with grade 4 hyponatremia also had grade 3 hypopituitarism on day 195, which led to treatment interruption; hyponatremia ultimately resolved with saline. The patient with intracranial tumor hemorrhage had a pituitary gland tumor, which is known to have an increased incidence of intralesional bleeding compared with those of other intracranial tumors 15, 16, and displayed symptoms before the study that were possibly related to this event (ongoing headache and dizziness 17). The patient with increased blood creatine phosphokinase interrupted treatment and had grade 3 hyponatremia (on day 30); whereas the latter was ongoing at data cutoff, the increased blood creatine phosphokinase event regressed to grade 2 without medication, and treatment was resumed. On day 35, the same patient had grade 3 hypoacusis (potentially immune‐related adverse event [irAE]) that responded well to prednisolone but ultimately led to study discontinuation. Rates of irAEs, infusion‐related reactions, and TRAEs leading to study discontinuation were low, with no treatment‐related deaths or TGF‐β–related skin adverse events in the dose‐escalation and HCC cohorts.
Among the two patients with a partial response (PR), the first patient (Fig. 3A) was treated with bintrafusp alfa at 3 mg/kg and had colorectal cancer associated with Lynch syndrome 18; this patient had a durable and ongoing response (25.0+ months) and was still receiving treatment as of the data cutoff. The second PR was documented in a patient with clear cell ovarian cancer (Fig. 3B) who received bintrafusp alfa 20 mg/kg; the PR occurred after treatment discontinuation (due to grade 3 hypoacusis), with no further anticancer therapy, persisting for 4.7 months until eventual progression due to a nontarget lesion, which was rated as PD. In the HCC safety‐assessment cohort, stable disease was recorded in 1 patient, corresponding to a confirmed DCR of 11.1%. Furthermore, a median PFS of 1.3 months (95% confidence interval [CI], 0.8–2.7 months) and a median OS of 4.4 months (95% CI, 2.1–14.4 months) were observed (Fig. 4), which was comparable to that of the dose‐escalation cohort (Fig. 5). In both cohorts, the unconfirmed efficacy data were identical to the confirmed data.
Figure 3.

Scans of target lesions from patients with a partial response (PR) treated with bintrafusp alfa. Case 1: (A) patient with colon cancer who had best overall response of PR. Case 2: (B) patient with ovarian cancer with best overall response of PR.
Figure 4.

Kaplan‐Meier analyses in the HCC cohort. Kaplan‐Meier analysis of (A) PFS assessed by the investigator and (B) OS in the HCC cohort.Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; OS, overall survival; PFS, progression‐free survival.
Figure 5.

Kaplan‐Meier analyses in the dose‐escalation cohort. Kaplan‐Meier analysis of (A) progression‐free survival (PFS) assessed by the investigator and (B) overall survival (OS) in the dose‐escalation cohort.Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression‐free survival.
Overall, the pharmacokinetic profile of bintrafusp alfa in Asian patients (Table 2) was similar to that in the global study 8, 9. Based on an integrated analysis of clinical activity and safety, the pharmacokinetic profile of bintrafusp alfa observed in the dose‐escalation phase of both studies, the pharmacodynamic profile of bintrafusp alfa from the global study, and reported data from cohorts of these studies exploring 500‐mg and 1200‐mg Q2W dosing 12, 13, 14, 19, a dose level of 1,200 mg was evaluated in multiple additional expansion cohorts 8, 20.
Table 2.
Pharmacokinetics of bintrafusp alfa after the first dose
| Dose level, mg/kg | Cmax, μg/mL | Cmax/dose, (μg/mL)/(mg/kg) | Ctrough, μg/mL | Ctrough/dose, (μg/mL)/(mg/kg) | AUCtau, h·μg/mL | AUCtau/dose, (h·μg/mL)/(mg/kg) | t1/2, h | CL, mL/h/kg | Vz, mL/kg |
|---|---|---|---|---|---|---|---|---|---|
| 3 | |||||||||
| GM | 54.6 | 18.1 | 7.97 | 2.64 | 7940 | 2630 | 132 | 0.314 | 59.8 |
| %CV | 17.9 | 18.6 | 27.9 | 28.4 | 16.7 | 17.1 | 12.8 | 15.6 | 22.5 |
| n | 7 | 7 | 6 | 6 | 7 | 7 | 7 | 7 | 7 |
| 10 | |||||||||
| GM | 211 | 21.1 | 34.6 | 3.46 | 29200 | 2910 | 138 | 0.290 | 57.8 |
| %CV | 21.7 | 21.8 | 36.8 | 36.9 | 25.3 | 25.4 | 17.4 | 29.8 | 20.7 |
| n | 9 | 9 | 9 | 9 | 8 | 8 | 7 | 7 | 7 |
| 20 | |||||||||
| GM | 331 | 16.4 | 68.5 | 3.39 | 51300 | 2550 | 150 | 0.327 | 70.8 |
| %CV | 19.2 | 19.8 | 26.3 | 26.5 | 24.7 | 25.2 | 24.4 | 26.6 | 20.0 |
| n | 7 | 7 | 7 | 7 | 7 | 5 | 5 | 5 | 5 |
Abbreviations: AUC, area under the concentration‐time curve; AUCtau, AUC over the dosing interval (tau = 336 h); Cmax, maximum concentration; Ctrough, trough concentration; CL, clearance; CV, coefficient of variation; GM, geometric mean; t1/2, terminal half‐life: Vz, volume of distribution during terminal phase mean.
Bintrafusp alfa is a bifunctional fusion protein designed to simultaneously target TGF‐β and PD‐L1 pathways, potentially leading to restored activation of the body's own tumor immune responses and tumor regression. Although the small sample size precludes any meaningful conclusions from being drawn with regard to treatment outcomes, the overall findings from the present phase I study in heavily pretreated patients shows a manageable safety profile and promising preliminary efficacy in solid tumors, including HCC, with no or limited treatment options.
Disclosures
Yutaka Fujiwara: Merck Serono, Inc, Toyko, Japan, an affiliate of Merck KGaA, Darmstadt, Germany (RF); Takafumi Koyama: Chugai, Sysmex (SAB); Masafumi Ikeda: Bayer, Eisai, Merck Sharp & Dohme, ASLAN (C/A), Bayer, Eisai, Ono, j‐Pharma, Merck Serono, Inc, Toyko, Japan, an affiliate of Merck KGaA, Darmstadt, Germany (RF), Bayer, Eisai, Yakult (H); Christoph Helwig: Merck KGaA (E, OI); Morihiro Watanabe: Merck Serono, Inc, Toyko, Japan, an affiliate of Merck KGaA, Darmstadt, Germany (E); Yulia Vugmeyster: EMD Serono Research & Development Institute, Inc., Billerica, MA, USA; a business of Merck KGaA, Darmstadt, Germany (E, IP); Masatoshi Kudo: Bayer, Eisai, Ono, Merck Sharp & Dohme, Bristol‐Myers Squibb, Eli Lilly (C/A), Daiichi Sankyo, Medico's Hirata, Otsuka, Taiho, Astellas Pharma, Chugai, Abbvie, Bristol‐Myers Squibb, EA Pharma, Takeda, Gilead, Eisai (RF), Bayer, Eisai, Merck Sharp & Dohme, Eli Lilly (H), Bayer, Eisai, Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche, AstraZeneca (SAB). Toshihiko Doi indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Table and Figures
Acknowledgments
The authors thank the patients and their families, investigators and coinvestigators, and study teams at each of the participating sites and at EMD Serono (Billerica, MA), a business of Merck KGaA (Darmstadt, Germany), and Merck KGaA (Darmstadt, Germany). This study was funded by Merck KGaA (Darmstadt, Germany) and is part of an alliance between Merck KGaA and GlaxoSmithKline. Medical writing support was provided by Marjorie Rummelt, Ph.D., ClinicalThinking Inc (Hamilton, NJ), which was also funded by Merck KGaA and GlaxoSmithKline in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02699515
- Sponsors: Merck KGaA, Darmstadt, Germany, and GlaxoSmithKline
- Principal Investigator: Toshihiko Doi
- IRB Approved: Yes
References
- 1. Li MO, Flavell RA. Contextual regulation of inflammation: A duet by transforming growth factor‐β and interleukin‐10. Immunity 2008;28:468–476. [DOI] [PubMed] [Google Scholar]
- 2. Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 2012;11:790–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi Y, Massagué J. Mechanisms of TGF‐β signaling from cell membrane to the nucleus. Cell 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 4. Vanella V, Festino L, Strudel M et al. PD‐L1 inhibitors in the pipeline: Promise and progress. OncoImmunology 2018;7:e1365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018;118:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lan Y, Zhang D, Xu C et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD‐L1 and TGF‐β. Sci Transl Med 2018;10:eaan5488. [DOI] [PubMed] [Google Scholar]
- 8. Strauss J, Heery CR, Schlom J et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD‐L1 and TGFβ, in advanced solid tumors. Clin Cancer Res 2018;24:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilkins J, Vugmeyster Y, Dussault I et al. Population pharmacokinetic analysis of bintrafusp alfa in different cancer types. Adv Ther 2019;36:2414–2433. [DOI] [PubMed] [Google Scholar]
- 10. David JM, Dominguez C, McCampbell KK et al. A novel bifunctional anti‐PD‐L1/TGF‐β trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology 2017;6:e1349589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strauss J, Gatti‐Mays ME, Redman J et al. Safety and activity of M7824, a bifunctional fusion protein targeting PD‐L1 and TGF‐β, in patients with HPV associated cancers. J Clin Oncol 2018; 36(suppl 15):3007a.29733771 [Google Scholar]
- 12. Paz‐Ares LG, Kim TM, Vicente Baz D et al. Results from a second‐line (2L) NSCLC cohort treated with M7824 (MSB0011359C), a bifunctional fusion protein targeting TGF‐β and PD‐L1. J Clin Oncol 2018;36(suppl 15):9017a. [Google Scholar]
- 13. Barlesi F, Isambert N, Felip E et al. Initial results from phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD‐L1 and TGF‐β, in patients with NSCLC refractory or resistant to prior anti–PD‐1/anti–PD‐L1 agents. J Immunother Cancer 2017;5(suppl 2):O14a Available at https://jitc.biomedcentral.com/articles/10.1186/s40425-017-0289-3. [Google Scholar]
- 14. Kopetz S, Spira AI, Wertheim M et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD‐L1 and TGF‐β, in patients with heavily pretreated CRC: Preliminary results from a phase I trial. J Clin Oncol 2018; 36(suppl 4):764a. [Google Scholar]
- 15. Goyal P, Utz M, Gupta N et al. Clinical and imaging features of pituitary apoplexy and role of imaging in differentiation of clinical mimics. Quant Imaging Med Surg 2018;8:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wakai S, Fukushima T, Teramoto A, Sano K. Pituitary apoplexy: Its incidence and clinical significance. J Neurosurg 1981;55:187–193. [DOI] [PubMed] [Google Scholar]
- 17. Giraldo EA. Intracerebral hemorrhage ‐ Neurologic disorders. Merck Manual Professional version. Available at https://www.merckmanuals.com/professional/neurologic‐disorders/stroke‐cva/intracerebral‐hemorrhage. Accessed September 26, 2019. [Google Scholar]
- 18. Rumilla K, Schowalter KV, Lindor NM et al. Frequency of deletions of EPCAM (TACSTD1) in MSH2‐associated Lynch syndrome cases. J Mol Diagn 2011;13:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang YK, Doi T, Kondo S et al. M7824 (MSB0011359C), a bifunctional fusion protein targeting PD‐L1 and TGF‐β, in Asian patients with pretreated recurrent or refractory gastric cancer: Preliminary results from a phase I trial. J Clin Oncol 2018;36(suppl 4):100a. [Google Scholar]
- 20. Vugmeyster Y, Wilkins J, Harrison‐Moench E et al. Selection of the recommended phase 2 dose (RP2D) for M7824 (MSB0011359C), a bifunctional fusion protein targeting TGF‐β and PD‐L1. J Clin Oncol 2018;36(suppl 15):2566a.29945529 [Google Scholar]


