Abstract
Background
Controversy exists over whether there has been a true increase in the occurrence of thyroid cancer or overdiagnosis secondary to imaging practices. Because cancer overdiagnosis is associated with detection of indolent disease, overdiagnosis can be associated with perceived improvement in survival.
Materials and Methods
Surveillance, Epidemiology, and End Results‐Medicare linked database was used to determine the relationship between type of imaging leading to thyroid cancer diagnosis and survival. Disease‐specific and overall survival were evaluated in 11,945 patients aged ≥66 years with differentiated thyroid cancer diagnosed between January 1, 2001, and September 30, 2015, who prior to their cancer diagnosis initially underwent thyroid ultrasound versus other imaging capturing the neck. Analyses were performed using the Kaplan‐Meier method and Cox proportional hazards model with propensity score.
Results
Patients who underwent thyroid ultrasound as compared with other imaging had improved disease‐specific and overall survival (p < .001, p < .001). However, those who underwent thyroid ultrasound were less likely to have comorbidities (p < .001) and more likely to be younger (p < .001), be female (p < .001), have localized cancer (p < .001), and have tumor size ≤1 cm (p < .001). After using propensity score analysis and adjusting for tumor characteristics, type of initial imaging still correlated with better overall survival but no longer correlated with better disease‐specific survival.
Conclusion
There is improved disease‐specific survival in patients diagnosed with thyroid cancer after thyroid ultrasound as compared with after other imaging. However, better disease‐specific survival is related to these patients being younger and healthier and having lower‐risk cancer, suggesting that thyroid ultrasound screening contributes to cancer overdiagnosis.
Implications for Practice
The findings from this study have implications for patients, physicians, and policy makers. Patients who have thyroid ultrasound as their initial imaging are fundamentally different from those who are diagnosed after other imaging. Because patients undergoing ultrasound are younger and healthier and are diagnosed with lower‐risk thyroid cancer, they are less likely to die of their thyroid cancer. However, being diagnosed with thyroid cancer can lead to cancer‐related worry and create risks for harm from treatments. Thus, efforts are needed to reduce inappropriate use of ultrasound, abide by the U.S. Preventive Services Task Force recommendations, and apply nodule risk stratification tools when appropriate.
Keywords: Thyroid neoplasms, Ultrasonography, Diagnosis, Survival
Short abstract
Because cancer overdiagnosis can be associated with both earlier detection and the detection of slower‐growing tumors, overdiagnosis can lead to perceived improvement in survival. This article reports on the relationship between type of imaging and survival, using SEER‐Medicare data to assess the initial imaging associated with cancer detection and disease‐specific and overall survival.
Introduction
Cancer overdiagnosis occurs when a cancer fulfills pathologic criteria for cancer but does not go on to cause symptoms or death or the cancer progresses so slowly that a patient dies of other causes prior to developing symptoms 1, 2. Because cancer overdiagnosis can be associated with both earlier detection and detection of slower‐growing tumors, overdiagnosis can lead to perceived improvement in survival 3.
Since 1996, the U.S. Preventive Services Task Force (USPSTF) has recommended against thyroid cancer screening in asymptomatic individuals by either physical examination or ultrasound 4, 5. Because over half the adult population has thyroid nodules, screening can lead to a diagnostic cascade, with a large number of thyroid nodules undergoing biopsy and some indolent thyroid cancers being diagnosed 6. Suggesting overdiagnosis, in 2006 Davies and Welch noted that from 1973 to 2002 there was a 2.4‐fold increase in the incidence of low‐risk thyroid cancer with mortality remaining stable 7. Prior work evaluating longitudinal imaging patterns found that between 2002 and 2013, despite USPSTF recommendations, area‐level use of thyroid ultrasound as initial imaging increased and was associated with a rise in thyroid cancer incidence, including a rise in low‐risk thyroid cancer 8. Similarly, in a survey of patients affiliated with Surveillance, Epidemiology, and End Results (SEER) and diagnosed with differentiated thyroid cancer, patients with small cancer size were more likely than those with larger cancer size to report thyroid ultrasound as the initial imaging test that led to their cancer diagnosis 9. However, controversy still exists, as a recent study found that although death from thyroid cancer was rare, there was a small increase in mortality over time, implying there could be a true rise in thyroid cancer incidence 10.
Understanding the relationship between the imaging initiating the thyroid cancer diagnostic cascade and survival from thyroid cancer is critical to determining the relationship between overdiagnosis and the thyroid cancer epidemic. We hypothesized that patients who had thyroid ultrasound as their initial imaging, as opposed to other imaging tests such as computed tomography (CT) of the neck, magnetic resonance imaging (MRI) of the neck, or positron emission tomography (PET), would have better disease‐specific and overall survival, largely because these would be healthier patients who had indolent disease diagnosed. To determine the relationship between type of imaging and survival, we used SEER‐Medicare to assess the initial imaging associated with cancer detection and disease‐specific and overall survival.
Materials and Methods
Data Source and Study Population
SEER‐Medicare complements the detailed, high‐quality clinical data provided from SEER with claims from Medicare 11. It is a well‐recognized, population‐based source of information on cancer, which includes details on cancer diagnosis, pathology, and disease‐specific and overall survival 12. We restricted the SEER‐Medicare cohort to older adults because older patients are most at risk for death and because close to 97% of patients age 65 and older are included in Medicare after age 65 13, 14. As we were interested in imaging sequence in this cohort, we further narrowed the cohort to patients age 66 and older to accurately capture imaging sequence in the 12 months prior to cancer diagnosis. Thus, the study cohort included SEER‐Medicare patients age 66 and older with differentiated thyroid cancer, that is, papillary, follicular, or Hürthle cell, diagnosed from January 1, 2001, to September 30, 2015, and enrolled in Medicare Part A & B and non‐Health Maintenance Organization (HMO) for at least 11 months during the time span including month of diagnosis and the year prior to diagnosis. Because the primary comparison was thyroid ultrasound versus other imaging, we excluded patients in SEER‐Medicare who did not have Medicare claims data for thyroid ultrasound or other imaging that would capture the neck (n = 1,632) or alternatively their diagnostic sequence did not start with thyroid ultrasound or other imaging that captures the neck (n = 1,338). The final analytic cohort (n = 11,945) was followed up to 2015, with a median follow‐up of 60 months.
Institutional review board approval was not required because this study involves research using publicly available data and cannot be tracked to human subjects.
Measures
SEER‐Medicare was used to obtain details on patient demographics, clinical characteristics, and survival. Patient sex and age at diagnosis were measured at the individual level with age at diagnosis divided into the following categories: 66–70, 71–75, 76–80, 81–85, and 86 and above. Charlson‐Deyo comorbidity index was also measured at the individual level through the time that the patient was diagnosed with thyroid cancer. Income and urbanicity, defined as metro, urban/rural and adjacent to metro, or urban/rural and not adjacent to metro 8, were measured at the zip code level.
Patients’ imaging sequence was documented in the year prior to thyroid cancer diagnosis. Patients were categorized as having “thyroid ultrasound” as initial imaging if there were claims data for thyroid ultrasound prior to their thyroid cancer diagnosis and an absence of claims data for another imaging test that would capture the neck prior to the thyroid ultrasound. If a patient had another imaging test that would capture the thyroid first, they were categorized as having “other imaging” as their initial imaging. “Other imaging” could include CT of neck, chest, or C‐spine, or MRI of neck, chest, or C‐spine, carotid duplex, maxillofacial CT, or body PET/CT. The mortality data reported by SEER were provided by the National Cancer for Health Statistics 12, 15. Overall survival was the time interval from diagnosis to death from any cause or censoring. Disease‐specific survival is the time interval from diagnosis to death from differentiated thyroid cancer or time of censoring. SEER uses algorithms to process cause of death from death certificates and determine the underlying etiology.
Statistical Analysis
Univariate analyses were performed using chi‐square tests to determine whether the patients who had thyroid ultrasound as initial imaging versus other imaging differed by demographics and clinical and tumor characteristics. Kaplan‐Meier method was used to estimate the survival function, and log‐rank test was used to compare survival in patients who underwent thyroid ultrasound versus other imaging.
Because there were differences in the cohort undergoing thyroid ultrasound versus other imaging, we used propensity score analysis as a quasi‐randomization technique to balance the two groups in terms of covariate distribution. In the first stage, we fitted a logistic regression model to predict the likelihood of thyroid ultrasound (versus other imaging) as a function of sex, age, comorbidity (i.e., covariates that are known prior to diagnosis and were significant in univariate analysis). The predicted probabilities (propensity scores) were incorporated into a Cox regression model to assess the effects of initial imaging, histology, SEER stage, and tumor size on both disease‐specific and overall survival (two separate models for the two outcomes).
All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC). Two sided tests were used, with p < .05 considered statistically significant.
Results
As demonstrated in Figures 1 and 2, patients who had thyroid ultrasound as their initial imaging had significantly better disease‐specific survival (p < .001) and overall survival (p < .001).
Figure 1.
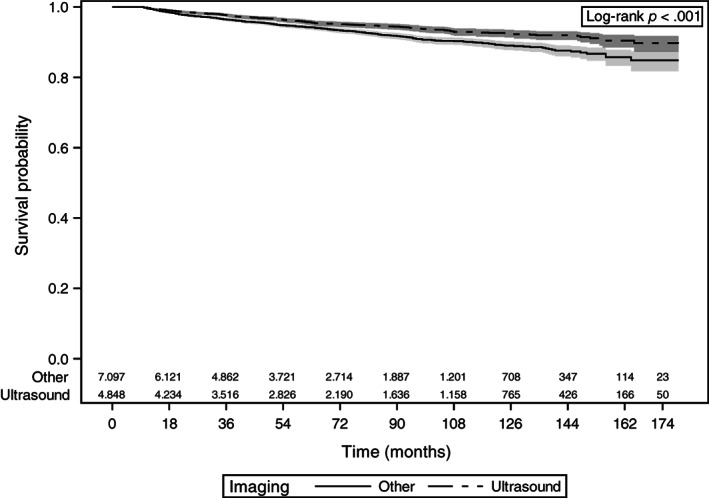
Kaplan‐Meier curves of disease‐specific survival with 95% confidence limits and number at risk at the given time interval. Patients with thyroid cancer who underwent thyroid ultrasound as their initial imaging are compared with patients who underwent other imaging including computed tomography (CT) of neck, magnetic resonance imaging of neck, or positron emission tomography/CT.
Figure 2.
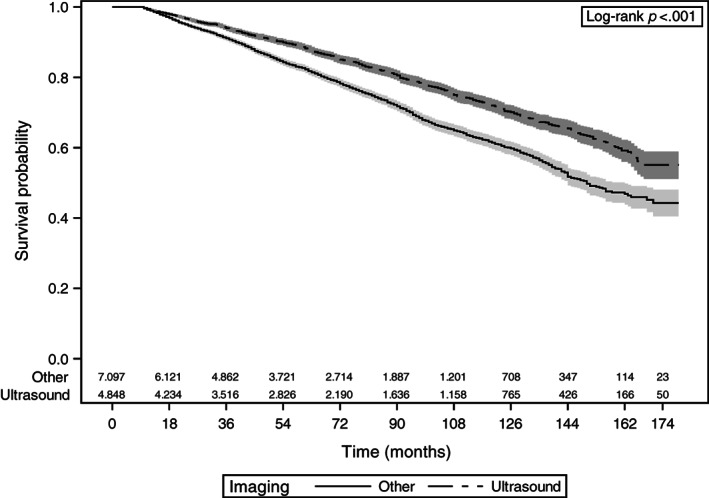
Kaplan‐Meier curves of overall survival with 95% confidence limits and number at risk at the given time interval. Patients with thyroid cancer who underwent thyroid ultrasound as their initial imaging are compared with patients who underwent other imaging including computed tomography (CT) of neck, magnetic resonance imaging of neck, or positron emission tomography/CT.
Table 1 provides background information on the two cohorts. Patients who had thyroid ultrasound as initial imaging as compared with other imaging were more likely to be female (75.3% vs. 65.1%, p < .001) and younger (43.9% age 66–70 vs. 32.6%, p value < .001). Those who had thyroid ultrasound as initial imaging were also more likely to have no comorbidities (21.9% vs. 8.1%, p < .001), localized disease (73.2% vs. 67.6%, p < .001), and tumor size ≤1 cm and > 4 cm (37.3% vs. 34.1% and 15.1% vs. 13.2%, respectively, p < .001). Patients who had thyroid ultrasound as initial imaging as compared with other imaging died less frequently from thyroid cancer (4.6% vs. 6.0%, p < .001) or from any cause (17.8% vs. 22.8%, p < .001).
Table 1.
Patient clinical characteristics and survival
| Characteristics | Other imaging, n = 7,097 (59.4%) | Thyroid ultrasound, n = 4,848 (40.6%) | p value |
|---|---|---|---|
| Sex | <.001 | ||
| Male | 2,480 (34.9) | 1,196 (24.7) | |
| Female | 4,617 (65.1) | 3,652 (75.3) | |
| Age at diagnosis, years | <.001 | ||
| 66–70 | 2,312 (32.6) | 2,127 (43.9) | |
| 71–75 | 2,102 (29.6) | 1,353 (27.9) | |
| 76–80 | 1,481 (20.9) | 789 (16.3) | |
| 81–85 | 852 (12.0) | 421 (8.7) | |
| 86 and above | 350 (4.9) | 158 (3.2) | |
| Urbanicity | .862 | ||
| Metro | 6,048 (85.2) | 4,140 (85.4) | |
| Urban/rural, adjacent to metro | 602 (8.5) | 407 (8.4) | |
| Urban/rural, not adjacent to metro | 446 (6.3) | 301 (6.2) | |
| Median household income, $ | .050 | ||
| <35,000 | 1,119 (15.8) | 715 (14.7) | |
| 35,000–59,000 | 2,749 (38.7) | 1,834 (37.8) | |
| ≥60,000 | 2,821 (39.8) | 2,034 (42.0) | |
| Unknown/missing | 408 (5.7) | 265 (5.5) | |
| Comorbidity | <.001 | ||
| 0 | 576 (8.1) | 1,064 (21.9) | |
| 1 | 898 (12.7) | 847 (17.5) | |
| 2 or more | 5,623 (79.2) | 2,937 (60.6) | |
| Histology | .227 | ||
| Papillary | 6,161 (86.8) | 4,156 (85.7) | |
| Follicular | 568 (8.0) | 415 (8.6) | |
| Hürthle cell | 368 (5.2) | 277 (5.7) | |
| SEER stage | <.001 | ||
| Localized | 4,796 (67.6) | 3,550 (73.2) | |
| Regional | 1,617 (22.8) | 1,012 (20.9) | |
| Distant | 514 (7.2) | 219 (4.5) | |
| Unknown | 170 (2.4) | 67 (1.4) | |
| Tumor size, cm | <.001 | ||
| ≤1 | 2,418 (34.1) | 1,807 (37.3) | |
| >1 and ≤2 | 1,744 (24.6) | 1,043 (21.5) | |
| >2 and ≤4 | 1,486 (20.9) | 1,005 (20.7) | |
| >4 | 936 (13.2) | 734 (15.1) | |
| Unknown | 513 (7.2) | 259 (5.4) | |
| Disease‐specific death | <.001 | ||
| Alive | 6,670 (94.0) | 4,626 (95.4) | |
| Dead | 427 (6.0) | 222 (4.6) | |
| Overall death | <.001 | ||
| Alive | 5,476 (77.2) | 3,983 (82.2) | |
| Dead | 1,621 (22.8) | 865 (17.8) |
Data are presented as n (%).Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
As shown in Table 2, after using propensity score and controlling for histology, SEER stage, and tumor size, thyroid ultrasound was no longer associated with improved disease‐specific death. Hürthle cell cancer (hazard ratio [HR] 1.38, 95% confidence interval [CI] 1.03–1.84), advanced SEER stage (regional stage HR 2.49, 95% CI 2.05–3.04, distant stage HR 8.09, 95% CI 6.57–9.95), and larger tumor size (>2 and ≤ 4 cm HR 1.52, 95% CI 1.17–1.96 and > 4 cm HR 2.14, 95% CI 1.64–2.79) were associated with increased likelihood of death from thyroid cancer.
Table 2.
Cox regression for disease‐specific death
| Characteristics | Hazard ratioa (95% CI) |
|---|---|
| Initial imaging | |
| Thyroid ultrasound | 0.95 (0.80–1.12) |
| Other imaging | ref |
| Histology | |
| Papillary | ref |
| Follicular | 1.12 (0.88–1.43) |
| Hürthle cell | 1.38 (1.03–1.84) |
| SEER stage | |
| Localized | ref |
| Regional | 2.49 (2.05–3.04) |
| Distant | 8.09 (6.57–9.95) |
| Unknown | 2.43 (1.55–3.81) |
| Tumor size, cm | |
| ≤1 | ref |
| >1 and ≤2 | 1.16 (0.89–1.52) |
| >2 and ≤4 | 1.52 (1.17–1.96) |
| >4 | 2.14 (1.64–2.79) |
| Unknown | 2.09 (1.54–2.84) |
Model adjusted for propensity scores.
Abbreviations: CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
In contrast, as shown in Table 3, patients who underwent thyroid ultrasound as initial imaging still had improved overall survival (HR 0.89, 95% CI 0.82–0.97) even after propensity score adjustment and controlling for histology, stage, and tumor size. Similar to disease‐specific death, Hürthle cell cancer (HR 1.23, 95% CI 1.05–1.39), advanced‐stage disease (regional stage HR 1.26, 95% CI 1.14–1.39, distant stage HR 2.59, 95% CI 2.29–2.93), and tumor size >4 cm (HR 1.59, 95% CI 1.40–1.81) were associated with increased likelihood of overall death.
Table 3.
Cox regression for overall death
| Characteristics | Hazard ratioa (95% CI) |
|---|---|
| Initial imaging | |
| Thyroid ultrasound | 0.89 (0.82–0.97) |
| Other imaging | ref |
| Histology | |
| Papillary | ref |
| Follicular | 1.12 (0.99–1.28) |
| Hürthle cell | 1.23 (1.05–1.39) |
| SEER stage | |
| Localized | ref |
| Regional | 1.26 (1.14–1.39) |
| Distant | 2.59 (2.29–2.93) |
| Unknown | 1.21 (0.94–1.55) |
| Tumor size, cm | |
| ≤1 | ref |
| >1 and ≤2 | 0.98 (0.87–1.11) |
| >2 and ≤4 | 1.12 (0.99–1.26) |
| >4 | 1.59 (1.40–1.81) |
| Unknown | 1.55 (1.33–1.81) |
Model adjusted for propensity score.
Abbreviations: CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
Discussion
Patients with thyroid cancer who underwent thyroid ultrasound as their initial imaging had better disease‐specific and overall survival as compared with those whose cancer was diagnosed as a result of other imaging. However, patients who underwent thyroid ultrasound as initial imaging also had lower‐risk cancer and were healthier in general, based on lower number of comorbidities and younger age. After using propensity score analyses and controlling for tumor characteristics, type of imaging was no longer associated with better disease‐specific survival but remained associated with better overall survival.
The diagnosis of thyroid cancer starts with first identifying a thyroid nodule. Nodules are detected in up to 65% of the population, with the highest prevalence seen in older adults 16. Nodules can be identified by palpation or imaging, with small nodules less likely to be palpable 16. Compressive symptoms from a large nodule are an accepted indication for thyroid ultrasound; however, this study found that not only was thyroid ultrasound as initial imaging associated with a higher percentage of cancers with tumor size >4 cm, a tumor size more likely to be associated with compressive symptoms, ultrasound use was also associated with a higher percentage of cancers ≤1 cm. Cancers ≤1 cm are unlikely to cause symptoms.
In asymptomatic patients, nodules can be incidental findings when a patient has imaging for another reason and the imaging captures the neck. Alternatively, in asymptomatic patients, nodules can be identified by screening with thyroid ultrasound. After a nodule is identified, patients often undergo a fine‐needle aspiration (FNA)/biopsy of their nodule; then, if cancer is diagnosed, standard treatment typically includes surgery. Since 1996, the USPSTF has recommended against thyroid cancer screening in asymptomatic individuals by either physical examination or ultrasound 4, 5. It has been recognized that screening can lead to a diagnostic cascade, with a large number of thyroid nodules undergoing biopsy and some indolent thyroid cancers being diagnosed 6.
Despite USPSTF recommendations against ultrasound screening in asymptomatic patients, prior work using SEER‐Medicare data suggested that imaging played a key role in the rise in thyroid cancer incidence, as increased area‐level use of thyroid ultrasound correlated with thyroid cancer incidence 8. In addition, through patient surveys linked to SEER data, it was demonstrated in a population‐based cohort that thyroid cancers ≤1 cm are more likely to be initially detected with thyroid ultrasound than cancers >1 cm 9. However, novel to prior work, our current study provides key information about the relationship between initial imaging and disease‐specific and overall survival.
Our study findings of perceived improvement in survival with thyroid ultrasound suggest thyroid ultrasound use contributes to the overdiagnosis of indolent cancer. Cancer overdiagnosis occurs when a cancer fulfills pathologic criteria for cancer but does not go on to cause symptoms or death or the cancer progresses so slowly that a patient dies of other causes prior to developing symptoms 1, 2. Because cancer overdiagnosis can be associated both with disease being detected earlier and with slower‐growing tumors having a greater likelihood of being detected early, overdiagnosis is associated with perceived improvement in survival 3.
Strengths of this study include the use of high‐quality cancer data, the population‐based cohort, and the analytic approach. However, despite the thorough study design, limitations do exist. Similar to other studies using claims data, there is a risk of miscoding or care received elsewhere. We minimized this risk by focusing on patients enrolled in Medicare Part A & B and non‐HMO for at least 11 months during the time span including month of diagnosis and the year prior to diagnosis, by excluding patients without imaging or with diagnostic sequence not preceded by thyroid ultrasound or by other imaging capturing the neck, and by focusing on patients age 66 and older who are likely to have at least 1 year of Medicare imaging claims. Although restricting the cohort to patients at higher risk of death and those likely to have the most comprehensive claims data is optimal both clinically and analytically, a limitation is that this restriction impacts translatability of the findings to younger cohorts. However, overdiagnosis trends in younger adults parallel those in older adults, making it probable that the findings from this study are applicable to younger patients. In addition, we recognize that thyroid cancer is heterogeneous, and although most patients do well and are at risk for overdiagnosis, there are a few patients in whom early identification of disease could reduce harm. Finally, another limitation is the fact that despite controlling for relevant covariates and using propensity score analysis to address potential confounding, there remained a relationship between imaging and overall survival. Propensity score can only address confounding due to measured covariates; however, because overall survival is a less specific outcome than disease‐specific survival, it is possible that additional unmeasured covariates, such as severity of cardiovascular disease or pulmonary disease, affect this outcome. Despite this limitation, disease‐specific survival is the more precise outcome for patients with well‐differentiated thyroid cancer, and our data suggest that although thyroid ultrasound is associated with better disease‐specific survival, it is likely due to overdiagnosis of thyroid cancer in healthier, younger patients who undergo thyroid ultrasound.
This study complements prior studies and adds novel information on survival 6, 7, 8, 9. This study is the foundation needed for future studies focusing on better understanding of why physicians order ultrasound as well as more research on where to intervene to decrease overdiagnosis. It is not known if the best approach for decreasing thyroid cancer overdiagnosis is through more optimal ultrasound use, a reduction in unnecessary FNA after a nodule is identified by ultrasound, or a reduction in surgery after FNA.
Conclusion
The findings from this study have implications for patients, physicians, and policy makers. Patients who have thyroid ultrasound as their initial imaging are fundamentally different from those who are diagnosed after other imaging. Because patients undergoing ultrasound are younger and healthier and are diagnosed with lower‐risk thyroid cancer, they are less likely to die of their thyroid cancer. However, being diagnosed with thyroid cancer will lead to cancer‐related worry and create risks for harm from treatments 17, 18, 19, 20. Thus, efforts are needed to reduce inappropriate use of ultrasound, abide by the USPSTF recommendations, apply nodule risk stratification tools when appropriate, and continue to improve the quality of research on cancer overdiagnosis.
Author Contributions
Conception/design: Megan R. Haymart, Elaine Caoili, Edward C. Norton, Mousumi Banerjee
Provision of study material or patients: Megan R. Haymart
Collection and/or assembly of data: Megan R. Haymart, David Reyes‐Gastelum, Mousumi Banerjee
Data analysis and interpretation: Megan R. Haymart, David Reyes‐Gastelum, Elaine Caoili, Edward C. Norton, Mousumi Banerjee
Manuscript writing: Megan R. Haymart, Mousumi Banerjee
Final approval of manuscript: Megan R. Haymart, David Reyes‐Gastelum, Elaine Caoili, Edward C. Norton, Mousumi Banerjee
Disclosures
The authors indicated no financial relationships.
Acknowledgments
We acknowledge Brittany Gay for her assistance with manuscript preparation and formatting. The funding source had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The ideas and opinions expressed herein are those of the authors, and endorsement by the National Cancer Institute and the Agency for Healthcare Research and Quality is not intended nor should be inferred. This work was supported by Agency for Healthcare Research and Quality Grant R01 HS024512. M.R.H. is also supported by R01 CA201198 from the National Cancer Institute.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010;102:605–613. [DOI] [PubMed] [Google Scholar]
- 2. Esserman LJ, Thompson IM Jr, Reid B. Overdiagnosis and overtreatment in cancer: An opportunity for improvement. JAMA 2013;310:797–798. [DOI] [PubMed] [Google Scholar]
- 3. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med 1993;328:1237–1243. [DOI] [PubMed] [Google Scholar]
- 4. US Preventive Services Task Force , Bibbins‐Domingo K, Grossman DC et al. Screening for thyroid cancer: US Preventive Services Task Force recommendation statement. JAMA 2017;317:1882–1887. [DOI] [PubMed] [Google Scholar]
- 5. US Preventive Services Task Force. Baltimore, MD: Williams & Wilkins, 1996. [Google Scholar]
- 6. Ahn HS, Kim HJ, Welch HG. Korea's thyroid‐cancer "epidemic"–Screening and overdiagnosis. N Engl J Med 2014;371:1765–1767. [DOI] [PubMed] [Google Scholar]
- 7. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973‐2002. JAMA 2006;295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 8. Haymart MR, Banerjee M, Reyes‐Gastelum D et al. Thyroid ultrasound and the increase in diagnosis of low‐risk thyroid cancer. J Clin Endocrinol Metab 2019;104:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esfandiari NH, Hughes DT, Reyes‐Gastelum D et al. Factors associated with diagnosis and treatment of thyroid microcarcinomas. J Clin Endocrinol Metab 2019;104:6060–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim H, Devesa SS, Sosa JA et al. Trends in thyroid cancer incidence and mortality in the United States, 1974‐2013. JAMA 2017;317:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute, Division of Cancer Control and Population Sciences. SEER‐Medicare database. 2019. Available at https://healthcaredelivery.cancer.gov/seermedicare/overview/. Accessed May 20, 2019.
- 12.National Cancer Institute. Surveillance, Epidemiology, and End Results program. Available at http://seer.cancer.gov/. Accessed May 20, 2019.
- 13. Moon M. What Medicare has meant to older Americans. Health Care Financ Rev 1996;18:49–59. [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Centers for Medicare & Medicaid Services . The official U.S. Government site for Medicare. Available at https://www.medicare.gov/. Accessed May 20, 2019.
- 15. Centers for Disease Control and Prevention . National Center for Health Statistics. Available at www.cdc.gov/nchs/. Accessed May 20, 2019.
- 16. Durante C, Grani G, Lamartina L et al. The diagnosis and management of thyroid nodules: A review. JAMA 2018;319:914–924. [DOI] [PubMed] [Google Scholar]
- 17. Papaleontiou M, Reyes‐Gastelum D, Gay BL et al. Worry in thyroid cancer survivors with a favorable prognosis. Thyroid 2019;29:1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Welch HG, Doherty GM. Saving thyroids ‐ Overtreatment of small papillary cancers. N Engl J Med 2018;379:310–312. [DOI] [PubMed] [Google Scholar]
- 19. Papaleontiou M, Hughes DT, Guo C et al. Population‐based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab 2017;102:2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kovatch KJ, Reyes‐Gastelum D, Hughes DT et al. Assessment of voice outcomes following surgery for thyroid cancer. JAMA Otolaryngol Head Neck Surg 2019;145:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]


