Short abstract
With advances in precision oncology, liquid biopsies have shown promise as a minimally invasive means to diagnose cancer and guide treatment decisions. This commentary presents an assessment of the potential and the challenges of widespread use of liquid biopsy testing, based on the 2019 Accelerating Anticancer Agent Development Workshop.
Precision oncology has become increasingly important in the diagnosis and management of patients with cancer. With these advances, liquid biopsies have shown promise as a minimally invasive means to diagnose cancer, detect resistance mutations and monitor tumor evolution, predict relapse, provide prognosis, and guide treatment decisions.
Although many potential liquid biopsy analytes exist, including circulating tumor RNA, cell‐free micro RNA, and exosomes, circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) have emerged as the most common analytes currently tested in clinical care [1]. Measurement of ctDNA via a liquid biopsy test offers a minimally invasive means to obtain valuable information on a tumor without depending on invasive procedures such as tissue biopsies that are not always possible to perform safely [2]. In addition, liquid biopsies can potentially capture multiple tumor clones and acquired mutations over the course of therapy and offer a faster turnaround time relative to traditional tissue biopsy or imaging methods. Currently, there are two U.S. Food and Drug Administration (FDA)–approved ctDNA‐based companion diagnostic tests essential for the safe and effective use of certain drugs: the cobas epidermal growth factor receptor (EGFR) mutation test V2 (Roche) to detect EGFR mutations in non‐small cell lung cancer and the therascreen PIK3CA RGQ PCR kit (QIAGEN, Hilden, Germany) to detect PIK3CA mutations in breast cancer.
However, key uncertainties with liquid biopsies still remain, including determination of standardized preanalytical criteria (e.g., timing of collection, collection tubes, extraction methods) and analytical criteria (e.g., determination of ctDNA or CTC positivity, assay cutoffs), the extent to which samples accurately reflect the heterogeneity of a tumor and its subclones, reproducibility of results across different platforms, different shedding based on tumor type and stage of disease, potential high false positive rate because of clonal hematopoiesis of indeterminate potential variants, and applicability to guide treatment decisions. Understanding the differences between normal genetic variation and potential precancer variant levels will also be essential if these tests are to be used for screening applications. Despite all the progress in liquid biopsy technology, a recent joint review from the American Society of Clinical Oncology and the College of American Pathologists in 2018 concluded that there was still insufficient evidence of clinical validity and utility for the majority of ctDNA assays in advanced cancer [3]. Currently, preanalytical variables are not standardized, and analytical validity and clinical utility of liquid biopsies vary across testing platform and disease type.
Potential applications of liquid biopsy in disease management include early cancer detection [4], assessment of residual disease [5, 6], monitoring for recurrence for those in remission [7], use in selection of patients for drugs [8, 9], and monitoring clonal evolution or response in the metastatic setting [10]. However, there is a range of level of development of liquid biopsies for use in these different clinical scenarios, with some more nascent in technology and clinical use (e.g., screening) and some already with companion diagnostics approved (e.g., patient selection).
To address these complexities, the 2019 Accelerating Anticancer Agent Development Workshop assembled a panel of experts for an in‐depth discussion session entitled “Liquid Biopsy: State of the Science and Future Directions.” This panel presented assessments of the potential and challenges faced by various stakeholders such as the FDA, clinicians, industry, and patients for the widespread use of liquid biopsy testing.
Challenges and Efforts to Standardize ctDNA
Stakeholders in the oncology community have recognized the importance of standardizing procedures and guidelines involved with liquid biopsy technology. For example, the Foundation for the National Institutes of Health manages The Biomarker Consortium, whose mission is to encourage collaboration between the public and private sector to accelerate the development of biomarker‐based technologies [11]. Their ctDNA quality control material project has the intent to develop and validate control materials for testing to ultimately enable its use to assess the performance of a laboratory's ctDNA reagents, technology, and methodology to assure analytical validity of results. In addition, the generated materials could potentially serve as a reference for comparison with other labs or assays. The Blood Profiling Atlas in Cancer Consortium has a goal to develop standardized analytical validation protocols and preanalytical variables that will aid in the development and validation of these emerging technologies. Both consortia have consulted with the FDA to help identify pathways toward achieving these objectives.
The ultimate goal for the standardization of liquid biopsy technology is to provide more confidence in interpreting ctDNA assay results across various tests. This would be a key step to ensuring more effective regulatory evaluation and clinical certainty for the use of these assays.
Regulatory and Clinical Trial Considerations
From the regulatory and clinical trial perspective, liquid biopsies such as CTCs or ctDNA can be used as a biomarker (measured as an indicator of normal biologic processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions) [12]. Use of liquid biopsy as a diagnostic biomarker would entail detection or confirmation of a disease or condition of interest based on the biopsy result. Use as a prognostic biomarker would identify the likelihood of a clinical event (e.g., disease recurrence or progression), thus aiding in the predetermination of the natural history of disease. For example, a prognostic biomarker of ctDNA in an adjuvant cancer setting might allow for use as a stratification factor or as selection of an enriched high‐risk population in which drug development could be pursued. Use of liquid biopsy as a putative surrogate endpoint or efficacy‐response biomarker would require a large body of evidence demonstrating biologic plausibility, demonstration of prognostic value of the surrogate endpoint for the clinical outcome, and evidence from clinical trials (or meta‐analysis) that the treatment effects on the biomarker correlate to the clinical outcome. Another use as an efficacy‐response biomarker could be for go or no‐go decision making earlier on in drug development. A predictive biomarker would allow identification of patients who would be likely to demonstrate a favorable or unfavorable effect from a drug. If results of a clinical study demonstrate that detection of a biomarker is essential for the safe and effective use of such a drug, then a companion diagnostic would be required. Diagnostic tests may also be appropriate when research demonstrates that a biomarker is not essential but would aid in the risk benefit determination of a drug. A monitoring biomarker would use a liquid biopsy result measured serially to detect a change in the extent or degree of disease or could also allow for resistance mutation detection.
Analytic validation of an assay detecting a liquid biopsy analyte is critical in the regulatory assessment of any test or drug development program and includes various performance characteristics of the assay including but not limited to the assay specificity and sensitivity, how reproducible and precise the test is, limit of detection of the assay, how accurate the test is when compared with a validated reference/orthogonal method, and the stability of reagents and specimen. Clinical validation of a given assay depends on the specific assay claims. For a companion diagnostic (CDx) claim, where a test is essential for the safe and effective use of the corresponding therapeutic, if a clinical trial assay is used for selection of patients in the trial and if this assay is different from the final CDx assay, then a bridging study is necessary to demonstrate that the final CDx assay is safe and effective for use of the drug.
Outlook on Liquid Biopsies
Liquid biopsies undoubtedly will continue to become more important in the management of malignancies and in the support of drug approval. Future applications of ctDNA are expected to include early cancer detection and screening, (neo)adjuvant treatment selection for high‐risk patients, prognosis and monitoring of residual disease and recurrence, and use as a tool to follow clonal evolution (Fig. 1). However, there remains uncertainty of the biological levels of ctDNA among different tumor types, in early‐ versus late‐stage patients, and other various clinical scenarios in which these levels have not yet been characterized. There is also much work to be accomplished to standardize procedures and guidelines related to liquid assay design and meeting rigorous regulatory standards. As these areas are further developed, the true potential of ctDNA to inform clinical decision making may be realized.
Figure 1.
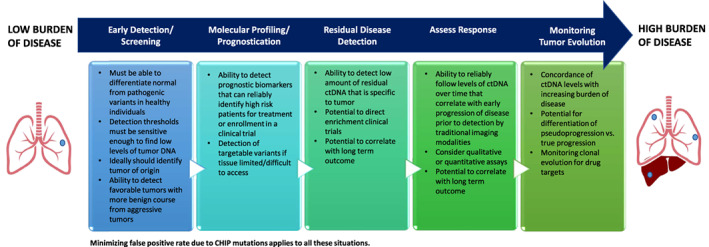
Potential applcations of ctDNA assays and regulatory considerations.
Abbreviations: CHIP, clonal hematopoiesis of indeterminate potential; ctDNA, circulating tumor DNA.
Disclosures
J. Carl Barrett: AstraZeneca (E); Justin I. Odegaard: Guardant Health (E); Geoffrey R. Oxnard: AstraZeneca, Abbvie, Blueprint, Janssen, Inivata, Loxo, Grail, Takeda, Illumina, Sysmex (C/A), Foundation Medicine, Guardant (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Heitzer E, Haque IS, Roberts CES et al. Current and future perspectives of liquid biopsies in genomics‐driven oncology. Nat Rev Genet 2019;20:71–88. [DOI] [PubMed] [Google Scholar]
- 2. Rossi G, Ignatiadis M. Promises and pitfalls of using liquid biopsy for precision medicine. Cancer Res 2019;11:2798–2804. [DOI] [PubMed] [Google Scholar]
- 3. Merker JD, Oxnard GR, Compton C et al. Circulating tumor DNA analysis in patients with Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol 2018;16:1631–1641. [DOI] [PubMed] [Google Scholar]
- 4. Aravanis AM, Lee M, Klausner RD. Next‐generation sequencing of circulating tumor DNA for early cancer detection. Cell 2017;168:571–574. [DOI] [PubMed] [Google Scholar]
- 5. Abbosh C, Birkbak NJ, Wilson GA et al. Phylogenetic ctDNA analysis depicts early‐stage lung cancer evolution. Nature 2017;7655:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tie J, Cohen JD, Wang Y et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol 2019;12:1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia‐Murillas I, Schiavon G, Weigelt B et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015;7:303ra133. [DOI] [PubMed] [Google Scholar]
- 8.Premarket Approval (PMA): Cobas EGFR Muation Test V2. [about 4 screens]. U.S. Food and Drug Administration. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150044. Accessed April 15, 2020.
- 9.The therascreen PIK3CA RGQ PCR Kit – P190001 and P190004. U.S. Food and Drug Administration. Available at https://www.fda.gov/medical‐devices/recently‐approved‐devices/therascreen‐pik3ca‐rgq‐pcr‐kit‐p190001‐and‐p190004. Accessed April 15, 2020.
- 10. Siravegna G, Mussolin B, Venesio T et al. How liquid biopsies can change clinical practice in oncology. Ann Oncol 2019;30:1580–1590. [DOI] [PubMed] [Google Scholar]
- 11. Parkinson DR, Dracopoli N, Petty GB et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 2012;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Food and Drug Administration; National Institutes of Health Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource. Silver Spring, MD: U.S. Food and Drug Administration; and Bethesda, MD: National Institutes of Health; 2018. [Google Scholar]


