Abstract
Background
Patients with diffuse large B‐cell lymphoma (DLBCL) with concurrent hepatitis B surface antigen (HBsAg)‐positive hepatitis B virus (HBV) infection have distinct clinical features. Nevertheless, the prognostic value of HBsAg in DLBCL in the rituximab era remains unclear.
Materials and Methods
We conducted a retrospective cohort study to investigate the clinical relevance of HBsAg in immunocompetent patients with DLBCL treated with homogeneous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone between 2002 and 2016.
Results
Among 416 analyzed patients, 98 (23.6%) were HBsAg positive. HBsAg positivity was associated with a younger age and more advanced stage at diagnosis, more frequent hepatic impairment during perichemotherapy, and a trend of higher National Comprehensive Cancer Network‐International Prognostic Index (NCCN‐IPI) score at diagnosis. Compared with the HBsAg‐negative patients, the HBsAg‐positive patients had a lower overall response rate (76.5% vs. 85.5%, p = .043), poorer 5‐year overall survival (OS) rate (57.2% vs. 73.5%, p < .001), and shorter 5‐year progression‐free survival (PFS) rate (47.2% vs. 60.7%, p = .013). Multivariate analyses showed that HBsAg positivity was an independent unfavorable prognostic indicator for OS and PFS. A scoring system incorporating HBsAg positivity, the NCCN‐IPI score, and serum albumin levels proved to be useful for stratifying prognostically relevant subgroups of patients with DLBCL.
Conclusion
This study demonstrated that HBV infection is uniquely relevant to DLBCL. HBsAg might serve as a novel biomarker to improve clinical risk stratification of patients with DLBCL in areas with high prevalence of HBV infection. Further research investigating the etiopathogenesis of HBV infection in DLBCL is imperative.
Implications for Practice
A considerable disparity exists regarding the prognostic relevance of hepatitis B surface antigen (HBsAg)‐positive hepatitis B virus (HBV) infection in patients with diffuse large B‐cell lymphoma (DLBCL). In this large, retrospective cohort study from an area with high prevalence of HBV infection, the authors demonstrated that HBsAg was an independent unfavorable factor significantly associated with survival, highlighting its potential as a novel prognostic indicator to improve the risk stratification of patients with DLBCL in the rituximab era.
Keywords: Diffuse large B‐cell lymphoma, Hepatitis B virus infection, Hepatitis B surface antigen, Risk stratification, Prognosis
Short abstract
Hepatitis B virus infection remains a major public health problem in endemic areas. This article reports a new scoring system for risk stratification for patients with diffuse large B‐cell lymphoma in endemic areas for HBV infection.
Introduction
Infectious pathogens cause not only chronic inflammation but also tumorigenesis in targeted organs. DNA viruses are the most prominent of such infectious pathogens, with well‐known associations including those of hepatitis B virus (HBV) with hepatocellular carcinoma (HCC) and human papillomavirus with cervical cancer. HBV, a hepatotropic DNA virus, is one of the most common viral infections in people. The global prevalence of chronic HBV infection is heterogeneous, with the highest, intermediate, and lowest levels of endemicity being observed in the African and Western Pacific, Southeast Asian and European, and North American regions, respectively 1. Currently, HBV infection remains a major public health problem in endemic areas.
HBV infection is a leading risk factor for the development of HCC. Numerous case–control or cohort studies 2, 3, 4, 5, 6 and meta‐analyses 7, 8 have also demonstrated a positive association between chronic hepatitis B surface antigen (HBsAg)‐positive HBV infections and B‐cell non‐Hodgkin lymphomas (NHLs), with HBsAg‐positive patients having a 2‐ to 3‐fold higher risk of developing B‐cell NHLs compared with noninfected patients. A recent meta‐analysis that included 58 studies with a total of 53,714 NHL cases and more than 1.7 million controls supported the positive association between HBV infection and B‐cell NHL development 8. Furthermore, HBV infection was significantly associated with diffuse large B‐cell lymphoma (DLBCL), with the corresponding summary odds ratio being 2.06 (95% confidence interval [CI], 1.48–2.88). Notably, countries with a high HBV prevalence were reported to have increased odds of developing DLBCL 7.
Studies have reported that HBsAg‐positive patients with DLBCL had a younger median onset age and more advanced disease at diagnosis 9, 10, 11, 12. Besides, many studies have focused on the effect of HBV infection on the clinical outcomes of patients with DLBCL. However, a considerable disparity has been observed among different studies. For instance, a cohort study of 262 patients with DLBCL showed similar response rates and median overall survival (OS) duration between patients with and without HBsAg positivity 9. Two retrospective studies performed in Singapore 13 and Hong Kong 14 have also shown the same results. By contrast, several retrospective cohort studies conducted in China and Saudi Arabia show that HBsAg‐positive patients had significantly poorer outcomes compared with HBsAg‐negative patients 10, 11, 12, 15, 16, 17, 18. Nevertheless, their treatment strategies for DLBCL were heterogeneous.
HBV infection is endemic in Taiwan. Before the launch of the universal hepatitis B vaccination in 1984, the prevalence of HBsAg in the general Taiwanese population was nearly 11%–20%, which was found to be the highest prevalence worldwide 19. This high prevalence offered the unique opportunity to study the association between HBV infection and DLBCL. In this study, we conducted a retrospective analysis to investigate the clinical characteristics and prognostic effects of HBV infection in a large cohort of immunocompetent patients with newly diagnosed DLBCL treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP). Furthermore, we built a new scoring system for initial risk stratification for patients with DLBCL in endemic areas for HBV infection and validated this scoring system using an external validation cohort.
Materials and Methods
Patient Selection and Clinical Data Collection
Patients aged 20 years or older with treatment‐naïve DLBCL without concurrent human immunodeficiency virus infection at National Taiwan University Hospital (NTUH) between January 2002 and December 2016 were considered for enrollment. Moreover, all patients enrolled had received at least one cycle of frontline R‐CHOP.
We performed a retrospective chart review to collect data on clinical characteristics, treatment responses, and outcomes. The cell‐of‐origin (COO) subtypes of DLBCL were determined based on the Hans algorithm 20. The National Comprehensive Cancer Network‐International Prognostic Index (NCCN‐IPI) score was obtained as previously reported 21. Routine liver function and coagulation tests were performed perichemotherapy to evaluate hepatic impairment in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Version 4.0. HBV reactivation was defined as a 10‐fold or greater increase in HBV DNA levels from baseline. For HBsAg‐negative and hepatitis B core antibody (HBcAb)‐positive patients, positive conversion of HBsAg was defined as HBV reactivation. Furthermore, all patients underwent hepatitis C virus (HCV) antibody tests. Follow‐up data were collected until death, loss to follow‐up, or the end of the study period (i.e., December 31, 2018).
The external validation cohort comprised immunocompetent adults aged 20 years or older with newly diagnosed DLBCL at Tri‐Service General Hospital (TSGH) from February 2014 to May 2019. These patients were treated with homogeneous R‐CHOP as those in the NTUH cohort and were used to validate the scoring system.
Antiviral Prophylaxis
All HBsAg‐positive patients received prophylactic antiviral therapy, starting at the initiation of chemotherapy and stopping 6 months after the completion of chemotherapy. The type of antiviral prophylaxis was determined according to the decision of consulting hepatologists.
Treatment Response Assessment
Tumor responses were assessed through computed tomography in all patients after two or three cycles of chemotherapy as well as at treatment completion. Positron emission tomography was performed in patients with ambiguous evaluations. Complete remission (CR), partial remission (PR), progressive disease (PD), and stable disease were estimated using the revised response criteria for malignant lymphoma 22. The overall response rate (ORR) was defined as the proportion of patients whose best response was either CR or PR.
Statistical Analysis
A chi‐square test or Fisher's exact test was used to compare categorical data. The Mann‐Whitney U test was used to compare the medians of the continuous variables. OS was measured from the date of the first diagnosis to the end of the follow‐up period, death from any cause, or the date of the last known follow‐up examination. Progression‐free survival (PFS) was measured from the date of the first diagnosis until the end of the follow‐up period, the date of relapse or PD, death from any cause, or the last known follow‐up examination, whichever came first. Lymphoma‐specific survival (LSS) was measured from the time from the first diagnosis until death due to lymphoma. The binary logistic regression analysis was performed to identify the independent risk factors associated with ORR. The Kaplan‐Meier method was used to estimate the OS, the PFS, and the LSS, and the log‐rank test was used to examine the significance of between‐group differences. Hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards regression models to determine independent risk factors associated with survival in the multivariate analyses. A two‐sided p value of <.05 was considered to indicate a statistically significant difference.
Results
Patient Characteristics
Between January 2002 and December 2016, a total of 416 patients with DLBCL were evaluated; the median age in the cohort was 59.3 years (range, 20.9–82.6 years), and the sex ratio was 1.26 to 1. Among the patients, 267 (63.9%) had extranodal involvement and 58 (13.9%) had bone marrow involvement at the time of diagnosis. Furthermore, 27 (6.5%) had an Eastern Cooperative Oncology Group performance status of ≥2, 220 (52.9%) had Ann Arbor stage III/IV, and 174 (41.8%) belonged to the high‐intermediate/high NCCN‐IPI risk groups. The 5‐year PFS and OS rates were 57.5% and 69.6%, respectively (supplemental online Fig. 1). In general, patients in the NTUH cohort had comparable prognoses to those reported by other international studies 23, 24, 25.
The results revealed that 98 patients (23.6%) were HBsAg positive and 22 (5.3%) had positive HCV antibody tests before treatment. Among the HBsAg‐positive patients, 81 were tested for hepatitis B e antigen (HBeAg) and 77 had plasma HBV DNA loads before treatment. All patients that were HBsAg positive had received prophylactic antiviral therapy, including 37 with lamivudine, 49 with entecavir, 4 with telbivudine, and 8 with tenofovir. Regarding HBsAg‐negative patients (n = 318), 229 were measured for the presence of HBcAbs before treatment, for which 165 were positive and 64 were negative.
Clinical Features in Patients with DLBCL with HBsAg Positivity
Compared with the patients in the HBsAg‐negative group, those in the HBsAg‐positive group had a younger median onset age, more advanced stage, higher incidence of hepatic impairment before or during chemotherapy, trend of higher serum lactate dehydrogenase (LDH) levels at the time of diagnosis, trend of higher incidence of lymphopenia, and trend of higher NCCN‐IPI scores before treatment (Table 1). Notably, the percentage of patients who underwent autologous stem cell transplantation was similar between the two groups.
Table 1.
Patient characteristics
| Variables | HBsAg‐positive patients (n = 98) | HBsAg‐negative patients (n = 318) | p value |
|---|---|---|---|
| Age, years | 55.9 (26.2–79.7) | 60.7 (20.9–82.6) | .008 |
| Sex | .486 | ||
| Male | 58 (59.2) | 174 (54.7) | |
| Female | 40 (40.8) | 144 (45.3) | |
| Ann Arbor stage | .021 | ||
| Stage I/II | 36 (36.7) | 160 (50.3) | |
| Stage III/IV | 62 (63.3) | 158 (49.7) | |
| B symptoms | .808 | ||
| Present | 35 (35.7) | 108 (34) | |
| Absent | 63 (64.3) | 210 (66) | |
| ECOG performance status | .241 | ||
| 0, 1 | 89 (90.8) | 300 (94.3) | |
| ≥2 | 9 (9.2) | 18 (5.7) | |
| Extranodal involvement | .811 | ||
| Present | 64 (65.3) | 203 (63.8) | |
| Absent | 34 (34.7) | 115 (36.2) | |
| BM involvement | .869 | ||
| Present | 14 (14.3) | 44 (13.8) | |
| Absent | 84 (85.7) | 274 (86.2) | |
| Bulky lesions | .845 | ||
| Present | 10 (10.2) | 30 (9.4) | |
| Absent | 88 (90.6) | ||
| Subtypea | .621 | ||
| GCB | 17 (34.7) | 73 (39.7) | |
| Non‐GCB | 32 (65.3) | 111 (60.3) | |
| LDH | .064 | ||
| Elevated | 57 (58.2) | 149 (46.9) | |
| Normal | 41 (41.8) | 169 (53.1) | |
| ALC | .187 | ||
| ≤1,000/uL | 30 (30.6) | 76 (23.9) | |
| >1,000/uL | 68 (69.4) | 242 (76.1) | |
| AMC | .788 | ||
| ≥630/uL | 25 (25.5) | 76 (23.9) | |
| <630/uL | 73 (74.5) | 242 (76.1) | |
| Hepatic impairment | .02 | ||
| Present | 75 (76.5) | 202 (63.5) | |
| Absent | 23 (23.5) | 116 (36.5) | |
| Albumin | .452 | ||
| <3.5 g/dL | 20 (20.4) | 54 (17) | |
| ≥3.5 g/dL | 78 (79.6) | 264 (83) | |
| NCCN‐IPI risk groups | .062 | ||
| Low/Low‐intermediate | 49 (50) | 193 (60.7) | |
| High‐intermediate/High | 49 (50) | 125 (39.3) | |
| ASCT | 14 (14.3) | 34 (10.7) | .366 |
Data are presented as either number of patients (%) or median (range).
The calculation was based on 233 samples with available data of cell‐of‐origin subtypes.
Abbreviations: ALC, absolute lymphocyte count; AMC, absolute monocyte count; ASCT, autologous stem cell transplant; BM, bone marrow; ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B‐cell‐like; HBsAg, hepatitis B surface antigen; LDH, lactate dehydrogenase; NCCN‐IPI, National Comprehensive Cancer Network‐International Prognostic Index.
In addition, no significant difference was found in the clinical background comparisons between HBsAg‐positive patients with and without high HBV DNA loads (>1,000 IU/mL) before treatment.
Effect of HBV Infection on Treatment Responses and Clinical Outcomes
The treatment responses to R‐CHOP are presented in supplemental online Table 1. The ORRs were 76.5% and 85.5% for patients with and without HBsAg positivity, respectively (p = .043). Moreover, age less than 60 years, limited stage, pretreatment serum albumin levels more than or equal to 3.5 g/dL, and negative HBsAg are four independent favorable risk factors associated with ORR in our cohort (supplemental online Table 2).
The univariate analysis of clinical characteristics associated with OS and PFS is presented in Table 2. Compared with the patients in the HBsAg‐negative group, those in the HBsAg‐positive group had a poorer 5‐year OS rate after a median follow‐up of 68.6 months (57.2% vs. 73.5%, p < .001, Fig. 1A) and a shorter 5‐year PFS rate (47.2% vs. 60.7%, p = .013, Fig. 1B). Among the patients with available data of COO subtypes (n = 233), we found that in the germinal center B‐cell‐like (GCB) subgroup, the HBsAg‐positive patients had a significantly worse outcome compared with the HBsAg‐negative patients (5‐year OS rates, 45.4% vs. 82.7%, p < .001, supplemental online Fig. 2A; 5‐year PFS rates, 39.2% vs. 72.1%, p = .001, supplemental online Fig. 2B). Nevertheless, HBsAg positivity had no significant effect on OS and PFS in patients with the non‐GCB subtype (supplemental online Fig. 2C, 2D).
Table 2.
Univariate analysis of clinical characteristics associated with overall survival and progression‐free survival in patients with DLBCL
| Variable | No. of patients | 5‐year overall survival | 5‐year progression‐free survival | ||
|---|---|---|---|---|---|
| % | p value | % | p value | ||
| Age, years | .003 | .019 | |||
| ≤60 | 217 | 75.6 | 60.9 | ||
| >60 | 199 | 63.2 | 53.9 | ||
| Sex | .585 | .63 | |||
| Male | 232 | 69.5 | 56.2 | ||
| Female | 184 | 69.7 | 59.2 | ||
| Ann Arbor stage | <.001 | <.001 | |||
| I/II | 196 | 83 | 76 | ||
| III/IV | 220 | 57.7 | 41.4 | ||
| No. of extranodal sites | <.001 | <.001 | |||
| 0, 1 | 319 | 75.4 | 64.1 | ||
| ≥2 | 97 | 50.5 | 36.2 | ||
| ECOG | .001 | <.001 | |||
| 0, 1 | 389 | 70.5 | 59 | ||
| ≥2 | 27 | 55.3 | 36.4 | ||
| LDH | <.001 | <.001 | |||
| Elevated | 206 | 58.6 | 45.6 | ||
| Normal | 210 | 80.2 | 69.3 | ||
| B symptoms | .009 | <.001 | |||
| Present | 143 | 61.9 | 42.9 | ||
| Absent | 273 | 73.5 | 64.9 | ||
| Bulky lesion | .157 | .308 | |||
| Present | 40 | 62.3 | 53.3 | ||
| Absent | 376 | 70.4 | 58 | ||
| BM involvement | .063 | <.001 | |||
| Present | 58 | 61.3 | 31.7 | ||
| Absent | 358 | 70.9 | 61.8 | ||
| ALC, uL | <.001 | <.001 | |||
| ≤1,000 | 106 | 49.9 | 35.2 | ||
| >1,000 | 310 | 76.6 | 65.5 | ||
| AMC, uL | .029 | .002 | |||
| ≥630 | 101 | 62.3 | 44.8 | ||
| <630 | 315 | 72 | 61.7 | ||
| Albumin, g/dL | <.001 | <.001 | |||
| <3.5 | 74 | 51.3 | 36.5 | ||
| ≥3.5 | 342 | 73.5 | 62.1 | ||
| Hepatic impairment | .024 | .077 | |||
| Present | 277 | 67.9 | 55.9 | ||
| Absent | 139 | 73.4 | 60.9 | ||
| HBsAg | <.001 | .013 | |||
| Present | 98 | 57.2 | 47.2 | ||
| Absent | 318 | 73.5 | 60.7 | ||
| NCCN‐IPI risk groups | <.001 | <.001 | |||
| Low/Low‐intermediate | 242 | 78.6 | 67.4 | ||
| High‐intermediate/High | 174 | 56.9 | 43.7 | ||
Abbreviations: ALC, absolute lymphocyte count; AMC, absolute monocyte count; BM, bone marrow; DLBCL, diffuse large B‐cell lymphoma; ECOG, Eastern Cooperative Oncology Group; HBsAg, hepatitis B surface antigen; LDH, lactate dehydrogenase; NCCN‐IPI, National Comprehensive Cancer Network‐International Prognostic Index.
Figure 1.
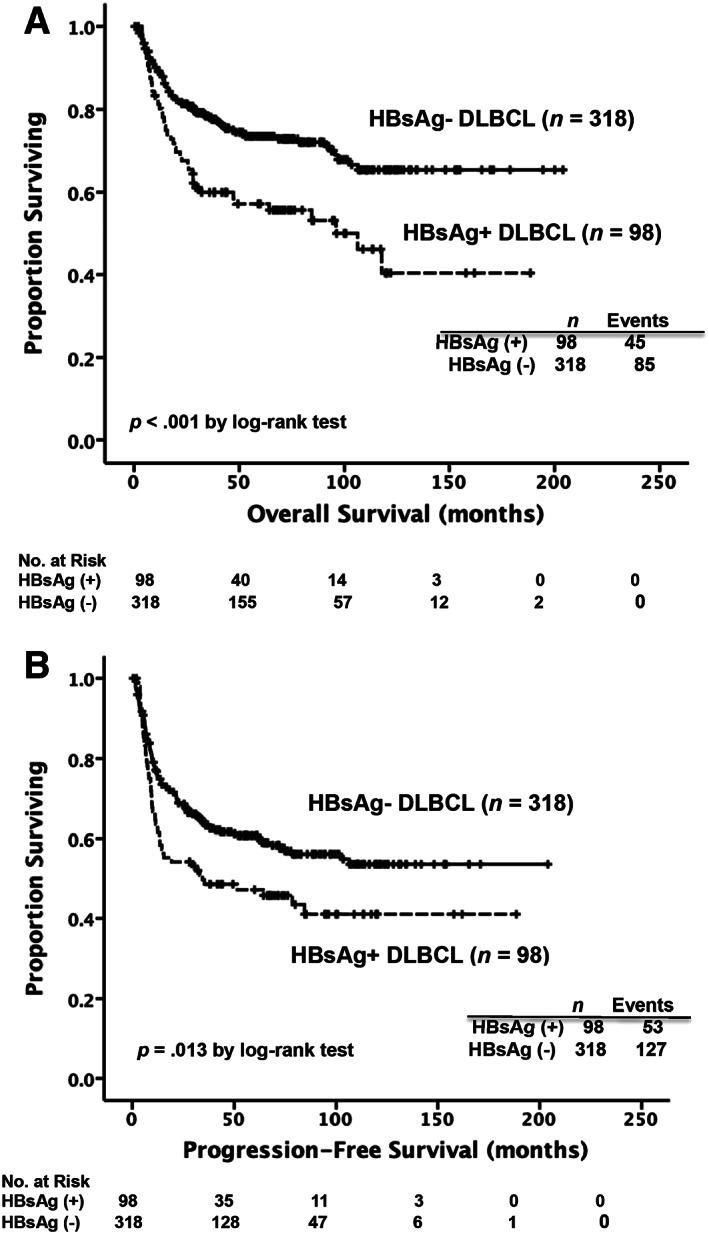
Kaplan‐Meier survival curves in patients with DLBCL, stratified by the presence of HBsAg at diagnosis. Patients in the HBsAg‐positive group had worse results in overall survival (A) and progression‐free survival (B) compared with those in the HBsAg‐negative group.
Abbreviations: DLBCL, diffuse large B‐cell lymphoma; HBsAg, hepatitis B surface antigen.
Multivariate analysis of variables significantly associated with clinical outcome in univariate analysis (Table 3) identified HBsAg as an independent unfavorable prognostic factor for OS (HR, 1.788; 95% CI, 1.244–2.568; p = .002) and PFS (HR, 1.425; 95% CI, 1.033–1.966; p = .031) in patients with DLBCL. Notably, the survival analysis also showed that patients in the HBsAg‐positive group exhibited a poorer 5‐year LSS rate compared with those in the HBsAg‐negative group (69.6% vs. 80.6%, p = .02, supplemental online Fig. 3).
Table 3.
Multivariate Cox regression analysis of overall survival and progression‐free survival in patients with DLBCL
| Variables | Overall survival | Progression‐free survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| NCCN‐IPIa | 2.102 | 1.448–3.052 | <.001 | 1.773 | 1.275–2.464 | .001 |
| B symptomsb | 1.398 | 1.013–1.929 | .042 | |||
| Albuminc | 1.711 | 1.137–2.575 | .01 | 1.637 | 1.143–2.346 | .007 |
| HBsAgd | 1.788 | 1.244–2.568 | .002 | 1.425 | 1.033–1.966 | .031 |
Variables that have statistical significance in univariate analysis were selected for multivariate analysis using backward elimination method (Wald test).
High‐intermediate/high versus low/low‐intermediate.
Present versus absent.
Less than 3.5 g/dL versus more than or equal to 3.5 g/dL.
HBsAg positivity versus HBsAg negativity.
Abbreviations: CI, confidence interval; DLBCL, diffuse large B‐cell lymphoma; HBsAg, hepatitis B surface antigen; HR, hazard ratio; NCCN‐IPI, National Comprehensive Cancer Network‐International Prognostic Index.
We then separated the HBsAg‐negative patients into two groups by the presence of HBcAbs. The survival analysis showed that compared with the patients in the HBcAb‐negative group (n = 64), those in the HBcAb‐positive group (n = 165) had a trend of poorer 5‐year OS rates (71% vs. 77.7%, p = .141, Fig. 2A), significantly shorter 5‐year PFS rates (54.4% vs. 71.3%, p = .03, Fig. 2B), and a trend toward poorer 5‐year LSS rates (78.8% vs. 86.6%, p = .117, supplemental online Fig. 4). Additionally, multivariate analysis showed that HBcAb positivity is an independent unfavorable prognostic factor for PFS (HR, 1.693; 95% CI, 1.01–2.839; p = .046, supplemental online Table 3) in HBsAg‐negative patients, irrespective of two well‐known prognostic factors, the NCCN‐IPI score at diagnosis and pretreatment serum albumin levels. Regarding HCV infection, no difference was observed in OS and PFS between patients with and without HCV antibody titers (supplemental online Fig. 5A, 5B).
Figure 2.
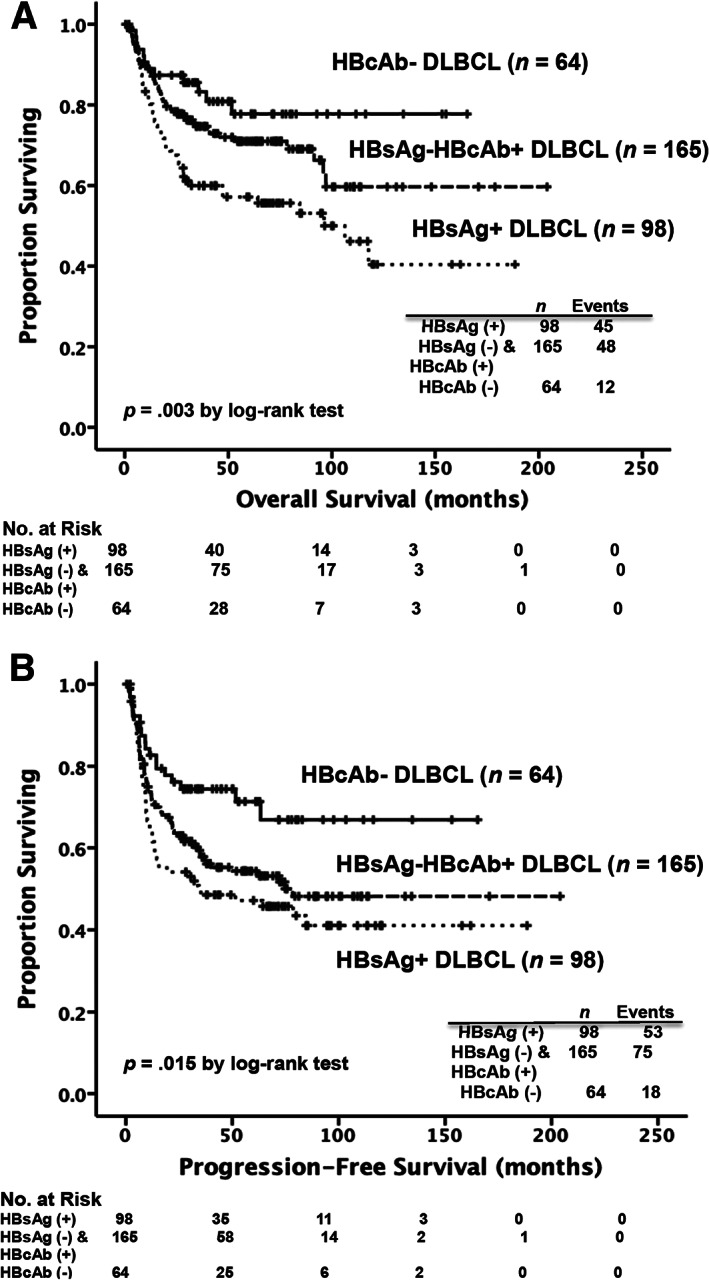
Kaplan‐Meier survival curves in patients with DLBCL, stratified by the presence of HBsAg and HBcAb at diagnosis. Eighty‐nine patients without data of HBcAb at diagnosis were excluded from the analysis. Patients in the HBsAg‐negative/HBcAb‐positive group had a trend of worse results in overall survival (A) and significantly poorer results in progression‐free survival (B) compared with those in the HBcAb‐negative group.
Abbreviations: DLBCL, diffuse large B‐cell lymphoma; HBcAb, hepatitis B core antibody; HBsAg, hepatitis B surface antigen.
To better stratify patients with DLBCL into different risk groups, a scoring system incorporating three independent prognostic factors identified by the multivariate analysis, including HBsAg positivity, pretreatment serum albumin levels, and the NCCN‐IPI score at diagnosis, into survival analysis was formulated. A point of 1 was assigned for the presence of HBsAg, so was for low pretreatment serum albumin levels. A point of 0, 1, 2, and 3 was assigned for low, low‐intermediate, high‐intermediate, and high NCCN‐IPI risk groups, respectively. A final score was calculated for each patient by the algebraic summation of these points corresponding to his or her risk factors. The patients were then divided into six subgroups according to the score, which ranged from 0 to 5. Survival estimates for the six subgroups were used to define five groups with significantly different clinical outcomes (p < .001 for OS, Fig. 3A; p < .001 for LSS, Fig. 3B).
Figure 3.
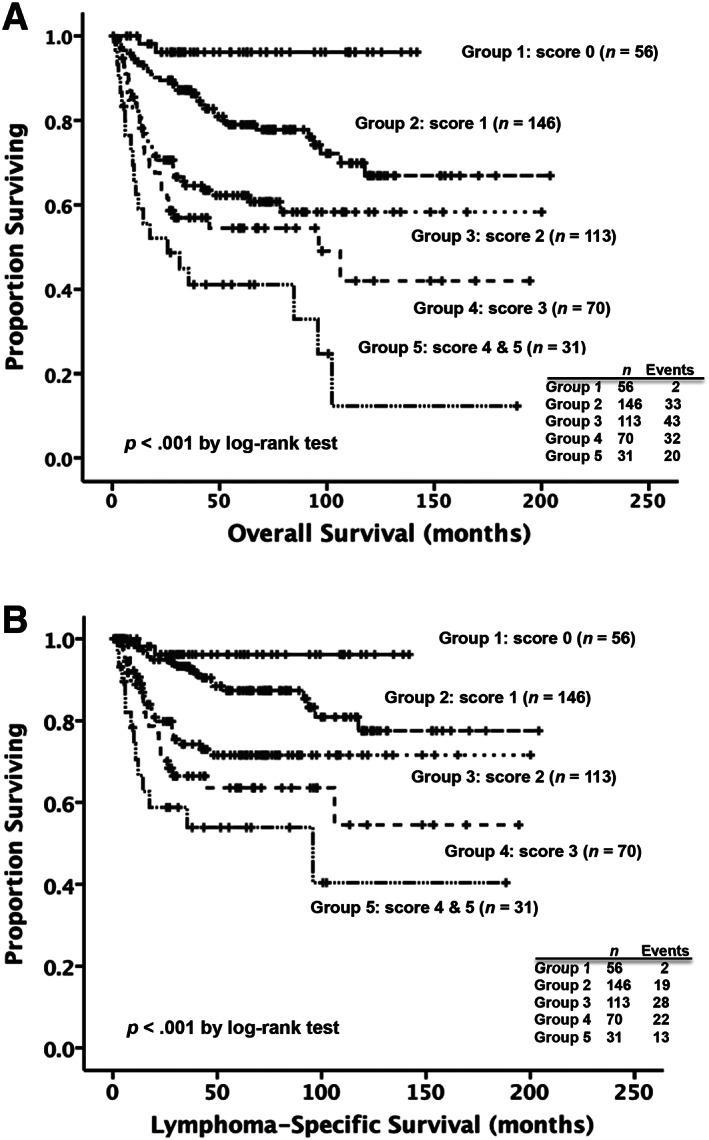
Kaplan‐Meier survival curves in patients with diffuse large B‐cell lymphoma, according to the scoring system. The risk score was determined by adding up the points for each of the following independent prognostic factors: hepatitis B surface antigen positivity (1 point), pretreatment serum albumin levels less than 3.5 g/dL (1 point), and the National Comprehensive Cancer Network‐International Prognostic Index (NCCN‐IPI) risk groups at diagnosis (A point of 0, 1, 2, and 3 was assigned for low, low‐intermediate, high‐intermediate, and high NCCN‐IPI risk groups, respectively). The patients were then divided into six subgroups on the basis of the score, which ranged from 0 to 5. Survival estimates for the six subgroups were used to define five groups with significantly different clinical outcomes. (A): The 5‐year overall survival rates of the patients in the group 1, group 2, group 3, group 4 and group 5 were 96.2%, 79%, 62.3%, 54.5%, and 41.1%, respectively. (B): The 5‐year lymphoma‐specific survival rates of the patients in group 1, group 2, group 3, group 4, and group 5 were 96.2%, 87.3%, 71.6%, 63.5%, and 53.9%, respectively.
Validation of the Scoring System in the External Validation Cohort
The TSGH external validation cohort consisted of 91 patients with DLBCL. The median age of the cohort was 59 years (range, 27–77 years). Among 91 analyzed patients, 50 (54.9%) had Ann Arbor stage III/IV and 38 (41.7%) belonged to the high‐intermediate/high NCCN‐IPI risk groups. Furthermore, 19 (20.9%) patients were HBsAg positive. After a median follow‐up time of 43 months, the 3‐year OS and LSS rates were 67.5% and 74.6%, respectively.
The scoring system still divided the patients in the external validation cohort into five groups with significant different clinical outcomes (p < .001 for OS, supplemental online Fig. 6A; p < .001 for LSS, supplemental online Fig. 6B).
Effects of HBeAg Levels, HBV Viral Loads, and Prophylactic Antiviral Therapy on Clinical Outcomes
We investigated whether the poor prognostic significance of patients with HBsAg was related to HBV disease activity. Nevertheless, neither the presence of HBeAg at diagnosis nor high HBV DNA loads before treatment had significant prognostic implications for OS (supplemental online Fig. 7A, 7B). In addition, the type of antiviral prophylaxis had no significant effect on OS in the HBsAg‐positive patients (supplemental online Fig. 8).
Hepatitis B Reactivation and Hepatic Impairment
Among the HBsAg‐positive patients, 75 (76.5%) had hepatic impairment based on the NCI CTCAE before or during treatment, including 48 (49%) with an NCI CTCAE of greater than or equal to grade 2. HBV reactivation was observed in 27 patients (27.6%). Notably, 3 patients developed HBV reactivation at the time of disease relapse, and all of them died of lymphoma.
In the HBsAg‐negative and HBcAb‐positive group (n = 165), the incidence of hepatic impairment greater than or equal to NCI CTCAE grade 2 was 32.1%, which was lower than that in the HBsAg‐positive group (p = .009). Twenty‐one patients (12.7%) developed HBV reactivation. This incidence was also significantly lower than that in the HBsAg‐positive patients (p = .005). Notably, four patients developed HBV reactivation at the time of disease relapse, and two of them died of lymphoma.
We further investigated the prognostic effects of HBV reactivation. Among the patients with HBcAb positivity (n = 263), no difference was observed in OS and PFS between those with and without HBV reactivation (5‐year OS rates, 63.3% vs. 66.5%, p = .966; 5‐year PFS rates, 47.5% vs. 52.8%, p = .674).
Discussion
Several epidemiologic and clinical studies have suggested that patients with DLBCL with concurrent HBV infection might constitute a unique subgroup according to distinct clinical characteristics. Nonetheless, a considerable disparity exists among previous studies regarding the prognostic effect of HBV infection. According to our review of the literature, to date, this is the largest cohort study to attempt to clarify the prognostic implications of HBV infection in patients with DLBCL with similar R‐CHOP treatments. Among the 416 patients analyzed in this study, 98 (23.6%) were found to be HBsAg positive before treatment. The HBsAg‐positive patients were characterized by a significantly younger age and more advanced stage at diagnosis, more frequent hepatic impairment during perichemotherapy, trend of higher incidence of lymphopenia, and a trend of higher NCCN‐IPI scores at diagnosis (Table 1). These patients’ clinical presentations are highly similar to those described in previous studies 10, 11, 12. In addition, the HBsAg‐positive patients exhibited a lower ORR and a significantly poorer outcome compared with the HBsAg‐negative patients. Moreover, HBsAg positivity was an independent unfavorable prognostic factor for OS and PFS in patients with DLBCL, regardless of a variety of well‐known prognostic factors 26. Intriguingly, a scoring system incorporating HBsAg positivity, the NCCN‐IPI score at diagnosis, and pretreatment serum albumin levels into survival analysis was powerful to separate patients with DLBCL into different prognostic groups. It was also validated using an external validation cohort and might be a useful tool for initial risk stratification for routine clinical use in areas with high prevalence of HBV infection.
One possible explanation for the poorer outcome of the HBsAg‐positive patients with DLBCL is the disease activity of chronic HBV infection and associated hepatic impairment and HBV reactivation during antilymphoma therapy, which could lead to deterioration of liver reserves, a delay in chemotherapy, and even progressive hepatic failure and death 27, 28, 29. Our study demonstrated that no significant differences in clinical characteristics and OS existed between HBsAg‐positive patients with and without high HBV DNA loads before chemotherapy. This result is consistent with a previous report by Deng et al. 10. Furthermore, HBeAg, a marker indicating chronic active hepatitis with active viral replication, had no prognostic implication in the HBsAg‐positive patients in our cohort. Regarding hepatic impairment and HBV reactivation, our study revealed a positive association between HBsAg and hepatic impairment during perichemotherapy. Hepatic impairment also engendered a poorer outcome for OS. In addition, 27.6% of the HBsAg‐positive patients developed HBV reactivation, and nine of these patients died of progressive hepatic failure. These results could negatively affect the outcome of patients with DLBCL with HBV infection. Nevertheless, patients in the HBsAg‐positive group had a poorer 5‐year LSS rate compared with those in the HBsAg‐negative group. Additionally, HBV reactivation had no prognostic significance in our cohort. By incorporating HBsAg results, hepatic impairment, and other prognostic factors simultaneously into our multivariate survival analysis, we observed that HBsAg continued to be an independent unfavorable factor for outcomes. Therefore, the negative effect of HBV infection on the patients’ prognoses could be attributable to unconfirmed pathways beyond hepatitis activity, hepatic impairment, and HBV reactivation.
Apart from patient and tumor characteristics, factors related to host immunity and the tumor microenvironment significantly affect the prognosis of DLBCL. Among them, the absolute monocyte count (AMC) and absolute lymphocyte count (ALC) at diagnosis are two simple and easily applicable surrogate markers 30. In our study, both high AMC and low ALC at diagnosis were proved to be unfavorable prognostic factors. However, they had no prognostic significance in multivariate analysis. Interestingly, we found that the incidence of low ALC tended to be higher in the HBsAg‐positive patients compared with the HBsAg‐negative patients. Yan et al. also showed lower levels of peripheral lymphocyte‐to‐monocyte ratios in HBV‐infected patients with DLBCL compared with non‐HBV‐infected patients 12. Lymphocytes play a crucial role in immune surveillance in NHLs, and lymphopenia is considered a surrogate marker of host immunologic incompetence 31 and a poor prognostic factor in DLBCL 32, 33. In addition, lymphocytes are crucial mediators of antibody‐dependent cellular cytotoxicity, which accounts for the rituximab efficacy in DLBCL 34. Lymphopenia could impair the ability of rituximab to destroy malignant B cells. Consequently, the adverse prognostic impact of HBsAg positivity in patients with DLBCL could be partially explained by the positive association between HBV infection and lymphopenia.
Several studies have sought to identify other factors responsible for the unfavorable outcomes of patients with DLBCL with concurrent HBV infection. One of the most interesting issues is COO subtype, which is associated with different prognostic effects and treatment considerations in patients with DLBCL. Nevertheless, previous studies have not shown a significant difference in COO subtypes between patients with and without HBsAg positivity 10, 11, 35, 36. Our study also showed the same result. Intriguingly, a significantly shorter OS and PFS were observed in HBsAg‐positive patients with the GCB subtype but not in those with the non‐GCB subtype. This finding shown by our study and others 11, 36 suggested that HBV infection mainly incurred a worse prognosis in patients with GCB‐type DLBCL.
Apart from the negative effect of HBsAg on clinical outcome, we found that in the HBsAg‐negative group, the HBcAb‐positive patients had significantly shorter 5‐year PFS rates and a trend of poorer 5‐year OS and LSS rates compared with the HBcAb‐negative patients. Multivariate analysis also showed that HBcAb positivity is an independent unfavorable prognostic factor for PFS. These findings re‐emphasize the prognostic value of HBV infection in patients with DLBCL, even in those with serologic evidence of resolved HBV infection. Nevertheless, the contribution of occult HBV infection to clinical outcomes is unclear owing to the lack of measured HBV DNA loads in this group of patients before treatment.
The positive association between HBV and DLBCL and distinct clinical features of HBV‐associated DLBCL strongly suggest a causal relationship between HBV infection and DLBCL development. Our study also showed that some patients developed HBV reactivation at the time of disease relapse, which might indeed indicate the contribution of HBV in lymphomagenesis. Currently, several possible mechanisms for the oncogenic role of HBV in DLBCL have been proposed. The first is chronic antigenic stimulation, which is similar to the explanation for HCV‐driven lymphomagenesis. This explanation was supported by Deng et al., who suggested that HBV‐associated DLBCL might arise from HBV antigen–selected B cells based on the use of certain biased immunoglobulin genes 10. However, Ren et al. could not replicate their results and did not support this model 11. Neoplastic B‐lymphocyte transformation as a result of HBV infection is another possible mechanism 37, 38. Furthermore, Ren et al. explored the mutational profiles observed in HBV‐associated DLBCL and suggested that infection of B cells by HBV could induce a hyperactive status that results in enhanced mutagenesis 11. Finally, integration of HBV DNA into the chromosome of lymph node cells had been identified 39. Thus, similar to HBV‐induced HCC, HBV DNA might integrate into the lymphoma genome and contribute to lymphomagenesis. Although Ren et al. did not detect HBV gene integration 11, whether HBV DNA integration into the lymphoma genome occurs and whether hotspot integration stimulates or suppresses the expression of specific cellular genes flanking the integration site need to be further investigated.
The main limitation of our study is its retrospective design. Data were incomplete regarding HBcAb, HBV DNA, and HBeAg levels before treatment. The prophylactic antiviral treatments varied in HBsAg‐positive patients. All of these factors could bias our results. Nevertheless, most studies on this subject have also applied retrospective designs. In addition, the type of antiviral prophylaxis used in this study has no prognostic significance and thus contributed little to the outcome analyses. In the literature, our study analyzed the largest cohort comprising patients with DLBCL treated similarly with R‐CHOP and a significant portion of HBsAg‐positive patients. The length of follow‐up (median, 68.6 months) was also sufficient to draw conclusions.
Conclusion
Our study comprehensively explored the clinical relevance of HBV infection in patients with DLBCL in an HBV‐endemic area in the rituximab era. In combination with two well‐established prognostic factors, the NCCN‐IPI score at diagnosis and pretreatment serum albumin levels, the presence of HBsAg can more effectively stratify patients with DLBCL into different risk groups. HBsAg positivity might serve as a new prognostic factor for predicting clinical outcomes in patients with DLBCL in endemic areas for HBV infection. Further research exploring the etiopathogenic role of HBV in DLBCL is imperative to develop new treatment strategies for this group of patients.
Author Contributions
Conception/design: Chieh‐Lung Cheng, Wei‐Quan Fang, Tung‐Hung Su
Provision of study material or patients: Chieh‐Lung Cheng, Jia‐Hong Chen, Chang‐Tsu Yuan, Jia‐Hau Liu, Ming‐Kai Chuang, Hwei‐Fang Tien
Collection and/or assembly of data: Chieh‐Lung Cheng, Sheng‐Chuan Huang, Chao‐Hung Wei
Data analysis and interpretation: Chieh‐Lung Cheng, Wei‐Quan Fang
Manuscript writing: Chieh‐Lung Cheng, Wei‐Quan Fang
Final approval of manuscript: Chieh‐Lung Cheng, Sheng‐Chuan Huang, Jia‐Hong Chen, Chao‐Hung Wei, Wei‐Quan Fang, Tung‐Hung Su, Chang‐Tsu Yuan, Jia‐Hau Liu, Ming‐Kai Chuang, Hwei‐Fang Tien
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.
Acknowledgments
This work was partially sponsored by Grant 108‐S4178 from the Department of Medical Research, National Taiwan University Hospital.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Michele Merli, Marco Frigeni, Laurent Alric et al. Direct‐Acting Antivirals in Hepatitis C Virus‐Associated Diffuse Large B‐cell Lymphomas. The Oncologist 2019;24:e720–e729.
Implications for Practice: Hepatitis C virus (HCV)‐associated diffuse large B‐cell lymphomas (DLBCLs) represent a great therapeutic challenge, especially in terms of hepatic toxicity during immune‐chemotherapy (I‐CT) and long‐term hepatic complications. The advent of highly effective and toxicity‐free direct‐acting antivirals (DAAs) created an exciting opportunity to easily eradicate HCV shortly after or in concomitance with first‐line immunochemotherapy (usually R‐CHOP). This retrospective international study reports the real‐life use of the combination of these two therapeutic modalities either in the concurrent or sequential approach (DAAs after I‐CT) in 47 patients. The favorable reported results on long‐term outcome seem to support the eradication of HCV with DAAs in all patients with HCV‐positive DLBCL. Moreover, the results from the concurrent approach were effective and safe and displayed an advantage in preventing hepatic toxicity during I‐CT.
References
- 1. Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis 2002;2:395–403. [DOI] [PubMed] [Google Scholar]
- 2. Kim JH, Bang YJ, Park BJ et al. Hepatitis B virus infection and B‐cell non‐Hodgkin's lymphoma in a hepatitis B endemic area: A case‐control study. Jpn J Cancer Res 2002;93:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non‐Hodgkin lymphoma in South Korea: A cohort study. Lancet Oncol 2010;11:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcucci F, Mele A, Spada E et al. High prevalence of hepatitis B virus infection in B‐cell non‐Hodgkin's lymphoma. Haematologica 2006;91:554–557. [PubMed] [Google Scholar]
- 5. Chen MH, Hsiao LT, Chiou TJ et al. High prevalence of occult hepatitis B virus infection in patients with B cell non‐Hodgkin's lymphoma. Ann Hematol 2008;87:475–480. [DOI] [PubMed] [Google Scholar]
- 6. Su TH, Liu CJ, Tseng TC et al. Chronic hepatitis B is associated with an increased risk of B‐cell non‐Hodgkin's lymphoma and multiple myeloma. Aliment Pharmacol Ther 2019;49:589–598. [DOI] [PubMed] [Google Scholar]
- 7. Dalia S, Chavez J, Castillo JJ et al. Hepatitis B infection increases the risk of non‐Hodgkin lymphoma: A meta‐analysis of observational studies. Leuk Res 2013;37:1107–1115. [DOI] [PubMed] [Google Scholar]
- 8. Li M, Gan Y, Fan C et al. Hepatitis B virus and risk of non‐Hodgkin lymphoma: An updated meta‐analysis of 58 studies. J Viral Hepat 2018;25:894–903. [DOI] [PubMed] [Google Scholar]
- 9. Wang F, Xu RH, Luo HY et al. Clinical and prognostic analysis of hepatitis B virus infection in diffuse large B‐cell lymphoma. BMC Cancer 2008;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deng L, Song Y, Young KH et al. Hepatitis B virus‐associated diffuse large B‐cell lymphoma: Unique clinical features, poor outcome, and hepatitis B surface antigen‐driven origin. Oncotarget 2015;6:25061–25073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren W, Ye X, Su H et al. Genetic landscape of hepatitis B virus‐associated diffuse large B‐cell lymphoma. Blood 2018;131:2670–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan X, Zhou M, Lou Z et al. Diffuse large B‐cell lymphoma with concurrent hepatitis B virus infection in the MabThera era: Unique clinical features and worse outcomes. J Cancer Res Ther 2018;14:S248–S253. [DOI] [PubMed] [Google Scholar]
- 13. Lim ST, Fei G, Quek R et al. The relationship of hepatitis B virus infection and non‐Hodgkin's lymphoma and its impact on clinical characteristics and prognosis. Eur J Haematol 2007;79:132–137. [DOI] [PubMed] [Google Scholar]
- 14. Law MF, Lai HK, Chan HN et al. The impact of hepatitis B virus (HBV) infection on clinical outcomes of patients with diffuse large B‐cell lymphoma. Eur J Cancer Care (Engl) 2015;24:117–124. [DOI] [PubMed] [Google Scholar]
- 15. Wei Z, Zou S, Li F et al. HBsAg is an independent prognostic factor in diffuse large B cell lymphoma patients in rituximab era: Result from a multicenter retrospective analysis in China. Med Oncol 2014;31:845. [DOI] [PubMed] [Google Scholar]
- 16. Liu Z, Li S, Liu Y et al. PD1 is highly expressed in diffuse large B‐cell lymphoma with hepatitis B virus infection. PLoS One 2017;12:e0180390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie W, Zhou D, Hu K et al. Clinical analysis and prognostic significance of hepatitis B virus infections for diffuse large B‐cell lymphoma with or without rituximab therapy. Exp Ther Med 2013;6:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Mansour MM, Alghamdi SA, Alsubaie MA et al. Negative effect of hepatitis in overall and progression‐free survival among patients with diffuse large B‐cell lymphoma. Infect Agent Cancer 2018;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu HY, Chang MH, Chen DS et al. Baseline seroepidemiology of hepatitis B virus infection in children in Taipei, 1984: A study just before mass hepatitis B vaccination program in Taiwan. J Med Virol 1986;18:301–307. [DOI] [PubMed] [Google Scholar]
- 20. Hans CP, Weisenburger DD, Greiner TC et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–282. [DOI] [PubMed] [Google Scholar]
- 21. Zhou Z, Sehn LH, Rademaker AW et al. An enhanced International Prognostic Index (NCCN‐IPI) for patients with diffuse large B‐cell lymphoma treated in the rituximab era. Blood 2014;123:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 23. Sehn LH, Donaldson J, Chhanabhai M et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B‐cell lymphoma in British Columbia. J Clin Oncol 2005;23:5027–5033. [DOI] [PubMed] [Google Scholar]
- 24. Habermann TM, Weller EA, Morrison VA et al. Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. J Clin Oncol 2006;24:3121–3127. [DOI] [PubMed] [Google Scholar]
- 25. Coiffier B, Thieblemont C, Van Den Neste E et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melchardt T, Troppan K, Weiss L et al. A modified scoring of the NCCN‐IPI is more accurate in the elderly and is improved by albumin and beta2 ‐microglobulin. Br J Haematol 2015;168:239–245. [DOI] [PubMed] [Google Scholar]
- 27. Matsue K, Kimura S, Takanashi Y et al. Reactivation of hepatitis B virus after rituximab‐containing treatment in patients with CD20‐positive B‐cell lymphoma. Cancer 2010;116:4769–4776. [DOI] [PubMed] [Google Scholar]
- 28. Pei SN, Chen CH, Lee CM et al. Reactivation of hepatitis B virus following rituximab‐based regimens: A serious complication in both HBsAg‐positive and HBsAg‐negative patients. Ann Hematol 2010;89:255–262. [DOI] [PubMed] [Google Scholar]
- 29. Kim SJ, Hsu C, Song YQ et al. Hepatitis B virus reactivation in B‐cell lymphoma patients treated with rituximab: Analysis from the Asia Lymphoma Study Group. Eur J Cancer 2013;49:3486–3496. [DOI] [PubMed] [Google Scholar]
- 30. Wilcox RA, Ristow K, Habermann TM et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high‐risk patients in diffuse large‐B‐cell lymphoma. Leukemia 2011;25:1502–1509. [DOI] [PubMed] [Google Scholar]
- 31. Porrata LF, Markovic SN. Timely reconstitution of immune competence affects clinical outcome following autologous stem cell transplantation. Clin Exp Med 2004;4:78–85. [DOI] [PubMed] [Google Scholar]
- 32. Kim DH, Baek JH, Chae YS et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B‐cell lymphoma. Leukemia 2007;21:2227–2230. [DOI] [PubMed] [Google Scholar]
- 33. Cox MC, Nofroni I, Laverde G et al. Absolute lymphocyte count is a prognostic factor in diffuse large B‐cell lymphoma. Br J Haematol 2008;141:265–268. [DOI] [PubMed] [Google Scholar]
- 34. Weiner GJ. Rituximab: Mechanism of action. Semin Hematol 2010;47:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Z, Li S, Guo W et al. MYC gene rearrangements are closely associated with poor survival of diffuse large B cell lymphoma with hepatitis B virus infection. Biomed Res Int 2017;2017:1967648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, Guo X, Xing L et al. HBV infection potentiates resistance to S‐phase arrest‐inducing chemotherapeutics by inhibiting CHK2 pathway in diffuse large B‐cell lymphoma. Cell Death Dis 2018;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Wang H, Pan S et al. Capable infection of hepatitis B virus in diffuse large B‐cell lymphoma. J Cancer 2018;9:1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pontisso P, Vidalino L, Quarta S et al. Biological and clinical implications of HBV infection in peripheral blood mononuclear cells. Autoimmun Rev 2008;8:13–17. [DOI] [PubMed] [Google Scholar]
- 39. Umeda M, Marusawa H, Seno H et al. Hepatitis B virus infection in lymphatic tissues in inactive hepatitis B carriers. J Hepatol 2005;42:806–812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.


