Abstract
Background
Delays to cancer diagnosis exist, resulting in worse survival outcomes for many cancers. Interventions targeting delays and barriers to cancer diagnosis and treatment have been investigated, but mostly in high‐income countries. We conducted a systematic literature review to identify and characterize the interventions studied across cancers, within low‐ and middle‐income countries (LMICs).
Methods
This systematic review forms part two of a wider study examining solutions to delays and barriers in cancer early diagnosis in LMICs. A comprehensive literature search was conducted on November 27, 2017, encompassing published studies from the preceding 15 years. We extracted study design, population, and intervention, and reported outcome measures from each study. Results were presented by target of interventions (general vs. health care professionals). A narrative synthesis was used to summarize intervention efficacy.
Results
Of 10,193 abstracts returned, 25 were included, consisting of studies across World Health Organization geographical regions, examining breast, cervix, childhood, prostate, head and neck, and gastric cancers. Altogether, 11 intervention studies targeted the general population, 12 targeted health care professionals, and 2 targeted both. The majority (17/25) of studies reported interventions focusing on patient and diagnosis‐related barriers early in the cancer care pathway. Most studies reported knowledge score as primary outcome measure (17/25); few (6/25) reported on clinically relevant measures such as reducing disease stage at presentation or diagnostic time interval. Effectiveness of interventions was demonstrated for some cancers only.
Conclusion
More interventions reporting clinically relevant measures and using standardized methods and outcomes are required to improve our ability to effectively improve cancer early diagnosis in LMICs.
Implications for Practice
Prior to this study, the extent of intervention literature in cancer early diagnosis in low‐ and middle‐income countries had not been characterized. This study aimed to outline and characterize interventions across all cancer types and across all countries. This systematic review demonstrated that interventions have been investigated targeting both the general population and health care professionals. Furthermore, this review demonstrates that the majority of studies report knowledge as an outcome measure, rather than clinically significant measures that improve cancer‐related outcomes, such as delay intervals or downstaging of disease. Future interventions should address clinically relevant measures to better assess efficacy of interventions.
Keywords: Interventions, Cancer, Low‐ and middle‐income countries, Early diagnosis, Delay interval, Barriers
Short abstract
This review reports on interventions that address delays and barriers in diagnosis and treatment of cancer, summarizing published studies from low‐ and middle‐income countries.
Introduction
Cancers represent a significant burden of disease and are responsible for approximately one in six deaths worldwide 1. In particular, there is a large but poorly documented cancer burden throughout low‐ and middle‐income countries (LMICs) that is estimated to be over 70% of global cancer deaths 1. In order to effectively address this, the World Health Organization (WHO) has focused increasing attention toward the improvement of early diagnosis and treatment of cancers to reduce the number of cancer deaths 2. It is important to recognize that this strategy focuses on the early diagnosis and treatment of patients with symptomatic disease rather than screening programs among asymptomatic persons.
Early diagnosis of cancer and appropriate treatment have demonstrated efficacy in reducing cancer deaths for select cancer types by ensuring that the disease does not progress to advanced stages at which prognosis is worse 3. The study of early diagnosis relies on examining where delays may occur throughout patients’ cancer diagnosis and management course 4. Three phases of the pathway can be described: (a) patients’ awareness and accessing care; (b) clinical evaluation, diagnosis, and staging; and (c) access to subsequent treatment 5. These phases represent particular moments at which barriers may exist and delay patients from being managed promptly before or after a cancer diagnosis. Examples of barriers include poor health literacy, poor health service coordination, and limited diagnostic or treatment services.
To date, many interventions to effectively address barriers to cancer early diagnosis have been studied in various settings within the different phases of the cancer care continuum; however, a majority of these studies have been done in high‐income countries, and it is unknown if these interventions can be applied effectively in LMICs. In this study we systematically review interventions that address delays in diagnosis and treatment of symptomatic cancer to summarize published studies from LMICs. This review excluded studies of cancer screening in asymptomatic individuals.
Materials and Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guideline. This systematic review has been registered on the PROSPERO systematic reviews database (CRD42017083868). This is the second report from a comprehensive systematic review of studies of early diagnosis of cancers in LMICs. The first report focused on delays and barriers to early diagnosis 4. A comprehensive overview of the search strategy has been presented previously and is briefly described below.
Inclusion of Studies
This review included any published full‐text article that reported on early diagnosis strategies for any cancer in LMICs. The definition of LMICs was obtained from the World Bank, based on per capita gross national income in 2016 6. The included articles were required to contain the following: (a) an intervention or program implemented that aimed to impact on the timing of diagnosis of any cancer and (b) at least one reported outcome measure to describe the efficacy of the studied intervention. The outcome measure was not limited to any particular type in order to capture as many relevant studies as possible. Outcome measures might include improvements in knowledge, stage of disease at presentation, reduction in delay intervals, or survival.
This search was conducted in English without language restriction; any article in a language other than English was translated using a Web‐based translation tool 7. A search filter for LMICs was adapted from the Cochrane Effective Practice and Organization of Care. Dates of publication were restricted to a recent 15‐year period (January 1, 2002, to November 27, 2017) to emphasize the most relevant data available in this field that reflect the current situation in countries. Studies that evaluated cancer screening or the diagnosis of cancer in an asymptomatic population were excluded to distinguish this review's focus, early diagnosis, from cancer screening. Only published original studies were included for analysis. This review excluded case reports, comments, letters, conference abstracts, and editorials.
Search Strategy
The search was performed on November 27, 2017, and initiated on Ovid MEDLINE with keywords including “cancer,” “interval,” “barrier,” “downstaging,” “early diagnosis,” and “delayed diagnosis” (supplemental online Table 1). The search was adapted to the following additional databases: Ovid EMBASE, Global Index Medicus, CINAHL Ebsco, and the Cochrane Library (consisting of Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, and the National Health Service Economic Evaluation Database). A manual bibliographic search was additionally performed by examining references of included articles.
Table 1.
Characteristic of studies included in the review by WHO region and by cancer type
| Total number of studies | Number of countries | Study design | Target population | ||||
|---|---|---|---|---|---|---|---|
| Randomized controlled trials | Quasi‐experimental | Cross‐sectional | General population | Health care professionals | |||
| WHO region | |||||||
| AFR | 5 | 5 | 2 | 2 | 1 | 3 | 2 |
| AMR | 4 | 4 | 1 | 3 | 0 | 2 | 2 |
| EMR | 6 | 3 | 3 | 3 | 0 | 3 | 4 |
| EUR | 2 | 1 | 2 | 0 | 0 | 0 | 2 |
| SEAR | 4 | 3 | 3 | 1 | 0 | 2 | 2 |
| WPR | 4 | 2 | 1 | 3 | 0 | 3 | 2 |
| Total | 25 | 18 | 12 | 12 | 1 | 13 | 14 |
| Cancers studied | |||||||
| Breast | 14 | 10 | 10 | 4 | 0 | 7 | 7 |
| Childhood | 4 | 4 | 1 | 3 | 0 | 2 | 2 |
| Othera | 3 | 3 | 0 | 3 | 0 | 2 | 1 |
| Multipleb | 4 | 4 | 1 | 2 | 1 | 2 | 4 |
| Total | 25 | 18c | 12 | 12 | 1 | 13 | 14 |
Includes prostate, oral, and gastric cancers.
Includes at least three types of cancers studied or studies of cancers not specified.
Sum of countries does not add because of different cancer types being studied in same country.
Abbreviations: AFR, African Region; AMR, Region for the Americas; EMR, Eastern Mediterranean Region; EUR, European Region; SEAR, South‐East Asia Region; WHO, World Health Organization; WPR, Western Pacific Region.
Data Collection
Two independent reviewers, N.R.B. and L.G.Q., screened the returned abstracts for inclusion eligibility. The eligibility of each study was independently determined by each reviewer and then corroborated between the two reviewers. Disagreements regarding inclusion were resolved through discussion with a third reviewer, A.M.I. Full‐text articles of each eligible abstract were examined for final inclusion. Full texts were searched for across three independent medical journal libraries. Contacting authors was not attempted in this review.
Data extracted from each study included study origin, design, population, cancer type studied, and details relating to the study intervention and outcomes. The type of interventions and reported outcomes were classified according to the three essential steps of early cancer diagnosis as specified in the WHO Guide to Cancer Early Diagnosis (supplemental online Fig. 1) 2. These three steps are Step 1, awareness of symptoms and accessing care; Step 2, clinical evaluation, diagnosis, and staging; and Step 3, access to treatment.
Figure 1.
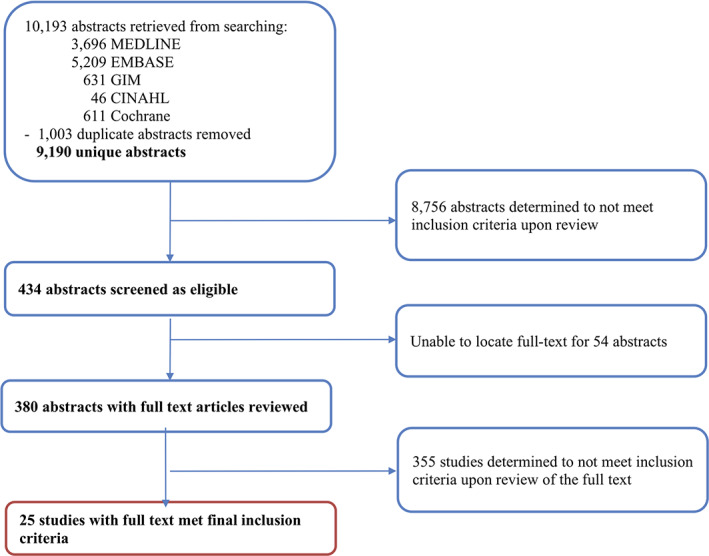
Flowchart of abstracts and full‐text articles reviewed, January 1, 2002, to November 27, 2017. The original search returned 10,193 abstracts in total. Only 25 studies met final inclusion criteria and were analyzed in this study.Abbreviation: GIM, Global Index Medicus.
The types of intervention studied were summarized and presented through a descriptive overview including duration of the intervention and its target population (health care professionals or general population), location, and method. In addition, the type of reported outcome measure was recorded and classified into the reporting of knowledge, stage of disease, time interval, treatment access, or other. The primary findings of each study were captured and summarized, describing the significance and efficacy of the studied intervention.
Data Analysis
Because of the wide‐scoped nature of this review, the included studies have varied methodology, study design, intervention type, target population, and outcome measures. The significant study heterogeneity limited our ability to conduct a meta‐analysis. The summarized meta‐data have been reported based on WHO region (African Region, Region for the Americas, Eastern Mediterranean Region, European Region, South‐East Asia Region, and Western Pacific Region), cancers studied, and study design. A narrative synthesis was conducted according to cancer type to describe the efficacy of the various interventions, outcomes reported, and target population (general population or health care professionals).
Assessment of Study Quality
The quality of each study included was assessed by N.R.B., L.G.Q., and A.M.I. using a tool developed by the U.S. National Heart, Lung, and Blood Institute. Study quality was grouped into three categories: high, intermediate, or low quality 8, 9. Low‐quality studies were included throughout this review in order to capture as many interventions as possible.
Results
The search of the five electronic databases yielded 10,193 abstracts, of which 1,003 duplicate abstracts were removed (Fig. 1). Upon review of title and abstract, 8,756 articles were determined ineligible, and an additional 54 were excluded because of unavailability of a published full‐text article. Of the 380 full‐text articles reviewed, 355 were determined to be ineligible and excluded because (a) the study did not assess an intervention to reduce diagnostic delays in cancer, (b) the study site was in a high‐income country, (c) the study focused on cancer screening, or (iv) the study was not primary research. In summary, 25 studies were eligible for inclusion.
Of the 25 included studies, 10 were determined to be of high quality, 13 of average quality, and 2 of low quality.
Characteristics of Studies Included in the Review
Studies included were conducted across all six WHO regions (Table 1, top). Of the six WHO regions and 18 countries reflected in this review, there was some variation in the number of studies per region (range: 2–6), and countries represented per region (range: 1–5; Fig. 2). The WHO region with the most studies included in this review was the Eastern Mediterranean Region (n = 6), whereas the WHO African Region had the most countries (n = 5) represented.
Figure 2.
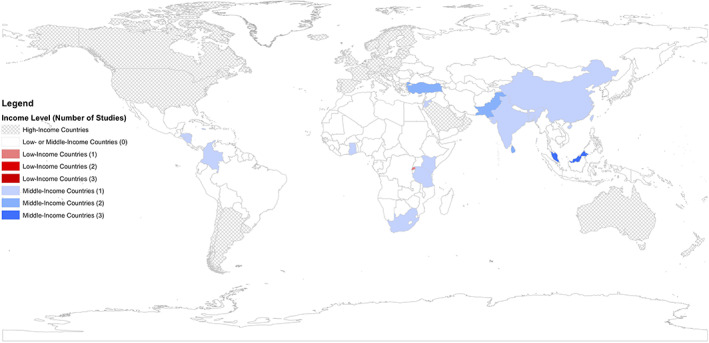
Geographic distribution of studies (n = 25) published by country income level. World map depicting countries of included studies based on income level and number of studies published.
The majority of included studies focused on interventions addressing one cancer type (21/25, 84%); breast cancer was most common (14/25, 56%; Table 1, bottom), followed by studies of childhood cancer (4/25, 16%). Four studies examined multiple (at least three cancer types) cancers together or cancers in general. Other studies investigated prostate (1), gastric (1), and oral cancers (1). Childhood cancers included hematological malignancies and solid tumors such as retinoblastoma, neuroblastoma, and hepatoblastoma.
The most common study designs used by the included studies (Table 1) were randomized controlled trials that compared different interventions (n = 12) and quasi‐experimental studies (n = 12) that assessed pre‐ and post‐intervention changes. One study used a descriptive cross‐sectional design.
Of 25 studies, 11 targeted the general population, 12 targeted health care professionals, and 2 targeted both the general population and health care professionals.
When examined by WHO Guide to Cancer Early Diagnosis steps, there was a predominance (19/25) of interventions that addressed barriers in Step 1 (Fig. 3). Most (16/19) of the interventions targeted the general population and addressed community engagement, reducing cancer stigma, and increasing general health literacy. Most (9/12) of the studies that addressed Step 2 targeted health care professionals and addressed improved counseling and care, early referral systems, strengthened diagnostic services, and improved primary care capacity. None of the included studies addressed Step 3 of the WHO Guide to Cancer Early Diagnosis pathway (Fig. 3).
Figure 3.
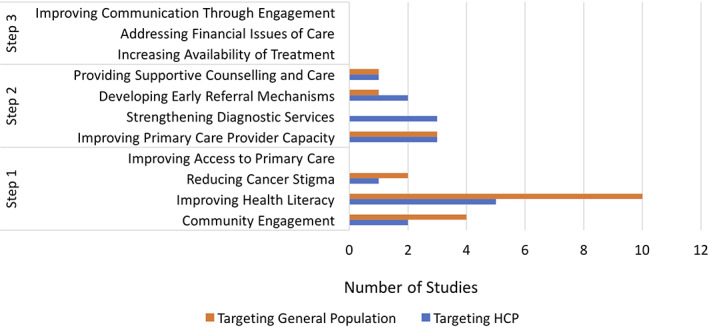
Number of studies that addressed specific interventions according to the World Health Organization (WHO) Guide to Cancer Early Diagnosis steps, by study target population (general population or health care professionals). A total of 13 and 14 studies focused on targeting the general public and health professionals, respectively. The various steps addressed in the studied interventions have been tabulated, as described in the WHO Guide to Cancer Early Diagnosis. No studies addressed any outcomes listed under Step 3.*, Number of studies totals more than 25 because of some studies reporting on more than one intervention. Abbreviation: HCP, health care professional.
The outcome measure most commonly reported was knowledge score (Fig. 4), regardless of study design or target population. There was no consistent knowledge score adopted; each study reported knowledge using its own individually internally validated score. Of the 16 studies that investigated improvement in knowledge scores, only 2 controlled intervention trials reported knowledge scores of at least 1 year post‐intervention. Other studies reported short‐term change in score 10, 11.
Figure 4.
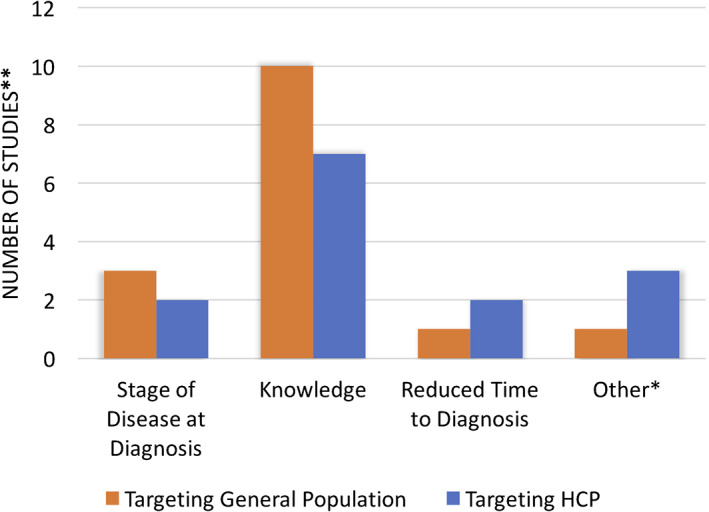
Number of studies by reported outcome measures and target population (general population or health care professionals). The reported outcome measures in included studies have been represented as absolute and proportional values. A total of 25 studies (14 targeting health care professionals and 13 targeting general population) were included in this analysis. Some studies reported multiple outcome measures. Two studies investigated interventions targeting both health care professionals and the general population.*, Includes treatment access, referrals, survival/abandonment, and detection rate. **, Number of studies totals more than 25 because of some studies reporting on more than one intervention. Abbreviation: HCP, health care professional.
Only a minority of studies reported an outcome measure bearing clinical significance, such as downstaging disease or reducing time interval delays. Four studies reported on reducing stage of disease on presentation, involving breast, cervical, nasopharyngeal, and childhood cancers 12, 13, 14, 15. Two studies reported on reducing time to diagnosis from symptom onset, whereas another study reported the median time interval from receipt of specimen to pathology reporting of final diagnosis 12, 16, 17. Other outcome measures included improved treatment access, increased number of referrals, improved survival or decreased abandonment rates, and improved detection rates 13, 18, 19, 20.
Interventions Targeting General Population
Altogether, 13 studies targeted the general population, and most focused on interventions improving knowledge outcomes (Table 2). Four controlled intervention studies investigated the benefits of an educational program to improve women's breast cancer knowledge 10, 21, 22, 23. Educational programs included practical examination workshops, instructional sessions, and audio‐visual presentations. The use of phone texting and pamphlets was also investigated in one study. All four trials demonstrated significantly improved knowledge scores with the respectively studied intervention 10, 21, 22, 23.
Table 2.
Published intervention studies targeting the general population, by study design
| Author | Country and WHO region | Cancer | Study design | Sample size | Population | Intervention(s) | Duration of intervention | Testing | Outcome measure | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Controlled intervention studies targeting general population | ||||||||||
|
Akhtari‐Zavare 2014 10 |
Malaysia WPR |
Breast cancer | Controlled intervention study/RCT | 370 | Female undergraduate students from two universities |
Educational program vs. control Lecture on breast awareness, and workshop on BSE |
16 × 2‐hour workshops |
Pre‐intervention
Post‐intervention at 6 and 12 months |
Knowledge | Mean knowledge scores (p < .003) and BSE knowledge and practice in the intervention group were significant higher in comparison with the control group at 6 and 12 months post‐intervention. |
|
Safari 2014 21 |
Iran EMR |
Breast cancer | Controlled intervention study/RCT | 90 | Female participants visiting health care service of Kamyaran city |
SMS vs. pamphlet vs. control SMS or pamphlet instructions on breast cancer knowledge, preventive methods, BSE, and screening |
10 × instruction sessions over 1 month | Post‐intervention (immediately after) | Knowledge | The pamphlet group performed significantly better than control in breast cancer knowledge (p = .025), whereas the SMS group had significantly better attitude compared with control (p = .004). |
|
Kisuya 2015 22 |
Kenya AFR |
Breast cancer | Controlled intervention study/RCT | 532 | Female participants in three communities in western Kenya |
Educational presentation vs. control Causes, signs, risk factors, screening, BSEs, and treatment options |
0.5‐hour sessions run over 1 month |
Pre‐intervention
Post‐intervention (immediately after) |
Knowledge | A 2.80 point (95% CI, 2.38–3.22) significant improvement in knowledge was noted about breast cancer after the educational session. |
|
Vithana 2015 23 |
Sri Lanka SEAR |
Breast cancer | Controlled intervention study/RCT | 520 | Female participants in Gampaha district |
Educational intervention vs. control Breast cancer checklist taught by public health midwives to improve risk assessment, examination, early detection, and referrals |
During clinics and home visits over 6 months |
Pre‐intervention
Post‐intervention at 6 months |
Knowledge | Median knowledge, attitude, and practice scores improved significantly from 54% to 77% in the intervention arm. No improvements were observed in the control arm. |
| Quasi‐experimental studies targeting general population | ||||||||||
|
Mena 2014 24 |
Ghana AFR |
Breast cancer | Quasi‐experimental study | 233 | Female participants in rural communities in Ghana |
Breast cancer awareness program vs. control Community lecture and examination workshop |
1‐hour lectures and workshops, run over 1 month | Post‐intervention at 10 months | Knowledge | Participants who attended the program were significantly more likely to obtain higher knowledge scores (OR, 2.10l; 95% CI, 1.14–3.86). |
|
Rao 2005 25 |
India SEAR |
Breast cancer | Quasi‐experimental study | 360 | Female participants in a coastal village of southern India |
Educational intervention Workshops teaching breast health and BSEs |
1‐day sessions run over 1 year | Post‐intervention at 1 and 3 months | Knowledge | Significant increase in overall breast cancer awareness (p < .001) and in BSE performance 321/342 (93%). |
|
Taha 2010 26 |
Jordan EMR |
Breast cancer | Quasi‐experimental study | 2,554 | Female participants from five governorates in Jordan |
Jordan Breast Cancer Program Lectures on statistics, risk factors, clinical features, BSE, diagnosis, and treatment |
2 × 45‐minute sessions run over 1 month |
Pre‐intervention
Post‐intervention (immediately after) |
Knowledge | Mean knowledge score from the questionnaire increased significantly from 10.9 to 13.5/15 between pre‐ and post‐intervention. |
|
McCree‐Hale 2012 27 |
Jamaica AMR |
Prostate cancer | Quasi‐experimental study | 188 | Men aged >40 years attending clinics in Western Jamaica |
Educational health intervention Computer‐based presentation on prostate cancer risk factors, clinical features, and testing |
45‐minute computer presentations run over 3 months |
Pre‐intervention
Post‐intervention (immediately after) |
Knowledge | Significant improvement in knowledge of risk factors, symptoms, and diagnostic tests (p < .05). |
|
Saleh 2012 29 |
Malaysia WPR |
Oral cancer | Quasi‐experimental study | 777 | Adults approached by e‐mail, across Malaysia |
Mass media campaign Television advertisement about signs of oral cancer and two once‐off talk shows |
Campaign ran over 32 consecutive days |
Pre‐intervention
Post‐intervention |
Knowledge | Significant increase in proportion of participants aware of oral cancer. There was no significant difference in knowledge of signs and symptoms pre‐ and post‐intervention. |
|
Ali |
Pakistan EMR |
Breast, colon, intestinal | Quasi‐experimental study | 281 | Students, teachers, community members, and health care workers in Karachi |
Educational sessions Presentations and examination models about cancers, their diagnosis, and treatment |
15 × educational sessions conducted over 1 year |
Pre‐intervention
Post‐intervention |
Knowledge | Increased proportion of participants with greater general knowledge for cancer and less cancer stigma. There was no reported change in identifying signs and symptoms. |
|
Leander 2017 12 |
Honduras AMR |
Childhood cancer | Quasi‐experimental study | 82 | Community visitors of Honduran government clinics |
Education program Program linked to national vaccination campaign using posters and flyers in government clinics |
1‐month campaign run annually for 3 years |
Pre‐intervention Retrospective data analysis over 8 years
Post‐intervention Retrospective data analysis 3 years after |
Stage of disease
Time interval |
Retinoblastoma: Extraocular disease at presentation was in 73% pre‐campaign and 35% post‐campaign (p = .002). Median diagnostic lag time was 7.2 months pre‐campaign and 5.5 months post‐campaign (p = .6). |
|
Poyiadjis 2011 13 |
South Africa AFR |
Childhood cancer | Quasi‐experimental study | 1,597 | Patients attending treatment unit for the North West Province and southern Gauteng |
Educational campaign Lectures to providers, posters, newspapers, radio interviews, clinic pamphlets, and toll‐free phone service to educate the public and the primary health workers on the Saint Siluan early warning signs of cancer in children |
52 × lectures and media campaign run over 6 months |
Pre‐intervention Retrospective data analysis over 12 years
Post‐intervention Retrospective data analysis over 6 years |
Stage of disease
Other (referrals) |
Intervention was ineffective in achieving referral at earlier stages of disease. However, there was a significant increase in the number of new patients referred in the 6 years after the campaign (p < .001). |
|
Devi |
Malaysia WPR |
Breast, cervix, and nasopharyngeal | Quasi‐experimental study | Not reported | General population attending 154 rural clinics and 18 hospitals in Sarawak |
Early cancer surveillance program Staff training to improve early detection; raising public awareness through poster and pamphlet distribution |
2‐day staff training and once‐off poster and pamphlet distribution |
Pre‐intervention Retrospective data analysis over 1 year
Post‐intervention at 4 years |
Stage of disease | Stage III and IV was significantly reduced for breast cancer (35% vs. 60%, p < .0001) and cervical (26% vs. 60%, p < .0001). No reduction was observed for nasopharyngeal cancer. |
A comprehensive breakdown of intervention and outcomes of the included studies are described, according to their study design and outcome measure.
Study intervention targeted both the general population and health care professionals and has been listed in both Tables 2 and 3.
Abbreviations: AFR, African Region; AMR, Region for the Americas; BSE, breast self‐examination; CI, confidence interval; EMR, Eastern Mediterranean Region; OR, odds ratio; RCT, randomized controlled trial; SEAR, South‐East Asia Region; SMS, short messaging service; WHO, World Health Organization; WPR, Western Pacific Region.
Of the six quasi‐experimental studies that examined interventions to improve knowledge among the general population, three studies tested lecture and workshop series to improve breast cancer knowledge, and all demonstrated significant improvement in knowledge scores 24, 25, 26. McCree‐Hale et al. tested a computer‐based educational intervention for prostate cancer and reported significantly improved knowledge of risk factors, symptoms, and investigations immediately after versus before the intervention (p < .05) 27. Ali et al. demonstrated an improvement in knowledge of cancers in general through the use of educational sessions but reported no change in ability to identify cancer signs and symptoms 28. A 32‐day mass media campaign on oral cancers showed that, although awareness increased significantly, there was no improvement in the specific knowledge of signs and symptoms of oral cancer after the intervention 29.
Three studies that targeted the general population measured lower stage of disease at diagnosis, reduced time interval to diagnosis, and number of referrals 12, 13, 14. A comprehensive intervention program in South Africa tested the effectiveness of educational presentations and a broad media campaign targeting newspapers and national radio broadcasts for disease downstaging and improved referral rates 13. At 6 years post‐intervention, no evidence was found that the intervention resulted in referrals at earlier stage disease of any childhood cancer, but there was significant increase in new referral rates (p < .001). In Honduras, Leander et al. reported significantly reduced presentation retinoblastoma at advanced stages (35% vs. 75%, p = .002) after implementing an intervention of distributed posters and flyers at government health clinics, linked to the national vaccination campaign 12. However, there was no reduction in the median diagnostic interval after campaign implementation (5.5 vs. 7.2 months, p = .6). Devi et al. investigated in Malaysia an intervention that targeted both the general population, through poster and pamphlet distribution, and health care professionals, through a 2‐day staff training program 14. The intervention significantly reduced late stage presentation of breast (60% vs. 35%, p < .0001) and cervical (60% vs. 26%, p < .001) but not nasopharyngeal cancer.
Interventions Targeting Health Care Professionals
Fourteen studies examined interventions targeting health care professionals, including eight controlled intervention studies, five quasi‐experimental studies, and one descriptive cross‐sectional study (Table 3). Health care professionals included doctors, nurses, midwives, dental professionals, and student health care professionals.
Table 3.
Published intervention studies targeting health care professionals, by study design
| Author | Country and WHO region | Cancer | Study design | Sample size | Population | Intervention(s) | Duration of intervention | Testing | Outcome measure | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Controlled intervention studies targeting health care professionals | ||||||||||
|
Moshfeghi 2010 30 |
Iran EMR |
Breast cancer | Controlled intervention study/RCT | 128 | Randomly sampled physicians in Arak city |
Video training vs. systematic review Histology, anatomy, epidemiology, assessment, and diagnosis of breast cancer |
0.5‐hour video vs. distributed printed handouts |
Pre‐intervention
Post‐intervention (immediately after) |
Knowledge | Mean significant increase in total knowledge score before and after both interventions. No significant difference between the two educational interventions. |
|
Ceber 2010 11 |
Turkey EUR |
Breast cancer | Controlled intervention study/RCT | 291 | Nurses and midwives from two health districts in rural Izmir |
Breast cancer educational program vs. control Presentations and videos on statistics, risk factors, symptoms, BSE, screening, and guidelines |
Not reported | Post‐intervention at 1 year | Knowledge | Significant improvement of total knowledge score compared with control. |
|
Torbaghan |
Iran EMR |
Breast cancer | Controlled intervention study/RCT | 130 | Female medical employees of a university |
Educational intervention vs. control Lectures, questions and answers, videos, booklet and digital disk on breast cancer awareness, screening, prevention, and barriers |
3 × 1–1.5‐hour sessions |
Pre‐intervention
Post‐intervention at 1 month |
Knowledge | Significant improvements for awareness, susceptibility, benefits, barriers, and behavior constructs. |
|
Karadag |
Turkey EUR |
Breast cancer | Controlled intervention study/RCT | 69 | Nursing students in a health college only |
Traditional lecturing method vs. Six Learning Hats method Lecture vs. active approach on knowledge and beliefs of breast cancer and BSE |
Not reported |
Pre‐intervention
Post‐intervention at 15 days and 3 months |
Knowledge | Knowledge score significantly increased for traditional learning method (9.32 to 14.41, p < .001), and for Six Thinking Hats method (9.20 to 14.73, p < .001). |
|
Vithana 2015 33 |
Sri Lanka SEAR |
Breast cancer | Controlled intervention study/RCT | 85 | Public health midwives only |
Training program vs. control Didactic lectures, discussions, practical sessions, and role‐plays |
2‐day training program |
Pre‐ intervention
Post‐intervention at 1 and 6 months |
Knowledge | Statistically significant increase in knowledge, attitudes, and practices of midwives who received intervention compared with those who did not. |
|
Ginsburg 2014 18 |
Bangladesh SEAR |
Breast cancer | Controlled intervention study/RCT | 22,337 | Case‐finding for female participants in Khulna |
mHealth vs. mHealth and Navigation vs. control Smart phone application and video to guide interview, report data, inform, and offer appointment. Navigation training was provided to health workers in second arm |
Case‐finding program run over 4 months | Post‐intervention (immediately after) | Other (treatment access) | mHealth demonstrated no significant increase in subsequent care attendance compared with control (63% vs. 53%). However, patient navigation showed significant improvements in care attendance compared with without navigation (63% vs. 43%, p < .0001). |
|
Ngoma 2015 15 |
Tanzania AFR |
Breast, cervix, and other not specified | Controlled intervention study/RCT | 10,979 | Village residents of two randomly chosen Tanzanian villages |
Case‐finding vs. control Village navigators were trained to actively seek cases and refer to health care to decrease advanced cancer rates |
3‐day health aide training for program run over 3 years | Post‐intervention at 1, 2, and 3 years | Stage of disease | The intervention village had significant downstaging to stage I and II disease (23% to 51% to 74%, p < .001), whereas no significant downstaging was observed in the control village (11% to 22% to 37%, p = not significant). |
| De Angelis 2012 16 |
Nicaragua AMR |
Childhood cancer | Controlled intervention study | Not reported | Physicians working in first or second level health care centers in pediatric oncology |
Training program vs. control Program focused on upskilling diagnosis and treatment of childhood oncological diseases |
Not reported |
Post‐intervention Retrospective data analysis over 3 years |
Time interval | Median time from symptom onset to diagnosis was decreased in districts with a training program implemented (20.5 vs. 40 days, p = .0019). |
| Quasi‐experimental studies targeting health care professionals | ||||||||||
|
Khokher 2015 34 |
Pakistan EMR |
Breast cancer | Quasi‐experimental study | 146 | Female participants in Lahore; mostly students (74%), including health science students (36%) |
Audio‐visual educational activity Early detection, examination, and treatment options |
3 × 20‐minute sessions run over 1 day |
Pre‐intervention
Post‐intervention (immediately after) |
Knowledge | There was a 66% increase in participants’ knowledge overall between pre‐ and post‐intervention questionnaire. |
|
Ali |
Pakistan EMR |
Breast, colon, intestinal | Quasi‐experimental study | 281 | Students, teachers, and community members, and health care workers in Karachi |
Educational sessions Presentations and examination models about cancers, their diagnosis, and treatment |
15 × educational sessions conducted over 1 year |
Pre‐intervention
Post‐intervention |
Knowledge | Increased proportion of participants with greater general knowledge for cancer and less cancer stigma. There was no reported change in identifying signs and symptoms. |
|
Devi |
Malaysia WPR |
Breast, cervix and nasopharyngeal | Quasi‐experimental study | Not reported | General population attending 154 rural clinics and 18 hospitals in Sarawak |
Early cancer surveillance program Staff training to improve early detection, and raising public awareness through poster and pamphlet distribution |
2‐day staff training and once‐off poster and pamphlet distribution |
Pre‐intervention Retrospective data analysis over 1 year
Post‐intervention at 4 years |
Stage of disease | Stage III and IV was significantly reduced for breast cancer (35% vs. 60%, p < .0001) and cervical (26% vs. 60%, p < .0001). No reduction was observed for nasopharyngeal cancer. |
|
Suarez 2015 19 |
Colombia AMR |
Childhood cancer | Quasi‐experimental study | 280 | Children diagnosed with acute lymphoblastic leukemia |
Multifaceted intervention Improved treatment protocol, increased health care worker capacity, health care worker educational program, and social worker support |
Intervention implemented over 3 years |
Pre‐intervention Retrospective data analysis over 10 years
Post‐intervention Retrospective data analysis over 4 years |
Other (survival, abandonment) | The study shows that after implementing the intervention, there was a significant improvement in complete remission survival (p = .005) and in abandonment rates (p < .001). |
|
Zhang 2015 20 |
China WPR |
Gastric cancer | Quasi‐experimental study | 48 | Patients referred to a single endoscopy center in Southern China |
Endoscopy training vs. control Training program to improve detection, through weekly case discussions, journal reviews, and video studies |
Weekly program run over 2 years |
Post‐intervention Retrospective data analyzed over 2 years |
Other (detection rate) | Significant improvement in the detection rate of early gastric cancer between the training and nontraining group (0.7% vs. 0.06%, p < .01). |
| Cross‐sectional studies targeting health care professionals | ||||||||||
|
Mpunga 2014 17 |
Rwanda AFR |
Not specified | Descriptive cross‐sectional study | 437 | Patient tissue specimens sent to a pathology service at a district hospital |
Anatomic pathology lab service Implementation of anatomic pathology at a hospital through personnel and infrastructure |
Implementation of service over 6 months | Post‐intervention at 6 months | Time interval | Median pathology interval 32 days; 72 specimens processed median per month. |
A comprehensive breakdown of intervention and outcomes of the included studies are described, according to their study design and outcome measure.
Study was deemed to be of low quality in study quality assessment.
Study intervention targeted both the general population and health care professionals and has been listed in both Tables 2 and 3.
Abbreviations: AFR, African Region; AMR, Region for the Americas; BSE, breast self‐examination; EMR, Eastern Mediterranean Region; EUR, European Region; RCT, randomized controlled trial; SEAR, South‐East Asia Region; WHO, World Health Organization; WPR, Western Pacific Region.
Of the seven studies that examined knowledge as the primary outcome, five controlled intervention studies demonstrated improvement in knowledge of health care professionals through video training, presentations, discussions, role‐plays, and learning through the review of academic papers and booklet material 11, 30, 31, 32, 33. Two quasi‐experimental studies that used audio‐visual educational activities demonstrated significant improvement in knowledge scores for breast cancer 28, 34.
Two studies investigated interventions to lower stage of disease at diagnosis. Ngoma et al. worked with village navigators for case‐finding and showed significant downstaging of cancers in a Tanzanian village across 3 successive years (p < .001) 15. Devi et al. showed that using a staff training and public awareness campaign led to significant downstaging of breast and cervical cancers but not of nasopharyngeal cancer 14.
In a controlled trial in Nicaragua, De Angelis et al. investigated the use of a training program for pediatric oncologists to improve the median diagnostic interval 16, which was significantly decreased (20.5 vs. 40 days, p = .0019) after 3 years of implementation. A Rwanda study reported on the impact of establishing anatomic pathology laboratory services at district hospitals to receive patient tissue specimen 17 and described the ability to implement and deliver a service with a median pathology interval of 32 days and the ability to process a median of 72 tissue specimens per month.
Three studies investigated treatment access, detection rates, and survival rates. The use of an mHealth smart phone application alongside upskilling patient navigators for breast cancer was investigated in Bangladesh in a controlled trial by Ginsburg et al. 18, with the proportion of patients attending subsequent health care services and treatment as primary outcome measures. The investigators reported significant improvements on attendance with the use of patient navigation (63% vs. 43%, p < .0001) but not with the mHealth application. A study of gastric cancer in China demonstrated a significant improvement in the detection of early gastric cancer (0.7% vs. 0.06%, p < .01) among endoscopists who underwent the intervention 20. A multifaceted intervention study conducted in Colombia showed that increasing health care worker capacity and health care worker education was associated with significant improvement in complete remission survival rates (p = .005) and reduced treatment abandonment rates (p < .001) among patients with acute lymphoblastic leukemia 19.
Discussion
To our knowledge, this is the first systematic literature review of public health interventions to improve earlier cancer diagnosis across LMICs. Despite the limited number of published studies, we identified studies that yielded evidence‐based strategies to improve early detection of cancer in Steps 1 and 2 of the WHO Guide to Cancer Early Diagnosis steps. The small number of published studies and limited number of LMICs represented highlight the urgent need for research on effective interventions to improve cancer early diagnosis in LMICs.
Although a variety of strategies have been reported in the literature reviewed, the majority of interventions employed approaches addressing health literacy through the use of lectures, presentations, or workshops. The reported significant improvements in knowledge scores reveal some important findings and caveats. Firstly, publication bias may exist in the reporting of the study results, as we found an absence of published studies that reported limited or no effect of the educational intervention tested. Secondly, each study reported different knowledge scores, but all demonstrated significant improvement in different domains, such as general awareness, awareness of cancer risk factors, or disease signs and symptoms. The heterogeneous reporting of measures of knowledge impedes the interpretation of the most effective or externally valid interventions. Finally, the lack of long‐term post‐intervention measurements of knowledge across studies likely reflects an overstatement of the impact of the interventions studied; most studies tended to measure outcomes immediately or soon after the intervention. At best, two studies included in this review reported knowledge scores measured at 1 year after program implementation. Future studies that measure long‐term change or retention of knowledge scores are needed to document impact. The use of health promotion campaigns through posters and flyers aimed at the general population reported varying efficacy in reducing stage of disease. Significant improvements were seen in programs for cancers of the breast and cervix but not of the nasopharynx. Childhood cancers were inconsistently downstaged in two studies: one demonstrated significantly reduced extraocular disease, but another study did not show increased referrals at earlier stages of disease 12, 13. Although study designs varied substantially and the number of studies was small, the implementation of public awareness programs may be beneficial in reducing late stage of disease at presentation for some cancers, particularly those with screening recommendations for asymptomatic individuals.
Training interventions to improve clinical skills of health care professionals were shown to improve time to diagnosis, treatment access, increased detection rates, and patient survival rates. Employed in studies of a range of cancer types, the upskilling of pediatric oncologists for childhood cancers, endoscopists for gastric cancers, community patient navigators for breast cancers, and organized programs for specimen transport and pathology review all demonstrated consistent improvements in measured outcomes. Although it is difficult to draw conclusions because of potential publication bias and heterogeneity in study design and methods, these findings reflect the important role of interventions that focus on upskilling health care professionals in LMICs.
The use of different outcome measures in studies complicated the interpretation of study results and definition of an effective intervention. Although knowledge scores have been widely reported, these are not measures that directly translate to improved clinical outcomes. Alternatively, reporting reduced time intervals suggest a direct benefit of the intervention for leading to earlier diagnosis of cancers. Measuring the number of patients referred to further treatment is also another indicator of improved earlier diagnosis, in which greater access means more patients may undergo investigation for cancers. The reporting of disease downstaging is a strong indicator of reducing the number of presentations of advanced cancers and, with appropriate treatment, reduced mortality. Future intervention studies should measure outcomes that are clinically relevant, such as downstaging of disease at diagnosis or improved and timely access to health care services. However, it is recognized that these studies require longer patient follow‐up and the availability of pathologic services.
Surprisingly, none of the included study interventions reviewed addressed Step 3 of the WHO Guide to Cancer Early Diagnosis steps: access to treatment. Possible explanations for the lack of such studies may include difficulty in measuring treatment availability, financial access, and issues with care communication. Another possible explanation may be that our literature search did not focus on these terms. Studies with clinically relevant indicators, such as downstaging or reducing time interval, may be reported in studies different from those examining interventions to improve treatment availability or affordability. Our review highlights the critical need for studies that address Step 3 of the WHO guidelines in LMICs to better understand and ensure high quality and affordable cancer treatment accessibility.
Because of the significant variation among studies reviewed, we were unable to directly compare intervention efficacy across different cancer types. However, some insight may be gained from the study by Devi et al. that reported significant improvement in downstaging of breast and cervical but not of nasopharyngeal cancers as a result of both public awareness campaign and health worker training 14. These findings, although from one study, suggest (a) interventions that combine targeting both the general public and health care workers may be more effective, particularly for cancers with screening recommendations, and (b) interventions should be tailored to a specific cancer type, rather than all cancer types. The intervention may be less useful for cancers that are less easily recognized, even in Malaysia, where subgroups of the population are at higher risk of nasopharyngeal cancer.
Our review has a number of limitations, mainly resulting from the heterogeneity of the studies reviewed. Firstly, nearly half of the studies reviewed used the quasi‐experimental study design. These studies are limited because of potential selection bias, uncontrolled confounding, and limited external validity. Randomized controlled intervention studies are less likely to be affected by these biases. Although we have attempted to summarize the different types of interventions, cancer types, target populations, and outcome measures, the findings of this review are difficult to generalize to other LMICs that have not been included in this review. Given that our previously reported systematic review found over 300 studies reporting delays and barriers to cancer early diagnosis in LMICs, we were surprised that only 25 of these studies tested interventions to address delays and barriers 4. As with other reviews of the published literature, our study may be susceptible to publication bias, as nonefficacious interventions were likely not published and therefore not included in our review. Although our search strategy did not specifically include “intervention,” we believe all relevant studies were captured, as the included search words “delays” and “barriers” reflect the targets for these interventions. The limited number of studies, interventions, and cancer types reveals a serious deficiency in available evidence on effective interventions to address barriers to cancer early diagnosis in LMICs. Our review included only papers published in or prior to November 2017, as we aimed to investigate the landscape for early cancer diagnosis research prior to the release of the updated WHO Guide to Cancer Early Diagnosis in 2017. Our study excluded interventions that focused primarily on screening of asymptomatic individuals in order to identify research in line with WHO's recommendations on early diagnosis and treatment of cancer in LMICs. This may have limited our ability to study the effect of screening interventions that may have had a component of community awareness and education. Nevertheless, this review highlights that much‐needed attention must be drawn toward the study of interventions in LMICs that use standardized methods, outcome measures, and control of potential biases.
In summary, our review showed that intervention studies aimed at increasing health literacy should measure long‐term post‐intervention outcomes using externally validated scoring systems. We found that health promotion campaigns in the general population combined with training of health care workers may be effective in downstaging some cancers, particularly for cancers with screening recommendation. Although some interventions targeting health care workforce upskilling showed significant improvement in a range of outcomes, more studies that measure and report clinically relevant outcomes are needed. Our review highlights the paucity and urgent need for research across intervention types, study populations, and cancer types to identify effective interventions to overcome barriers to cancer early diagnosis, particularly studies that address treatment accessibility and affordability in LMIC.
Conclusion
This review describes published interventions for improving cancer early diagnosis in LMICs, with most interventions using educational campaigns and focusing on breast cancer. There is urgent need for research conducted in more LMICs, cancer types, types of interventions, and especially studies that address treatment access and affordability.
Author Contributions
Conception/design: Liang G. Qu, Nathan R. Brand, André M. Ilbawi
Provision of study material or patients: Liang G. Qu, Nathan R. Brand, André M. Ilbawi
Collection and/or assembly of data: Liang G. Qu, Nathan R. Brand
Data analysis and interpretation: Liang G. Qu, Nathan R. Brand, Ann Chao, André M. Ilbawi
Manuscript writing: Liang G. Qu, Nathan R. Brand
Final approval of manuscript: Liang G. Qu, Nathan R. Brand, Ann Chao, André M. Ilbawi
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Table
Acknowledgments
This work was supported by funding from the U.S. National Cancer Institute.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Guide to Cancer Early Diagnosis. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 3. Neal RD, Tharmanathan P, France B et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112(suppl 1):S92–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brand NR, Qu LG, Chao A et al. Delays and barriers to cancer care in low‐ and middle‐income countries: A systematic review. The Oncologist 2019;24:e1371–e1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weller D, Vedsted P, Rubin G et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fantom N, Serajuddin U. The World Bank's Classification of Countries by Income (English). Policy Research Working Paper no. WPS 7528. Washington, DC: World Bank Group, 2016. [Google Scholar]
- 7. DocTranslator Web site . Available at https://www.onlinedoctranslator.com/. Accessed November 27, 2017.
- 8. Study quality assessment tools . National Heart, Lung, and Blood Institute Web site. Available at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed January 7, 2018.
- 9. CASP Qualitative Study Checklist . Oxford, UK: Critical Appraisal Skills Programme, 2018. Available at https://casp-uk.net/casp-tools-checklists/. Accessed January 7, 2018.
- 10. Akhtari‐Zavare M, Juni MH, Said SM et al. Result of randomized control trial to increase breast health awareness among young females in Malaysia. BMC Public Health 2016;16:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ceber E, Turk M, Ciceklioglu M. The effects of an educational program on knowledge of breast cancer, early detection practices and health beliefs of nurses and midwives. J Clin Nurs 2010;19:2363–2371. [DOI] [PubMed] [Google Scholar]
- 12. Leander C, Fu LC, Pena A et al. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer 2007;49:817–819. [DOI] [PubMed] [Google Scholar]
- 13. Poyiadjis S, Wainwright L, Naidu G et al. The Saint Siluan warning signs of cancer in children: impact of education in rural South Africa. Pediatr Blood Cancer 2011;56:314–316. [DOI] [PubMed] [Google Scholar]
- 14. Devi BC, Tang TS, Corbex M. Reducing by half the percentage of late‐stage presentation for breast and cervix cancer over 4 years: A pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol 2007;18:1172–1176. [DOI] [PubMed] [Google Scholar]
- 15. Ngoma T, Mandeli J, Holland JF. Downstaging cancer in rural Africa. Int J Cancer 2015;136:2875–2879. [DOI] [PubMed] [Google Scholar]
- 16. De Angelis C, Pacheco C, Lucchini G et al. The experience in Nicaragua: Childhood leukemia in low income countries‐the main cause of late diagnosis may be “medical delay.” Int J Pediatr 2012;2012:129707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mpunga T, Tapela N, Hedt‐Gauthier BL et al. Diagnosis of cancer in rural Rwanda: Early outcomes of a phased approach to implement anatomic pathology services in resource‐limited settings. Am J Clin Pathol 2014;142:541–545. [DOI] [PubMed] [Google Scholar]
- 18. Ginsburg OM, Chowdhury M, Wu W et al. An mHealth model to increase clinic attendance for breast symptoms in rural Bangladesh: Can bridging the digital divide help close the cancer divide? The Oncologist 2014;19:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suarez A, Pina M, Nichols‐Vinueza DX et al. A strategy to improve treatment‐related mortality and abandonment of therapy for childhood ALL in a developing country reveals the impact of treatment delays. Pediatr Blood Cancer 2015;62:1395–1402. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Q, Chen ZY, Chen CD et al. Training in early gastric cancer diagnosis improves the detection rate of early gastric cancer: an observational study in China. Medicine (Baltimore) 2015;94:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Safari YA, Afzali B, Ghasemi S et al. Comparing the effect of short message service (SMS) and pamphlet instruction methods on women's knowledge, attitude and practice about breast cancer (2014). Acta Med Mediterr 2016;32:1927. [Google Scholar]
- 22. Kisuya J, Wachira J, Busakhala N et al. Impact of an educational intervention on breast cancer knowledge in western Kenya. Health Educ Res 2015;30:786–796. [DOI] [PubMed] [Google Scholar]
- 23. Vithana PC, Ariyaratne M, Jayawardana P. Educational intervention on breast cancer early detection: Effectiveness among target group women in the district of Gampaha, Sri Lanka. Asian Pac J Cancer Prev 2015;16:2547–2553. [DOI] [PubMed] [Google Scholar]
- 24. Mena M, Wiafe‐Addai B, Sauvaget C et al. Evaluation of the impact of a breast cancer awareness program in rural Ghana: A cross‐sectional survey. Int J Cancer 2014;134:913–924. [DOI] [PubMed] [Google Scholar]
- 25. Rao RS, Nair S, Nair NS et al. Acceptability and effectiveness of a breast health awareness programme for rural women in India. Indian J Med Sci 2005;59:398–402. [PubMed] [Google Scholar]
- 26. Taha H, Halabi Y, Berggren V et al. Educational intervention to improve breast health knowledge among women in Jordan. Asian Pac J Cancer Prev 2010;11:1167–1173. [PubMed] [Google Scholar]
- 27. McCree‐Hale R, Hale TM, Rutley KR et al. Evaluating a theory‐based health education intervention to improve awareness of prostate cancer among men in Western Jamaica. West Indian Med J 2012;61:580–586. [PubMed] [Google Scholar]
- 28. Ali TS, Baig S. Evaluation of a cancer awareness campaign: Experience with a selected population in Karachi. Asian Pac J Cancer Prev 2006;7:391–395. [PubMed] [Google Scholar]
- 29. Saleh A, Yang YH, Wan Abd Ghani WM et al. Promoting oral cancer awareness and early detection using a mass media approach. Asian Pac J Cancer Prev 2012;13:1217–1224. [DOI] [PubMed] [Google Scholar]
- 30. Moshfeghi K, Mohammadbeigi A. Comparison the effects of two educational methods on knowledge, attitude and practices of Arak physicians about breast cancer. Pak J Biol Sci 2010;13:901–905. [DOI] [PubMed] [Google Scholar]
- 31. Torbaghan AE, Farmanfarma KK, Moghaddam AA et al. Improving breast cancer preventive behavior among female medical staff: The use of educational intervention based on health belief model. Malays J Med Sci 2014;21:44–50. [PMC free article] [PubMed] [Google Scholar]
- 32. Karadag M, Iseri O, Etikan I. Determining nursing student knowledge, behavior and beliefs for breast cancer and breast self‐examination receiving courses with two different approaches. Asian Pac J Cancer Prev 2014;15:3885–3890. [DOI] [PubMed] [Google Scholar]
- 33. Vithana PV, Ariyaratne MA, Jayawardana PL. Effectiveness of an educational intervention among public health midwives on breast cancer early detection in the district of Gampaha, Sri Lanka. Asian Pac J Cancer Prev 2015;16:227–232. [DOI] [PubMed] [Google Scholar]
- 34. Khokher S, Qureshi MU, Fatima W et al. Impact of a breast health awareness activity on the knowledge level of the participants and its association with socio‐ demographic features. Asian Pac J Cancer Prev 2015;16:5817–5822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Supplemental Table


