Abstract
Background
There are few studies on breast cancer outcomes in the Caribbean region. This study identified a retrospective cohort of female patients with nonmetastatic breast cancer in Haiti and conducted survival analyses to identify prognostic factors that may affect patient outcomes.
Methods
The cohort included 341 patients presenting between June 2012 and December 2016. The primary endpoint was event‐free survival (EFS), defined as time to disease progression, recurrence, or death. Descriptive summaries of patient characteristics and treatments were reported. Survival curves were plotted using Kaplan‐Meier estimation. Multivariate survival analyses were performed using Cox proportional hazards regression.
Results
Median age at diagnosis was 49 years, with 64.2% being premenopausal. Most patients (55.1%) were staged as locally advanced. One hundred and sixty patients received neoadjuvant therapy: 33.3% of patients with early stage disease and 61.2% of those with locally advanced stage disease. Curative‐intent surgery was performed in 278 (81.5%) patients, and 225 patients received adjuvant therapy. Adjuvant endocrine therapy was used in 82.0% of patients with estrogen receptor–positive disease. During the follow‐up period, 28 patients died, 77 had disease recurrence, and 10 had progressive disease. EFS rates at 2 years and 3 years were 80.9% and 63.4%, respectively. After controlling for multiple confounders, the locally advanced stage group had a statistically significant adjusted hazard ratio for EFS of 3.27 compared with early stage.
Conclusion
Patients with nonmetastatic breast cancer in Haiti have more advanced disease, poorer prognostic factors, and worse outcomes compared with patients in high‐income countries. Despite several limitations, curative treatment is possible in Haiti.
Implications for Practice
Patients with breast cancer in Haiti have poor outcomes. Prior studies show that most Haitian patients are diagnosed at later stages. However, there are no rigorous studies describing how late‐stage diagnosis and other prognostic factors affect outcomes in this population. This study presents a detailed analysis of survival outcomes and assessment of prognostic factors in patients with nonmetastatic breast cancer treated in Haiti. In addition to late‐stage diagnosis, other unfavorable prognostic factors identified were young age and estrogen receptor‐negative disease. The study also highlights that the availability of basic breast cancer treatment in Haiti can lead to promising early patient outcomes.
Keywords: Breast cancer outcomes, Global oncology, Cancer in low‐resource settings, Haiti, Caribbean region
Short abstract
Disparities in global breast cancer outcomes are well established; however, few studies describe potential contributors to poor outcomes in Haiti and other countries in the Caribbean region. This article assesses patient outcomes and explores predictors of poor outcomes based on a retrospective cohort of patients with nonmetastatic breast cancer seen at a tertiary care facility in Haiti.
Introduction
Disparities in global breast cancer outcomes are well established [1, 2]. However, there are few rigorous studies describing potential contributors to poor outcomes in Haiti and in other countries in the Caribbean region. There are limited reliable national cancer statistics and no active population tumor registries in Haiti. Hence, statistics on cancer epidemiology are based on extrapolation from population demographics in surrounding Caribbean countries [3, 4, 5, 6, 7]. Based on these estimates, breast cancer is the most commonly occurring cancer and the leading cause of cancer mortality in Haitian women. Breast cancer mortality to incidence ratio in Haitian women is over 60%, the highest of any country in the Caribbean region [4]. In comparison, mortality to incidence ratios are 18%, 21%, and 46% in North America, Western Europe, and the neighboring Dominican Republic, respectively [2, 3, 4, 8]. Previous breast cancer descriptive reports from the Caribbean region indicate that patients with breast cancer present at younger ages and with more advanced disease compared with patients in the U.S. [9, 10, 11]. One prior study from Haiti indicated that 83.9% of patients had stage III or IV disease at presentation; however, this study had limited treatment and outcome data [12].
In order to assess patient outcomes and explore predictors of poor outcomes, we assembled a retrospective cohort of patients with nonmetastatic breast cancer seen at a tertiary care facility in Haiti.
Materials and Methods
Study Setting
The setting of the study was the University Hospital Mirebalais (HUM), a 350‐bed public tertiary care government facility located in the Central Plateau region of Haiti, and one of only two centers that provide comprehensive breast cancer treatment in Haiti, a country of 11 million people [13, 14, 15, 16]. The HUM oncology department started in 2013, when patients and clinicians relocated from a previous oncology program at a smaller hospital facility in change [14]. HUM receives administrative and financial support through collaboration with Zanmi Lasante, as Partners In Health (an international nonprofit organization) is known locally in Haiti [13]. Dana‐Farber Cancer Institute also provides educational, technical, and financial support to the oncology program [17].
At HUM, most of the cancer‐related care is at no direct cost to the patient. Patients with breast cancer have access to curative surgical treatment with modified radical mastectomy and axillary sampling. Systemic therapy options available are limited; cytotoxic medications include doxorubicin, cyclophosphamide, 5‐fluorouracil, methotrexate, and paclitaxel, and available endocrine therapy agents include tamoxifen and letrozole. However, human epidermal growth factor receptor 2 (HER2)–directed therapy, targeted therapies, breast conserving surgery, and radiation therapy are unavailable.
Study Population
The study population cohort included female patients with nonmetastatic breast cancer treated at HUM who presented to the program between June 2012 and December 2016. HUM has an electronic medical record system run on the Open Medical Record System (OpenMRS) platform, which includes coded diagnoses associated with patient visits [18]. An electronic list of 1,372 individuals with a coded diagnosis of “breast cancer” was generated. Of these individuals, 966 patients had a confirmed breast cancer diagnosis based on review of clinical assessments in the medical records and on pathology reports. The cancer stage was ascertained from clinical examination and imaging reports. There were 374 (38.7%) patients with distant metastatic disease at diagnosis who were excluded. Likewise, those who did not initiate treatment at HUM or received most of their cancer care at a different facility were also excluded. The final resultant cohort included 341 eligible patients (Fig. 1).
Figure 1.
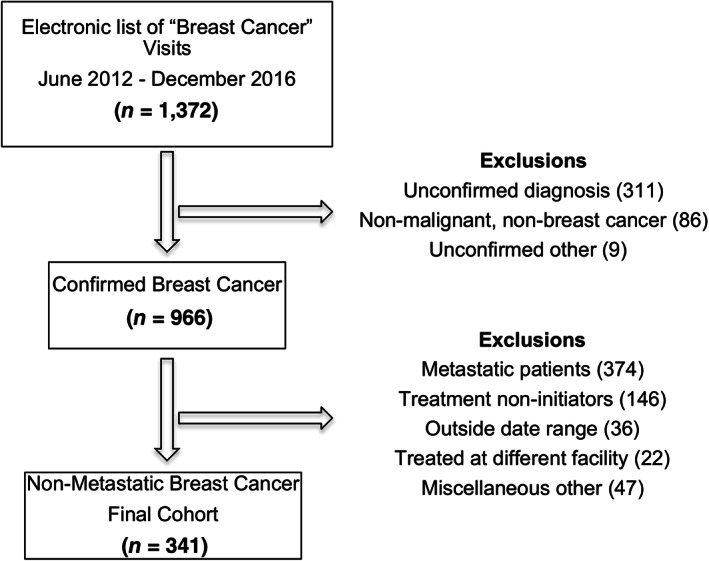
Cohort derivation.
Research and ethical approvals were obtained from the institutional review boards at Zanmi Lasante in Haiti, which governs local research at HUM, and from the Dana‐Farber/Harvard Cancer Center in Boston, MA.
Study Variables and Covariates
Comprehensive data were collected on patient characteristics at presentation, diagnostic information, treatments received, and outcomes including disease progression, recurrence, death, and loss to follow‐up. Study covariate data were manually abstracted from the medical records. The primary independent variable was stage at presentation, based on Union for International Cancer Control TNM staging, 7th edition classification [19]. Clinical stage I and stage II were classified as “early” and stage III as “locally advanced.” Patients with nonmetastatic disease with insufficient documentation in the medical records to classify were categorized as “unclear.” Other covariates of interest fell into the following categories: patient demographics, geographical information, prior medical and social history, pathologic classification, and treatment details.
Menopausal status was determined from recorded report of loss of periods for at least 12 months prior to presentation; for individuals with missing status (7.4%) age greater than 50 was deemed postmenopausal, based on menopausal age estimates in the Caribbean region [20]. Urban and rural residence classification was determined by the patient's recorded home residence location; this residence location was linked to a World Bank database, which classifies residence locations as urban or rural based on population density estimates [21]. Performance status was measured by the Eastern Cooperative Oncology Group scale [22]. Human immunodeficiency virus (HIV) positivity was determined from the medical records, as all patients with cancer were routinely tested for HIV. Smoking history and family history of cancers were obtained from the physicians’ notes. Pathologic subtype and grade were obtained from pathology reports based on World Health Organization classifications [23]. Estrogen receptor (ER) status was deemed positive for 1% cell positivity and higher as documented in pathology reports. Presentation date was defined as date of initial breast cancer clinical consultation at HUM. Time to definitive treatment date was defined as time from presentation date to start of earliest treatment, either definitive surgery or initiation of neoadjuvant therapy. Based on prior observational studies, delayed treatment initiation was defined as greater than 12 weeks [24, 25, 26].
Study Endpoints
The primary endpoint was event‐free survival (EFS), defined as time from presentation date to tumor recurrence, occurrence of a new breast cancer, progression of disease, or death from any cause. Overall survival (OS) was also assessed and defined as time from presentation date to death from any cause. Disease recurrence or progression was based on clinician documentation in the medical records, as determined from clinical examination, imaging, or pathologic confirmation. Individuals who had disease growth during or after neoadjuvant therapy, and as a result were unable to receive curative surgery, were deemed to have progressive disease. Death information was obtained from the medical records and from the HUM death registry.
Statistical Analysis
Baseline patient characteristics were described, and proportions were estimated within each covariate category. Similarly, treatment summaries were reported with estimated proportions within relevant clinical subgroups. Kaplan‐Meier methods were used to generate survival curves for the cohort. Log‐rank tests were used to compare survival curves between groups [27]. Cox proportional hazards regression analysis was used to determine the simultaneous effect of other variables potentially associated with the primary outcome [28]. The initial unadjusted model included the primary independent variable, disease stage. The final model further adjusted for potential confounders including menopausal status (premenopausal vs. postmenopausal), age (dichotomized at 50), pathologic grade (categorized as well‐ or moderately differentiated vs. poorly differentiated), ER status (positive vs. negative), time to definitive treatment (categorized as surgery prior to presentation, treatment within 12 weeks, and treatment after 12 weeks), and home location (urban vs. rural). The selection of covariates for the final model was based on clinical significance and previous studies. Except as otherwise noted, covariates with missing values were coded as a separate “unknown” category. A sensitivity analysis was performed to confirm the robustness of the findings; this analysis excluded pathologic grade covariate from the model because of the high proportion of missing values, as well as excluded individuals who obtained surgery prior to presentation and those with missing values in the other covariates.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All p values are two‐sided, and a threshold level of p < .05 was considered statistically significant. Data used for the analyses were abstracted between May 2018 and December 2018 and stored securely in a Research Electronic Data Capture (REDCap) database; deidentified data were electronically exported for analysis.
Results
Baseline Patient Characteristics
Baseline patient characteristics are described in Table 1. The median age at diagnosis was 49 years, with 64.2% being premenopausal at diagnosis. Patients represented all 10 administrative regions in the country, with a majority of patients (61.9%) coming from the West region, which includes Port‐au‐Prince, the country's capital and largest city. The Central Plateau region, where the hospital is located, accounted for 9.1% of the patients. Most patients (61.6%) lived in urban areas. Of those with known values, most patients were nonsmokers (93.5%) and were HIV negative (95.9%). The most common presenting symptoms were breast mass (89.4%) and breast pain (26.4%). The majority of patients (93.3%) had at least one form of staging imaging. A summary of imaging modalities used is presented in supplemental online Table 1; the most commonly performed staging imaging studies were chest X‐ray (49.9%), chest computed tomography (CT) scan (34.9%), and abdominal ultrasound (51.6%). A large proportion (55.1%) of patients were staged as locally advanced, whereas the staging was “unclear” in 17.6%. Pathology reports were present in the medical records of most patients (82.7%), and the most common histology was invasive ductal carcinoma. There was a substantial amount of missing data on breast biopsy pathology reports: 41.1% did not specify pathologic grade, and 44.9% were missing hormone receptor status. When taking into account both diagnostic and surgical resection specimens, hormone receptor status was known in 257 (75.4%) patients; of those, 165 (64.2%) were ER positive. Because of financial constraints, testing for progesterone receptor (PR) status and HER2 amplification were not routinely performed in Haiti.
Table 1.
Patient characteristics
| Characteristics | Total cohort (n = 341), n (%) |
|---|---|
| Age, median (IQR) | 49 (42–58) |
| Premenopausal | 219 (64.2) |
| Regional home location | |
| Artibonite | 41 (12.0) |
| Centre | 31 (9.1) |
| Grande‐Anse | 7 (2.1) |
| Nippes | 2 (0.6) |
| North | 18 (5.3) |
| North East | 3 (0.9) |
| North West | 9 (2.6) |
| West | 211 (61.9) |
| South | 13 (3.81) |
| South East | 6 (1.7) |
| Urban home location | 210 (61.6) |
| Never smokers | 319 (93.5) |
| Performance status (ECOG) a | |
| 0–1 | 71 (20.8) |
| 2–4 | 1 (0.3) |
| Unknown | 269 (78.8) |
| HIV status | |
| Positive | 5 (1.5) |
| Negative | 327 (95.9) |
| Unknown | 9 (2.6) |
| Family history of cancer | |
| Breast cancer b | 11 (3.2) |
| Other cancers | 4 (1.2) |
| Presenting symptoms c | |
| Breast mass | 305 (89.4) |
| Breast pain | 90 (26.4) |
| Breast discharge | 4 (1.2) |
| Other | 12 (3.5) |
| Staging imaging c | |
| Chest X‐ray | 170 (49.9) |
| CT scan of chest | 119 (34.9) |
| Abdominal ultrasound | 176 (51.6) |
| CT scan of abdomen | 4 (1.2) |
| No imaging | 23 (6.7) |
| T stage d | |
| T1–2 | 79 (23.2) |
| T3 | 169 (49.6) |
| T4 | 13 (3.8) |
| Unknown | 80 (23.5) |
| N stage e | |
| N0 | 109 (32.0) |
| N1 | 130 (38.1) |
| N2 | 28 (8.2) |
| N3 | 9 (2.6) |
| Unknown | 65 (19.1) |
| Final disease stage | |
| Early | 93 (27.3) |
| Locally advanced | 188 (55.1) |
| Unclear | 60 (17.6) |
| Diagnostic pathology report | 282 (82.7) |
| Diagnostic biopsy hormone receptor status | |
| ER positive | 122 (35.8) |
| ER negative | 66 (19.4) |
| ER unknown | 153 (44.9) |
| Final hormone receptor status f | |
| ER positive | 165 (48.4) |
| ER negative | 92 (27.0) |
| ER unknown | 84 (24.6) |
| Diagnostic biopsy grade | |
| Well‐ or moderately differentiated | 76 (22.3) |
| Poorly differentiated | 125 (36.7) |
| Unknown | 140 (41.1) |
| Biopsy histologic subtype | |
| Invasive ductal | 224 (65.7)) |
| Invasive lobular | 12 (3.5) |
| Other g | 46 (13.5) |
| Unknown | 59 (17.3) |
ECOG performance statuses are as follows: ECOG 0, Fully active, able to carry on all pre‐disease performance without restriction; ECOG 1, Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; ECOG 2, Ambulatory and capable of all self‐care but unable to carry out any work activities; up and about more than 50% of waking hours; ECOG 3, Capable of only limited self‐care; confined to bed or chair more than 50% of waking hours; ECOG 4, Completely disabled; cannot carry on any self‐care; totally confined to bed or chair.
Breast cancer in four mothers, two sisters, and five aunts.
Not mutually exclusive categories.
T stages are as follows: T1, Tumor ≤20 millimeters in greatest dimension; T2, Tumor >20 millimeters but ≤50 millimeters in greatest dimension; T3, Tumor >50 millimeters in greatest dimension; T4, Tumor of any size with direct extension to the chest wall and/or to the skin.
N stages are as follows: N0, No regional lymph node metastases; N1, Metastases to movable ipsilateral level I, II axillary lymph node(s); N2, Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted; or in clinically detected ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastases; N3, Metastases in ipsilateral infraclavicular (level III axillary) or in clinically detected ipsilateral internal mammary lymph node(s) with clinically evident level I, II axillary lymph node metastases or metastases in ipsilateral supraclavicular lymph node(s).
Receptor status from diagnostic biopsy or surgery.
Mixed ductal/lobular carcinoma (n = 8), spindle cell (n = 5), Paget's disease (n = 2), invasive mucinous (n = 1), and adenocarcinoma unspecified (n = 5).
Abbreviations: CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HIV, human immunodeficiency virus; IQR, interquartile range; N, regional nodal stage; T, primary tumor stage.
Treatment Received
Curative‐intent surgical resections were performed in 278 patients (81.5%; supplemental online Table 2.) Loss to follow‐up prior to surgery occurred in 46 patients and was the most common reason (71.8%) for not receiving a surgical resection. Some patients (12.3%) had surgical resection at other facilities prior to presentation.
Systemic treatments were classified as neoadjuvant therapy and adjuvant therapy, as well as endocrine therapy and chemotherapy (Table 2). Overall, 160 (46.9%) patients received neoadjuvant therapy, 31 (33.3%) of those with early stage disease and 115 (61.2%) of those with locally advanced disease. In the neoadjuvant setting, 49 patients received endocrine therapy, 121 patients received chemotherapy, and 10 patients received both. Of the patients who underwent curative surgery, 70 (85.4%) of those with early stage disease and 105 (71.9%) of those with locally advanced disease subsequently received adjuvant therapy. In the adjuvant setting, 166 patients received endocrine therapy, 164 patients received chemotherapy, and 105 patients received both. Adjuvant endocrine therapy was initiated in 114 (82.0%) of the patients with ER‐positive disease, 42 (68.8%) of those with ER‐unknown disease, and 10 (12.8%) of those with ER‐negative disease (supplemental online Table 3). Lastly, treatment initiation delays of more than 12 weeks occurred in 142 (41.6%) patients.
Table 2.
Systemic therapy details by stage
| Systemic therapy | Total, n | Early stage, n (%) | Locally advanced, n (%) | Stage unclear, n (%) |
|---|---|---|---|---|
| Neoadjuvant therapy a | (n = 341) | (n = 93) | (n = 188) | (n = 60) |
| Total | 160 | 31 (33.3) | 115 (61.2) | 14 (23.3) |
| Hormonal | 49 | 11 (11.8) | 32 (17.0) | 6 (10.0) |
| Chemotherapy | 121 | 22 (23.7) | 90 (47.8) | 9 (15.0) |
| Both | 10 | 2 (2.2) | 7 (3.7) | 1 (1.7) |
| Adjuvant therapy a | (n = 278) | (n = 82) | (n = 146) | (n = 50) |
| Total | 225 | 70 (85.4) | 105 (71.9) | 50 (100) |
| Hormonal | 166 | 54 (68.6) | 75 (51.4) | 37 (74.0) |
| Chemotherapy | 164 | 53 (64.6) | 80 (54.8) | 31 (62.0) |
| Both | 105 | 37 (45.1) | 50 (34.2) | 18 (36.0) |
Not mutually exclusive categories.
Outcomes
Median follow‐up time from presentation was 24.2 months with longest follow‐up time of 66.7 months. During the follow‐up period, 77 patients had disease recurrence or new breast cancer, 10 had progressive disease, and 28 patients died; 8 of the deaths occurred in patients without documented recurrence or progression. EFS and OS curves for the entire cohort are presented in supplemental online Figures 1 and 2, respectively. EFS rates at 2‐ and 3‐year time points were 80.9% and 63.4%, respectively.
Because of the relatively short follow‐up time, median EFS from Kaplan‐Meier estimates was not reached for the patients with early stage breast cancer. Median EFS for patients with locally advanced disease was 39.6 months (95% confidence interval [CI], 33.5 to unreached). The difference in survival estimates by disease stage was statistically significant with p < .0001 (Table 3). Kaplan‐Meier survival estimates by ER status showed that patients with ER‐negative disease had statistically significant shorter median EFS compared with individuals with ER‐positive disease (Table 3). Unadjusted EFS curves by disease stage and ER status are presented in Figures 2 and 3, respectively. Unadjusted OS estimates were similarly plotted by disease stage and ER status in supplemental online Figures 3 and 4, respectively. Because of the short follow‐up time, median OS estimates were not reached for the individual subgroups. Patients with locally advanced disease had shorter OS than those with early stage disease (p = .035); likewise, patients with ER‐negative disease had shorter survival that those with ER‐positive disease (p = .0025). Unadjusted EFS curves for patients with ER‐positive disease, grouped by whether or not adjuvant endocrine therapy was initiated, are shown in supplemental online Figure 5; median EFS in the noninitiators was markedly shorter at 28.3 months (95% CI, 18.1–39.6) compared with the initiators (median not unreached).
Table 3.
Event‐free survival outcomes by stage and estrogen receptor status
| Characteristic | Events/At risk, n (%) | EFS, median (95% CI), months | Log‐rank p value |
|---|---|---|---|
| Total cohort | 95/341 (27.9) | 49.5 (43.3, unreached) | |
| Stage | |||
| Early stage | 15/93 (16.1) | Unreached | <.0001 |
| Locally advanced | 75/188 (39.9) | 39.6 (33.5–49.0) | |
| Stage unclear | 5/60 (8.3) | Unreached | |
| ER status | |||
| ER positive | 41/165 (24.8) | 53.3 (44.2, unreached) | .004 |
| ER negative | 35/92 (38.0) | 36.8 (30.4, 65.0) | |
| ER unknown | 19/85 (22.6) | 50.1 (40.3, unreached) |
Abbreviations: CI, confidence interval; EFS, event‐free survival; ER, estrogen receptor.
Figure 2.
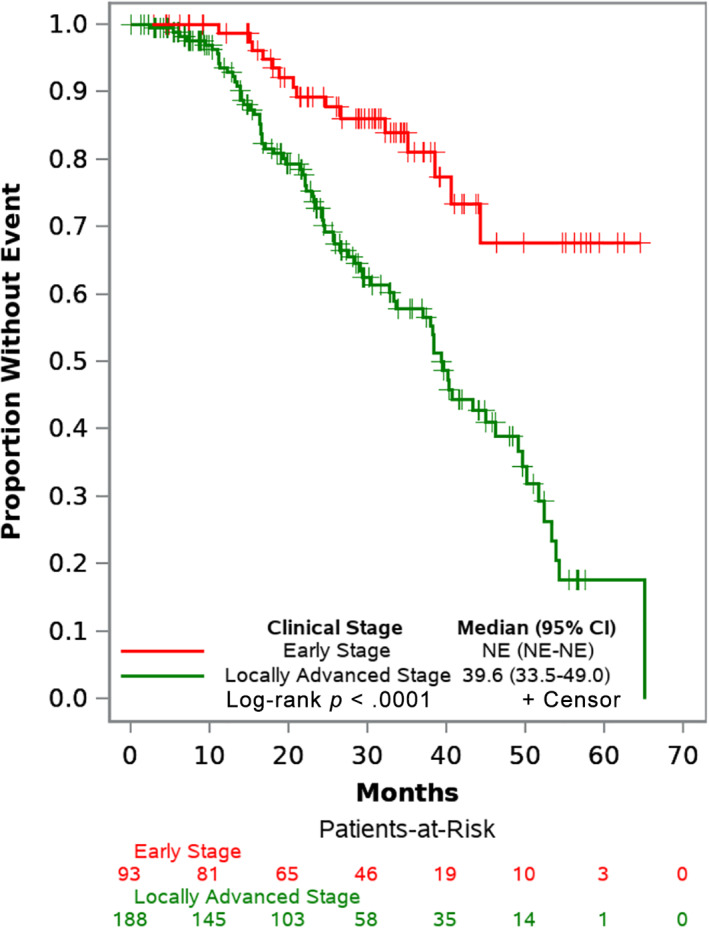
Event‐free survival curves by stage. Abbreviations: CI, confidence interval; NE, unreached and unable to estimate.
Figure 3.
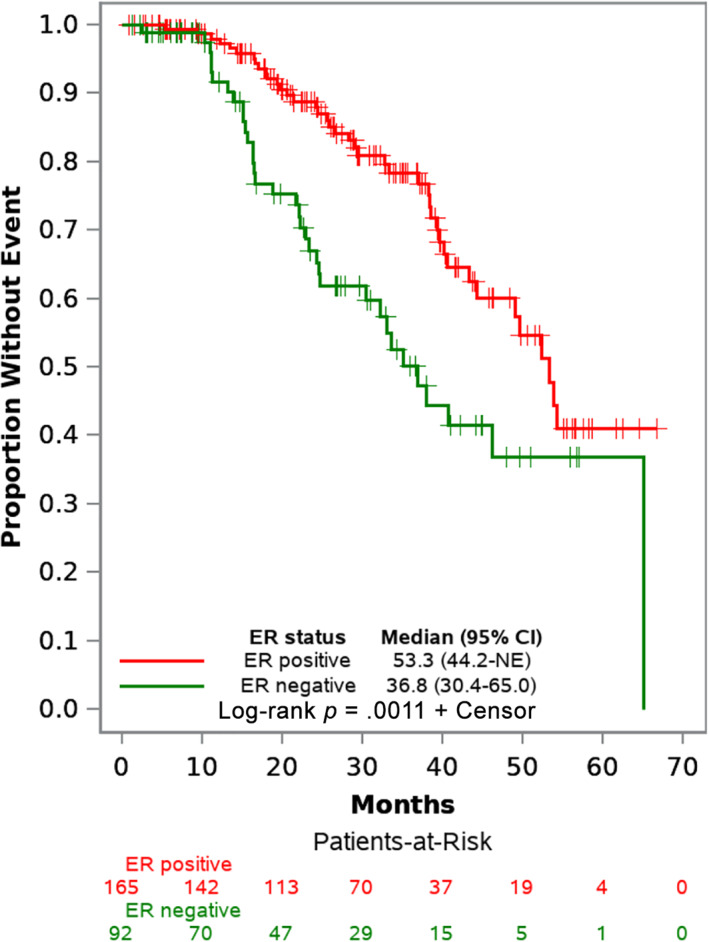
Event‐free survival curves by ER status. Abbreviations: CI, confidence interval; ER, estrogen receptor; NE, unreached and unable to estimate.
Furthermore, after controlling for menopausal status, age, pathologic grade, ER status, time to treatment, and home location, individuals with locally advanced stage experienced an adjusted hazard ratio (HR) for EFS of 3.27 (95% CI, 1.86–5.76; p < .0001) compared with individuals with early stage disease. In exploratory multivariate analyses, ER‐negative disease had an association with worse outcomes and adjusted HR for EFS of 1.91 (95% CI, 1.16–3.14; p = .01); age less than 50 at diagnosis had a borderline statistically significant association with worse outcomes and adjusted HR for EFS of 1.73 (95% CI, 0.99–3.03; p = .05). There was no statistically significant association between EFS and the other factors explored (Table 4).
Table 4.
Exploratory Cox proportional hazards regression analyses of factors associated with event‐free survival outcome
| Factors | Hazard ratio (95% CI) | Multivariate p value |
|---|---|---|
| Disease stage | ||
| Early stage | Reference | |
| Locally advanced stage | 3.27 (1.86–5.76) | <.0001 |
| Estrogen receptor status | ||
| ER positive | Reference | |
| ER negative | 1.91 (1.16–3.14) | .01 |
| Age at diagnosis | ||
| 50 and older | Reference | |
| Less than 50 | 1.73 (0.99–3.03) | .05 |
| Menopausal status | ||
| Postmenopausal | Reference | |
| Premenopausal | 0.78 (0.43–1.42) | .42 |
| Pathology grade | ||
| Well and moderately differentiated | Reference | |
| Poorly differentiated | 1.03 (0.54–1.98) | .93 |
| Home location | ||
| Urban dwellers | Reference | |
| Rural dwellers | 0.71 (0.44–1.12) | .14 |
| Timing of definitive treatment | ||
| Within 12 weeks | Reference | |
| After 12 weeks | 1.24 (0.81–1.90) | .32 |
| Surgery prior to presentation | 0.96 (0.36–2.61) | .94 |
Abbreviations: CI, confidence interval; ER, estrogen receptor.
The sensitivity analysis, excluding individuals with missing values in the relevant covariates, showed similar HR estimates (supplemental online Table 4). In this analysis, care initiation delays also resulted in a higher adjusted HR for EFS of 1.87 (95% CI, 1.12–3.14; p = .017) compared with individuals who initiated care within 12 weeks.
Discussion
This study describes care delivery and early survival outcomes in a cohort of patients with nonmetastatic breast cancer treated in Haiti. The median age at diagnosis of patients included in the cohort was relatively young at 49 years. This finding is consistent with prior studies that have reported younger onset breast cancer in Caribbean women compared with women in the U.S. Despite several limitations to making cross‐study comparisons because of varying study methodologies, highlighting themes in similar regional and international cohorts may provide a useful context for understanding our results. Other breast cancer studies reported median ages at diagnosis of 49 and 50 in native Haitians [12, 29], 54 in Haitians living in the U.S. [29], and a range from 52 to 61 in Latin American countries [30]. In contrast, the median age at diagnosis in the overall U.S. breast cancer population is 62, whereas it is slightly younger at 59 in black Americans [31]. The younger median age may be reflective of an overall younger population in Haiti; most recent estimates place the median age of the Haitian population at 22.7 years compared with 37.6 years in the U.S. [32]. In addition, the age differential at breast cancer diagnosis may reflect differences in disease biology, which need to be further characterized.
The cohort also highlighted the high proportion of patients with advanced disease at presentation. During the cohort derivation, 39% of women with confirmed breast cancer had metastatic disease and were excluded. Of those included, more than 95% of the patients had symptomatic disease at presentation, with more than half of the patients having masses greater than 5 cm and staged as locally advanced disease. The rate of advanced disease at presentation in the Haitian population appears higher than rates from other published studies on Caribbean populations [10, 12, 29, 33, 34, 35]. However, in a recent systematic review of studies from low‐resource settings in sub‐Saharan Africa, some countries had even higher rates of advanced disease, with some exceeding 90% [36]. Advanced disease is thought to be primarily driven by delays in presentation after symptom onset. A prior study in Haiti highlighted the following factors as being associated with delayed presentation in patients with breast cancer: lower educational status, failure to recognize significance of symptoms, and fear of treatment cost [15]. Another probable contributory factor is the lack of national breast cancer early detection programs in Haiti, as is the case in many low‐resource settings.
Limited diagnostic pathology capacity is also a recognized barrier in the delivery of breast cancer care in low‐resource settings [37, 38]. Diagnostic pathology capacity in Haiti was centralized to a few labs in the capital city of Port‐au‐Prince until 2016. These labs had limited capacity to perform even basic tissue processing functions such as making tissue blocks, mounting slides, and hematoxylin and eosin staining, whereas more advanced testing, including immunohistochemistry and molecular diagnostics, was unavailable anywhere in the country. In order to mitigate these deficiencies, HUM developed a formal collaboration with external partners, including Dana‐Farber Cancer Institute and Brigham and Women's Hospital, whereby many patients treated at HUM had their tissue samples sent outside the country for diagnostic pathology [39]. A pathology lab became functional at HUM in 2016. Through mentoring and capacity building, the lab can now provide basic diagnostic pathology services. The cohort population highlights the need for basic pathology and immunohistochemistry testing for ER status, which directly affects treatment options for patients. At the time of the study, HER2 testing was not routinely performed on breast pathology samples because of the unavailability of HER2‐directed therapy. Unfortunately, PR testing was also not routinely performed; hence, the rates of triple‐negative disease are unknown.
The treatment paths for patients with breast cancer at HUM were based on a joint decision between the oncology clinicians and general surgeons. Most patients with early breast cancer as assessed by the surgeon proceeded directly to surgery, and pathologic features after resection determined further adjuvant therapy. In contrast, most patients with locally advanced disease were deemed poor resection candidates and received neoadjuvant therapy. The rate of neoadjuvant therapy in our overall cohort (47%) is markedly higher than rates in the U.S. (17%–23%), which is reflective of the higher proportion of advanced breast cancer stages diagnosed in Haiti [40, 41]. Interestingly, the rate of neoadjuvant chemotherapy use among patients with locally advanced disease was still only 47.8%. This rate is lower than expected, as most international treatment guidelines would recommend neoadjuvant chemotherapy for this group of patients.
Similarly, there was suboptimal staging imaging for patients with locally advanced disease with only 44.2% having documented chest and abdominal imaging evaluation by X‐ray, abdominal ultrasound, or CT scans. Advanced imaging techniques, including nuclear studies, magnetic resonance imaging, and positron emission tomography scan, are not readily available in Haiti. The reasons for the underuse of the available imaging modalities in these patients were not elucidated in this study but may be related to periodic radiologic equipment failure at HUM and variation in clinician practice patterns.
Surgery completion rates were, likewise, suboptimal, as almost 20% of patients in the overall cohort did not obtain definitive surgery; most of these patients were lost to follow‐up. A few patients received partial mastectomies, and some had no axillary sampling. No patients in the cohort received radiation therapy, as this modality is unavailable in Haiti. With the lack of radiation therapy, modified radical mastectomy is the procedure of choice. The stigma around loss of the whole breast likely contributed to the loss to follow‐up before mastectomy [42, 43].
Use of adjuvant endocrine therapy was appropriately dictated by ER status. The rate of adjuvant endocrine therapy initiation in patients with ER‐positive tumors was 82%, comparable to the rates reported in U.S. cohorts [44, 45, 46]. Adjuvant endocrine therapy is a vital component in the care of these patients, and, as expected, therapy initiation was associated with longer EFS. Interestingly, neoadjuvant endocrine therapy was used in some patients. This practice has been reported in other low‐resource settings [47, 48]. There were no explicit criteria for neoadjuvant endocrine therapy use; anecdotally, HUM clinicians noted use in patients for whom there was a concern for poor tolerance of chemotherapy because of significant comorbidities.
The primary analysis endpoint, EFS, was chosen because of the relatively short length of follow‐up time as well as the challenges with death ascertainment from the lack of a reliable centralized national death registry. There are limitations to comparing the study survival estimates with others in similar low‐resource settings, as most studies report OS and few segregate out patients with nonmetastatic disease. There were no directly comparable studies from the Caribbean region, but two studies from sub‐Saharan Africa had similar endpoints. In comparison with the 3‐year EFS rate of 63.4% reported in this Haiti cohort, a South African study reported a 3‐year disease‐free survival rate of 72%, whereas the other study from Ethiopia reported a 3‐year metastasis‐free survival rate of 46% [49, 50]. Moreover, 5‐year OS rates for patients with nonmetastatic breast cancer in high‐income countries like the U.S. are over 90% [51].
As expected, advanced stage disease was associated with shorter EFS, and the association persisted after controlling for several other factors that may confound the association. The exploratory multivariate and sensitivity analyses also revealed that ER‐negative status, age less than 50, and treatment initiation delays of more than 12 weeks were each associated with shorter EFS. These analyses did not show any associations with other possible covariates. Because of the sample size these exploratory analyses were likely not sufficiently powered to detect some true associations with smaller magnitude. The prognostic factors are largely consistent across other populations in the Caribbean region, within African cohorts, as well as within cohorts from high‐income countries [12, 29, 35, 49, 52, 53, 54].
There are several strengths of our study. This study cohort, to our knowledge, is the largest and most comprehensive assessment of treatment and clinical outcomes of patients with nonmetastatic breast cancer in Haiti. Identifying poor prognostic factors in patients with nonmetastatic disease is particularly important as these are potentially curable patients who can benefit significantly from optimizing care delivery. The analyses allowed us to control for multiple confounders in our attempt to uncover associations between variables of interest and EFS. Lastly, the patient cohort will be followed over time with updates to survival endpoints and serve as a basis for future exploratory studies.
Moreover, there are limitations to the study. The follow‐up time was relatively short, with a median of approximately 2 years, which limits robust assessment of OS outcomes. Subsequent analyses of the cohort after longer follow‐up may allow for more accurate OS analyses. In addition, as is the case with retrospective studies, there is a potential for selection bias in the identification of the cohort. There may be systematic differences in individuals who were excluded from the study or those who did not get care at HUM. As a result, findings from this cohort may not be generalizable across patients with breast cancer in all of Haiti. However, patients from every region in the country were represented within the cohort, and HUM offers the most comprehensive level of oncology care currently available in Haiti.
In addition, because of the retrospective nature of the study, there were some missing covariate data. For covariates with missing data we created a separate variable category as detailed in the Materials and Methods section. This method was chosen over imputation because of limitations with assessing pattern and randomness of missing data. In order to preserve the sample size, patients with missing data were not excluded from the primary analysis. Missing data variable categories were included within the regression models, which may introduce uncertainty into the models. However, we performed a sensitivity analysis excluding patients with missing data, and the association trends between EFS and the relevant predictors remained largely unchanged. Lastly, although we controlled for several known confounders and clinically significant factors, there remains a possibility that unmeasured covariates may confound the associations that were reported.
Conclusion
This study outlines the delivery of breast cancer care for patients with nonmetastatic disease at a tertiary care facility in Haiti, a low‐resourced country. The study shows that the availability of basic breast cancer treatments can lead to promising early outcomes. Some poor prognostic factors identified within this cohort included young age, advanced disease at presentation, and ER‐negative status. Rigorous context‐appropriate studies are needed to understand the underlying determinants of these poor prognostic factors.
Furthermore, the study identified some potential areas for improvement even within the confines of the limited available diagnostic and treatment capacity. Strategies for optimizing breast cancer care at this facility may focus on increasing surgical resection rates, increasing the use of neoadjuvant chemotherapy among patients with locally advanced disease, and encouraging the initiation of adjuvant endocrine therapy in those with ER‐positive disease. Despite the gaps and limitations, delivery of curative breast cancer treatment in Haiti is possible.
Author Contributions
Conception/design: Temidayo Fadelu, Ruth Damuse, Elizabeth Pecan, Timothy Rebbeck, Lawrence N. Shulman
Provision of study material or patients: Temidayo Fadelu, Ruth Damuse, Joarly Lormil, Viergela Pierre
Collection and/or assembly of data: Temidayo Fadelu, Ruth Damuse, Elizabeth Pecan, Cyrille Dubuisson, Viergela Pierre
Data analysis and interpretation: Temidayo Fadelu, Ruth Damuse, Elizabeth Pecan, Timothy Rebbeck, Lawrence N. Shulman
Manuscript writing: Temidayo Fadelu, Ruth Damuse, Joarly Lormil, Elizabeth Pecan, Cyrille Dubuisson, Viergela Pierre, Timothy Rebbeck, Lawrence N. Shulman
Final approval of manuscript: Temidayo Fadelu, Ruth Damuse, Joarly Lormil, Elizabeth Pecan, Cyrille Dubuisson, Viergela Pierre, Timothy Rebbeck, Lawrence N. Shulman
Disclosures
Lawrence N. Shulman: Breast Cancer Research Foundation, Celgene (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.
Acknowledgments
We acknowledge the financial and administrative support of the Center for Global Cancer Medicine at Dana‐Farber Cancer Institute. We also acknowledge the administrative support from Zanmi Lasante executive and clinical leadership and Partners In Health in Boston. The views expressed in the submitted article are those of the authors and not an official position of their affiliated institutions or funders. We are grateful to the following individuals for their contributions: Lori Buswell, Scott Triedman, Lauren Greenberg, Christine Brown, Joia Mukherjee, Mary Clisbee, and all the clinicians and staff at the University Hospital Mirebalais oncology department. Lastly, we are very grateful to the patients. Preliminary results were presented as an e‐poster at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2019.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. DeSantis CE, Bray F, Ferlay J et al. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev 2015;24:1495–1506. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4.Population fact sheets. International Agency for Research on Cancer Web site. Available at https://gco.iarc.fr/today/fact-sheets-populations. Accessed April 26, 2018.
- 5. Razzaghi H, Quesnel‐Crooks S, Sherman R et al. Leading causes of cancer mortality ‐ Caribbean region, 2003–2013. MMWR Morb Mortal Wkly Rep 2016;65,:1395–1400. [DOI] [PubMed] [Google Scholar]
- 6. Curado MP, Bezerra de Souza DL. Cancer burden in Latin America and the Caribbean. Ann of Glob Health 2014;80:370–377. [DOI] [PubMed] [Google Scholar]
- 7. Sierra MS, Soerjomataram I, Antoni S et al. Cancer patterns and trends in Central and South America. Cancer Epidemiol 2016;44(suppl 1):S23–S42. [DOI] [PubMed] [Google Scholar]
- 8. Justo N, Wilking N, Jönsson B et al. A review of breast cancer care and outcomes in Latin America. The Oncologist 2013;18:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ragin C, Banydeen R, Zhang C et al. Breast cancer research in the Caribbean: Analysis of reports from 1975 to 2017. J Glob Oncol 2018;4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. George SH, Donenberg T, Akbari M et al. Breast cancer in the Caribbean – A six‐country cohort. Cancer Epidemiol Biomarkers Prev 2016;25(suppl 3):B50a. [Google Scholar]
- 11. Hennis AJ, Hambleton IR, Wu SY et al. Breast cancer incidence and mortality in a Caribbean population: Comparisons with African‐Americans. Int J Cancer 2009;124:429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeGennaro V, Jiwani F, Patberg E et al. Epidemiological, clinical, and histopathological features of breast cancer in Haiti. J Glob Oncol 2018;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.After earthquake, university hospital is transforming lives in Haiti [news release]. Boston, MA: Partners In Health, January 12, 2014. Available at https://www.pih.org/article/on‐earthquake‐anniversary‐university‐hospital‐is‐transforming‐lives‐in‐hait. Accessed July 24, 2019.
- 14.The evolution of cancer care in Haiti [news release]. Boston, MA: Partners In Health, March 19, 2019. Available at https://www.pih.org/article/evolution-cancer-care-haiti. Accessed July 24, 2019.
- 15. Sharma K, Costas A, Damuse R et al. The Haiti Breast Cancer Initiative: Initial findings and analysis of barriers‐to‐care delaying patient presentation. J Oncol 2013;2013:e206367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeGennaro V, Libby R, Patberg E et al. Development of a breast cancer treatment program in Port‐au‐Prince, Haiti: Experiences from the field. J Glob Oncol 2016;2:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Global Cancer Medicine: Haiti Clinical Care. Dana‐Farber Cancer Institute Web site. Available at https://www.dana-farber.org/center-for-global-cancer-medicine/haiti-clinical-care/. Accessed March 2, 2020.
- 18. OpenMRS Web site. Available at https://openmrs.org/. Accessed July 24, 2019. [Google Scholar]
- 19. Edge SB, Byrd DR, Compton CC. et al., eds.; American Joint Committee on Cancer: AJCC Cancer Staging Manual, 7th ed New York, NY: Springer, 2010. [Google Scholar]
- 20. Vélez MP, Alvarado B, Lord C et al. Life course socioeconomic adversity and age at natural menopause in women from Latin America and the Caribbean. Menopause 2010;17:552–559. [PubMed] [Google Scholar]
- 21. Lozano‐Gracia N, Lozano MG, eds. Haitian Cities: Actions for Today with an Eye on Tomorrow. Washington, DC: International Bank for Reconstruction and Development/The World Bank, 2017. Available at http://documents.worldbank.org/curated/en/709121516634280180/pdf/122880‐V1‐WP‐P156561‐OUO‐9‐FINAL‐ENGLISH.pdf. Accessed July 24, 2019. [Google Scholar]
- 22. Zubrod CG, Schneiderman M, Frei E et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis 1960;11:7–33. [Google Scholar]
- 23. Lakhani SR, Ellis IO, Schnitt SJ et al., eds. WHO Classification of Tumours of the Breast, 4th ed. Lyon, France: International Agency for Research on Cancer, 2012. [Google Scholar]
- 24. Richards MA, Westcombe AM, Love SB et al. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet 1999;353:1119–1126. [DOI] [PubMed] [Google Scholar]
- 25. Richards MA, Smith P, Ramirez AJ et al. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer 1999;79:858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shin DW, Cho J, Kim SY et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol 2013;20:2468–2476. [DOI] [PubMed] [Google Scholar]
- 27. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 28. Cox DR, Oakes D. Analysis of Survival Data. Boca Raton, FL: CRC Press, 1984. [Google Scholar]
- 29. Gomez A, DeGennaro V, George SHL et al. Presentation, treatment, and outcomes of Haitian women with breast cancer in Miami and Haiti: Disparities in breast cancer—a retrospective cohort study. J Glob Oncol 2016;3:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villarreal‐Garza C, Aguila C, Magallanes‐Hoyos MC et al. Breast cancer in young women in Latin America: An unmet, growing burden. The Oncologist 2013;18:26–34. [DOI] [PubMed] [Google Scholar]
- 31. DeSantis CE, Ma J, Sauer AG et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439–448. [DOI] [PubMed] [Google Scholar]
- 32.World Population Prospects 2019 [custom data acquired via Web site]. United Nations Population Division Web site. Available at https://population.un.org/wpp. Accessed April 6, 2020.
- 33. Chin SN, Green CMA, Gordon‐Strachan GM et al. Locally advanced breast cancer in Jamaica: Prevalence, disease characteristics and response to preoperative therapy. Asian Pac J Cancer Prev 2014;15:3323–3326. [DOI] [PubMed] [Google Scholar]
- 34. Chin SN, Green C, Strachan GG et al. Clinicopathologic characteristics of breast cancer in Jamaica. Asian Pac J Cancer Prev 2014;15:3319–3322. [DOI] [PubMed] [Google Scholar]
- 35. Deloumeaux J, Gaumond S, Bhakkan B et al. Incidence, mortality and receptor status of breast cancer in African Caribbean women: Data from the cancer registry of Guadeloupe. Cancer Epidemiol 2017;47:42–47. [DOI] [PubMed] [Google Scholar]
- 36. Jedy‐Agba E, McCormack V, Adebamowo C et al. Stage at diagnosis of breast cancer in sub‐Saharan Africa: A systematic review and meta‐analysis. Lancet Glob Health 2016;4:e923–e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martei YM, Pace LE, Brock JE et al. Breast cancer in low‐ and middle‐income countries. Clin Lab Med 2018;38:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pace LE, Shulman LN. Breast cancer in Sub‐Saharan Africa: Challenges and opportunities to reduce mortality. The Oncologist 2016;21:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orozco JD, Greenberg LA, Desai IK et al. Building laboratory capacity to strengthen health systems: The Partners In Health experience. Clin Lab Med 2018;38:101–117. [DOI] [PubMed] [Google Scholar]
- 40. Mougalian SS, Soulos PR, Killelea BK et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015;121:2544–2552. [DOI] [PubMed] [Google Scholar]
- 41. Killelea BK, Yang VQ, Wang SY et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: Results from the National Cancer Data Base. J Clin Oncol 2015;33:4267–4276. [DOI] [PubMed] [Google Scholar]
- 42. Akuoko CP, Armah E, Sarpong T et al. Barriers to early presentation and diagnosis of breast cancer among African women living in sub‐Saharan Africa. PLoS One 2017;12:e0171024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martei YM, Vanderpuye V, Jones BA. Fear of mastectomy associated with delayed breast cancer presentation among Ghanaian women. The Oncologist 2018;23:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farias AJ, Du XL. Racial differences in adjuvant endocrine therapy use and discontinuation in association with mortality among Medicare breast cancer patients by receptor status. Cancer Epidemiol Biomarkers Prev 2017;26:1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farias AJ, Du XL. Ethnic differences in initiation and timing of adjuvant endocrine therapy among older women with hormone receptor‐positive breast cancer enrolled in Medicare Part D. Med Oncol 2016;33:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Daly B, Olopade OI, Hou N et al. Evaluation of the quality of adjuvant endocrine therapy delivery for breast cancer care in the United States. JAMA Oncol 2017;3:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Agrawal LS, Pereira ML, Mayer IA et al. Neoadjuvant endocrine therapy in locally advanced hormone receptor positive (HR+) breast cancer (BC) in a low‐resource, middle‐income setting (Guatemala). J Clin Oncol 2015;33(suppl 15):e17594a. [Google Scholar]
- 48. Carlson RW, Anderson BO, Chopra R et al. Treatment of breast cancer in countries with limited resources. Breast J 2003;9(suppl 2):S67–S74. [DOI] [PubMed] [Google Scholar]
- 49. Cubasch H, Dickens C, Joffe M et al. Breast cancer survival in Soweto, Johannesburg, South Africa: A receptor‐defined cohort of women diagnosed from 2009–11. Cancer Epidemiol 2018;52:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gakwaya A, Kigula‐Mugambe JB, Kavuma A et al. Cancer of the breast: 5‐year survival in a tertiary hospital in Uganda. Br J Cancer 2008;99:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Howlader N, Noone AM, Krapcho M. et al., eds. SEER Cancer Statistics Review, 1975‐2016. Bethesda, MD: National Cancer Institute. Based on November 2018 SEER data submission and posted to the SEER Web site April 2019. Available at https://seer.cancer.gov/csr/1975_2016/.
- 52. Camacho‐Rivera M, Ragin C, Roach V et al. Breast cancer clinical characteristics and outcomes in Trinidad and Tobago. J Immigr Minor Health 2015;17:765–772. [DOI] [PubMed] [Google Scholar]
- 53. Eber‐Schulz P, Tariku W, Reibold C et al. Survival of breast cancer patients in rural Ethiopia. Breast Cancer Res Treat 2018;170:111–118. [DOI] [PubMed] [Google Scholar]
- 54. Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early‐stage breast cancer. The Oncologist 2004;9:606–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Figures.
Appendix S2. Tables.


