Abstract
Background and aims
Emerging data have linked the presence of cardiac injury with a worse prognosis in novel coronavirus disease 2019 (COVID-19) patients. However, available data cannot clearly characterize the correlation between cardiac injury and COVID-19. Thus, we conducted a meta-analysis of recent studies to 1) explore the prevalence of cardiac injury in different types of COVID-19 patients and 2) evaluate the association between cardiac injury and worse prognosis (severe disease, admission to ICU, and mortality) in patients with COVID-19.
Methods and results
Literature search was conducted through PubMed, the Cochrane Library, Embase, and MedRxiv databases. A meta-analysis was performed with Stata 14.0. A fixed-effects model was used if the I2 values ≤ 50%, otherwise the random-effects model was performed. The prevalence of cardiac injury was 19% (95% CI: 0.15–0.22, and p < 0.001) in total COVID-19 patients, 36% (95% CI: 0.25–0.47, and p < 0.001) in severe COVID-19 patients, and 48% (95% CI: 0.30–0.66, and p < 0.001) in non-survivors. Furthermore, cardiac injury was found to be associated with a significant increase in the risk of poor outcomes with a pooled effect size (ES) of 8.46 (95% CI: 3.76–19.06, and p = 0.062), severe disease with an ES of 3.54 (95% CI: 2.25–5.58, and p < 0.001), admission to ICU with an ES of 5.03 (95% CI: 2.69–9.39, and p < 0.001), and mortality with an ES of 4.99 (95% CI: 3.38–7.37, and p < 0.001).
Conclusions
The prevalence of cardiac injury was greatly increased in COVID-19 patients, particularly in patients with severe disease and non-survivors. COVID-19 patients with cardiac injury are more likely to be associated with poor outcomes, severity of disease, admission to ICU, and mortality.
Keywords: COVID-19, SARS-CoV-2, Cardiac injury, Prevalence, Outcome
Highlights
-
•
We found that the pooled prevalence of cardiac injury was 19% in all the COVID-19 patients, 36% in severe COVID-19 patients, and 48% in non-survivors.
-
•
Cardiac injury was found to be associated with a significant increase in the mortality of patients with COVID-19.
-
•
COVID-19 patients with cardiac injury are more likely to be associated with poor outcome; therefore, more intensive attention and care should be paid to these patients.
Introduction
The recent outbreak of novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) continues to spread worldwide [1]. Up to now, July 13, 2020, at least 12,768,307 people have confirmed diagnosis and more than 566,654 infected cases have died across the globe (https://www.who.int). As there are no effective treatments, COVID-19 has now become one of the deadliest pandemics in modern history [2].
On the basis of recent researches, SARS-CoV-2 enters cells through the direct binding of the virus's spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor [3]. ACE2 has been identified, which has expressed predominantly in the lungs but also throughout the cardiovascular system [3]. Thus, though the most common consequences of COVID-19 are pulmonary manifestations, which cause severe pneumonia and respiratory distress syndrome, accumulating evidence suggests the increased frequency of a variety of cardiovascular complications in patients infected with this virus [[4], [5], [6], [7]]. Cardiac injury, defined as an increase in the troponin level above the 99th percentile's upper reference limit, is the most reported cardiac abnormality in COVID-19 patients [8,9]. Furthermore, there was evidence that COVID-19 patients with cardiac injury may face a greater risk of fatal outcomes. Therefore, we need to pay more attention to such patients and give them comprehensive management [4,9].
As a novel disease, the shortage of clinical data has greatly limited our understanding of the linkage between the cardiac injury and clinical outcome in COVID-19 patients. Moreover, data available provide wide variations of results and may have resulted in relatively poor conclusions. Therefore, we conducted a systemic review and meta-analysis to explore cardiac injury's prevalence and its connection with prognosis in patients with COVID-19.
Methods
Search strategy and study selection
The systematic review and meta-analysis were accomplished based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We performed a comprehensive systematic literature search from the following medical electronic database: Medline, Embase, PubMed, Medrvix, and Cochrane Central Databases with the search terms: (“COVID-19” OR “SARS-CoV-2”) AND (“heart injury” OR “cardiac injury” OR “myocardial injury”). The studies were retrieved from inception to June 5, 2020. In addition, we also manually reviewed the reference lists of relevant articles for potential studies. After an initial search, the duplicate results were then removed. The remaining articles were screened for relevance by their titles and abstracts by two authors independently (Guojun Zhao and Pan Huang). All selected potential articles were then comprehensively reviewed by the remaining investigators to ensure their eligibility for inclusion in this review. Disagreements about the eligibility of the literature were resolved by consensus based on the agreements of all investigators.
Eligibility criteria
We include all research studies that meet the following criteria: (1) cross-sectional studies or cohort studies and (2) investigating the prevalence and outcomes of cardiac injury among patients with COVID-19. Exclusion criteria were as follows: (1) children or pregnant, (2) review, and (3) language of studies with non-English.
Data extraction and quality assessment
Data extraction was performed by two independent authors using the standardized form. The following information was extracted: region, sample number, percentage of male subjects, age, study design, the definition of cardiac injury, and clinical outcome. Any disagreements were resolved through discussions or referral to a third author.
For quality assessment, the Newcastle–Ottawa Scale was applied to evaluate the quality and risk of bias of all the selected studies. The studies with 7 points or more were considered as high quality.
Statistical analysis
The statistical analysis was carried out using Stata 14.0 (Stata Corp, College Station, TX). The heterogeneity in included studies was assessed by using the Cochran's Q chi-square and I 2 statistic analysis. The fixed-effects model was used when the I 2 values ≤ 50%, otherwise the random-effects model was used. To investigate the association between disease severity and cardiac injury, we evaluated the pool prevalence in the severe patients and non-survivors. For clinical outcomes, we calculated the pooled effect size (ES) for the association of cardiac injury with all-cause mortality, admission to ICU, and disease severity. Publication bias were evaluated by the Egger test and Begger test.
Results
Study selection and characteristics
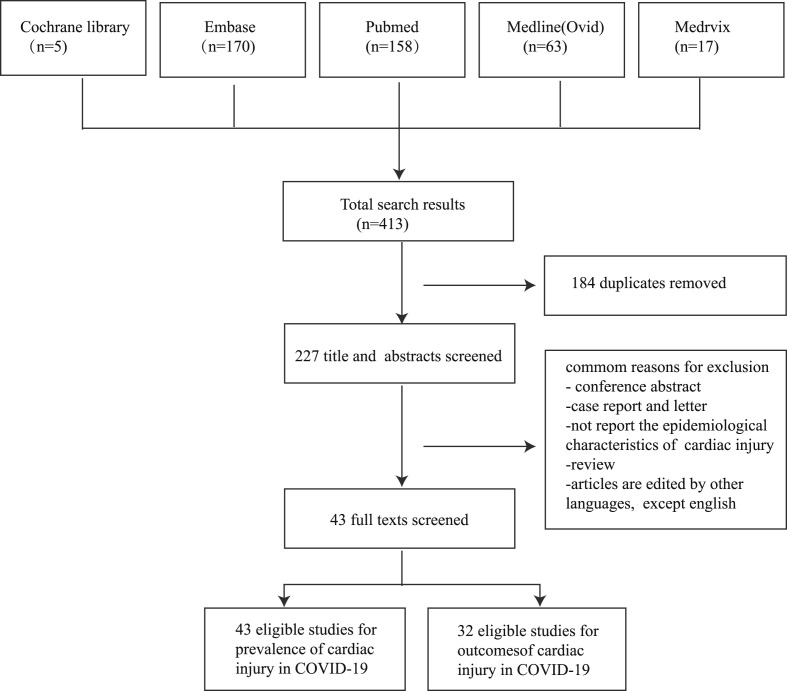
A total of 413 articles were found in the initial database search. Of these, 227 studies were remaining after removing duplicate publications and then screening through title and abstract. Among those, we identified 43 articles for full text review. Ultimately, all the 43 studies were included for review [2,4,9,[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]]. All of these eligible studies were enrolled for analyzing the prevalence of cardiac injury in patients with COVID-19, and 32 of them were selected for analyzing the outcomes of COVID-19 patients who have cardiac injury. The workflow of the process of study selection is demonstrated in Fig. 1 .
Figure 1.
Flow diagram for study selection.
Essential characteristics of the included studies are outlined in Table 1 . A total of 43 studies that involved 9475 patients were included in this meta-analysis. Among those studies, 40 were carried out in China, one in Korea, and two in the USA. The sample size of studies varied from 21 to 2737 patients, whilst the clinical outcome was defined as poor outcomes in three studies, the severity of disease in eight studies, ICU admission in seven studies, and death in 20 studies. Of all 43 studies, 27 were retrospective cohort studies and the remaining 16 were cross-sectional studies. Most of the included studies defined cardiac injury as TnI elevation above 99th percentile. There are studies that did not specify their definition of cardiac injury, however, these studies may presumably use a definition similar to the existing studies.
Table 1.
The characteristics of included studies.
| Study | Region | Sample | Male (%) | Age | Design | Definition of cardiac injury | Outcomes | NOS score |
|---|---|---|---|---|---|---|---|---|
| Ni WT 2020 [10] | China | 176 | 57.4% | 67 ± 2.7 | retrospective cohort study | TnI above the 99th percentile | death [adjusted OR:6.93 (95%CI 1.83,26.22)] | 8 |
| Wei JF 2020 [11] | China | 101 | 53.5% | 49 ± 4.7 | retrospective cohort study | hs-TnT>14 pg/mL | death [unadjusted OR:44.3 (95%CI 2.17,906.88)] severity [unadjusted OR:2.55 (95%CI 1.65,3.94)] admission to ICU [unadjusted OR:2.53 (95%CI 1.49,4.3)] |
8 |
| Yang F 2020 [12] | China | 92 | 53.3% | 69.8 ± 14.5 | cross-sectional study | increased myocardial enzymes | – | 7 |
| Yang QX 2020 [13] | China | 136 | 48.5% | 56 ± 3.3 | retrospective cohort study | unclear | severity [unadjusted OR:3.64 (95%CI 2.2,6.02)] | 5 |
| Shi SB 2020 [14] | China | 416 | 49.3% | 64 ± 12.3 | retrospective cohort study | hs-TNI above the 99th percentile | death [adjusted HR:3.41 (95%CI 1.62,7.16)] | 9 |
| Guo T 2020 [9] | China | 187 | 48.7% | 58.5 ± 14.7 | cross-sectional study | elevated TnT levels | death [unadjusted OR:15.13 (95%CI 6.72,34.06)] | 7 |
| Shi SB 2020 [14] | China | 671 | 48.0% | 63 ± 3.7 | retrospective cohort study | cardiac biomarkers above the 99th percentile | death [adjusted HR:1.25 (95%CI 1.07,1.46)] | 9 |
| Yang F 2020 [16] | China | 52 | 53.8% | 63 ± 16 | retrospective cohort study | unclear | severity [unadjusted OR:2.54 (95%CI 1.38,4.66)] | 7 |
| Lei SQ 2020 [17] | China | 34 | 41.2% | 55 ± 5 | cross-sectional study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | admission to ICU [unadjusted OR:2.62 (95%CI 1.52,4.51)] | 6 |
| Zheng Y 2020 [18] | China | 34 | 67.6% | 66 ± 4.5 | cross-sectional study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | – | 6 |
| Chen T 2020 [19] | China | 274 | 62.0% | 62 ± 5.7 | retrospective cohort study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | death [unadjusted OR:3.59 (95%CI 2.69,4.79)] | 8 |
| Deng Y 2020 [20] | China | 225 | 67.0% | 69 ± 2 | retrospective cohort study | unclear | death [unadjusted OR:3.34 (95%CI 2.6,4.28)] | 5 |
| Zhao XY 2020 [21] | China | 91 | 53.8% | – | retrospective cohort study | elevated cardiac biomarkers | severity [unadjusted OR:1.92 (95%CI 1.08,3.41)] | 7 |
| Wang DW 2020 [22] | China | 107 | 53.3% | 51 ± 4.8 | retrospective cohort study | cardiac biomarkers above the 99th percentile or new abnormalities in echocardiography. | death [unadjusted OR:5.76 (95%CI 2.9,11.42)] | 7 |
| Yang XB 2020 [23] | China | 52 | 67.0% | 59.7 ± 13.3 | retrospective cohort study | hsTNI>28 pg/mL | death [unadjusted OR:1.74 (95%CI 1.13,2.68)] | 6 |
| Hong KS 2020 [24] | Korea | 98 | 38.8% | 55.4 ± 17.1 | cross-sectional study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | admission to ICU [unadjusted OR:17.8 (95%CI 6.57,48.22)] | 8 |
| Zhan GQ 2020 [25] | China | 221 | 48.9% | 55 ± 4.6 | cross-sectional study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | severity [unadjusted OR:4.92 (95%CI 3.62,6.69)] | 9 |
| Wu J 2020 [26] | China | 101 | 54.5% | 62 ± 3.8 | cross-sectional study | TnI above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | – | 8 |
| Huang CL 2020 [27] | China | 41 | 73.0% | 41 ± 4.25 | cross-sectional study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | admission to ICU [unadjusted OR:3.2 (95%CI 1.56,6.55)] | 7 |
| Zhang L 2020 [28] | China | 143 | 51.7% | 63 ± 14 | retrospective cohort study | hs-TNI above the 99th percentile | – | 8 |
| Yu Y 2020 [29] | China | 226 | 61.5% | 64 ± 2.2 | cross-sectional study | hs-TnI > 28 ng/L or TnI > 0.3 ng/mL | – | 7 |
| Yang RR 2020 [30] | China | 212 | 43.0% | – | retrospective cohort study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | death [unadjusted OR:2.73 (95%CI 1.22,6.1)] | 8 |
| Li DZ 2020 [31] | China | 182 | 54.4% | 62.4 ± 5.7 | cross-sectional study | hs-TNI above the 99th percentile | – | 8 |
| Lala A 2020 [2] | USA | 2736 | 59.6% | – | cross-sectional study | TnI>0.03 ng/mL | death [adjusted HR:3.23 (95%CI 2.59,4.02)] | 9 |
| Zhang F 2020 [32] | China | 110 | 54.5% | 64.0 ± 16.5 | retrospective cohort study | hs-TNI above the 99th percentile | death [adjusted HR:10.902 (95%CI 1.279,92.927)] | 7 |
| Liu YB 2020 [33] | China | 291 | 45.7% | 48.1 (34–62) | retrospective cohort study | TnI above the 99th percentile | death [unadjusted OR:51.93 (95%CI 2.2,1225.04)] severity [unadjusted OR:11.24 (95%CI 6.55,19.32)] admission to ICU [unadjusted OR:13.49 (95%CI 7.56,24.08)] |
8 |
| Shi Q 2020 [34] | China | 101 | 59.4% | 71 ± 3.5 | retrospective cohort study | hs-TNI above the 99th percentile | death [unadjusted OR:1.27 (95%CI 0.83,1.93)] | 8 |
| Feng XB 2020 [35] | China | 114 | 62.3% | 64.0 ± 13.4 | retrospective cohort study | cardiac troponin I ≥ 26.2 | poor outcome [adjusted HR:5.02 (95%CI 1.92,13.14)] | 7 |
| Hu L 2020 [36] | China | 323 | 51.4% | 61 ± 11.3 | retrospective cohort study | hs-TnI >0.04 pg/mL | poor outcome [unadjusted OR:6.52 (95%CI 4.80,8.87)] severity [unadjusted OR:9.66 (95%CI 2.31,40.39)] |
7 |
| Liu R 2020 [37] | China | 41 | 41.5% | 39.1 ± 9.2 | retrospective cohort study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography | – | 8 |
| Wu CM 2020 [38] | China | 188 | 63.3% | 51.9 ± 14.3 | retrospective cohort study | TnI above the 99th percentile | death [unadjusted OR:5.25 (95%CI 2.9,9.5)] admission to ICU [unadjusted OR:2.19 (95%CI 1.4,3.45)] |
7 |
| Hou W 2020 [39] | China | 101 | 43.6% | 50.9 ± 20.1 | retrospective cohort study | unclear | poor outcome [unadjusted OR:34.53 (95%CI 8.423,141.589)] | 6 |
| Li YM 2020 [40] | China | 120 | 48.0% | 61 ± 14 | retrospective cohort study | abnormality in cardiac biomarkers and electrocardiography | – | 8 |
| Liu K 2020 [41] | China | 56 | 55.4% | 53.7 ± 10.3 | cross-sectional study | unclear | – | 5 |
| Mercuro NJ 2020 [42] | Massachusetts | 90 | 51.1% | 60.1 ± 16.7 | retrospective cohort study | unclear | – | 5 |
| Wan SX 2020 [43] | China | 135 | 53.3% | 47 ± 3.2 | cross-sectional study | unclear | severity [unadjusted OR:0.83 (0.232,3.00)] | 6 |
| Yang LH 2020 [44] | China | 200 | 49.0% | 55 ± 17.1 | cross-sectional study | unclear | admission to ICU [unadjusted OR:9.64 (95%CI 5.49,16.93)] | 6 |
| Chen G 2020 [15] | China | 21 | 81.0% | 56.8 ± 3.8 | cross-sectional study | cardiac biomarkers above the 99th percentile or new abnormalities in electrocardiography and echocardiography. | – | 6 |
| Li KY 2020 [46] | China | 101 | 59.0% | 58 (40–70) | retrospective cohort study | Cardiac troponin I ≥ 34.2 pg/mL | death [unadjusted OR:6.69 (95%CI 2.61,17.17)] | 7 |
| Luo XM 2020 [47] | China | 403 | 38.9% | 43 (33–56) | cross-sectional study | Cardiac troponin I ≥ 40 pg/mL | death [unadjusted OR:10.94 (95%CI 6.83,17.52)] | 8 |
| Zhou F 2020 [50] | China | 191 | 62.0% | 56 (46–67) | retrospective cohort study | hs-TNI above the 99th percentile | death [unadjusted OR:81.19 (95%CI 11.38,579.41)] | 7 |
| Cao J 2020 [49] | China | 102 | 52.0% | 54 (37–67) | retrospective cohort study | unclear | death [unadjusted OR:65.6 (95%CI 13.86,310.39)] | 5 |
| Du RH 2020 [45] | China | 179 | 54.2% | 57.6 ± 13.7 | prospective cohort Study | Cardiac troponin I ≥ 0.05 ng/mL | death [unadjusted OR:7.2 (95%CI 1.518,34.139)] | 8 |
Abbreviations: hs-TNI, high sensitivity troponin I; TnI, troponin I; NOS, Newcastle Ottawa Scale; ICU, intensive care unit; OR, odd ratio; and HR, hazard ratio.
Prevalence of cardiac injury in patients with COVID-19
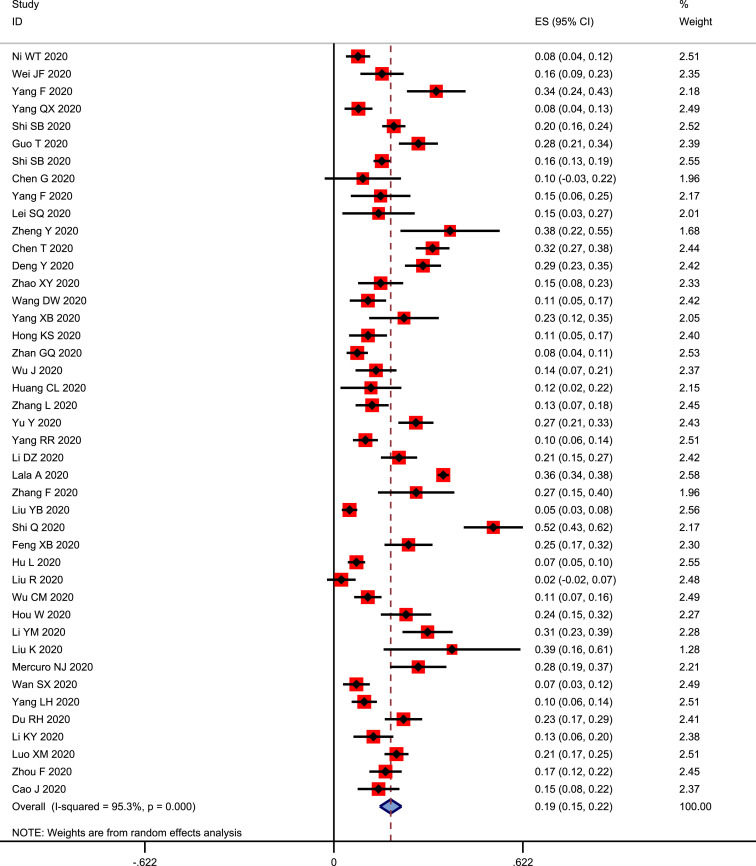
As cardiac injury is commonly recorded in patients with COVID-19, it is particularly important to estimate the prevalence of cardiac injury in COVID-19 patients. Thus, we first analyzed the overall prevalence of cardiac injury in COVID-19 patients. Forty-three studies that reported cardiac injury in patients with COVID-19 were included in this analysis [2,4,9,[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]]. Our pooled analysis revealed a 19% (95% CI: 0.15–0.22 and p < 0.001) prevalence of cardiac injury in total COVID-19 patients (Fig. 2 ).
Figure 2.
Forest plot showing prevalence cardiac injury in total patients.
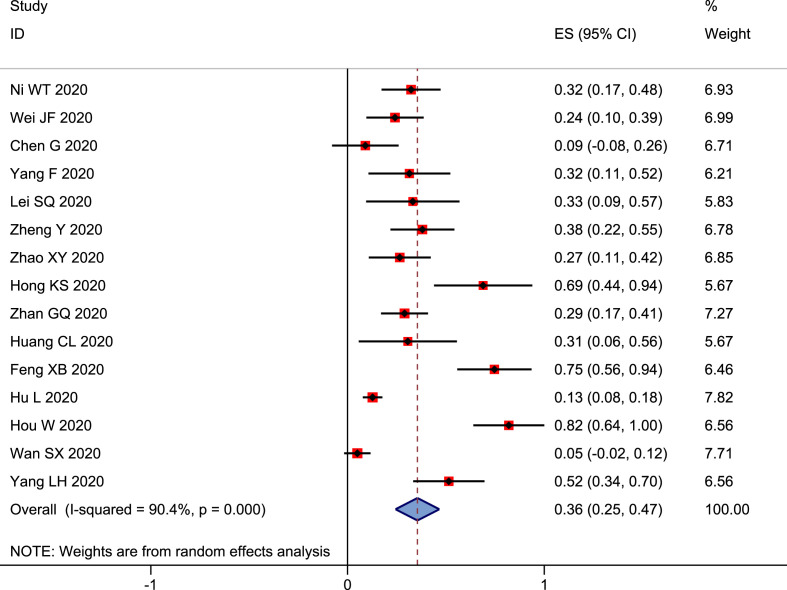
Furthermore, it appears that the COVID-19 patients with cardiac injury have a higher risk of severe disease and mortality according to some retrospective studies. We next conducted the subgroup analysis to evaluate the prevalence of cardiac injury in severe COVID-19 patients or non-survivors. Fifteen studies reported data on cardiac injury in severe COVID-19 patients [11,13,[15], [16], [17], [18],21,24,25,27,35,36,39,44]. A pooled analysis result showed 36% (95% CI: 0.25–0.47 and p < 0.001) prevalence of cardiac injury in severe COVID-19 patients (Fig. 3 ).
Figure 3.
Forest plot showing prevalence cardiac injury in severe patients.
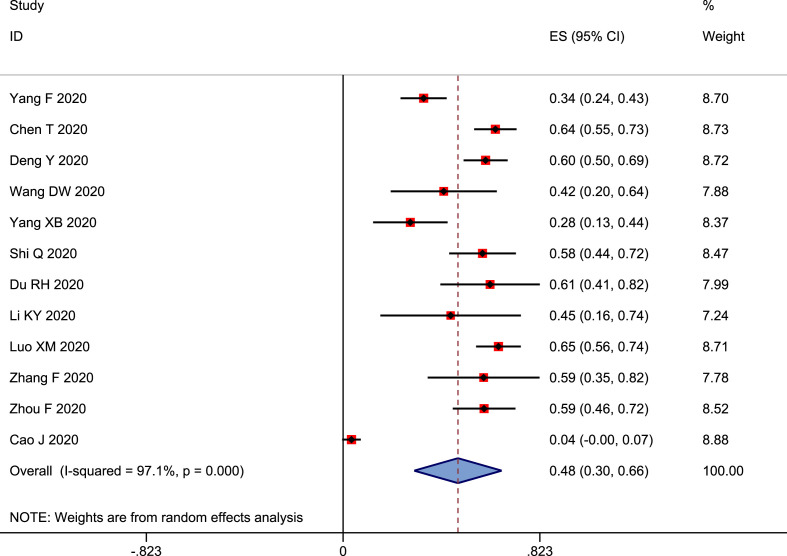
Twelve studies were included for the analysis of prevalence of cardiac injury in nonsurvivors [12,19,20,22,23,32,34,[45], [46], [47],49,50]. According to the pooled analysis result, the prevalence of cardiac injury was 48% (95% CI: 0.30–0.66 and p < 0.001) in the nonsurvivors (Fig. 4 ). Obviously, the prevalence of cardiac injury was greatly increased in patients with COVID-19, particularly in patients with severe disease and non-survivors.
Figure 4.
Forest plot showing prevalence cardiac injury in non-survivors.
The subgroup analysis according to the quality of included studies indicated a 19% (95% CI: 0.14–0.23 and p < 0.001) prevalence of cardiac injuries in the high quality group and 19% (95% CI: 0.13–0.24, p < 0.001) in the low quality group (Supplementary Table 1).
Clinical outcomes of cardiac injury in patients with COVID-19
As there was a high prevalence of cardiac injury in patients with COVID-19, we conducted a systematic analysis of association between cardiac injury and the common adverse outcomes (poor outcomes, severity of disease, need for ICU care, and mortality) in such patients.
We first analyzed the relationship between cardiac injury and poor outcome. A total of three studies reported data on the association between cardiac injury and poor outcome in patients with COVID-19 [35,36,39]. In the pooled analysis, cardiac injury was found to be associated with a significantly increased risk of poor outcomes in COVID-19 patients (ES = 8.46, 95% CI: 3.76–19.06, I 2 = 63.9%, and p = 0.062), (Supplementary Fig. 1).
Additionally, there were eight studies that reported data on the association between cardiac injury and COVID-19 severity [11,13,16,21,25,33,36,43]. In the pooled analysis, cardiac injury was found to be associated with a significantly increased risk of a severe form of COVID-19 (ES = 3.54, 95% CI: 2.25–5.58, I 2 = 80.3%, and p < 0.001), (Supplementary Fig. 2).
Moreover, we identified seven studies reporting data on the association between cardiac injury and admission to ICU in COVID-19 patients [11,17,24,27,33,38,44]. A pooled analysis of these seven studies found that patients with cardiac injury were five times more liable to require ICU admission (ES = 5.03, 95% CI: 2.69–9.39, I 2 = 87.2%, and p < 0.001), (Supplementary Fig. 3).
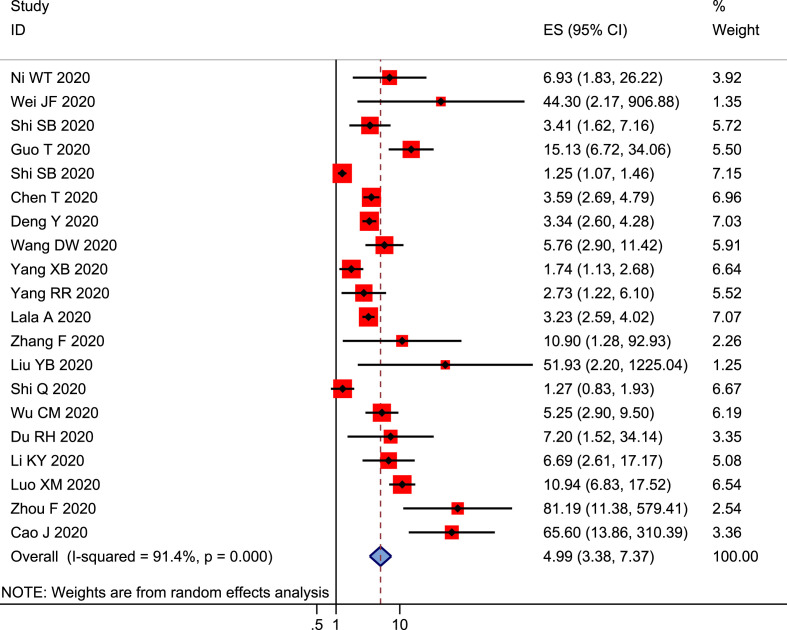
Finally, 20 studies were enrolled in the pooled analysis to explore the association between cardiac injury and mortality in COVID-19 patients [2,4,9,10,11,14,19,20,22,23,30,[32], [33], [34],38,[45], [46], [47],49,50]. Pooled analysis results showed that patients with cardiac injury had an approximately a five-fold higher risk of mortality (ES = 4.99, 95% CI: 3.38–7.37, I 2 = 91.4%, and p < 0.001), (Fig. 5 ).
Figure 5.
Forest plot for association between cardiac injuries and mortality.
The subgroup analysis according to unadjusted or adjusted for covariates indicated that cardiac injuries were significantly associated with increased all-cause mortality with 3.06 (95% CI: 1.52–6.16, I 2 = 93.1%, and p = 0.002) for adjusting covariates and 5.83 (95% CI: 3.74–9.08, I 2 = 86.4%, and p < 0.001) for unadjusting covariates.
Discussion
COVID-19 has resulted in 12,768,307 confirmed infections and 566,654 deaths worldwide over the past few months according to the World Health Organization (WHO) (https://www.who.int). Although the main clinical characteristics of COVID-19 were dry cough, fever, and shortness of breath, cardiac injury has been reported to be highly prevalent in patients affected by this virus [4,51]. Our study evaluated the prevalence of cardiac injury in patients with COVID-19 and investigated the association between cardiac injury and prognosis in these patients. Findings from this meta-analysis indicated that the prevalence of cardiac injury was increased in patients with COVID-19, particularly correlated with the severity of the disease. Furthermore, we found that cardiac injury is significantly connected with poor outcomes and mortality in patients with COVID-19. Together, our meta-analysis results indicated that COVID-19 patients with cardiac injury are more likely to develop severe disease, and more prone to require ICU care or death.
Cardiac injury, usually defined as an elevation of troponin above the 99th percentile of the upper reference, is commonly recorded in hospitalized patients with COVID-19 [8,52]. The earliest retrospective study, involving 41 patients with COVID-19, cardiac injury was detected in five patients (12%) [51]. Since then, several retrospective studies have reported an increased prevalence of cardiac injury in patients with COVID-19, with the rate ranging from 5% to 28% [4,9,11,50,51,53]. Additionally, patients with previous or underlying cardiovascular diseases (CVD) showed a higher risk of cardiac injury during COVID-19. Studies have demonstrated that the prevalence of coronary artery disease and hypertension among patients with cardiac injury is estimated to be up to 20%–30% and 45%–65%, respectively [ 4, 9].
Our meta-analysis that enrolled 34 studies, showed that the prevalence of cardiac injury was 19% (95% CI: 15%–22%) among patients with COVID-19, which might represent the average incidence of cardiac injury in patients with COVID-19. Numerous studies have indicated that cardiac TnI increased significantly in severe cases than in mild cases [[54], [55], [56], [57]]. Furthermore, there was also a significant increase of TnI in the end-stage group when compared with the severe group, which indicates that the prevalence of cardiac injury might increase with the severity of the disease [55,57]. Based on these retrospective studies, our meta-analysis revealed that the prevalence of cardiac injury is 36% (95% CI: 25%–47%) among severe patients with COVID-19. Severe patients usually have worse outcomes and higher death rate; therefore, the investigators sought to evaluate the association of myocardial injury with mortality. Zhou et al. found that there was a rapid rise of troponin in nonsurvivors starting from day 10, which was not observed in survivors [50]. Besides, Ruan et al. showed that the top causes of death in the nonsurvivors were respiratory failure, combination of heart and respiratory failure, and heart failure, which account for 53%, 33%, and 7% of all the death, respectively [58]. Furthermore, the occurrence of acute cardiac injury in hospitalized patients was associated with an increased risk of mortality (ES = 4.99, 95% CI: 3.38–7.37, and p < 0.001) [9]. It is plausible to explain why there was up to 44% incidence of cardiac injury in non-survivors with COVID-19 according to our meta-analysis results. Thus, our results support the notion that cardiac injury is very common in patients with COVID-19 and the prevalence of cardiac injury increased with the severity of the disease.
Although the association between cardiac injury and prognosis in patients with COVID-19 has been consistently described, data on its independent prognostic role remain largely unexplored. Therefore, we systematically conducted a meta-analysis to evaluate the outcome (poor outcomes, severe disease, ICU admission, and mortality) in consecutive patients with COVID-19 with and without troponin elevation. In our meta-analysis, a pooled analysis of the association between cardiac injury and poor outcomes revealed that cardiac injury was associated with poor outcomes in COVID-19 patients (ES = 8.46, 95% CI: 3.76–19.06, and p = 0.062). Our result was in accordance with a previous brief meta-analysis, which showed that troponin levels were significantly higher in patients with severe disease as compared to those with mild forms of the disease [52]. Therefore, it is reasonable to hypothesize that if cardiac troponin is tested immediately after admission, it may help to evaluate the severity of the illness and the extent of injury in the heart. In a single-center case series study performed by Huang et al., four out of five patients with cardiac injury required ICU admission [27]. Similarly, in another single-center case series study of 138 patients, 36 of them were reported to have had a higher level of TnT and CK-MB and required ICU admission [53]. Thus, it appears that (COVID-19) patients with cardiac injury are more likely to be admitted to the ICU. Then, we analyzed the association between cardiac injury and ICU admission and found ICU admission is truly associated with cardiac injury (ES = 5.03, 95% CI: 2.69–9.39, I 2 = 87.2%, and p < 0.001). Studies have shown that myocardial injury is associated with a higher risk of mortality and an overall fatal outcome of COVID-19. Of the enrolled 416 COVID-19 patients, a case series study revealed that patients with cardiac injury had a much higher risk of death than those without cardiac injury during the time from admission to study endpoint (HR: 3.41 and 95% CI: 1.62–7.16) [9]. Similarly, a retrospective cohort study, including 188 patients found that patients with high levels of hs-TnI had a remarkably higher death rate (50%) than that of patients with low or moderated levels of hs-TnI (9.1% or 10%). Furthermore, hs-TnI levels were negatively correlated with the patients’ survival time (r = −0.42 and p = 0.005) [38]. Our meta-analysis results further reinforced the conclusion that patients with COVID-19 infection and cardiac injury have a significantly high risk of mortality. Accordingly, our meta-analysis results are of prognostic importance, because patients with cardiac injury have a higher risk of severe disease and mortality and are more prone to require ICU care. Thus, they deserve more clinical attention.
Up to now, the precise etiology of cardiac injury in patients with COVID-19 remains under investigation, the following potential mechanisms have been suggested. One potential mechanism is that SARS-CoV-2 uses ACE2 as a receptor to enter target cells and causes direct damage to the heart [59,60]. According to a previous research, SARS-CoV genome was detected in 35% of autopsied hearts obtained from the SARS-CoV-infected patients [61]. As reported, ACE2 is highly expressed in the heart, thus the SARS- CoV uses the ACE2 receptor for entry into the host cells and downregulates ACE2 expression and subsequently inhibits protective signaling pathways in cardiac myocytes [62,63]. As SARS-CoV-2 and SARS-CoV are highly homologous in the genome, thus suggesting that SARS-CoV-2 may share the same mechanism with SARS-CoV [64,65]. Then, COVID-19-induced cardiac injury might potentially be mediated by ACE2. The most recent researches address the marked affinity of SARS-CoV-2 to the host ACE2 receptor, raising the possibility of direct damage of cardiomyocytes by the virus [66]. Furthermore, another research further identified that SARS-CoV-2 uses ACE2 as their receptor for entry into the targeted cell [67]. All of these data further suggested that the SARS- CoV-2 can have a direct invasion and damage role to the myocardium. Viral infections could trigger the activation of the immune-mediated host antiviral response, including the activation of macrophages, natural killer cells, and virus-related T lymphocytes, which have been recognized as the most frequent causes of cardiac injury [68]. Several studies have highlighted that there exists a severe systemic inflammatory response, including elevated interleukin levels, C-reactive protein, neutrophil and leukocyte counts, and globulin [4,9]. Systemic inflammatory response after infection may further cause the overexpression of tissue-resident macrophages and leukocyte adhesion molecule, causing reduction in the coronary blood flow, decreases in oxygen supply, and destabilization of the coronary artery [69,70]. Similarly, Guo et al. revealed that in patients with COVID-19, the hs-CRP levels were significantly positively correlated with plasma TnT levels, which indicated that cardiac injury may be closely associated with an inflammatory response [9]. Huang et al. have found that in patients with COVID-19, the plasma levels of cytokines, including MCP-1 (monocyte chemoattractant protein-1), interleukins (IL- 2, IL-7, and IL-10), MIP-1α (macrophage inflammatory protein 1-alpha), and TNF-α (tumor necrosis factor α) were higher in patients who were admitted to the ICU [51]. Elevated plasma cytokines can further activate the proliferation of lymphocytes and macrophages, provoke the dysfunction of the coronary microvasculature, activate the microvascular endothelium, and the consequent cardiac injury [4,71]. Another suggested mechanism is an imbalance between myocardial oxygen supply and demand. Severe hypoxia due to acute lung injury and potential subsequent systemic complications can result in a mismatch between myocardial oxygen supply and demand and hence cause myocardial injury [72,73]. Further, hypoxia may also contribute to the development of systematic inflammatory response, which can be transformed into myocardial ischemia and injury [74]. Additionally, the current data based on up-to-date evidence suggests that patients with previous or underlying CVD were susceptible to suffer from cardiac injury. Patients with coronary artery disease had a high potential risk of coronary plaque rupture secondary to virus infection induced by systemic inflammation [75]. Coagulation abnormalities and arrhythmias are also common in COVID-19 patients, though the mechanism is poorly understood [76,77]. Furthermore, volume evidence has indicated the presence of both preexisting CVD and cardiac injury was associated with the highest death rate, while patients with CVD but without elevated troponin levels had a relatively favorable prognosis [9,78].
To the best of our knowledge, this systematic review is a comprehensive analysis of existing literature to explore the prevalence of cardiac injury in patients with COVID-19 and the clinical outcomes in COVID-19 patients with cardiac injury. There were some limitations in this meta-analysis. First, the limited number of studies available and the interpretation of our findings might be restricted by the small sample size. Second, the enrolled studies are mainly from China, and the results of this meta-analysis may restrict a more precise estimation in the context of other regions of the world. Third, we could not distinguish if the cardiac injury in COVID-19 patients was preexisting or caused by a virus infection. Four, there existed a large heterogeneity in our included studies. Fifth, there were significant differences in adjusting confoundings in studies investigating the association of cardiac injury with clinical outcomes, which may weaken the reliability of our result. Fortunately, the results from subgroup analysis according to studies with unadjusting or adjusting for covariates, cardiac injuries were significantly associated with increased all-cause mortality.
It is concluded that COVID-19 has been associated with an increased prevalence of cardiac injury, even more so in patients with severe disease. The presence of cardiac injury may act as a marker of adverse outcomes or a risk of mortality in patients with COVID-19. Further studies are warranted to elucidate the predominant etiology of cardiac injury in patients with COVID-19 and thus to promote targeted treatment programs and preventive strategies to improve patients’ prognosis.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Handling Editor: A. Siani
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2020.09.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Salahuddin N. The COVID-19 pandemic. J Pak Med Assoc. 2020;70(Suppl 3):S4–S6. doi: 10.5455/JPMA.02. [DOI] [PubMed] [Google Scholar]
- 2.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng J.-H., Liu Y.-X., Yuan J., Wang F.-X., Wu W.-B., Li J.-X. First case of COVID- 19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020 doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imazio M., Klingel K., Kindermann I., Brucato A., De Rosa F.G., Adler Y. British Cardiac Society); 2020. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. [DOI] [PubMed] [Google Scholar]
- 9.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni W., Yang X., Liu J., Bao J., Li R., Xu Y. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J.-F., Huang F.-Y., Xiong T.-Y., Liu Q., Chen H., Wang H. British Cardiac Society; 2020. Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Q., Xie L., Zhang W., Zhao L., Wu H., Jiang J. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J Clin Pharm Therapeut. 2020 doi: 10.1111/jcpt.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25972. [DOI] [PubMed] [Google Scholar]
- 17.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y., Sun L-j, Xu M., Pan J., Zhang Y.T., Fang XLl. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ - Sci B. 2020;21:378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ Br Med J (Clin Res Ed) 2020:368. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng Y., Liu W., Liu K., Fang Y.-Y., Shang J., Zhou L. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chinese Med J. 2020;133:1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X.-Y., Xu X.-X., Yin H.-S., Hu Q.-M., Xiong T., Tang Y.-Y. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020:20. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24 doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J., Shu H., Xia Ja, Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong K.S., Lee K.H., Chung J.H., Shin K.-C., Choi E.Y., Jin H.J. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020:127. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J., Li J., Zhu G., Zhang Y., Bi Z., Yu Y. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol: CJASN. 2020 doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J. Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24 doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang R., Gui X., Zhang Y., Xiong Y. The role of essential organ-based comorbidities in the prognosis of COVID-19 infection patients. Expet Rev Respir Med. 2020 doi: 10.1080/17476348.2020.1761791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D., Chen Y., Jia Y., Tong L., Tong J., Wang W. SARS-CoV-2-Induced immune dysregulation and myocardial injury risk in China: insights from the ERS-COVID-19 study. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317070. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F., Yang D., Li J., Gao P., Chen T., Cheng Z. 2020. Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: a single center retrospective cohort study. [Google Scholar]
- 33.liu y., Li J., liu D., Song H., chen C., lv M. Clinical features and outcomes of 2019 novel coronavirus-infected patients with cardiac injury. medRxiv. 2020 doi: 10.1101/2020.03.11.20030957. [DOI] [Google Scholar]
- 34.Shi Q., Zhao K., Yu J., Jiang F., Feng J., Zhao K. Clinical characteristics of 101 COVID-19 nonsurvivors in Wuhan, China: a retrospective study. medRxiv. 2020 doi: 10.1101/2020.03.04.20031039. [DOI] [Google Scholar]
- 35.Feng X., Li P., Ma L., Liang H., Lei J., Li W. Clinical characteristics and short- term outcomes of severe patients with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.04.24.20078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu L., Chen S., Fu Y., Gao Z., Long H., Wang J.-M. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis : Offl Pub Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R., Ming X., Xu O., Zhou J., Peng H., Xiang N. Association of cardiovascular manifestations with in-hospital outcomes in patients with COVID-19: a hospital staff data. medRxiv. 2020 doi: 10.1101/2020.02.29.20029348. [DOI] [Google Scholar]
- 38.Wu C., Hu X., Song J., Du C., Xu J., Yang D. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) medRxiv. 2020:2020. doi: 10.1101/2020.02.26.20028589. [DOI] [Google Scholar]
- 39.Hou W., Zhang W., Jin R., Liang L., Xu B., Hu Z. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis. 2020;52:498–505. doi: 10.1080/23744235.2020.1759817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Li H., Zhu S., Xie Y., Wang B., He L. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC (J Am Coll Cardiol): Cardiovascular Imaging. 2020:3423. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:E14–E18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L., Liu J., Zhang R., Li M., Li Z., Zhou X. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol : Off Publ Pan Am Soc Clin Virol. 2020;129:104475. doi: 10.1016/j.jcv.2020.104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li K., Chen D., Chen S., Feng Y., Chang C., Wang Z. Radiographic findings and other predictors in adults with covid-19. medRxiv. 2020 doi: 10.1101/2020.03.23.20041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo X., Xia H., Yang W., Wang B., Guo T., Xiong J. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 48.Qi X., Liu Y., Fallowfield J.A., Wang J., Wang J., Li X. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicenter cohort study. medRxiv. 2020 doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao J., Tu W.-J., Cheng W., Yu L., Liu Y.-K., Hu X. Clinical features and short- term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis : Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lippi G., Lavie C.J., Sanchis-Gomar F. 2020. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Progress in cardiovascular diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He X.W., Lai J.S., Cheng J., Wang M.W., Liu Y.J., Xiao Z.C. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xinxueguanbing Zazhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200228-00137. E011-E. [DOI] [PubMed] [Google Scholar]
- 55.Zhou B., She J., Wang Y., Ma X. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. J Infect. 2020;81:147–178. doi: 10.1016/j.jinf.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xinxueguanbing Zazhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200225-00123. E008-E. [DOI] [PubMed] [Google Scholar]
- 57.Han H., Xie L., Liu R., Yang J., Liu F., Wu K. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 61.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M. SARS- coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu L., O'Kane A.M., Peng H., Bi Y., Motriuk-Smith D., Ren J. SARS-CoV-2 and cardiovascular complications: from molecular mechanisms to pharmaceutical management. Biochem Pharmacol. 2020:114114. doi: 10.1016/j.bcp.2020.114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang C., Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic-COVID-19 and cardiovascular diseases. JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.0934. [DOI] [PubMed] [Google Scholar]
- 67.Hoffmann M., Kleine-Weber H., Schroeder S., Krueger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fung G., Luo H., Qiu Y., Yang D., McManus B. Myocarditis. Circulation Res. 2016;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 69.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 70.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China (vol 395, pg 497, 2020) Lancet. 2020;395:496. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Libby P. The heart in COVID19: primary target or secondary bystander? JACC Basic Translat Sci. 2020 doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 73.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilcox I., Chan K.H., Lattimore J.-D. Hypoxia and inflammation. N Engl J Med. 2011;364:1976–1977. doi: 10.1056/NEJMc1103019. [DOI] [PubMed] [Google Scholar]
- 75.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:E438–E440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kochav S.M., Coromilas E., Nalbandian A., Ranard L.S., Gupta A., Chung M.K. Cardiac arrhythmias in COVID-19 infection. Cir Arrhythmia Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008719. e008719-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cosyns B., Lochy S., Luchian M.L., Gimelli A., Pontone G., Allard S.D. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020;21:709–714. doi: 10.1093/ehjci/jeaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.