Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is causing the current pandemic of coronavirus disease 2019 (COVID-19) that has killed nearly one million people so far. While this is a respiratory virus, surprisingly, it has been recognized that patients with cardiovascular disease are likely to be affected severely and die of COVID-19. This phenomenon cannot be explained by the generally accepted logic that the SARS-CoV-2 infection/replication is the sole determinant of the actions of the virus to define the fate of host cells. I herein propose the viral protein fragment theory of COVID-19 pathogenesis based on my observations in cultured human vascular cells that SARS-CoV-2 spike protein can activate cell signaling events without the rest of the viral components. It is generally thought that SARS-CoV-2 and other single-stranded RNA viruses attach to the host cells through the interactions between surface proteins of the viral capsid and the host cell receptors; the fusion and the entry of the viral components, resulting in the replication of the viruses; and the host cell responses are the consequence of these events. I hypothesize that, as humans are infected with SARS-CoV-2, the virus releases (a) fragment(s) of the spike protein that can target host cells for eliciting cell signaling without the rest of the viral components. Thus, COVID-19 patients are subjected to the intact virus infecting the host cells for the replication and amplification as well as the spike protein fragments that are capable of affecting the host cells. I propose that cell signaling elicited by the spike protein fragments that occur in cardiovascular cells would predispose infected individuals to develop complications that are seen in severe and fatal COVID-19 conditions. If this hypothesis is correct, then the strategies to treat COVID-19 should include, in addition to agents that inhibit the viral replication, therapeutics that inhibit the viral protein fragment-mediated cardiovascular cell signaling.
Introduction
Coronaviruses are positive sense single-stranded RNA viruses that often cause the common cold [1], [2]. Some coronaviruses can, however, be lethal; and currently the world is suffering from the pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3], [4]. So far, over 30 million people have been infected with SARS-CoV-2 and nearly 1 million people have died of COVID-19 worldwide, causing serious health, economical, and sociological problems. SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as the receptor to enter the host cells [5], [6]. SARS-CoV-2 and other single-stranded RNA viruses attach to the host cells through the interactions between surface proteins of the viral capsid and the host cell receptors; the fusion and the entry of the viral components, resulting in the replication of the viruses; and the host cell responses are often thought to be the consequence of these events. As a respiratory virus, lung cells are the primary targets of SARS-CoV-2, resulting in severe pneumonia and acute respiratory distress syndrome (ARDS). [7], [8]. However, it has been noted that certain populations of infected individual are more susceptible to being severely affected by and die of COVID-19. Elderly patients with cardiovascular diseases are particularly susceptible to developing severe and possibly fatal conditions [4], [9], [10]. These surprising observations that cardiovascular disease, but not respiratory disease, predisposes COVID-19 patients to develop severe and fatal conditions question the general consensus that biological responses by the human host cells are merely a consequence of the viral replication.
The hypothesis
Viruses invade host cells, resulting in cell death. The major target cells for SARS-CoV-2 should be respiratory cells. However, surprisingly, patients with cardiovascular disease, rather than respiratory disease, are predisposed to severe COVID-19 symptoms and death. To explain this phenomenon, I herein propose the viral protein fragment theory of COVID-19 pathogenesis (Fig. 1 ). This hypothesis is based on my experimental observations in cultured human vascular cells that the recombinant full length S1 subunit of SARS-CoV-2 spike protein can activate cell signaling events without the rest of the viral components [11]. Thus, the pathology of COVID-19 may not merely depend on the spike protein serving as a fusion protein to facilitate the viral entry and the infection. I propose a scenario that, as humans are infected with SARS-CoV-2, the virus releases (a) fragment(s) of the spike protein that can target host cells for eliciting cell signaling. Thus, infected patients would have at least two entities introduced in response to the SARS-CoV-2 infection. One is the intact virus that uses its spike protein to target ACE2, resulting in the entry into the host cells for the viral replication and amplification. The other is the circulating fragment(s) of the spike protein that independently elicit(s) actions, which ultimately lead to severe pathological conditions. My experiments using cultured vascular cells also showed that, while the full length S1 subunit of SARS-CoV-2 spike protein elicits cell signaling, the shorter protein that only contains the receptor binding domain (RBD) does not [11]. These results suggest that other regions of the S1 subunit are responsible for eliciting cell signaling in host cells. Thus, if the SARS-CoV-2 spike protein fragment(s) that has/have been released into the blood is/are responsible for the pathogenesis of severe COVID-19, therapies that target the viral replication such as inhibitors of RNA-dependent RNA polymerase alone would not work. Also, if the regions of spike protein other than the RBD are responsible for eliciting pathological cell signaling and if the fragment(s) is/are released quickly after the host is exposed to SARS-CoV-2, then vaccine strategies that target RBD would not affect the viral protein fragment-mediated cell signaling. Thus, therapeutic strategies to inhibit viral protein fragment-mediated cell signaling in cardiovascular cells, in addition to drugs that inhibit the viral replication, are necessary to reduce the COVID-19-associated death.
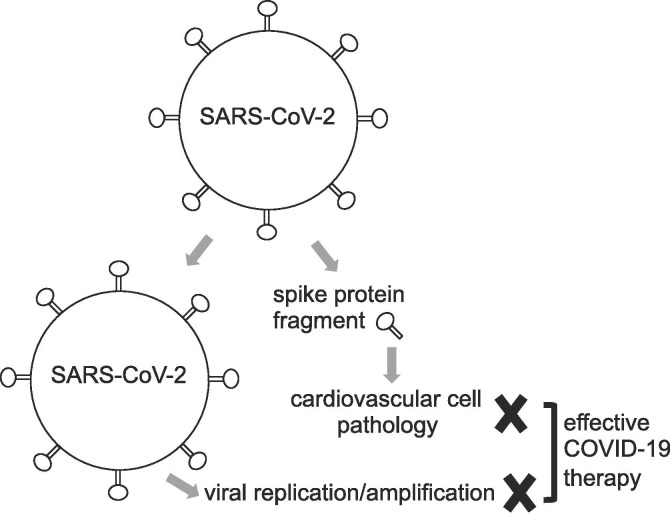
Fig. 1.
Proposed viral protein fragment theory of COVID-19 pathogenesis. According to my hypothesis, SARS-CoV-2 produces a fragment or fragments of spike protein that get(s) circulated in the blood. In addition to the intact virus targeting ACE2 receptor-containing host cells for the viral replication and amplification, the viral spike protein fragment elicits cell signaling events in cardiovascular cells. Thus, if infected patients have the cardiovascular comorbidity, viral protein fragment-mediated cell signaling and the viral amplification would together worsen the COVID-19 condition. For these patients, in addition to the anti-viral drugs or the vaccines that inhibits the viral replication, agents that inhibits the viral spike protein fragment-mediated cell signaling is needed in order to effectively reduce the mortality and morbidity associated with COVID-19.
Evaluation of the hypothesis
My specific hypothesis is that certain viruses shed fusion protein fragments that circulate in the blood and, in turn, elicit cell signal transduction, which makes the infected individuals with cardiovascular diseases predisposed to severe COVID-19 conditions and death. Thus, infected patients would have at least two entities that may cause complications when they are infected with SARS-CoV-2: (i) virus itself that gets incorporated into the host cells where the viral replication and amplification occur; and (ii) components of viral fusion proteins (i.e. spike protein for SARS-CoV-2) that get circulated to elicit distinct processes promoting pathologic conditions. This is based on my observations that recombinant SARS-CoV-2 spike protein (without the rest of the virus) is capable of eliciting cell signaling in cultured human vascular cells [11]. Interestingly, my experiments also showed that RBD-only containing segment of SARS-CoV-2 spike protein does not activate cell signaling, suggesting the role of other regions within the S1 subunit of SARS-CoV-2 spike protein to activate this cell signaling event. Thus, the first experiments that are needed to validate this hypothesis is to identify the regions of SARS CoV-2 spike protein that are responsible for activating cell signaling, perhaps by using cultured human cells. After identifying active protein regions of the S1 subunit of SARS-CoV-2 spike protein, the occurrence of protein fragments that possess such amino acid sequences should be detected in patients infected with SARS-CoV-2. Experimental animals should also be employed to determine whether the administration of such fragments promotes cardiovascular disease conditions or worsens the existing cardiovascular pathology.
Consequences of the hypothesis
I herein propose the viral protein fragment theory of COVID-19 pathogenesis, in which a fragment or fragments of SARS-CoV-2 spike protein is/are released into the blood circulation, predisposing patients with cardiovascular disease conditions to severe COVID-19 outcomes. Thus, COVID-19 deaths of patients with cardiovascular comorbidity are due to the death of host cells as a consequence of the viral infection and replication as well as the spike protein fragment-mediated cell signaling in human host cardiovascular cells. Currently, the development of therapeutic strategies against COVID-19 focuses on the vaccine development and searching for agents that inhibit viral replication. If my hypothesis is correct, it is critical to also inhibit the unexpected biological events that are elicited by SARS-CoV-2 such as the release of spike protein fragments, which affect cardiovascular cells in addition to inhibiting the viral replication in order to successfully reduce the mortality and morbidity associated with COVID-19 and to end the pandemic.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported in part by NIH (R21AI142649, R03AG059554, and R03AA026516) to Y.J.S. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
References
- 1.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satija N., Lal S.K. The molecular biology of SARS coronavirus. Ann N Y Acad Sci. 2007;1102:26–38. doi: 10.1196/annals.1408.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. pneumonia in Wuhan, China. JAMA Intern Med. 2019;2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki YJ. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. (Submitted for publication). [DOI] [PMC free article] [PubMed]