Abstract
The severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) has resulted in almost 28 million cases of COVID-19 (Corona virus disease-2019) and more than 900000 deaths worldwide since December 2019. In the absence of effective antiviral therapy and vaccine, treatment of COVID-19 is largely symptomatic. By making use of its spike (S) protein, the virus binds to its primary human cell receptor, angiotensin converting enzyme 2 (ACE2) which is present in the pulmonary epithelial cells as well as other organs. SARS-CoV-2 may cause a downregulation of ACE2. ACE2 plays a protective role in the pulmonary system through its Mas-receptor and alamandine-MrgD-TGR7 pathways. Loss of this protective effect could be a major component of COVID-19 pathogenesis. An attractive strategy in SARS-CoV-2 therapeutics would be to augment ACE2 either directly by supplementation or indirectly through drugs which increase its levels or stimulate its downstream players. In this semi-systematic review, we have analysed the pathophysiological interplay between ACE and ACE2 in the cardiopulmonary system, the modulation of these two proteins by SARS-CoV-2, and potential therapeutic avenues targeting ACE-Ang II and ACE2-Ang (1–7) axes, that can be utilized against COVID-19 disease progression.
Keywords: COVID-19, Angiotensin converting enzyme-2, Angiotensin II, Ang (1–7), RAAS, Diabetes
1. Introduction
The novel corona virus, Severe acute respiratory syndrome-corona virus-2 (SARS-CoV-2), that originated in China in November 2019, has spread worldwide affecting 213 countries. Disease caused by this virus (Corona virus disease-2019, COVID-19) has transformed into a feared global pandemic with 28.6 million confirmed cases and 917000 deaths as of September 13, 2020 (“Coronavirus Update”). In the absence of effective antiviral therapy and vaccine, treatment of COVID-19 is largely symptomatic. The primary receptor for SARS-CoV-2 in humans is the protein angiotensin converting enzyme-2 (ACE2), widely expressed in different tissues including epithelial cells of respiratory tract and gastrointestinal tract. The virus binds to ACE2 by making use of its spike (S) protein which has two functional domains S1 and S2; S1 contains receptor binding domain (RBD) which interacts with ACE2, and S2 is needed for membrane fusion (Hoffmann et al., 2020; Zhou et al., 2020). Compared to SARS-CoV, the RBD of SARS-CoV-2 binds to ACE2 with greater affinity as indicated by X-ray crystallographic studies and protein-protein binding assays using surface plasmon resonance, and it appears that the RBD of SARS-CoV-2 differs from that of SARS-CoV by several amino acid substitutions which actually stabilize the binding of SARS-CoV-2 with ACE2 (Shang et al., 2020). The fusion of SARS-CoV-2 with the host cell however requires a priming, in which a serine protease (TMPRSS2) present on the host cell membrane makes two proteolytic cleavages on the S protein, and this is followed by the internalization of the virus along with ACE2 (Hoffmann et al., 2020). Recently, several reviews have highlighted renin angiotensin aldosterone system (RAAS) blockers and anti-inflammatory drugs like ibuprofen as double-edged swords in COVID-19 progression. These drugs are known to increase the expression of ACE2, theoretically having a facilitatory role on the entry of the virus into host cells (Gurwitz, 2020; Kakodkar et al., 2020; Magrone et al., 2020; Rothuizen et al., 2020; Verdecchia et al., 2020; Zolk et al., 2020). On the other hand, there is a strong experimental evidence that ACE2 is protective in acute lung injury and that downregulation of ACE2 exacerbates lung inflammation (Imai et al., 2005; Kuba et al., 2005). Hence, drugs augmenting ACE2 may have a beneficial effect once viral damage has started.
While most reviews have focused on the beneficial or deleterious effects of ACE-inhibitors or angiotensin (Ang) receptor blockers, we decided to focus on the protective role of ACE2. We conducted a PubMed search on possible therapies that could activate or up regulate ACE2 directly or indirectly and further explored the relationship, if any, between known anti-inflammatory drugs and ACE2/ACE pathways. Since many patients with COVID-19 mortality suffer from underlying co-morbidities such as ischemic heart disease, hypertension, asthma and diabetes, the authors also conducted a PubMed search focusing on individual drugs prescribed in these conditions and their possible interplay with ACE2. In this review, we analyse the pathophysiological interplay between ACE and ACE2, their role in cardiopulmonary hemodynamics, the modulation of these two proteins by SARS-CoV-2, and potential therapeutic avenues targeting ACE-Ang II and ACE2-Ang (1–7) axes, that can be utilized against COVID-19 disease progression.
2. Search methodology for the review
A PubMed search was carried out using the keywords (ACE2 OR ACE-2) AND lung in Title/Abstract which yielded 270 results. Similarly, we searched PubMed using keywords (ACE2 OR ACE-2) AND pulmonary in Title/Abstract (29 results); using keywords Ang (1–7) AND lung in Title/Abstract (116 results); and using keywords Ang (1–7) AND pulmonary in Title/Abstract (88 results). Searches were carried out for individual drugs known to have some effect on the ACE-pathway or in lung injury. An expansive list of keywords used in these searches and the results obtained is provided in Supplementary Table 1. We also performed a search on ClinicalTrials.gov using the keywords ACE2 and COVID-19; and Ang (1–7) and COVID-19, to identify individual trials being carried out in this domain. The abstracts of the articles obtained were scrutinized by the primary author (UK) assisted by the second author (KA), and full texts were analysed in case the abstracts were found relevant. For articles without abstract or with less detailed abstracts, the full text was directly analysed. Once the findings were compiled, they were reviewed by the two corresponding authors (SSC and SC).
3. ACE, Ang II and ACE2 in the cardiopulmonary system
ACE is present in the vascular endothelium, predominantly of the lungs and converts angiotensin-I to the octapeptide angiotensin-II (Ang II). Levels of Ang II depend on many factors. These include ACE and non ACE enzymes such as chymase and cathepsin, renin and angiotensinogen, systemic blood pressure, renal blood flow, renal levels of ATP, prostaglandins and adenosine, systemic and renal sympathetic activity, plasma aldosterone levels and levels of ACE2, aminopeptidase A and neprilysin. Factors causing upregulation of ACE and downregulation of ACE2 and neprilysin would result in increments in Ang II levels (Arendse et al., 2019; Chappell, 2016). Ang II via the Gq type of angiotensin-1 (AT1) receptor, causes generalized vasoconstriction, increased peripheral vascular resistance, ventricular remodelling, and vascular and cardiac fibrosis. In lungs, Ang II mediated contraction of pulmonary endothelial cells increases the leaky space between these cells. Pulmonary smooth muscle contraction and fibrosis leads to pulmonary arterial hypertension. In conjunction with prostaglandin E2 (PGE2), prostacyclin (PGI2), and leukotriene C4 (LTC4), it also causes enhancement of pulmonary vessel permeability, leading to diffuse lung inflammation and injury (Imai et al., 2008). Ang II has been shown to impair airway fluid clearance (AFC) via the angiotensin AT1 receptor-mediated downregulation of the beta and gamma subunits of epithelial sodium channels (ENaC) (Deng et al., 2012). Further, the activation of NADPH oxidases (NOX1, NOX2, NOX4 and NOX5) by Ang II in cardiovascular tissues produces a state of oxidative stress, which then activates a multitude of inflammatory cytokines through mitogen activated protein kinase (MAPK) and nuclear factor-kappa beta (NF-Kβ) signalling (Nguyen Dinh Cat et al., 2013). Activation of tissue factor and endothelial dysfunction by Ang II promotes a thrombosis prone state in blood vessels. Ang II can also activate the angiotensin AT2 receptor, the signalling cascade of which is still not fully delineated. Angiotensin AT2 receptors are widely present in foetal tissues including lung, but their expression decreases drastically after birth with limited expression in endothelial cells, vascular smooth muscle cells, brain, adrenal gland, kidney, and reproductive tissues in adults. The expression of angiotensin-AT2 receptors increases on activated fibroblasts in fibrotic disease states of lung and limited expression has also been noticed on bronchial epithelial cells of lung cancer patients (Bullock et al., 2001; Jones et al., 2008). Angiotensin AT2 receptors in heterodimerization with bradykinin receptors (BK-2) activate nitric oxide synthase, resulting in vasodilatation. Activation of angiotensin-AT2 receptors has also been linked with decreased MAPK activity and decreased NF-κB signalling inside cells (Arendse et al., 2019; Nguyen Dinh Cat et al., 2013).
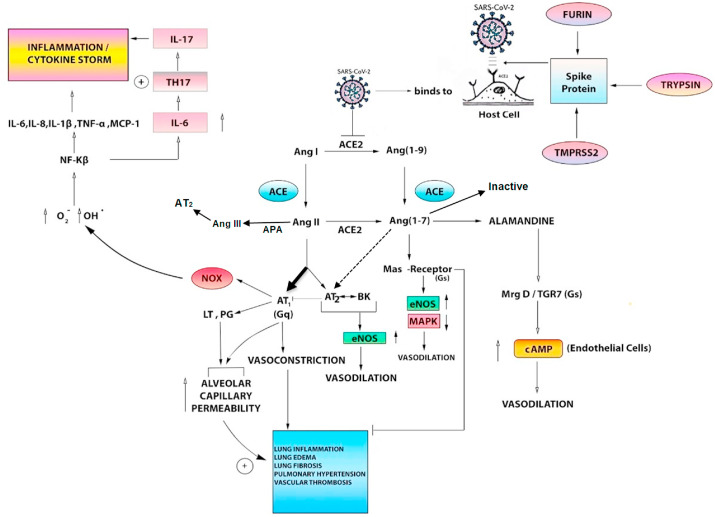
The normal physiological role of ACE2 in the lungs is not clear at present but under conditions of uncontrolled activation of the RAAS axis, ACE2 serves to counteract the actions of Ang II. High expression of ACE2 protein has been shown in cardiomyocytes, tubular epithelial cells of kidney and lung epithelial cells (Hamming et al., 2004). The protein expression has also been profiled in diverse other cell types such as enterocytes of the small intestine, endothelial cells, neurons and arterial smooth muscle cells of various visceral organs (Hamming et al., 2004). Unlike ACE which is a dipeptidase, ACE2 is a mono-carboxypeptidase and has higher affinity for Ang II compared to Ang I. Activation of ACE2 results in the production of the heptapeptide, Ang (1–7). The latter acts on multiple receptors such as Mas which is a Gs-linked G-protein coupled receptor (GPCR), the angiotensin-AT1 receptor, the angiotensin-AT2 receptor and MrgD which is a Mas related GPCR. Though ACE2 by acting on Ang II decreases the levels of Ang II, which is also a ligand of angiotensin-AT2 receptors, the production of Ang (1–7) by ACE2 seems to counteract the effect of AT2 deficiency in cardiopulmonary tissues. Another peptide produced through the ACE2 arm is alamandine which is a decarboxylated form of Ang (1–7) and acts primarily through the MrgD receptors (Arendse et al., 2019). Activation of Mas and MrgD receptors increases cAMP in endothelial cells, enhances phosphatidyl-inositol-3-kinase (PI3K) activity and decreases MAPK activity. Overall, the ACE2 arm through Ang (1–7)-Mas or alamandine-MrgD axis, is a major physiological regulator of the ACE-Ang II-AT1 receptor axis and protects cardiovascular, renal and pulmonary systems from the devastating effects of uncontrolled Ang II (Arendse et al., 2019; Schleifenbaum, 2019). Fig. 1 shows the various pathways mediated by ACE and ACE2 in the pulmonary system.
Fig. 1.
ACE and ACE2 in the pulmonary system and the tentative role of SARS-CoV-2
The top right part of the figure shows the activation of the Spike (S)-protein of SARS-CoV-2 by predominantly TMPRSS2 and also other host proteases such as trypsin and furin. The activated S-protein aids the virus to enter the host cells, mainly pulmonary epithelial cells, after it has bound to its cellular receptor- ACE2. SARS-CoV-2 on internaliz
ation into the host cells may bring about a downregulation of ACE2 expression, similar to SARS-CoV and other respiratory viruses.
The middle and top left part of the figure demonstrate the natural pathophysiologic role of the interdependent ACE and ACE2 pathways in the pulmonary system, including the vasculature and cytokine response. Normally, ACE converts Angiotensin I to Angiotensin II which exerts effects through both AT1 and AT2 receptors. In various disease states, the AT1 receptor pathway (Gq-receptor mediated) is the dominant pathway (bold black arrow) which brings about vasoconstriction, increase in alveolar capillary permeability and multiple other deleterious pulmonary effects (blue box). The AT1 receptor also stimulates leukotrienes and prostaglandins which increase alveolar capillary permeability. It also stimulates NOX (NADPH oxidase) in the endothelial cells, phagocytes and vascular smooth muscle cells which results in an increase in deleterious free radicals and an inflammatory cytokine storm (top left part of figure). A minor role is played by the protective AT2-receptor mediated pathway which in association with bradykinin, brings about an increase in endothelial nitric oxide synthase and causes vasodilatation. Angiotensin II is also converted to Angiotensin III by APA (aminopeptidase A) which is the predominant molecule acting on the AT2 receptor.
ACE2 normally plays a protective role in pulmonary pathophysiology by converting Ang I to Ang (1–
9) and Ang II to Ang (1–
7). Ang (1–
9) is also converted to Ang (1–
7) by ACE. Ang (1–
7) acts through the Mas-receptor (Gs) pathway and results in an increase in endothelial nitric oxide synthase (eNOS) and a decrease in MAPK (mitogen-activated protein kinase), resulting in protective vasodilatation. The Mas-receptor also has an inhibitory effect on the deleterious effects of Ang II on the pulmonary system (blue
box). Ang (1–
7) also acts through the Alamandine- MrgD-TGR7 (Gs)- pathway which increases cyclic adenosine monophosphate (cAMP) in endothelial cells, resulting in protective vasodilatation. Ang (1–
7) participates in cross-talk with AT2 receptor (dashed arrow), and is also inactivated by ACE. The balance between the ACE and ACE2 mediated actions determines the end result in disease states. SARS-CoV-2, by presumptively downregulating ACE2, may shift the balance towards the deleterious effects of the ACE pathway. On the other hand, it may be hypothesized that several drugs which act as direct and indirect activators of ACE2, may favourably shift the balance towards the Mas-receptor and Alamandine mediated protective pathways.
3.1. ACE2 and its interplay with other peptides-apelin and bradykinin
Apart from the substrate Ang II, ACE2 is involved in the metabolism of many other peptides like bradykinin, des-Arg-bradykinin (DABK), apelin and dynorphin (Arendse et al., 2019). The physiological consequences of ACE2 mediated inhibition of kinins and apelin cannot be underestimated. Whereas kinins are known for their inflammatory actions, apelin is thought to exert vasodilatory and anti-inflammatory actions such as endothelial nitric oxide synthase (eNOS) dependent vasodilatation, NF-Kβ inhibition and amelioration of mitochondrial dysfunction in endothelial and vascular smooth muscle cells (Andersen et al., 2011; Yan et al., 2020). Apelin, in cross-talk between its receptor APJ and the angiotensin-AT1 receptor, suppresses AT1 signalling and upregulates ACE2. As such, degradation of kinins may contribute to the anti-inflammatory actions of ACE2. However, the cardiopulmonary effects of ACE2 mediated metabolism of apelin may be overshadowed by Ang (1–7) generation by the enzyme. This is particularly important as Ang (1–7) infusion has shown reversal of apelin deficiency induced deleterious effects on cardiovascular system (Sato et al., 2013). Also, therapies enhancing ACE2 are being tried enthusiastically in cardiopulmonary diseases which are subsequently discussed.
4. Cardiopulmonary involvement in COVID-19
ARDS, myocardial injury, disseminated intravascular coagulation (DIC) especially of the pulmonary vasculature, are the major contributors to mortality due to SARS-CoV-2 (Chen C, Yan JT, Zhou N, Zhao JP, 2020; Guo et al., 2020; McGonagle et al., 2020; Xie and Chen, 2020). Pulmonary invasion by SARS-CoV-2 leads to diffuse alveolar damage, pulmonary interstitial inflammation, microvascular thrombosis and a cytokine storm like state. Cardiac involvement may be the result of these secondary manifestations, precipitation of underlying cardiovascular diseases by viral attack, direct viral mediated myocardial injury or indirect, viral mediated cardiac ACE/ACE2 disturbance. Myocardial injury estimated by raised levels of troponin-T (TnT) has been seen in around 28% patients with confirmed COVID-19. Around 8.5% patients can have elevated TnT levels in the absence of underlying cardiovascular disease. Elevated TnT levels are associated with high levels of creatine kinase, N-terminal pro-brain type natriuretic peptide (NT-pro BNP), high sensitivity C-reactive protein (hs-CRP), as also with elevated D-dimers (Guo et al., 2020). Further, the elevated cardiac enzymes are associated with higher chances of ICU admission, frequent need of mechanical ventilation, glucocorticoid use, development of arrythmias and overall mortality. Microvascular thrombosis seen in SARS-CoV-2 is believed to be an unfavourable outcome of a pulmonary specific pathology which now has been termed as diffuse pulmonary intravascular coagulopathy (DPIC) (McGonagle et al., 2020). Pulmonary hypertension produced by the action of Ang II on pulmonary vasculature and microvascular thrombosis produced as a consequence of DPIC can respectively lead to acute right ventricular dilatation and precipitation of cardiac events in patients with underlying diseases such as coronary artery disease, hypertension, diabetes mellitus and obesity (McGonagle et al., 2020). While SARS-CoV genome has been observed in myocardium of 35% SARS patients, the cardiac invasion of SARS-CoV-2 and evidence of viral myocarditis, confirmation of which requires endomyocardial biopsy or post mortem autopsy has not been demonstrated conclusively so far (Oudit et al., 2009).
5. SARS-CoV-2, respiratory viruses and ACE/ACE2 axis
SARS-CoV and SARS-CoV-2 bind to ACE2 on pulmonary epithelial cells through the S1 subunit of their S protein. ACE2 knock out mice show reduced susceptibility to SARS and anti-ACE2 antibody reduces the entry of SARS-CoV and SARS-CoV-2 in cultured Vero E6 cells (Hoffmann et al., 2020; Kuba et al., 2005; Li et al., 2003; Zhou et al., 2020). For SARS, some other receptors such as liver/lymph node-specific ICAM3-grabbing nonintegrin (L-SIGN) and dendritic cell specific ICAM3-grabbing nonintegrin (DC-SIGN) have been identified in the liver, lymph nodes, dendritic cells as also on pneumocytes and endothelial cells (Jeffers et al., 2004; Marzi et al., 2004). As of now, the alternate binding sites of SARS-CoV-2 are unknown although preprints have mentioned both L-SIGN and DC-SIGN.
Mice infected with SARS-CoV demonstrate a reduced expression of ACE2 in the lungs (Kuba et al., 2005). This is partly because SARS-CoV via certain proteases such as TMPRSS2 and ADAM-17 (A disintegrin and metalloproteinase-17), mediates ACE2 cleavage and shedding respectively (Heurich et al., 2014; Lambert et al., 2005). The reduced expression of ACE2 in turn enhances S protein mediated viral signalling and viral infectivity (Haga et al., 2008; Heurich et al., 2014).
Preclinical studies of H5N1, H1N1, RSV and H7N9 infections in animal models and human cell lines have also shown decreased levels of ACE2 and elevated levels of Ang II (Gu et al., 2016; Liu et al., 2014; Yang et al., 2014; Zou et al., 2014). Treatment with rACE2 or angiotensin-AT1 receptor blockers significantly attenuates lung injury in animals. Downregulation of ACE2, evident by elevated levels of Ang II has also been demonstrated in the plasma of patients of RSV, H7N9, H5N1, and SARS-CoV-2 (Gu et al., 2016; Liu et al., 2020; Yang et al., 2014; Zou et al., 2014). ACE2 gets reduced in established animal models of acute lung injury such as acid aspiration model, caecal ligation and perforation model and endotoxin challenge model (Imai et al., 2005). The mortality as well as the histopathological changes in lungs such as degree of inflammation, oedema, stiffness and hyaline membrane deposition are markedly high in ACE2 knock out mice subjected to potential stimuli of lung injury compared to wild type mice exposed to similar insults (Gu et al., 2016; Imai et al., 2005; Yang et al., 2014; Zou et al., 2014). The levels of Ang II have been found to be elevated in the plasma and lungs of ACE2 knockout mice and mice subjected to potential agents of lung injury. Considerably high levels of Ang II are observed in the lungs when ACE2 knock out is coupled with lung insulting stimuli like acid aspiration. The angiotensin-AT1 receptor is responsible for mediating the damaging actions of ACE or Ang II, whereas the angiotensin-AT2 receptor is protective against lung inflammation induced by potential threats. Supplementation of recombinant ACE2 and down regulation of ACE have been shown to attenuate the lung injury both in wild mice and ACE2 knock out mice exposed to deleterious stimuli (Imai et al., 2008, 2005).
Thus, it seems that ACE2 action in lung becomes more important under conditions of lung injury and overactivation of the renin-Ang II axis when it protects the pulmonary system from the hypoxic and inflammatory attacks of Ang II. As mentioned above, the cleaved and internalized ACE2 facilitates further viral signalling inside host cells. It may not be wrong to hypothesize that once viral infection has occurred, approaches restoring the soluble ACE2 levels or activity of ACE2 can competitively interfere with viral entry and at the same time offer cardiopulmonary protection. These can be novel weapons in the armamentarium against SARS-CoV-2. Tentative therapies would include the use of recombinant ACE2, viral mediated delivery of ACE2, Ang (1–7) based peptides, and agents augmenting the expression or activity of ACE2. Fig. 1 represents how SARS-CoV-2 has differential effects on the pathways mediated by ACE and ACE2 in the cardiopulmonary system.
6. Existing approved drugs with experimental evidence of modulating RAAS/ACE2
6.1. ACE inhibitors & angiotensin receptor blockers
ACE inhibitors (ACEIs) such as ramipril, lisinopril and angiotensin-AT1 receptor blockers (ARBs) such as olmesartan, losartan, by suppressing the RAAS-Ang II-AT1 axis, shift Ang I and Ang II metabolism to the ACE2-Ang (1–7)-Mas/MrgD axis. ACE inhibitors decrease the conversion of Ang I to Ang II. Ang I thereby is shifted by neprilysin to Ang (1–7) pathway. ACE through its N domain is also involved in the metabolism and inactivation of Ang (1–7). Angiotensin-AT1 receptor blockers, by increasing the levels of Ang II stimulate the angiotensin-AT2 receptor, activation pathway of which is unclear but resembles that of Mas receptor of Ang (1–7). Ang (1–7) levels are also increased by ARBs but the increase is less as compared to ACEIs (Arendse et al., 2019; Chappell, 2016). The use of angiotensin-AT1 receptor blockers increases the expression of cardiac ACE2 in spontaneously hypertensive rats (SHR) and animal models of myocardial infarction (Agata et al., 2006; Ishiyama et al., 2004). Likewise, treatment with eprosartan in a rat model of heart failure has been found to significantly increase the cardiac expression of ACE2 by more than 100% (Karram et al., 2005). A retrospective study assessing the impact of using ACEI/ARBs in hypertensive in-patients of COVID-19 observed a lower all-cause mortality in users of ACEI/ARBs compared to non-users. Adjusted hazard ratio for all-cause mortality was 0.42 with ACEI/ARB use. Also, a reduced adjusted hazard ratio of 0.3 was observed with ACEI/ARB group of antihypertensives compared to other anti-hypertensives (Zhang et al., 2020).
ACEI/ARBs may thus have a dual role in SARS-CoV-2 infection. Patients on chronic ACE inhibitor or angiotensin-AT1 receptor blocker therapy might be hypothetically at increased risk of infection by SARS-CoV-2 due to overexpression of ACE2. In contrast, once infection has been established, discontinuation of such agents would eliminate the protective role of ACE2-Ang (1–7) axis and can lead to a severe or potentially fatal course. Confirmation of both hypotheses, however, requires head to head comparison trials of patients on ACEIs/ARBs and controls with respect to differences in SARS-CoV-2 infection rate, symptoms, severity, and mortality. One prospective observational study is evaluating the impact of previous treatment with ARBs on outcome and severity of disease in SARS-CoV-2 patients (NCT04337190). Another trial is investigating the effect of continuation or discontinuation of ACEIs/ARBs on clinical features and mortality in patients hospitalized with COVID-19 (NCT04351581). The effect of supplementation of telmisartan and valsartan in COVID-19 patients is also being evaluated in randomized controlled clinical settings (NCT04335786, NCT04355936).
6.2. Aldosterone antagonists: spironolactone and eplerenone
Aldosterone causes upregulation of ACE and downregulation of ACE2 in cardiac and renal tissues (Bernardi et al., 2015; Yamamuro et al., 2008). Treatment with spironolactone has been shown to reduce cardiac collagen, decrease cardiac ACE by 41% and increase ACE2 activity in the heart by 42%, in an animal model of heart failure (Karram et al., 2005). Another study assessed the effect of spironolactone on expression and activity of ACE and ACE2 enzymes in macrophages of congestive heart failure patients. Spironolactone treatment for one month reduced ACE activity by 47% and increased ACE2 activity by 300%. The study also analysed the effect of eplerenone administration in mice on cardiac and macrophage ACE2 activity. Eplerenone was found to increase the ACE2 activity by more than 2.5 times (Keidar et al., 2005).
6.3. Other anti-hypertensives
Calcium channel blockers such as amlodipine and cilnidipine, when combined with valsartan induce higher elevations in Ang (1–7) levels and greater reductions in NOX-1 gene expression in vascular tissues compared to valsartan alone in stroke prone SHRs. In this study, ACE gene expression was observed to be the lowest and ACE2 gene expression maximum in the valsartan-cilnidipine group (Takai et al., 2013). Apart from amlodipine and cilnidipine, nifedipine also shows a protective effect on ACE2 as assessed in human aortic endothelial cells under mechanical stress setting (Iizuka et al., 2009). Low dose hydrochlorothiazide has been seen to reduce plasma Ang II/Ang I ratio and plasma ACE in SHR strains. In normotensive and non-SHR rats, hydrochlorothiazide increases cardiac ACE2. No difference was however observed in cardiac angiotensin-AT1 and Mas-receptor expression between SHR and normotensive rats following hydrochlorothiazide treatment (Jessup et al., 2008). The new drug valsartan-sacubitril combination, compared to valsartan alone, has shown better results with respect to blood pressure lowering and cardiac upregulation of ACE2 and angiotensin-AT2 receptor mRNA in SHRs (Zhao et al., 2019).
6.4. Antidiabetic drugs
Hyperglycaemia is known to augment the RAAS axis. In turn, the latter through unopposed Ang II and suppressed Ang (1–7) pathway contributes to the development of diabetes and its complications. RAAS blockers are known to prevent the development of new-onset diabetes (Bindom and Lazartigues, 2009). Thus, hyperglycaemia and RAAS share a vicious bidirectional relationship. Increased urinary ACE2 excretion is seen in patients with diabetes and is associated with deterioration of renal function, poor glycaemic control, and increased serum inflammatory markers (Gilbert et al., 2019). A hospital database study of SARS-CoV patients observed hyperglycaemia in as high as 50% patients in the absence of previous history of diabetes, chronic liver or kidney disease and steroid use. Since ACE2 is expressed on pancreatic beta cells, the authors proposed SARS mediated direct beta cell dysfunction as a probable cause of the new onset diabetes (Yang et al., 2010). Viral stress mediated hypothalamic-pituitary activation and cortisol production also leads to elevated blood sugar levels necessitating the need for anti-diabetic medications. We therefore performed a search for antidiabetic therapies that can possibly modulate the RAAS/ACE2 arms.
6.4.1. PPARγ agonists
Rosiglitazone stabilizes urinary ACE2, decreases renal ADAM-17, improves glycaemic control, and reduces urinary albumin excretion in diabetic mice models (Chodavarapu et al., 2013). The drug improves the response to natriuretic peptide and upregulates renal ACE2 in animal model of congestive heart failure (Goltsman et al., 2019). In a rat model of hypertension, rosiglitazone was shown to improve vascular reactivity, lower blood pressure and increase the antioxidant activity in blood vessels. This was accompanied by an increase in ACE2, Ang (1–7), Mas-receptor and angiotensin-AT2 receptor expression and was mediated via PPARγ stimulation (Sánchez-Aguilar et al., 2019). High fat diet (HFD) is known to increase plasma angiotensin II and blunt ACE2 activity in adipose tissue. It alters the tissue expression of ACE2 via ADAM-17 (Gupte et al., 2008). Pioglitazone has been shown to upregulate the expression of ACE2 in hepatocytes of rats maintained on HFD in addition to bringing improvement in blood glucose and lipid profile (Gupte et al., 2008). The drug also improved cardiac fibrosis, increased Ang (1–7) and ACE2 and reduced ACE/ACE2 ratio in the heart in an animal model of streptozotocin induced diabetes (Qiao et al., 2015).
6.4.2. Insulin
Insulin administration has also been shown to reduce urinary ACE2, renal ADAM-17 and renal ACE2 in various mouse models of type-1 diabetes (Riera et al., 2014; Salem et al., 2014). Insulin has additional immunomodulatory and anti-inflammatory roles such as blunting NF-Kβ signalling, reducing the levels of TNF-α and interfering with neutrophil chemotaxis (Honiden and Gong, 2009).
6.4.3. Drugs acting through GLP-1 receptor
Liraglutide, a glucagon-like peptide-1 (GLP-1) agonist which is approved for both diabetes and obesity, was found to improve lung development in pups of food deprived mother rats via ACE2-Ang (1–7)-Mas axis (Fandiño et al., 2018). The drug has also shown reversal of diabetes induced surfactant protein deficiency in lungs, and suppression of right ventricular hypertrophy, by augmenting ACE2 and Ang (1–7) levels in lungs and circulation (Romaní-Pérez et al., 2015). Liraglutide is also used in hepatic steatosis and non-alcoholic fatty liver disease (NAFLD). In animal models of NAFLD, potentiation of the ACE2-Ang (1–7)-Mas arm of RAAS, suppression of Ang II and activation of PI3K have been recently demonstrated as proposed mechanisms of the hepatoprotective action of liraglutide (Yang et al., 2020). Because of the wide distribution of GLP-1 receptors in lungs, GLP-1 agonists and DPP-IV inhibitors such as linagliptin and sitagliptin known to elevate the levels of GLP-1 should be explored in preclinical studies of acute lung injury. Linagliptin, like liraglutide, potentiates ACE2 activity, angiotensin-AT2 receptor expression and viability of myocardium in animals subjected to Ang II induced cardiac fibrosis (Zhang et al., 2015). Some DPP-4 inhibitors such as sitagliptin can also inhibit ACE. Whether they stimulate ACE2 too is yet to be deciphered (Abouelkheir and El-Metwally, 2019). Clinical trials evaluating the role of linagliptin in COVID-19 patients are ongoing (NCT04371978 and NCT04341935). Since liraglutide has been shown to improve cardiovascular outcomes in diabetic patients and carries a minimal risk of causing hypoglycaemia, its supplementation can be investigated in diabetic patients with COVID-19 illness (Marso et al., 2016). Exendin-4 has also been shown to stimulate ACE2-Ang (1–7)-Mas axis. Levels of intrarenal ACE and Ang II show a decline while those of ACE2 and Ang (1–7) increase with intraperitoneal administration of exendin in a mouse model of ureteric obstruction (Le et al., 2016).
6.4.4. Other antidiabetics-
Among other antidiabetic drugs, metformin and glyburide have been shown to be protective in animal models of acute lung injury through anti-inflammatory and antioxidant action, and inhibitory action on neutrophil migration. Whether these drugs or the newer class of SGLT-2 inhibitors can intervene in the ACE2 or renin-Ang II axis is also worth studying (Nakhleh and Shehadeh, 2020).
6.5. Other approved drugs
6.5.1. Resveratrol
Resveratrol upregulates ACE2, increases Ang (1–7), stimulates Mas-receptors, decreases Ang II levels and downregulates angiotensin-AT1 receptor expression in the ageing kidney and also in animal models of cardiac hypertrophy (Dorri Mashhadi et al., 2017; Jang et al., 2018). Similar changes have been observed in aortic tissues of ageing animals where the drug exerted anti-inflammatory, anti-fibrotic and antioxidant actions (Kim et al., 2018). Resveratrol has been found to increase ACE2 and reduce the development of abdominal aortic aneurysm in APOE knock out mice exposed to HFD and Ang II infusion (Moran et al., 2017). Sirtuin-6 attenuates Ang II mediated cardiac fibrosis in rats by activating AMP kinase and the ACE2 pathway (Zhang et al., 2017).
6.5.2. Lipid lowering drugs-
Rosuvastatin was found to reduce Ang II, upregulate ACE2 and increase Ang (1–7) in rats exposed to vascular balloon injury compared to controls not receiving statins (Li et al., 2013). Fluvastatin reduces diabetic cardiac fibrosis, improves cardiac function and enhances ACE2 expression in glucose-controlled diabetic rats (Shin et al., 2017). Similar findings with cardiac ACE2 preservation have been observed for pravastatin (Min et al., 2018). Likewise, atorvastatin inhibits TNF-α mediated suppression of ACE2 and upregulation of ACE in cultured vascular smooth muscle cells. ACE2 and Ang (1–7) levels improve after atorvastatin treatment (Suski et al., 2014). Among other lipid lowering drugs, fibrates such as fenofibrate have been shown to produce antioxidant action through blunting of Ang II/NOX4 signalling in animal model of myocardial ischemia and metabolic syndrome (Ibarra-Lara et al., 2016b). Clofibrate via PPAR-α stimulation reduces blood pressure and coronary vascular resistance in rats with coarctated aorta. Cardiac ACE2, AT2 receptors, eNOS and superoxide dismutase (SOD) increased after clofibrate treatment (Ibarra-Lara et al., 2016a).
6.5.3. Rho kinase inhibitors
Rho kinase inhibitors like fasudil have also been shown to upregulate ACE2 and increase Ang (1–9) in animal model of hypertension (“Fasudil hydrochloride hydrate,” 2020). Fasudil is approved in Japan for the treatment of cerebral vasospasm and is being tested for unstable angina, pulmonary hypertension and peripheral vascular disease (“Fasudil hydrochloride hydrate,” 2020).
6.5.4. Drugs amplifying the levels of intracellular NO
Drugs such as nitrates, sodium nitroprusside and nitroso acetyl penicillamine have been shown to reduce the expression of Ang II receptors in vascular smooth muscle cells of rats in vitro (Cahill et al., 1995). In this regard, inhalation of NO may also be useful, as NO in addition to being a vasodilator and bronchodilator, also exerts direct anti-viral effect on SARS-CoV replication in vitro (Ignarro, 2020).
6.5.5. Sildenafil
Sildenafil, a drug used in erectile dysfunction and pulmonary hypertension, modulates renin-Ang II axis in the myocardium and improves survival in pigs in induced model of ischemia-recovery. Reduced Ang II levels and reduced expression of angiotensin-AT1 receptors was seen in the myocardium of animals after treatment with sildenafil. Like nitrates, this suppressive action of sildenafil on Ang II receptors comes via NO production inside the cells as eNOS and inducible nitric oxide synthase (iNOS) enzyme levels are increased in myocardium with sildenafil treatment (Wang et al., 2015).
6.5.6. Oestrogen
Oestrogen has a stimulatory effect on ACE2 of adipose tissue, endothelial cells and heart (Bukowska et al., 2017; Gupte et al., 2012; Mompeón et al., 2016). Oestrogen has also been shown to down regulate angiotensin-AT1 receptor mRNA in lungs while having no effect on ACE2, ACE and angiotensin-AT2 receptor mRNAs (Brosnihan et al., 2008). Thus, oestrogen might exert a protective role against SARS-CoV-2 induced ACE2 suppression. This may also explain lower mortality trends observed in female patients of COVID-19 compared to males.
6.5.7. All trans retinoic acid (ATRA)
ATRA increases the cardiac and renal expression of ACE2, reduces blood pressure and myocardial damage in SHRs (Zhong et al., 2004). ATRA has been shown to enhance ACE2, SOD and glutathione (GSH) protein levels and negatively regulate ACE, Ang I and Ang II levels in renal tubular epithelial cells subjected to hypoxia and reoxygenation (Zhou et al., 2014). Improvement in renal morphology, increase in renal ACE2 protein and decrease in Ang I and Ang II are produced by ATRA treatment in glomerulosclerosis model of rats (Zhou et al., 2013). ATRA also ameliorates cardiac fibrosis induced by aortic constriction of rats through enhanced cardiac expression of ACE2 and decline in ACE, Ang II and angiotensin-AT1 receptor (Choudhary et al., 2008).
6.5.8. Activated protein C (APC)
APC deficiency seen in animal models of sepsis potentiates lung injury via activation of ACE, elevation of Ang II and downregulation of the ACE2 pathway. Supplementation of activated protein C improves pulmonary pathology by increasing ACE2. Recombinant activated protein C, drotrecogin alfa is already approved for sepsis associated multi inflammatory response. The same can be tried in patients of SARS-CoV-2 (Richardson et al., 2008). APC has demonstrated nephroprotective effect in lipopolysaccharide (LPS) model of acute renal injury in rats. mRNA expression levels of ACE, iNOS and levels of Ang II peptide was seen to decline while the expression of ACE2 was upregulated in renal tissues after APC treatment (Gupta et al., 2007).
6.5.9. The vitamin D analogue, calcitriol
Calcitriol has been observed to decrease LPS inflammation in pulmonary vascular endothelial cells as well as in vivo in rats by reducing the expression of ACE, angiotensin-AT1 receptor, renin and Ang II, and by increasing ACE2 (Xu et al., 2017). Compared to wild type mice, vitamin D receptor (VDR) knock out mice show increased severity and mortality with LPS induced acute lung injury (Kong et al., 2013). Calcitriol also attenuates diabetes induced downregulation of renal tubular ACE2 and upregulation of ACE by modulating MAPK/ERK pathways (Lin et al., 2016). A clinical trial assessing the immunomodulator role of vitamin D supplementation in prevention and treatment of COVID-19 illness is going on (NCT04334005). Considering diverse other roles of vitamin D supplementation in the elderly who are the major victims of COVID mortality, supplementation can be an attractive option.
6.5.10. Ibuprofen
Ibuprofen has been shown to reduce diabetes induced cardiac fibrosis by activation of the ACE2-Mas axis and downregulation of ACE-Ang II in animal models of type 1 diabetes (Qiao et al., 2015). A clinical trial is evaluating the effect of ibuprofen supplementation on severity of SARS-CoV-2 illness (NCT04383899). Ang II induced hypertension, NOX activity and oxidative stress has also been shown to be ameliorated by other anti-inflammatory drugs such as rofecoxib, nimesulide and aspirin (Wu et al., 2005). RAAS balance is disturbed in inflammation, with a decrease in ACE2 levels in various organs. NSAIDS such as rofecoxib, celecoxib, meloxicam and flurbiprofen have been shown to increase ACE2 and Ang (1–7) levels in the heart and kidney in animal models of adjuvant arthritis (Asghar et al., 2017). A family study assessed the predictors of ACE and ACE2 in circulation. Aspirin users were observed to have lower levels of circulating ACE. A further lowering of ACE levels was seen with combined use of ACEI and aspirin compared to ACEI alone. This suggests a negative association between aspirin use and ACE levels in circulation. The study also observed that higher percentage of patients (20%) with measurable ACE2 were using aspirin compared to those without measurable ACE2 (3.2%), pointing towards an aspirin guided ACE2 regulation. Whether the observations can be explained by direct inhibition of ACE or stimulation of ACE2 by aspirin is yet to be explored (Rice et al., 2006).
6.5.11. Etanercept
A TNF-α blocker used in rheumatoid arthritis has also been shown to reduce brain ACE-AT1 receptor axis & NOX activity and stimulate ACE2-Mas and AT2 receptors in Ang II induced hypertension in rats (Sriramula et al., 2013).
7. Unapproved compounds with experimental evidence of modulating RAAS/ACE2
Several other compounds have been shown in experimental models to have RAAS/ACE2 modulating properties. These are yet to gain drug regulatory approval and range from traditional Chinese medicinal products to novel molecules, herbal extracts, and repurposed compounds. A list of these compounds with their effects on ACE/ACE2 is provided in Table 1 (Castardeli et al., 2018; Chang et al., 2018; Q.-F. Chen et al., 2019; Chen et al., 2018; Y. Chen et al., 2019; Guo et al., 2010; Haber et al., 2014; Kadakol et al., 2015; Lee et al., 2015; Li et al., 2018; Liao et al., 2019; Lin et al., 2017; Liu et al., 2018; Lv et al., 2017; de Macedo et al., 2015; Martínez-Aguilar et al., 2016; Prata et al., 2017; Qaradakhi et al., 2020; Shi et al., 2013; Syed et al., 2016; Tain et al., 2016; Tikellis and Thomas, 2012; Wang et al., 2016, 2018; 2012; Q. Wang et al., 2015; Wei et al., 2015; Wu et al., 2014; Ye et al., 2008; Zhang et al., 2013; Zhao et al., 2015).
Table 1.
Novel compounds with experimental evidence of modulating RAAS/ACE2.
| Compound | Model of assessment | Properties | Modulation of ACE/ACE2 | Reference |
|---|---|---|---|---|
| Adamantan ureido dodecanoic acid (AUDA) | Offspring of high fructose fed maternal rats | Anti-hypertensive and reno-protective property. Increases renal PGE2. |
Activates renal ACE2. | Tain YL, 2016 |
| Astragali radix | High fat diet induced metabolic syndrome in rats | Increases antioxidant enzymes in renal tissue. | Activates ACE2 and Mas-receptor expression in renal tissue. | Wang QY, 2015 |
| Baicalin | HUVEC | Upregulates PI3K/eNOS. | Upregulates ACE2 and Ang (1–7). | Wei X, 2015 |
| Beta- Casomorphin-7 | Diabetic rats | Hypoglycemic, antioxidant, and cardio protective. | Activates renal and cardiac ACE2-Ang (1–7)-Mas axis. | Zhang W, 2013; Wang K, 2016 |
| Biejiajian Oral Liquid | CCl4 induced hepatic fibrosis in rats | Decreases Ang II. Suppresses mRNAs of renin, ACE, and AT1 receptor. | Activates ACE2-Ang (1–7)-Mas axis. | Li X, 2018 |
| BML-111 | LPS induced ALI in mice | Decreases ACE activity. | ACE2 activator. | Chen QF, 2019 |
| Catestatin | Cultured endothelial cells | Anti-hypertensive and anti-atherosclerotic action. Inhibits production of inflammatory cytokines. |
ACE2 activator. | Chen Y, 2019 |
| Curcumin | Cardiac fibrosis and hypertension in rats | Tissue fibrosis attenuated. | ACE2 enhancer. | Pang XF, 2015 |
| Diminazene aceturate (DIZE) | Bleomycin induced lung fibrosis in mice; MI-left ventricular dysfunction in rats; animal models of hypertension, diabetes & atherosclerosis; healthy animals |
Anti-trypanosomal drug in veterinary use. Improves functional capacity, metabolic profile. Reduces adipogenesis. However, neurological toxicity reported. |
ACE2 expression enhancer or independent of ACE2. |
Prata LO, 2017; Castardeli C, 2018; de Macedo SM, 2015; Qaradakhi T 2020; Haber PK, 2014; Qi Y 2013; Ruebush TK 1979 |
| Esculetin | Metabolic syndrome associated vascular dysfunction in diabetic rats | Decreases Ang I receptor expression in aorta. | Preserved ACE2. | Kadakol A, 2015 |
| FGF-21 | Ang II treated mice | Inhibits Ang II induced hypertension, has favorable effect on glucose metabolism, and stimulates PPARγ mediated actions of thiazolidinediones | Stimulates ACE2 in adipocytes and renal cells | Pan X, 2018 |
| IRW | SHR | Reduces blood pressure by activation of Mas receptor. Upregulation of ACE2. Decreases IL-6 and MCP-1. | ACE2 activator. | Liao W, 2019 |
| Lipoxin A4 | LPS induced ALI in mice | Decreases TNF-α, IL-1β, NF- Kβ. | Increases ACE2-Ang (1–7)-Mas pathway. | Chen QF, 2018 |
| Magnolol | Monocrotaline/pneumonectomy induced pulmonary hypertension in rats | Anti-inflammatory, antioxidant, anti-hypertensive, and antiplatelet property. Inhibits ACE, AT1 receptor, and ETA. Upregulates eNOS in lungs. |
Activates ACE2 in lungs. | Chang H, 2018 |
| NaHS | Carotid artery ligation animal model | Inhibits atherosclerosis. | Activates ACE2 and Ang (1–7). | Lin Y, 2017 |
| Osthole | LPS induced ALI in mice | Decreases levels of IL-6 and TNF-⍺. | Stabilization of ACE2 and Ang (1–7) in lungs. | Shi Y, 2013 |
| Panax notoginseng | MI rat model | Decreases cardiac TNF-⍺. | Activates ACE2. | Guo JW, 2010 |
| Puerarin | SHR | Cardioprotective, neuroprotective, anti-inflammatory, antioxidant, vasodilatory, and metabolic regulatory properties. Increases cardiac AT1 receptor mRNA. |
Activates cardiac ACE2. | Ye XY, 2008 |
| Qishenyiqi (QSYQ) | Coronary heart disease rat model | Decreases intracardiac renin and Ang II. | Increases intracardiac ACE2. | Wang Y, 2012 |
| Red Liriope platyphylla (ethanol extract) | SHR | Up-regulates ACE, ACE2, eNOS, and SOD in aorta. | Activates ACE2 in aorta. | Lee YJ, 2015 |
| Sini decoction (SND) | E. coli induced ALI in mice | Suppresses ACE-Ang II-AT1 receptor pathway. | Upregulates ACE2-Ang (1–7)-Mas axis. | Liu J, 2018 |
| Tanshinone | Bleomycin induced pulmonary fibrosis, paraquat induced ALI in rats | Anti-inflammatory. | Enhanced myocardial ACE2-Ang (1–7)-Mas axis. | Wu H, 2014; Wang Y, 2018 |
| Taurine | Stress induced hypertension in rats | Down-regulates ACE of hypothalamic-pituitary-adrenal axis. | Activates ACE2 in hypothalamic-pituitary-adrenal axis. | Lv Q, 2017 |
| TBTIF | SHR | Decreases blood pressure and abrogates vasoconstrictor response of Ang II in aortic rings. | Increases ACE2 mRNA in aortic rings. | Martínez-Aguilar L, 2016 |
| Ulmus wallichiana Planchon | Isoprenaline induced animal model of cardiac hypertrophy | Decreases expression of IL-6 and TNF-⍺. Decreases plasma ACE and Ang II levels. |
Activates cardiac ACE2 and Mas receptor. | Syed AA, 2016 |
| Xanthenone, Resorcinol naphthalene | Animal models of cardiovascular & pulmonary diseases | Blood pressure lowering, anti-inflammatory, cardio protective & antithrombotic actions. | ACE2 activators. | Tikellis C; 2012. |
[Abbreviations; ACE: Angiotensin converting enzyme, ALI: Acute lung injury, Ang: Angiotensin, BP: Blood pressure, CCl4: Tetrachloromethane, eNOS: Endothelial nitric oxide synthase, ETA: Endothelin receptor A, HUVEC: Human umbilical vein endothelial cells, IL-1β: Interleukin-1-beta, IL-6: Interleukin-6, LPS: Lipopolysaccharide, MCP-1: Monocyte chemoattractant protein-1, MI: Myocardial infarction, mRNA: messenger ribonucleic acid, NF-kB: Nuclear factor kappa beta, PGE2: Prostaglandin E2, PI3K: Phosphoinositide-3-kinase, PPARγ: peroxisome proliferator activated receptor gamma, SHR: Spontaneously hypertensive rats, SOD: Superoxide dismutase, TBTIF: Tert-butyl-thiomorpholinyl-methylphenol, TNFα: Tumor Necrosis Factor alpha].
8. Candidate drugs which directly affect the ACE2 arm
Considering the protective role of ACE2 in cardiopulmonary hemodynamics, Mas-receptor agonists such as Ang (1–7) have been hypothesized by many as important weapons in SARS-CoV-2 armamentarium (Magalhaes et al., 2020; Peiró and Moncada, 2020). Ang (1–7), glycosylated Ang (1–7) and a nonpeptide based compound AVE0991, have given successful results in preclinical studies testing their efficacy in cardiopulmonary diseases and diabetes (Patel et al., 2012; Pinheiro et al., 2004). Ang (1–7) peptide and its cyclodextrin-hydroxy propyl based oral formulation improve neurological outcomes in animal models of stroke (Bennion et al., 2018). Topical application of the Ang (1–7) analogue, DSC127 has given successful results in clinical trials enrolling diabetic foot ulcer patients (NCT00796744). Some Phase 2 clinical trials aim to elucidate the role of intravenous Ang (1–7) peptide on mortality and clinical outcomes in COVID-19 patients (NCT04375124, Status: Recruiting patients, NCT04332666 and NCT04401423, Status: Not yet recruiting). Certain cyclized derivatives of Ang (1–7) with introduction of a thioether ring have advantages such as better in vivo stability, increased resistance to degradation by ACE and higher magnitude of vasorelaxation compared to the natural Ang (1–7) peptide (Kluskens et al., 2009; Rink et al., 2010). AVE0991, a non-peptide Mas-receptor agonist, is being evaluated for various cardiovascular diseases (Pinheiro et al., 2004). The ACE2 axis can also be stimulated in vivo by administering recombinant ACE2. Soluble ACE2 administration has been shown to block the entry of SARS-CoV in sensitive cells (Li et al., 2003). Human recombinant soluble form of ACE2 (hrsACE2) has been shown to reduce SARS-CoV-2 infection in Vero-E6 cells by a factor of 1000–5000. The study also showed reduced SARS-CoV-2 infection of human blood vessel organoid and kidney organoid in the presence of hrsACE2 (Monteil et al., 2020). Very recently, ACE2 supplementation has been shown to reduce lung inflammation in mouse model of LPS-induced inflammation. LPS-TLR4 pathway of inflammation was attenuated with ACE2 supplementation through activation of Mas-receptors (Ye and Liu, 2020). ACE2 based therapies are novel candidates that can be explored in clinical trials of COVID-19 patients. Their safety and tolerability have already been assessed adequately in healthy individuals (NCT00886353). Recombinant ACE2 is also being evaluated in pulmonary arterial hypertension patients (NCT01884051, NCT03177603). B38-CAP is an ACE2 like enzyme derived from Paenibacillus species. B38-CAP decreases Ang II and increases Ang (1–7). The enzyme has been shown to reduce Ang II induced hypertension and cardiac fibrosis in mice (Minato et al., 2020). B38-CAP is being investigated as a promising strategy in trials involving COVID-19 patients (NCT04375046).
9. Conclusion
In this review, we have tried to compile the available literature on medical therapies that could possibly activate the ACE2/Mas-receptor pathway. Drugs used in diverse cardiopulmonary diseases and diabetes are especially relevant, as a greater fraction of patients succumbing to COVID-19 suffer from these co-morbidities. Among drugs used in cardiovascular diseases, experimental evidence for ACE2 upregulation is high for ACEIs and ARBs. Aldosterone antagonists like spironolactone and eplerenone have also shown the ability to upregulate ACE2. Other antihypertensives such as low dose hydro-chlorothiazide, calcium channel blockers and valsartan/sacubitril too have limited evidence of ACE2 upregulating properties. These and multiple other drugs such as lipid lowering agents, resveratrol etc may be easily tried in COVID-19 patients who have already been prescribed these drugs for underlying cardiovascular diseases. As far as anti-diabetes medications are concerned, experimental evidence for an ACE2 regulatory role is at present strong for GLP-1 agonists such as liraglutide. PPARγ agonists such as rosiglitazone and pioglitazone upregulate ACE2 in conditions such as diabetes, obesity, hypertension, and heart failure. Some ACE2 upregulating action has also been observed with linagliptin. Although insulin is the standard anti diabetic therapy during any acute illness, liraglutide and DPP4 inhibitors may be tried in diabetic patients with SARS-CoV-2 infection. Chances of hypoglycaemia are less with these medications and liraglutide is already known to improve cardiovascular outcomes in diabetic patients. Rosiglitazone and pioglitazone, however, have a propensity to induce fluid retention; hence should be best avoided in heart failure. Insulin exerts anti-inflammatory effects mainly via modulation of NF-Kβ signalling and whether cardiac or pulmonary ACE2 is affected by insulin administration has not been proven yet.
Among NSAIDS, ibuprofen, rofecoxib, celecoxib, flurbiprofen and meloxicam have been shown to correct the ACE2 imbalance of inflammation. Some of these agents are being explored in clinical trials of COVID-19 patients. The ACE2 expression-enhancing effects of celecoxib and rofecoxib should however be balanced with their propensity to induce vascular thrombosis and ischemic events. Although aspirin exerts anti-inflammatory and anti-platelet actions and blunts AT1 receptor signalling, its direct interaction with ACE/ACE2 pathways needs to be deciphered.
Calcitriol, the active form of vitamin D, has shown upregulation of ACE2 in acute lung injury. While the results of clinical trials on vitamin D supplementation are awaited, it may be added to standard of care in COVID-19 patients who have baseline vitamin D insufficiency or deficiency, along with careful monitoring of serum calcium and phosphate. Because of the favorable effects of oestrogen on ACE2 in various tissues, brief supplementation of this hormone can also be tried at least in post-menopausal women or women with premature menopause and COVID-19 illness.
Finally, potential direct activators of the ACE2/Mas-receptor system and unapproved drugs with the ability to increase ACE2 in experimental models may open therapeutic strategies for COVID-19 and even related viral illnesses with a similar pathogenesis. Compounds such as xanthenone, resorcinol naphthalene, DIZE, lipoxin A4, curcumin, beta casomorphin, catestatin, AUDA, TBTIF, FGF-21 and multiple traditional Chinese herbal compounds come in this category. These and direct participants in the ACE2 axis such as rACE2, B38-CAP and Mas-receptor agonists like Ang (1–7) and AVE0991 should be explored in controlled clinical trials. In the absence of effective anti-viral therapy and in the face of the continuing SARS-CoV-2 pandemic and its potential to transform into an endemic disease, drug repurposing seems a reasonable and time saving option, worthy to be explored.
Funding support
None.
CRediT authorship contribution statement
Upinder Kaur: Conceptualization, Investigation, Writing - original draft, Supervision. Kumudini Acharya: Investigation, Writing - original draft. Ritwick Mondal: Writing - original draft. Amit Singh: Writing - review & editing. Luciano Saso: Writing - review & editing. Sasanka Chakrabarti: Writing - review & editing. Sankha Shubhra Chakrabarti: Writing - review & editing, Supervision.
Declaration of competing interest
None.
Acknowledgement:
Prof S Chakrabarti wishes to thank the Indian Council of Medical Research and the Maharishi Markandeshwar (deemed to be) University for research support. Dr SS Chakrabarti wishes to thank the Indian Council of Medical Research for research support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejphar.2020.173545.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abouelkheir M., El-Metwally T.H. Dipeptidyl peptidase-4 inhibitors can inhibit angiotensin converting enzyme. Eur. J. Pharmacol. 2019;862:172638. doi: 10.1016/j.ejphar.2019.172638. [DOI] [PubMed] [Google Scholar]
- Agata J., Ura N., Yoshida H., Shinshi Y., Sasaki H., Hyakkoku M., Taniguchi S., Shimamoto K. Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertens. Res. 2006;29:865–874. doi: 10.1291/hypres.29.865. [DOI] [PubMed] [Google Scholar]
- Andersen C.U., Hilberg O., Mellemkjær S., Nielsen-Kudsk J.E., Simonsen U. Apelin and pulmonary hypertension. Pulm. Circ. 2011;1:334–346. doi: 10.4103/2045-8932.87299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendse L.B., Danser A.H.J., Poglitsch M., Touyz R.M., Burnett J.C., Llorens-Cortes C., Ehlers M.R., Sturrock E.D. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019;71:539–570. doi: 10.1124/pr.118.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar W., Aghazadeh-Habashi A., Jamali F. Cardiovascular effect of inflammation and nonsteroidal anti-inflammatory drugs on renin–angiotensin system in experimental arthritis. Inflammopharmacology. 2017;25:543–553. doi: 10.1007/s10787-017-0344-1. [DOI] [PubMed] [Google Scholar]
- Bennion D.M., Jones C.H., Donnangelo L.L., Graham J.T., Isenberg J.D., Dang A.N., Rodriguez V., Sinisterra R.D.M., Sousa F.B., Santos R.A.S., Sumners C. Neuroprotection by post-stroke administration of an oral formulation of angiotensin-(1-7) in ischaemic stroke. Exp. Physiol. 2018;103:916–923. doi: 10.1113/EP086957. [DOI] [PubMed] [Google Scholar]
- Bernardi S., Toffoli B., Zennaro C., Bossi F., Losurdo P., Michelli A., Carretta R., Mulatero P., Fallo F., Veglio F., Fabris B. Aldosterone effects on glomerular structure and function. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2015;16:730–738. doi: 10.1177/1470320315595568. [DOI] [PubMed] [Google Scholar]
- Bindom S.M., Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol. Cell. Endocrinol. 2009;302:193–202. doi: 10.1016/j.mce.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnihan K.B., Hodgin J.B., Smithies O., Maeda N., Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT 1/AT 2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-α knock-out mice. Exp. Physiol. 2008;93:658–664. doi: 10.1113/expphysiol.2007.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowska A., Spiller L., Wolke C., Lendeckel U., Weinert S., Hoffmann J., Bornfleth P., Kutschka I., Gardemann A., Isermann B., Goette A. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp. Biol. Med. 2017;242:1412–1423. doi: 10.1177/1535370217718808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock G.R., Steyaert I., Bilbe G., Carey R.M., Kips J., De Paepe B., Pauwels R., Praet M., Siragy H.M., de Gasparo M. Distribution of type-1 and type-2 angiotensin receptors in the normal human lung and in lungs from patients with chronic obstructive pulmonary disease. Histochem. Cell Biol. 2001;115:117–124. doi: 10.1007/s004180000235. [DOI] [PubMed] [Google Scholar]
- Cahill P.A., Redmond E.M., Foster C., Sitzmann J.V. Nitric oxide regulates angiotensin II receptors in vascular smooth muscle cells. Eur. J. Pharmacol. Mol. Pharmacol. 1995;288:219–229. doi: 10.1016/0922-4106(95)90197-3. [DOI] [PubMed] [Google Scholar]
- Castardeli C., Sartório C.L., Pimentel E.B., Forechi L., Mill J.G. The ACE 2 activator diminazene aceturate (DIZE) improves left ventricular diastolic dysfunction following myocardial infarction in rats. Biomed. Pharmacother. 2018;107:212–218. doi: 10.1016/j.biopha.2018.07.170. [DOI] [PubMed] [Google Scholar]
- Chang H., Chang C.-Y., Lee H.-J., Chou C.-Y., Chou T.-C. Magnolol ameliorates pneumonectomy and monocrotaline-induced pulmonary arterial hypertension in rats through inhibition of angiotensin II and endothelin-1 expression. Phytomedicine. 2018;51:205–213. doi: 10.1016/j.phymed.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Chappell M.C. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016;310:H137–H152. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Chinese J. Cardiovasc. Dis. 2020;48:E008. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- Chen Q.-F., Hao H., Kuang X.-D., Hu Q.-D., Huang Y.-H., Zhou X.-Y. BML-111, a lipoxin receptor agonist, protects against acute injury via regulating the renin angiotensin-aldosterone system. Prostag. Other Lipid Mediat. 2019;140:9–17. doi: 10.1016/j.prostaglandins.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Chen Q.-F., Kuang X.-D., Yuan Q.-F., Hao H., Zhang T., Huang Y.-H., Zhou X.-Y. Lipoxin A 4 attenuates LPS-induced acute lung injury via activation of the ACE2-Ang-(1-7)-Mas axis. Innate Immun. 2018;24:285–296. doi: 10.1177/1753425918785008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang X., Yang C., Su X., Yang W., Dai Y., Han H., Jiang J., Lu L., Wang H., Chen Q., Jin W. Decreased circulating catestatin levels are associated with coronary artery disease: the emerging anti-inflammatory role. Atherosclerosis. 2019;281:78–88. doi: 10.1016/j.atherosclerosis.2018.12.025. [DOI] [PubMed] [Google Scholar]
- Chodavarapu H., Grobe N., Somineni H.K., Salem E.S.B., Madhu M., Elased K.M. Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PloS One. 2013;8 doi: 10.1371/journal.pone.0062833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary R., Palm-Leis A., Scott R.C., Guleria R.S., Rachut E., Baker K.M., Pan J. All- trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am. J. Physiol. Circ Physiol. 2008;294:H633–H644. doi: 10.1152/ajpheart.01301.2007. [DOI] [PubMed] [Google Scholar]
- Coronavirus Update Www Document https://www.worldometers.info/coronavirus/ n.d. accessed 6.11.20.
- Deng J., Wang D.-X., Deng W., Li C.-Y., Tong J. The effect of endogenous angiotensin II on alveolar fluid clearance in rats with acute lung injury. Can. Respir. J. 2012;19:311–318. doi: 10.1155/2012/951025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorri Mashhadi F., Zavvar Reza J., Jamhiri M., Hafizi Z., Zare Mehrjardi F., Safari F. The effect of resveratrol on angiotensin II levels and the rate of transcription of its receptors in the rat cardiac hypertrophy model. J. Physiol. Sci. 2017;67:303–309. doi: 10.1007/s12576-016-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandiño J., Vaz A.A., Toba L., Romaní-Pérez M., González-Matías L., Mallo F., Diz-Chaves Y. Liraglutide enhances the activity of the ACE2/ang(1–7)/mas receptor pathway in lungs of male pups from food-restricted mothers and prevents the reduction of SP-A. Int J. Endocrinol. 2018 doi: 10.1155/2018/6920620. 2018, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasudil hydrochloride hydrate Www Document https://www.pharmacodia.com/yaodu/html/v1/chemicals/23e846638607adcbc730817a77581220.html n.d. accessed 5.7.20.
- Gilbert A., Liu J., Cheng G., An C., Deo K., Gorret A.M., Qin X. vol. 29. 2019. pp. 1–11. (A Review of Urinary Angiotensin Converting Enzyme 2 in Diabetes and Diabetic Nephropathy). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltsman I., Khoury E.E., Aronson D., Nativ O., Feuerstein G.Z., Winaver J., Abassi Z. Rosiglitazone treatment restores renal responsiveness to atrial natriuretic peptide in rats with congestive heart failure. J. Cell. Mol. Med. jcmm. 2019;14366 doi: 10.1111/jcmm.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Xie Z., Li T., Zhang S., Lai C., Zhu P., Wang K., Han L., Duan Y., Zhao Z., Yang X., Xing L., Zhang P., Wang Z., Li R., Yu J.J., Wang X., Yang P. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016;6:19840. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J.-W., Li L.-M., Qiu G.-Q., Deng Z.-J., Fu Y.-H., Yang M., Pan J.-Q., Liu R.-X. [Effects of Panax notoginseng saponins on ACE2 and TNF-alpha in rats with post-myocardial infarction-ventricular remodeling] Zhong Yao Cai. 2010;33:89–92. [PubMed] [Google Scholar]
- Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Rhodes G.J., Berg D.T., Gerlitz B., Molitoris B.A., Grinnell B.W. Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am. J. Physiol. Renal Physiol. 2007;293:F245–F254. doi: 10.1152/ajprenal.00477.2006. [DOI] [PubMed] [Google Scholar]
- Gupte M., Boustany-Kari C.M., Bharadwaj K., Police S., Thatcher S., Gong M.C., English V.L., Cassis L.A. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am. J. Physiol. Integr. Comp. Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte M., Thatcher S.E., Boustany-Kari C.M., Shoemaker R., Yiannikouris F., Zhang X., Karounos M., Cassis L.A. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57bl/6 mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev. Res. ddr. 2020;21656 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber P.K., Ye M., Wysocki J., Maier C., Haque S.K., Batlle D. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: studies in vivo, ex vivo, and in vitro. Hypertens. (Dallas, Tex. 2014;63:774–782. doi: 10.1161/HYPERTENSIONAHA.113.02856. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF- -converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF- production and facilitates viral entry. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honiden S., Gong M.N. Diabetes, insulin, and development of acute lung injury. Crit. Care Med. 2009;37:2455–2464. doi: 10.1097/CCM.0b013e3181a0fea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Lara L., Del Valle-Mondragón L., Soria-Castro E., Torres-Narváez J.C., Pérez-Severiano F., Sánchez-Aguilar M., Ramírez-Ortega M., Cervantes-Pérez L.G., Pastelín-Hernández G.S., Oidor-Chan V.H., Zarco-Olvera G., Sánchez-Mendoza A. Peroxisome proliferator-activated receptor-α stimulation by clofibrate favors an antioxidant and vasodilator environment in a stressed left ventricle. Pharmacol. Rep. 2016;68:692–702. doi: 10.1016/j.pharep.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Ibarra-Lara L., Sánchez-Aguilar M., Sánchez-Mendoza A., Del Valle-Mondragón L., Soria-Castro E., Carreón-Torres E., Díaz-Díaz E., Vázquez-Meza H., Guarner-Lans V., Rubio-Ruiz M. Fenofibrate therapy restores antioxidant protection and improves myocardial insulin resistance in a rat model of metabolic syndrome and myocardial ischemia: the role of angiotensin II. Molecules. 2016;22:31. doi: 10.3390/molecules22010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L.J. Inhaled NO and COVID‐19. Br. J. Pharmacol. bph. 2020;15085 doi: 10.1111/bph.15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka K., Kusunoki A., Machida T., Hirafuji M. Angiotensin II reduces membranous angiotensin-converting enzyme 2 in pressurized human aortic endothelial cells. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2009;10:210–215. doi: 10.1177/1470320309343710. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008;93:543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.-C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertens. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Jang I.-A., Kim E., Lim J., Kim M., Ban T., Yoon H., Park C., Chang Y., Choi B. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients. 2018;10:1741. doi: 10.3390/nu10111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., DeMartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup J.A., Brosnihan K.B., Gallagher P.E., Chappell M.C., Ferrario C.M. Differential effect of low dose thiazides on the Renin Angiotensin system in genetically hypertensive and normotensive rats. J. Am. Soc. Hypertens. 2008;2:106–115. doi: 10.1016/j.jash.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.S., Vinh A., McCarthy C.A., Gaspari T.A., Widdop R.E. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol. Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadakol A., Malek V., Goru S.K., Pandey A., Bagal S., Gaikwad A.B. Esculetin attenuates alterations in Ang II and acetylcholine mediated vascular reactivity associated with hyperinsulinemia and hyperglycemia. Biochem. Biophys. Res. Commun. 2015;461:342–347. doi: 10.1016/j.bbrc.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Kakodkar P., Kaka N., Baig M.N. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020;12 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram T., Abbasi A., Keidar S., Golomb E., Hochberg I., Winaver J., Hoffman A., Abassi Z. Effects of spironolactone and eprosartan on cardiac remodeling and angiotensin-converting enzyme isoforms in rats with experimental heart failure. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1351–H1358. doi: 10.1152/ajpheart.01186.2004. [DOI] [PubMed] [Google Scholar]
- Keidar S., Gamliel-Lazarovich A., Kaplan M., Pavlotzky E., Hamoud S., Hayek T., Karry R., Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ. Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- Kim E.N., Kim M.Y., Lim J.H., Kim Y., Shin S.J., Park C.W., Kim Y.-S., Chang Y.S., Yoon H.E., Choi B.S. The protective effect of resveratrol on vascular aging by modulation of the renin–angiotensin system. Atherosclerosis. 2018;270:123–131. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- Kluskens L.D., Nelemans S.A., Rink R., de Vries L., Meter-Arkema A., Wang Y., Walther T., Kuipers A., Moll G.N., Haas M. Angiotensin-(1-7) with thioether bridge: an angiotensin-converting enzyme-resistant, potent angiotensin-(1-7) analog. J. Pharmacol. Exp. Therapeut. 2009;328:849–854. doi: 10.1124/jpet.108.146431. [DOI] [PubMed] [Google Scholar]
- Kong J., Zhu X., Shi Y., Liu T., Chen Y., Bhan I., Zhao Q., Thadhani R., Li Y.C. VDR attenuates acute lung injury by blocking ang-2-tie-2 pathway and renin-angiotensin system. Mol. Endocrinol. 2013;27:2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y., Zheng Z., Xue J., Cheng M., Guan M., Xue Y. Effects of exendin-4 on the intrarenal renin-angiotensin system and interstitial fibrosis in unilateral ureteral obstruction mice: exendin-4 and unilateral ureteral obstruction. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2016;17 doi: 10.1177/1470320316677918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.-J., Koh E.-K., Kim J.-E., Go J., Song S.-H., Seong J.-E., Son H.-J., Kang B.-C., Hwang D.-Y. Beneficial effects of ethanol extracts of Red Liriope platyphylla on vascular dysfunction in the aorta of spontaneously hypertensive rats. Lab. Anim. Res. 2015;31:13. doi: 10.5625/lar.2015.31.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Peng Y., Bu X., Yao J., Yao L. Balancing effect of biejiajian oral liquid (鳖甲煎口服液) on ACE-ang II-AT1R Axis and ACE2-ang-(1–7)-mas Axis in rats with CCl4-induced hepatic fibrosis. Chin. J. Integr. Med. 2018;24:853–859. doi: 10.1007/s11655-017-2909-7. [DOI] [PubMed] [Google Scholar]
- Li Y.-H., Wang Q.-X., Zhou J.-W., Chu X.-M., Man Y.-L., Liu P., Ren B.-B., Sun T.-R., An Y. Effects of rosuvastatin on expression of angiotensin-converting enzyme 2 after vascular balloon injury in rats. J. Geriatr. Cardiol. 2013;10:151–158. doi: 10.3969/j.issn.1671-5411.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Fan H., Davidge S.T., Wu J. Egg white–derived antihypertensive peptide IRW (Ile‐Arg‐Trp) reduces blood pressure in spontaneously hypertensive rats via the ACE2/ang (1‐7)/mas receptor Axis. Mol. Nutr. Food Res. 2019;63:1900063. doi: 10.1002/mnfr.201900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Gao P., Zhao T., He L., Li M., Li Y., Shui H., Wu X. Calcitriol regulates angiotensin-converting enzyme and angiotensin converting-enzyme 2 in diabetic kidney disease. Mol. Biol. Rep. 2016;43:397–406. doi: 10.1007/s11033-016-3971-5. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zeng H., Gao L., Gu T., Wang C., Zhang H. Hydrogen sulfide attenuates atherosclerosis in a partially ligated carotid artery mouse model via regulating angiotensin converting enzyme 2 expression. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen Q., Liu S., Yang X., Zhang Y., Huang F. Sini decoction alleviates E. coli induced acute lung injury in mice via equilibrating ACE-AngII-AT1R and ACE2-Ang-(1-7)-Mas axis. Life Sci. 2018;208:139–148. doi: 10.1016/j.lfs.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Liu X., Yang N., Tang J., Liu S., Luo D., Duan Q., Wang X. Downregulation of angiotensin-converting enzyme 2 by the neuraminidase protein of influenza A (H1N1) virus. Virus Res. 2014;185:64–71. doi: 10.1016/j.virusres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li Jinxiu, Li Jianming, Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q., Yang Q., Cui Y., Yang J., Wu G., Liu M., Ning Z., Cao S., Dong G., Hu J. 2017. Effects of Taurine on ACE, ACE2 and HSP70 Expression of Hypothalamic-Pituitary-Adrenal Axis in Stress-Induced Hypertensive Rats; pp. 871–886. [DOI] [PubMed] [Google Scholar]
- Macedo S., Guimarares T., Andrade J., Guimaraes A., Paula A., Ferreira A., Santos S. Angiotensin converting enzyme 2 activator (DIZE) modulates metabolic profiles in mice, decreasing lipogenesis. Protein Pept. Lett. 2015;22:332–340. doi: 10.2174/0929866522666150209125401. [DOI] [PubMed] [Google Scholar]
- Magalhaes G.S., Rodrigues-Machado M. da G., Motta-Santos D., Campagnole-Santos M.J., Santos R.A.S. Activation of ang-(1-7)/mas receptor is a possible strategy to treat coronavirus (SARS-CoV-2) infection. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrone T., Magrone M., Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocr. Metab. Immune Disord. - Drug Targets. 2020 doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F.E., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., Steinberg W.M., Stockner M., Zinman B., Bergenstal R.M., Buse J.B. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Aguilar L., Lezama-Martínez D., Orozco-Cortés N.V., González-Espinosa C., Flores-Monroy J., Valencia-Hernández I. Antihypertensive properties of a novel morphologic derivative (4-tert-buthyl-2,6-bis(thiomorpholine-4-ilmethyl)phenol) J. Cardiovasc. Pharmacol. 2016;67:246–251. doi: 10.1097/FJC.0000000000000340. [DOI] [PubMed] [Google Scholar]
- Marzi A., Gramberg T., Simmons G., Möller P., Rennekamp A.J., Krumbiegel M., Geier M., Eisemann J., Turza N., Saunier B., Steinkasserer A., Becker S., Bates P., Hofmann H., Pöhlmann S. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004;78 doi: 10.1128/JVI.78.21.12090-12095.2004. 12090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.J., Shin B.-S., Lee J.-H., Jeon Y., Ryu D.K., Kim S., Shin Y.H. Effects of pravastatin on type 1 diabetic rat heart with or without blood glycemic control. J. Diabetes Res. 2018 doi: 10.1155/2018/1067853. 2018, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato T., Nirasawa S., Sato T., Yamaguchi T., Hoshizaki M., Inagaki T., Nakahara K., Yoshihashi T., Ozawa R., Yokota S., Natsui M., Koyota S., Yoshiya T., Yoshizawa-Kumagaye K., Motoyama S., Gotoh T., Nakaoka Y., Penninger J.M., Watanabe H., Imai Y., Takahashi S., Kuba K. B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction. Nat. Commun. 2020;11:1058. doi: 10.1038/s41467-020-14867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mompeón A., Lázaro-Franco M., Bueno-Betí C., Pérez-Cremades D., Vidal-Gómez X., Monsalve E., Gironacci M.M., Hermenegildo C., Novella S. Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1–7 production. Mol. Cell. Endocrinol. 2016;422:1–8. doi: 10.1016/j.mce.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.004. 905-913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C.S., Biros E., Krishna S.M., Wang Y., Tikellis C., Morton S.K., Moxon J.V., Cooper M.E., Norman P.E., Burrell L.M., Thomas M.C., Golledge J. Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin-converting enzyme 2. Arterioscler. Thromb. Vasc. Biol. 2017;37:2195–2203. doi: 10.1161/ATVBAHA.117.310129. [DOI] [PubMed] [Google Scholar]
- Nakhleh A., Shehadeh N. Interactions between antihyperglycemic drugs and the renin-angiotensin system: putative roles in COVID-19. A mini-review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:509–512. doi: 10.1016/j.dsx.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Dinh Cat A., Montezano A.C., Burger D., Touyz R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.B., Bodiga S., Fan D., Das S.K., Wang Z., Wang W., Basu R., Zhong J., Kassiri Z., Oudit G.Y. Cardioprotective effects mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin 1-7 in experimental heart failure in angiotensin-converting enzyme 2–null mice. Hypertension. 2012;59:1195–1203. doi: 10.1161/HYPERTENSIONAHA.112.191650. [DOI] [PubMed] [Google Scholar]
- Peiró C., Moncada S. Substituting angiotensin-(1-7) to prevent lung damage in SARS-CoV-2 infection? Circulation. 2020;141:1665–1666. doi: 10.1161/CIRCULATIONAHA.120.047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro S.V.B., Simões e Silva A.C., Sampaio W.O., de Paula R.D., Mendes E.P., Bontempo E.D., Pesquero J.B., Walther T., Alenina N., Bader M., Bleich M., Santos R.A.S. Nonpeptide AVE 0991 is an angiotensin-(1–7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44:490–496. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- Prata L.O., Rodrigues C.R., Martins J.M., Vasconcelos P.C., Oliveira F.M.S., Ferreira A.J., Rodrigues-Machado M. da G., Caliari M.V. Original Research: ACE2 activator associated with physical exercise potentiates the reduction of pulmonary fibrosis. Exp. Biol. Med. 2017;242:8–21. doi: 10.1177/1535370216665174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaradakhi T., Gadanec L.K., McSweeney K.R., Tacey A., Apostolopoulos V., Levinger I., Rimarova K., Egom E.E., Rodrigo L., Kruzliak P., Kubatka P., Zulli A. The potential actions of angiotensin‐converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin. Exp. Pharmacol. Physiol. 2020;47:751–758. doi: 10.1111/1440-1681.13251. [DOI] [PubMed] [Google Scholar]
- Qiao W., Wang C., Chen B., Zhang F., Liu Y., Lu Q., Guo H., Yan C., Sun H., Hu G., Yin X. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131:97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- Rice G.I., Jones A.L., Grant P.J., Carter A.M., Turner A.J., Hooper N.M. Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension. 2006;48:914–920. doi: 10.1161/01.HYP.0000244543.91937.79. [DOI] [PubMed] [Google Scholar]
- Richardson M.A., Gupta A., O'Brien L.A., Berg D.T., Gerlitz B., Syed S., Sharma G.R., Cramer M.S., Heuer J.G., Galbreath E.J., Grinnell B.W. Treatment of sepsis-induced acquired protein C deficiency reverses angiotensin-converting enzyme-2 inhibition and decreases pulmonary inflammatory response. J. Pharmacol. Exp. Therapeut. 2008;325:17–26. doi: 10.1124/jpet.107.130609. [DOI] [PubMed] [Google Scholar]
- Riera M., Márquez E., Clotet S., Gimeno J., Roca-Ho H., Lloreta J., Juanpere N., Batlle D., Pascual J., Soler M.J. Effect of insulin on ACE2 activity and kidney function in the non-obese diabetic mouse. PloS One. 2014;9 doi: 10.1371/journal.pone.0084683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink R., Arkema-Meter A., Baudoin I., Post E., Kuipers A., Nelemans S.A., Akanbi M.H.J., Moll G.N. To protect peptide pharmaceuticals against peptidases. J. Pharmacol. Toxicol. Methods. 2010;61:210–218. doi: 10.1016/j.vascn.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M., Santisteban P., González-Matías L.C., Vigo E., Mallo F. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156:3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- Rothuizen L.E., Livio F., Buclin T. Traitements aggravant une infection par le COVID-19 : vraiment ? Rev. Med. Suisse. 2020;16:852–854. [PubMed] [Google Scholar]
- Salem E.S.B., Grobe N., Elased K.M. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am. J. Physiol. Renal Physiol. 2014;306:F629–F639. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Aguilar M., Ibarra-Lara L., Del Valle-Mondragón L., Rubio-Ruiz M.E., Aguilar-Navarro A.G., Zamorano-Carrillo A., Ramírez-Ortega M., del C., Pastelín-Hernández G., Sánchez-Mendoza A. Rosiglitazone, a ligand to PPAR γ , improves blood pressure and vascular function through renin-angiotensin system regulation. PPAR Res. 2019 doi: 10.1155/2019/1371758. 2019, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Suzuki T., Watanabe H., Kadowaki A., Fukamizu A., Liu P.P., Kimura A., Ito H., Penninger J.M., Imai Y., Kuba K. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Invest. 2013;123:5203–5211. doi: 10.1172/JCI69608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifenbaum J. Alamandine and its receptor MrgD pair up to join the protective arm of the renin-angiotensin system. Front. Med. 2019;6:107. doi: 10.3389/fmed.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang B., Chen X.-J., Xu D.-Q., Wang Y.-X., Dong H.-Y., Ma S.-R., Sun R.-H., Hui Y.-P., Li Z.-C. Osthole protects lipopolysaccharide-induced acute lung injury in mice by preventing down-regulation of angiotensin-converting enzyme 2. Eur. J. Pharmaceut. Sci. 2013;48:819–824. doi: 10.1016/j.ejps.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Shin Y.H., Min J.J., Lee J.-H., Kim E.-H., Kim G.E., Kim M.H., Lee J.J., Ahn H.J. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Ves. 2017;32:618–627. doi: 10.1007/s00380-016-0936-5. [DOI] [PubMed] [Google Scholar]
- Sriramula S., Cardinale J.P., Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PloS One. 2013;8 doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suski M., Gębska A., Olszanecki R., Stachowicz A., Uracz D., Madej J., Korbut R. Influence of atorvastatin on angiotensin I metabolism in resting and TNF-α -activated rat vascular smooth muscle cells. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2014;15:378–383. doi: 10.1177/1470320313475907. [DOI] [PubMed] [Google Scholar]
- Syed A.A., Lahiri S., Mohan D., Valicherla G.R., Gupta A.P., Kumar S., Maurya R., Bora H.K., Hanif K., Gayen J.R. Cardioprotective effect of ulmus wallichiana planchon in β-adrenergic agonist induced cardiac hypertrophy. Front. Pharmacol. 2016;7 doi: 10.3389/fphar.2016.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain Y.-L., Lee W.-C., Wu K.L.H., Leu S., Chan J.Y.H. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016;38:86–92. doi: 10.1016/j.jnutbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Takai S., Jin D., Aritomi S., Niinuma K., Miyazaki M. Powerful vascular protection by combining cilnidipine with valsartan in stroke-prone, spontaneously hypertensive rats. Hypertens. Res. 2013;36:342–348. doi: 10.1038/hr.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012 doi: 10.1155/2012/256294. 2012, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhang Q., Yuan W., Wu J., Li C. Sildenafil protects against myocardial ischemia-reperfusion injury following cardiac arrest in a porcine model: possible role of the renin-angiotensin system. Int. J. Mol. Sci. 2015;16:27015–27031. doi: 10.3390/ijms161126010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Han D., Zhang Yujuan, Rong C., Zhang Yuanshu. [Protective effect and mechanism of β-CM7 on renin angiotensin system & diabetic cardiomyopathy] Sheng Wu Gong Cheng Xue Bao. 2016;32:195–203. [PubMed] [Google Scholar]
- Wang Q., Liang W., Jiang C., Li N., Xu H., Yang M., Lin X., Yu H., Chang P., Yu J. [Effect of Astragali Radix in improving early renal damage in metabolic syndrome rats through ACE2/Mas pathway] Zhongguo Zhongyao Zazhi. 2015;40:4245–4250. [PubMed] [Google Scholar]
- Wang Y., Li C., Ouyang Y., Yu J., Guo S., Liu Z., Li D., Han J., Wang W. Cardioprotective effects of qishenyiqi mediated by angiotensin II type 1 receptor blockade and enhancing angiotensin-converting enzyme 2. Evidence-based complement. Altern. Med. 2012 doi: 10.1155/2012/978127. 2012, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu H., Niu W., Chen J., Liu M., Sun X., Li Z. Tanshinone IIA attenuates paraquat-induced acute lung injury by modulating angiotensin-converting enzyme 2/angiotensin-(1-7) in rats. Mol. Med. Rep. 2018 doi: 10.3892/mmr.2018.9281. [DOI] [PubMed] [Google Scholar]
- Wei X., Zhu X., Hu N., Zhang X., Sun T., Xu J., Bian X. Baicalin attenuates angiotensin II-induced endothelial dysfunction. Biochem. Biophys. Res. Commun. 2015;465:101–107. doi: 10.1016/j.bbrc.2015.07.138. [DOI] [PubMed] [Google Scholar]
- Wu H., Li Y., Wang Y., Xu D., Li C., Liu M., Sun X., Li Z. Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis via modulating angiotensin-converting enzyme 2/angiotensin-(1-7) Axis in rats. Int. J. Med. Sci. 2014;11:578–586. doi: 10.7150/ijms.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Laplante M.-A., de Champlain J. Cyclooxygenase-2 inhibitors attenuate angiotensin II–induced oxidative stress, hypertension, and cardiac hypertrophy in rats. Hypertension. 2005;45:1139–1144. doi: 10.1161/01.HYP.0000164572.92049.29. [DOI] [PubMed] [Google Scholar]
- Xie M., Chen Q. Insight into 2019 novel coronavirus — an updated interim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro M., Yoshimura M., Nakayama M., Abe K., Sumida H., Sugiyama S., Saito Y., Nakao K., Yasue H., Ogawa H. Aldosterone, but not angiotensin II, reduces angiotensin converting enzyme 2 gene expression levels in cultured neonatal rat cardiomyocytes. Circ. J. 2008;72:1346–1350. doi: 10.1253/circj.72.1346. [DOI] [PubMed] [Google Scholar]
- Yan J., Wang A., Cao J., Chen L. Apelin/APJ system: an emerging therapeutic target for respiratory diseases. Cell. Mol. Life Sci. 2020;77:2919–2930. doi: 10.1007/s00018-020-03461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Ma X., Xuan X., Deng H., Chen Q., Yuan L. Liraglutide attenuates non-alcoholic fatty liver disease in mice by regulating the local renin-angiotensin system. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 2020;113:104350. doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- Ye X., Song H., Lu C. [Effect of puerarin injection on the mRNA expressions of AT1 and ACE2 in spontaneous hypertension rats] Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chinese J. Integr. Tradit. West. Med. 2008;28:824–827. [PubMed] [Google Scholar]
- Zhang L.-H., Pang X.-F., Bai F., Wang N.-P., Shah A.I., McKallip R.J., Li X.-W., Wang X., Zhao Z.-Q. Preservation of glucagon-like peptide-1 level attenuates angiotensin II-induced tissue fibrosis by altering AT1/AT2 receptor expression and angiotensin-converting enzyme 2 activity in rat heart. Cardiovasc. Drugs Ther. 2015;29:243–255. doi: 10.1007/s10557-015-6592-7. [DOI] [PubMed] [Google Scholar]
- Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J., Liu Y.-M., Zhao Y.-C., Huang Xuewei, Lin L., Xia M., Chen M.-M., Cheng X., Zhang X., Guo D., Peng Y., Ji Y.-X., Chen J., She Z.-G., Wang Y., Xu Q., Tan R., Wang H., Lin J., Luo P., Fu S., Cai H., Ye P., Xiao B., Mao W., Liu L., Yan Y., Liu M., Chen M., Zhang X.-J., Wang X., Touyz R.M., Xia J., Zhang B.-H., Huang Xiaodong, Yuan Y., Rohit L., Liu P.P., Li H. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Miao J., Wang S., Zhang Y. The protective effects of beta-casomorphin-7 against glucose -induced renal oxidative stress in vivo and vitro. PloS One. 2013;8 doi: 10.1371/journal.pone.0063472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-Z., Cheng Y.-W., Jin H.-Y., Chang Q., Shang Q.-H., Xu Y.-L., Chen L.-X., Xu R., Song B., Zhong J.-C. The sirtuin 6 prevents angiotensin II-mediated myocardial fibrosis and injury by targeting AMPK-ACE2 signaling. Oncotarget. 2017;8:72302–72314. doi: 10.18632/oncotarget.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Ma R., Yu X., Li N., Zhao X., Yu J. AHU377+Valsartan (LCZ696) modulates renin-angiotensin system (RAS) in the cardiac of female spontaneously hypertensive rats compared with valsartan. J. Cardiovasc. Pharmacol. Therapeut. 2019;24:450–459. doi: 10.1177/1074248419838503. [DOI] [PubMed] [Google Scholar]
- Zhao Z.-Q., Pang X.-F., Zhang L.-H., Bai F., Wang N.-P., McKallip R., Garner R. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des. Dev. Ther. 2015;6043 doi: 10.2147/DDDT.S95333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.-C., Huang D.-Y., Yang Y.-M., Li Y.-F., Liu G.-F., Song X.-H., Du K. Upregulation of angiotensin-converting enzyme 2 by all- trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44:907–912. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T.-B., Ou C., Rong L., Drummen G.P. Effect of all-trans retinoic acid treatment on prohibitin and renin–angiotensin–aldosterone system expression in hypoxia-induced renal tubular epithelial cell injury. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2014;15:243–249. doi: 10.1177/1470320314542727. [DOI] [PubMed] [Google Scholar]
- Zhou T.-B., Wu W.-F., Qin Y.-H., Yin S.-S. Association of all-trans retinoic acid treatment with the renin-angiotensin aldosterone system expression in glomerulosclerosis rats. J. Renin-Angiotensin-Aldosterone Syst. JRAAS. 2013;14:299–307. doi: 10.1177/1470320312465220. [DOI] [PubMed] [Google Scholar]
- Zolk O., Hafner S., Schmidt C.Q. German Society for Experimental and Clinical Pharmacology and Toxicology (DGPT), 2020. COVID-19 pandemic and therapy with ibuprofen or renin-angiotensin system blockers: no need for interruptions or changes in ongoing chronic treatments. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020 doi: 10.1007/s00210-020-01890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., Ju X., Liang Z., Liu Q., Zhao Y., Guo F., Bai T., Han Z., Zhu J., Zhou H., Huang F., Li C., Lu H., Li N., Li D., Jin N., Penninger J.M., Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.