Abstract
Background:
Despite advances in treatment, the recurrence rates for laryngeal cancer range from 16% to 40%.
Methods:
Patients with recurrent laryngeal cancer treated at Memorial Sloan Kettering (MSK) from 1999 to 2016 were reviewed. Survival outcomes were analyzed.
Results:
Of 241 patients, 88% were male; the median age was 67 years; 71% had primary glottic tumors. At initial treatment, 72% of patients were seen with early stage disease; primary treatment was radiation (68%), chemoradiation (29%), and surgery (3%). The most common salvage surgery was total laryngectomy (74%). Forty-seven percentage were upstaged at salvage surgery. The 2- and 5-year disease-specific survival (DSS) was 74% and 57%, respectively. Patients with cT4 disease treated with nonsurgical primary management had a 0% 5-year DSS. Independent predictors of DSS were tumor location, perineural invasion, margin, and stage.
Conclusions:
Salvage surgery results in acceptable oncologic outcomes. Stage, disease site, perineural invasion, and margins are associated with inferior DSS.
Keywords: head and neck neoplasm, larynx cancer, prognosis, salvage laryngectomy, treatment failure
1 |. INTRODUCTION
Failure of the primary curative-intent treatment for the patient with squamous cell carcinoma (SCC) of the larynx has significant implications. The function of the anatomy housing this disease process mandates that a considerable morbidity and quality-of-life burden is associated with salvage treatment. The traditional role of surgical resection as the first-line salvage therapy continues in the contemporary setting of evidence-based practice. With overall reported recurrence rates for SCC of the larynx being 16%–40%,1–3 salvage surgery is familiar to the tertiary head and neck surgeon. In 2018, approximately 13 000 new cases of laryngeal cancer were diagnosed in the United States, with over 175 000 new cases diagnosed worldwide.4 The overwhelming majority of these cancers are SCCs. Even though a slight decrease in mortality from laryngeal SCC has been observed in the past decade,5 its treatment, even when successful, is associated with substantial impact on the patient.6
Organ-preservation protocols for early stage larynx SCC have consistently shown oncological outcomes equal to more extensive (organ-sacrificing) surgery.7,8 Disease recurrence after organ-preservation treatment resulted in the rise of salvage resection as an eminent form of laryngeal surgery. First described in several series from the 1970s,9,10 these salvage surgeries pose therapeutic challenges, with a significant increase in both the technical difficulty encountered by treating surgeons and the rate of surgical complications experienced by patients.
Increasing recognition of patient autonomy in the clinical decision-making process has seen primary total laryngectomy (TL), which is now reserved for patients in a narrow window of clinical severity. Multiple studies have reported on the feasibility of open partial laryngeal surgery, and, more recently, of transoral laser micro (TLM)/endoscopic surgeries in the recurrent setting; however, most of these series are from high-volume centers with strict selection criteria.11,12 Thus, TL has retained its status as a gold standard, albeit evolving to salvage locally recurrent SCC of the larynx.
The dawn of the era of patient center medicine poses both therapeutic challenges and exciting opportunities in the framing of the salvage approach to laryngeal carcinoma recurrence. As this patient subset proportionally increases in size within the head and neck oncology population, we present our analysis of a high-volume, single-center experience aiming to identify prognostic factors related to patient outcomes.
2 |. PATIENTS AND METHODS
2.1 |. Patients
After obtaining approval from Memorial Sloan Kettering Cancer Center’s (MSK’s) Institutional Review Board, a retrospective chart review was conducted for all surgically treated patients with laryngeal cancer in our institution from 1999 to 2016. Patients with a biopsy-proven SCC recurrence in the larynx, without distant disease who required salvage surgery, were identified. Salvage surgery was defined as surgery after failure of initial treatment performed with curative intent.
Patients received their primary treatment at MSK or an outside institution. Primary treatments included definitive radiotherapy with or without chemotherapy and any partial surgical resection with curative intent. Four patients initially treated with induction chemotherapy who experienced progression during treatment were excluded from the study.
2.2 |. Data collection
Demographic data points were collected, such as age, sex, race, alcohol and tobacco consumption, and comorbidity status. The burden of concurrent comorbid disease status was assessed using the Washington University Head and Neck Comorbidity Index (WUHNCI).13 Identified subjects were staged according to the American Joint Committee on Cancer 8th edition at initial presentation, and then re-staged with definitive pathology after salvage surgery. Clinical extranodal extension (ENE) status was obtained from pretreatment images when available.14 Radiation therapy delivered and chemotherapeutic agents administered were detailed as part of primary treatment. Salvage treatment information included type of surgery (total or partial laryngectomy), presence and extent of neck dissection, reconstruction techniques, perioperative complications, and length of hospitalization. Variables from salvage surgical pathology data included surgical margin status, tumor dimensions, cartilage invasion, tumor histological differentiation, lymphovascular invasion (LVI), perineural invasion (PNI), number of positive lymph nodes, and ENE.
2.3 |. Statistical analysis
Primary endpoints of interest were overall survival (OS), calculated as the number of months from the date of salvage surgery to the date of last follow-up or death, and disease-specific survival (DSS), calculated from the date of salvage surgery to the date of last follow-up as assessed by a member of the disease management team or death from disease. Subsequent local recurrence-free survival (sLRFS), subsequent regional recurrence-free survival (sRRFS), and subsequent distant recurrence-free survival (sDRFS) were all calculated in months from the date of salvage surgery using the Kaplan-Meier method.
Disease-free interval (DFI) was defined as the time between completion of treatment for the initial tumor and the diagnosis of recurrence prior to salvage surgery. Second and third recurrences were calculated from salvage surgery date. Categorical variables were compared using chi-square analysis. To identify factors predictive of survival outcomes, the following variables were analyzed by univariate analysis: age, sex, WUHNCI, alcohol and tobacco consumption, PNI, LVI, margin status, site (glottic or non-glottic), and overall pathological stage at salvage.
All variables with P ≤ .05 on the univariate analysis were used for multivariable analysis using the Cox proportional hazard method. Statistical calculations were carried out using SPSS version 25.
3 |. RESULTS
3.1 |. Patient characteristics
Two-hundred forty-one patients with biopsy-proven recurrent SCC of the larynx who required salvage surgery were identified. The majority (88%) were male. The median age was 65 years, and 91% were current or former smokers. Alcohol consumption was recorded in 42% of patients. A minimal comorbidity profile was evidenced by a low WUHNCI (0–1) in 82% of patients. The most frequent tumor location was the glottic larynx (71%), followed by the supraglottic larynx (28%). At initial diagnosis, early stage (I/II) disease was identified in 72% of patients, while cervical nodal involvement was present in only 12%. Baseline characteristics are summarized in Table 1.
TABLE 1.
Patients characteristics
| N | 241 (%) |
|---|---|
| Mean age (range) | 67 (56–74) |
| Sex | |
| Female | 30 (12) |
| Male | 211 (88) |
| Tobacco | |
| Ever | 219 (91) |
| Never | 22 (9) |
| Alcohol | |
| Ever | 102 (42) |
| Never | 139 (58) |
| WUHNCI | |
| 0–1 | 198 (82) |
| ≥2 | 43(18) |
| Tumor location | |
| Glottic | 172 (71) |
| Supraglottic | 66 (27) |
| Subglottic | 3 (1) |
| Clinical T stage | |
| Tis | 6 (3) |
| T1 | 112 (47) |
| T2 | 66 (27) |
| T3 | 39 (16) |
| T4 | 18 (8) |
| Clinical N stage | |
| Nx-N0 | 213 (88) |
| N1 | 10 (4) |
| N2 | 15 (6) |
| N3 | 3 (1) |
| Clinical overall stage | |
| 0 | 6 (3) |
| I | 110 (46) |
| II | 57 (24) |
| III | 38 (16) |
| IV | 30 (12) |
| Pathological T stage | |
| T1 | 59 (25) |
| T2 | 60 (25) |
| T3 | 58 (24) |
| T4 | 64 (27) |
| Pathological N stage | |
| Nx-N0 | 209 (87) |
| N1 | 12 (5) |
| N2 | 7 (3) |
| N3 | 13 (5) |
| Overall stage | |
| Stage I | 55 (23) |
| Stage II | 54 (22) |
| Stage III | 56 (23) |
| Stage IV | 76 (32) |
| Tumor differentiation | |
| Well | 47 (19) |
| Moderate | 142 (59) |
| Poorly | 41 (17) |
| Spindle | 8 (3) |
| Verrucous | 3 (1) |
| Cartilage invasion | |
| No | 155 (64) |
| Yes | 86 (36) |
| Thyroid invasion | |
| No | 226 (94) |
| Yes | 15 (6) |
| PNI | |
| No | 135 (56) |
| Yes | 106(44) |
| LVI | |
| No | 184 (76) |
| Yes | 57 (24) |
| ENE | |
| No | 121 (82) |
| Yes | 19 (13) |
| Unknown | 7 (5) |
| Margins | |
| Negative | 181 (75) |
| Close | 37 (15) |
| Positive | 23 (10) |
| Median tumor diameter | 2.30 cm (0.10–9.00 cm) |
Abbreviations: ENE, extranodal extension; LVI, lymphovascular invasion; PNI, perineural invasion; WUHNCI, Washington University Head and Neck Comorbidity Index.
3.2 |. Primary curative-intent treatments
Primary treatment was radiotherapy alone for 68% of patients and chemoradiation in 29% of patients. The remaining 3% of patients underwent a primary partial laryngeal surgery. Most patients (71%) were initially treated at an outside institution. One-hundred twenty-seven (74%) patients who failed treatment at outside institutions had early stage (cT1-T2N0) disease. Induction cisplatin-based chemotherapy was used in 4% of patients.
3.3 |. Salvage treatment
Salvage therapy consisted of surgery alone in 95% of patients and surgery followed by radiation or chemoradiation in 5% of patients; nine patients were re-irradiated, most of whom had stage IV disease and a close or positive margin. TL was the most frequent salvage surgery, representing 74% of the surgeries at first recurrence. Partial open laryngectomies were performed in 38 (16%) of patients, and endoscopic resection was carried out in 10% of patients (Figure 1). In the cohort who received radiation as primary treatment (N = 165), of which 161 (98%) were T1/T2, 110 (67%) had a TL, 38 (23%) had an open partial laryngectomy, and 17 (10%) an endoscopic resection for salvage of recurrent tumor. In the cohort of patients treated primarily with chemoradiation (N = 69), of which 53 (77%) were T3/T4, 63 (91%) had salvage total laryngectomy (STL); three underwent TLM resection. Of those who failed primary surgical treatment (N = 7), three had a TL, and four had a salvage endoscopic surgery. The median DFI was 9.8 months (range 1.1–212 months).
FIGURE 1.
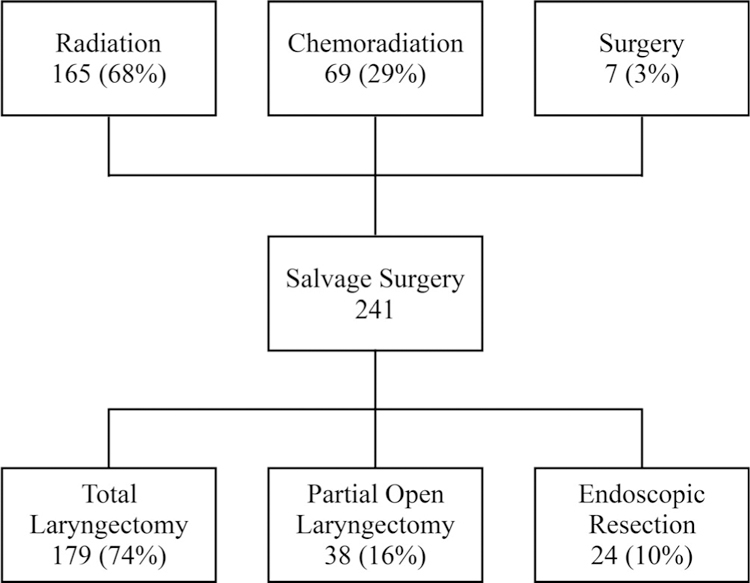
Salvage laryngectomy flow chart
3.4 |. Salvage total laryngectomy
TL was the salvage procedure in 179 patients, where 116 were recurrences from a primary lesion of the glottis. Partial or total pharyngectomy was required in 36 patients. One-hundred forty-six (82%) patients had a unilateral or bilateral neck dissection. Primary pharyngeal closure was achieved in 112 (63%) patients; primary pharyngeal closure and reinforcement using a regional flap were performed in 17 (9%) patients; regional flap reconstruction was performed in 32 (18%) patients; free flap reconstruction was performed in 18 (10%) patients. One gastric pull-up was performed. One-hundred-nineteen (66%) patients underwent primary tracheoesophageal puncture placement. Median hospitalization time was 13 days (3–71 days).
3.4.1 |. Open partial laryngectomy
Of 38 patients treated with open salvage partial laryngectomy, 11 (29%) had supracricoid laryngectomy, and 27 (71%) had a vertical partial laryngectomy. Nearly all (95%) of the tumor recurrences suitable for these less-radical surgeries originated in the glottic larynx, and most of them had an early stage recurrence (T1–T2). The median hospitalization time was 13 days (0–31 days). Most of these surgeries (84%) were performed before 2010. After 12 months, two patients had a percutaneous endoscopic gastrostomy tube, and six patients had tracheostomy.
3.4.2 |. Transoral laser salvage
Twenty-one patients were salvaged with a transoral endoscopic surgical resection. Tumors of the glottic larynx were almost exclusively involved (20 patients), and each patient had early stage recurrences. One patient had an ipsilateral modified radical neck dissection. Median hospitalization time for endoscopic surgery patients was 1 day (0–4 days). Four patients had failed previous endoscopic resection.
3.4.3 |. Histopathology of salvage specimens
The pathological analysis of surgical specimens from salvage procedures revealed that over 75% of recurrences were excised with adequate negative margins; an additional 15% were reported as negative but close. Open partial laryngeal surgeries achieved the highest rates of adequate negative margins. Most recurrent tumors were found to be moderately differentiated (59%). PNI and LVI were reported in 106 (44%) and 57 (24%) of the specimens, respectively. Regional nodal disease was present in 20% of patients, with 13% displaying ENE. Overall pathologic staging at the time of salvage was advanced (III-IV) in 55% of patients (Table 1).
3.4.4 |. Fistula complications of salvage surgery
Ninety-six patients experienced a postoperative complication, the most frequent event being pharyngocutaneous fistula, which occurred in 67 patients. Of the 67 patients who experienced a postoperative fistula, four had an open partial laryngectomy, 49 had a TL, and 14 had a TL with a partial or total pharyngectomy. Of the total number of fistulas, 15 (22%) required a second surgery, while the remaining patients were managed conservatively (78%). Fistulas were more common in patients treated primarily with chemoradiation than in patients treated with radiation alone (48% vs 21%, P < .001).
3.5 |. Correlates of survival
The median follow-up post-salvage surgery was 33 months (0–213). The 2- and 5-year OS rates were 67% and 49%, respectively. The corresponding DSS rates were 74% and 57%, respectively (Figure 2). One-hundred sixty-three patients had a DFI less than 2 years from initial treatment, and 35 patients had a DFI more than 2 years from initial treatment. A shorter DFI was not a predictor of worse OS outcome or recurrence-free survival (5-years OS 44% vs 54%, P = .83; 5-years recurrence-free survival 49% vs 52%, P = .63).
FIGURE 2.
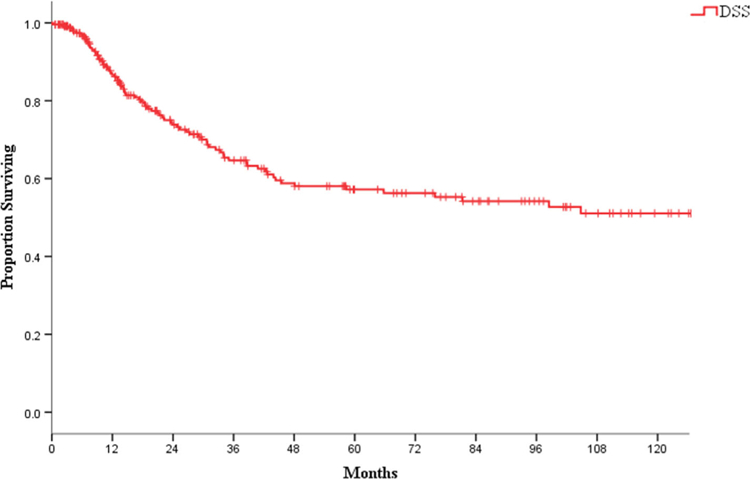
Disease-specific survival (DSS) for whole cohort [Color figure can be viewed at wileyonlinelibrary.com]
Positive margin status, LVI, PNI, non-glottic subsite, high overall pathological stage, and high WUHNCI were predictive of worse DSS on univariate analysis. The subgroup analysis of margins showed that the close and positive margin groups had similar outcomes compared with the negative margin groups. Therefore, positive and close margins were combined for analysis. On multivariate analysis, recurrent overall stage (stage II: HR, 2.12; CI 0.81–5.60; stage III HR, 4.52; CI 1.80–11.39; stage IV: HR, 4.63; CI, 1.89–11.37, P = .001), non-glottic location of disease (HR, 1.85; 95% CI, 1.15–2.99; P = .01), the presence of PNI (HR, 1.91; 95% CI, 1.16–3.14; P = .01), and positive margin status (HR, 1.70; 95% CI, 1.05–2.75; P = .03) were independent factors associated with inferior DSS. Univariate and multivariate analyses of potential factors associated with DSS are included in Table 2.
TABLE 2.
Univariate and multivariate analysis for DSS
| Factor | Variable | N | Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2-year DSS | 5-year DSS | HR | CI | P value | HR | CI | P value | |||
| Age | ≤60 | 82 | 74.3 | 60.1 | REF | |||||
| >60 | 159 | 73.4 | 55.3 | 1.134 | 0.716–1.798 | .59 | ||||
| Sex | Male | 211 | 72.8 | 57.1 | REF | |||||
| Female | 30 | 81.3 | 57.7 | 1.039 | 0.536–2.016 | .91 | ||||
| WUHNCI | 0–1 | 198 | 77.1 | 59.2 | REF | REF | ||||
| >2 | 43 | 54.4 | 44.5 | 1.813 | 1.045–3.146 | .03 | 1.660 | 0.940–2.930 | .08 | |
| Alcohol | Never | 139 | 76.6 | 63.8 | REF | |||||
| Ever | 102 | 70.2 | 48.0 | 1.497 | 0.965–2.322 | .07 | ||||
| Tobacco | Never | 22 | 95.0 | 75.5 | REF | |||||
| Ever | 219 | 71.6 | 55.2 | 2.543 | 0.930–6.956 | .07 | ||||
| Margin Status | Negative | 181 | 77.3 | 62.9 | REF | 1.326–3.342 | .002 | REF | ||
| Positive/close | 60 | 63.2 | 37.4 | 2.105 | 1.697 | 1.047–2.750 | .03 | |||
| LVI | No | 184 | 79.8 | 65.5 | REF | REF | ||||
| Yes | 57 | 54.5 | 30.5 | 2.468 | 1.558–3.910 | <.001 | 1.147 | 0.677–1.945 | .61 | |
| PNI | No | 135 | 85.7 | 71.6 | REF | REF | ||||
| Yes | 106 | 58.3 | 37.6 | 2.817 | 1.792–4.429 | <.001 | 1.905 | 1.156–3.142 | .01 | |
| Site | Glottic | 172 | 79.5 | 64.9 | REF | REF | ||||
| Non-Glottic | 69 | 59.6 | 38.7 | 2.346 | 1.504–3.658 | <.001 | 1.854 | 1.148–2.993 | .01 | |
| Overall pathological stage (8th edition) | I | 55 | 97.7 | 94.3 | REF | REF | ||||
| II | 54 | 80.0 | 67.2 | 2.477 | 0.951–6.451 | 2.129 | 0.809–5.604 | |||
| III | 56 | 65.7 | 30.3 | 6.586 | 2.717–15.965 | 4.520 | 1.797–11.369 | |||
| IV | 76 | 57.6 | 40.7 | 6.515 | 2.718–15.618 | <.001 | 4.632 | 1.886–11.374 | .001 | |
Abbreviations: CI, confidence interval; DSS, disease-specific survival; HR, hazard ratio; LVI, lymphovascular invasion; PNI, perineural invasion; REF, reference; WUHNCI, Washington University Head and Neck Comorbidity Index.
3.6 |. Laryngeal subsite
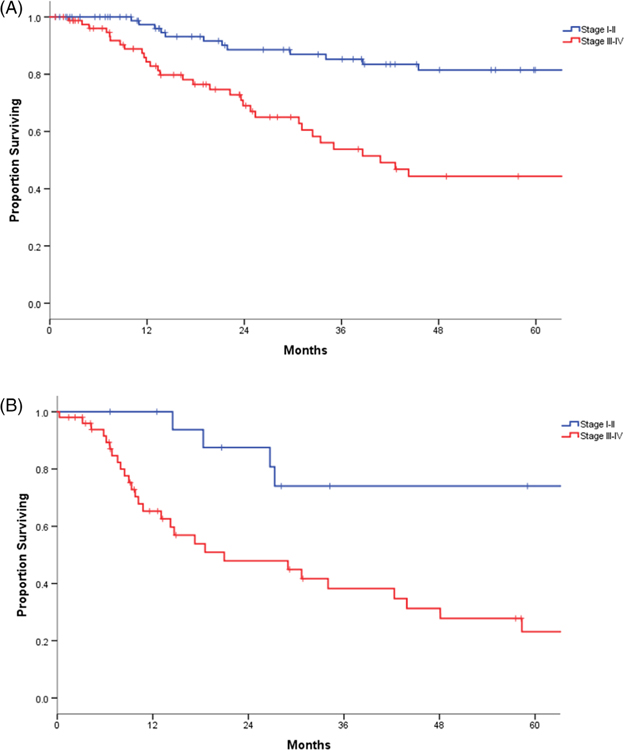
To further identify the factors in prognostication, survival was analyzed with respect to tumor location by subsites. A statistically significant superior 5-year DSS was demonstrated for patients with glottic subsites, compared with those with non-glottic tumors (65% vs 39%, P < .001). The subgroup analysis showed early stage (I-II) glottic subsite disease to have a slightly superior 5-year DSS compared with non-glottic disease (82% vs 74%, P < .001). Advanced stage disease (III-IV) worsened survival differences by subsite, with non-glottic DSS at 5 years declining to 23%, compared with 44% for glottic tumors (P < .001) (Figure 3A,B).
FIGURE 3.
(A) Disease-specific survival (DSS) early vs late stage in glottic tumors; (B) DSS early vs late stage in non-glottic tumors [Color figure can be viewed at wileyonlinelibrary.com]
3.7 |. Clinical T stage at initial presentation
The staging of the primary tumor at initial presentation was identified as an important indicator of DSS. Patients who had early stage (T1-T2) lesions, which were those most likely to have received single modality primary treatment, had significantly better survival outcomes than patients who had more advanced stage (T3-T4) disease. In the advanced stage group who failed nonsurgical primary management, 2- and 5-year DSS from salvage was 46% and 24%, respectively. Importantly, patients with cT4 disease treated with nonsurgical primary management had a 0% DSS at 5-year post-salvage (Figure 4). More than 75% of patients with early stage disease were alive 5 years after their diagnosis. In contrast, this number halved to 37% for 5-year DSS in the patients with advanced stage (stage III/IV) group.
FIGURE 4.
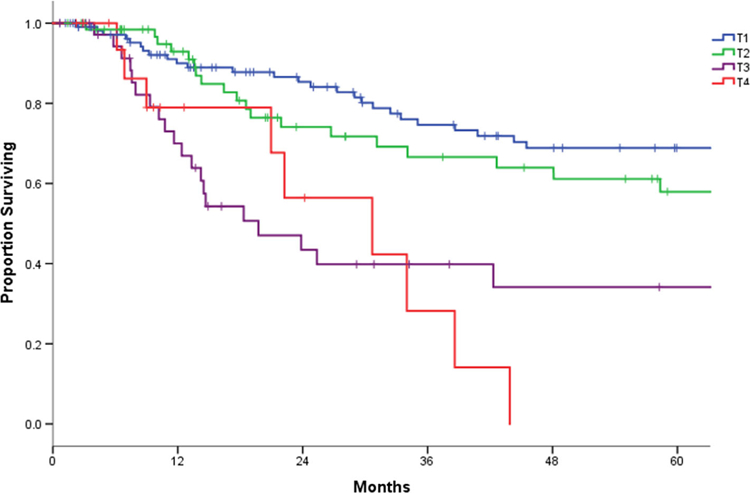
Post-salvage survival according to cT stage at diagnosis [Color figure can be viewed at wileyonlinelibrary.com]
When comparing clinical staging at initial diagnosis and pathological staging at salvage surgery, 47% of patients underwent upstaging. Thirty-nine patients (35%) were initially diagnosed, as cT1 patients changed to a pathological T3-T4 at salvage surgery; 33 patients with a cT2 were upstaged to a pT3-T4, and 12 cT3 were upstaged to a pT4 (Table 3).
TABLE 3.
Relationship between initial clinical staging and pathological staging at salvage surgery
| Total | pT1 | pT2 | pT3 | pT4 | |
|---|---|---|---|---|---|
| cTis | 6 | 4 | 1 | 0 | 1 |
| cT1 | 112 | 49 | 24 | 17 | 22 |
| cT2 | 66 | 4 | 29 | 19 | 14 |
| cT3 | 39 | 2 | 6 | 19 | 12 |
| cT4 | 18 | 0 | 0 | 3 | 15 |
3.8 |. Post-salvage recurrent disease
The 2- and 5-year subsequent recurrence-free survival rates were 58% and 49%, respectively. Of the 100 patients who experienced a subsequent recurrence, the median time from surgery to recurrence was 10 months (1–90 months). Two-and 5-year sLRFS were 71% and 65%, respectively. Subsites again displayed statistically significant differences in sLRFS (5-year sLRFS, glottic 69% vs non-glottic 55%, P = .03).
Regional recurrences were an infrequent event with a 2-and 5-year sRRFS of 91% and 88%, respectively. Regional control between glottic and non-glottic tumors did not differ. Eighty percentage of patients with non-glottic tumors had a neck dissection at salvage, compared with 53% who had glottic tumors. The 2- and 5-year sDRFS were 82% and 75%, respectively. Again, a significant difference was observed between glottic tumors and non-glottic tumors; 5-year sDRFS was 81% in glottic tumors vs 61% in non-glottic tumors (P = .002). A second surgery with curative intent was performed in 30 of the subsequent recurrence patients. At the time of analysis, 17 were alive with no evidence of disease.
4 |. DISCUSSION
This study provides a unique overview by analyzing all patients with recurrent laryngeal SCC managed with salvage surgery, independent of the initial curative-intent treatment modality. It shows that salvage laryngeal surgery provides acceptable oncological outcomes, with 57% DSS at 5-years post-salvage comparable to the published literature.15,16 Despite the intrinsic challenges of the surgical field in the posttreatment setting and higher rates of complications, salvage resection of recurrent laryngeal cancer has proved to be a safe undertaking.
TL is the most frequent salvage modality.11,15–17 In our cohort, 179 (74%) patients required a TL; 122 (68%) TLs were performed in those with early stage disease at initial presentation. This is in agreement with others publications that are conformed by initially diagnosed early glottic disease treated with radiation alone.16,18,19 Given most studies, including ours, analyze patients initially treated at outside facilities, this phenomenon is difficult to definitively explain. Treatment failures of early stage disease could be related to errors at initial clinical staging that consequently result in less intense treatment of reportedly lower stage tumors, variation in technical expertise with radiation delivery or, more simply, may be artifactual differences related to a large denominator of early stage disease treated with radiation. A National Cancer Database (NCDB) study differs with our findings; in a cohort of 726 patients with T1-T2 radiation failures, 24% received a TL, 35% received a open partial laryngectomy, and 41% received a endoscopic partial laryngectomy.20 However, surgical details on 952 patients are not reported. Another possible explanation is the known referral bias of unusual and high-risk cases. In addition, the NCDB only provides data on OS, rendering assessment of local control and the need for multiple surgeries including TL hard to determine.
In our cohort, a partial resection with the goal of conserving a functional larynx was performed in 38 patients. All patients had small recurrences, where a high rate of negative margin was achieved. Previous partial salvage laryngectomy experience in early glottic recurrent disease at MSK published by Ganly et al reported that 49% of patients were able to be salvaged with open partial resection.11 Ten years later in our cohort, the number of patients treated with open salvage partial laryngectomy has decreased due to the emerging use of TLM. An increasing volume of literature has supported acceptable outcomes in the treatment of early recurrent disease with TLM, which has a 5-year OS ranging from 76% to 91%.12,21
While a retrospective assessment limits any clinically significant conclusions, certain aspects within our analysis may aid future conversations around patient education, management, and disease prognostication. Organ-preservation protocols have had an overwhelmingly positive impact on the treatment of stage I-III laryngeal SCC for more than 40 years and have well-established survival endpoints reporting superior quality-of-life outcomes for their patients. Given patient reservations about the life-changes associated with radical laryngeal surgery, it would be the rare patients who would decline organ-preservation therapy when they were seen with outcome-equivalent treatment options. While being the accepted intervention for patients with T1-T2 disease, their use in advanced laryngeal cancer is more controversial. Historically, T3 lesions were those generating debate during the formulation of treatment recommendations from a multidisciplinary unit, requiring the most careful consideration of relevant patient factors, tumor factors, and treatment-institution factors. The oncologic efficacy of organ-preservation therapies in select T3 disease has become well established. However, studies over the past decade have shown the “bracket-creep” of organ-preservation treatments expanding to capture patients with T4 laryngeal disease.22–24
A recent study at The University of M. D. Anderson Cancer Center by Rosenthal et al and an NCDB analysis by Stokes et al have reiterated that upfront laryngectomy (± adjuvant radiotherapy) should remain the standard of care for treatment of T4 larynx cancer.25,26 The estimated DSS in our cohort of cT4 patients at initial diagnosis was 0% 4-year post-salvage surgery. The notion of “falling-back” on the option of surgery if organ-preservation treatment fails is likely not applicable in patients with cT4 larynx cancer, and the at-risk patient should be appropriately counseled to help them make an informed decision. These patients need to be appropriately identified and adequately informed prior to making decisions about, and ultimately consenting to, their desired treatment. Practitioners who specialize in each therapy should assess and counsel patients for whom different treatment options are available. They should not advocate for their specific treatment area of expertise but should facilitate robust informed consent and further aid in ongoing continuity and certainty of care for each patient. More specifically, patients with locally advanced T4 disease should be advised that if an organ-preservation treatment is commenced and if it fails, salvage surgery has poor oncologic efficacy and may become a palliative undertaking.
The concept of tailoring specific treatments to individual patients with their biologically unique malignant lesions emphasizes the importance of disease prognostication, a notion also furthered by laryngeal subsite analysis. Non-glottic disease has previously been identified as an adverse feature in recurrent laryngeal SCC; Sessions et al reported 5-year DSS of 17% for supraglottic tumors.27 Despite aggressive treatment in our cohort, only 39% of non-glottic patients were alive at 5 years. Again, the candidacy of patients for organ-preservation therapy needs to be rigorously assessed on a case-by-case basis, especially in locally advanced non-glottic disease. While various tumor-staging systems can provide guidelines for treatment modalities, the individual complexities of tumor specifics, like anatomic subsites, must all be considered in the establishment of each individualized treatment protocol. The specter of what a salvage surgery entails should form part of the pretreatment discussion for patients with even early stage lesions who may have identifiable adverse prognostic factors, such as an unfavorable subsite.
The utility of the multidisciplinary team in the treatment of cancer has been well described with positive effects on patient outcomes in the literature. The need for close observation by a multidisciplinary team following organ-preserving primary treatment for laryngeal SCC is paramount. The median time between initial treatment and detection of recurrent laryngeal SCC was 9.8 months, which is comparable to 8.7–12.1 months reported in the literature.18,19 Late recurrences were uncommon, and in some cases, it is difficult to differentiate a recurrence from a second primary, in our cohort a shorter DFI was not correlated with a worse OS or recurrence-free survival, nevertheless treatment modality was not the same at diagnosis. Interestingly, at recurrence, 47% of the patients were upstaged relative to the pretreatment clinical T stage. Development of aggressive behavior at recurrence could be explained by the biologic selection of aggressive radio-resistant disease. However, it may also be influenced by the difficulty of assessing recurrent disease in a previously radiated field where inflammatory and fibrotic changes are common. Expert endoscopy may be complemented by cross-sectional imaging in the event of ambiguity in this early posttreatment period.
In our multivariate analysis, several features were found to be associated with better DSS outcome, including glottic disease, absence of PNI, negative margins, and early stage recurrence. While literature on the impact of PNI on the DSS of recurrent laryngeal cancer is inconsistent and scarce, patients with PNI had a 2.5-fold increased risk of death in our series. Fletcher et al found an association between the presence of PNI and a worse OS outcome, with an unadjusted HR of 2.69 (P = .006).28 In contrast, Scharpf et al found that PNI was not a significant adverse event, albeit in a univariate analysis.16 The presence of positive margins is a well-known detrimental factor in laryngeal cancer.27,29 Patients with negative margins had a statistically significantly improved DSS, compared with patients with close or positive margins.
As was previously described in the literature, patients initially treated with radiation therapy had a high risk of postoperative complications after salvage surgery and had particularly high rates of pharyngocutaneous fistulas.30,31 Weber et al published outcomes for 129 patients who required an STL; the incidence of fistula was higher in the group treated initially with chemoradiation than in patients treated with radiation alone.7 This is in agreement with our findings where 48% of patients treated with chemoradiation had a postoperative fistula. At MSK, previous treatment with chemoradiation, in conjunction with extent of resection, is the most frequent indications for vascularized flap reconstruction.
Our study is one of the largest published series representing outcomes after salvage surgery for recurrent SCC of the larynx. As a single institutional, retrospective study, we acknowledge that this study has limitations. In our cohort, 71% of patients were treated in an outside institution; therefore, details regarding radiation fields are not available. Furthermore, this database spans a large number of years, and lack of standardization in regard to treatment and objective functional evaluation can be observed. The inclusion of nonsurgical and surgical patients with larynx recurrence makes this cohort a heterogeneous group, but simultaneously allows us to analyze differences in the approach to salvage surgery.
Future research should be focused on identifying individual factors that are able to predict recurrences, specifically in the group of patients who present with early stage disease. Tumor biology, including genomic profiling, may provide tissue-level sampling clues for lesions that are at risk of failing primary treatments and may identify factors suggesting potential radio-resistance. Counseling patients about the risk of recurrence is essential, even in those who present with early stage tumors. Assessment of the multiple options of treatment and their use in primary, adjuvant, and salvage settings should be used to give each patient a clear idea about possible outcomes, morbidity, and future treatment implications.
5 |. CONCLUSIONS
Identifiable risk factors, such as final overall stage at recurrence, margins status, site of the disease, and PNI, are associated with inferior DSS after salvage treatment. Salvage surgical treatment for laryngeal cancer recurrence results in good oncologic outcomes. However, the rate of total laryngectomies is high, even for patients who were initially seen with early stage disease. Treatment failure of patients who were seen with advanced stage III/IV disease is associated with poor survival outcomes. Importantly, outcome of salvage surgery in patients with cT4 disease treated with organ-preservation nonsurgical therapy is dismal. Such patients require careful counseling to make them aware of this poor outcome with primary nonsurgical treatment.
ACKNOWLEDGMENT
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
REFERENCES
- 1.Goodwin WJ Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110(3 Pt 2 Suppl 93):1–18. [DOI] [PubMed] [Google Scholar]
- 2.Haapaniemi A, Koivunen P, Saarilahti K, et al. Laryngeal cancer in Finland: a 5-year follow-up study of 366 patients. Head Neck. 2016;38(1):36–43. [DOI] [PubMed] [Google Scholar]
- 3.Ritoe SC, Verbeek AL, Krabbe PF, Kaanders JH, van den Hoogen FJ, Marres HA. Screening for local and regional cancer recurrence in patients curatively treated for laryngeal cancer: definition of a high-risk group and estimation of the lead time. Head Neck. 2007;29(5):431–438. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 5.Noone AM, Howlader N, Kraphco M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2015. [Google Scholar]
- 6.Singer S, Danker H, Guntinas-Lichius O, et al. Quality of life before and after total laryngectomy: results of a multicenter prospective cohort study. Head Neck. 2014;36(3):359–368. [DOI] [PubMed] [Google Scholar]
- 7.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91–11. Arch Otolaryngol Head Neck Surg. 2003;129(1):44–49. [DOI] [PubMed] [Google Scholar]
- 8.Wolf GT, Fisher SG, Hong WK, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685–1690. [DOI] [PubMed] [Google Scholar]
- 9.Ballantyne AJ, Fletcher GH. Surgical management of irradiation failures of nonfixed cancers of the glottic region. Am J Roentgenol Radium Ther Nucl Med. 1974;120(1):164–168. [DOI] [PubMed] [Google Scholar]
- 10.Shamboul K, Doyle-Kelly W, Bailey D. Results of salvage surgery following radical radiotheraphy for laryngeal carcinoma. J Laryngol Otol. 1984;98(9):905–907. [DOI] [PubMed] [Google Scholar]
- 11.Ganly I, Patel SG, Matsuo J, et al. Results of surgical salvage after failure of definitive radiation therapy for early-stage squamous cell carcinoma of the glottic larynx. Arch Otolaryngol Head Neck Surg. 2006;132(1):59–66. [DOI] [PubMed] [Google Scholar]
- 12.Weiss BG, Bertlich M, Canis M, Ihler F. Transoral laser microsurgery or total laryngectomy for recurrent squamous cell carcinoma of the larynx: retrospective analysis of 199 cases. Head Neck. 2017;39(6):1166–1176. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002;128(10):1172–1179. [DOI] [PubMed] [Google Scholar]
- 14.Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. Philadelphia, PA: Springer International Publishing; 2017. [Google Scholar]
- 15.Li M, Lorenz RR, Khan MJ, et al. Salvage laryngectomy in patients with recurrent laryngeal cancer in the setting of nonoperative treatment failure. Otolaryngol Head Neck Surg. 2013;149(2):245–251. [DOI] [PubMed] [Google Scholar]
- 16.Scharpf J, Ward M, Adelstein D, Koyfman S, Li M. Elucidation of salvage laryngectomy pathologic and clinical variables to guide further treatment intensification investigation. Laryngoscope. 2018;128(4): 823–830. [DOI] [PubMed] [Google Scholar]
- 17.Haapaniemi A, Vaisanen J, Atula T, Alho OP, Makitie A, Koivunen P. Predictive factors and treatment outcome of laryngeal carcinoma recurrence. Head Neck. 2017;39(3):555–563. [DOI] [PubMed] [Google Scholar]
- 18.van der Putten L, de Bree R, Kuik DJ, et al. Salvage laryngectomy: oncological and functional outcome. Oral Oncol. 2011;47(4):296–301. [DOI] [PubMed] [Google Scholar]
- 19.Sandulache VC, Vandelaar LJ, Skinner HD, et al. Salvage total laryngectomy after external-beam radiotherapy: a 20-year experience. Head Neck. 2016;38(Suppl 1):E1962–E1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheraghlou S, Kuo P, Mehra S, Yarbrough WG, Judson BL. Salvage surgery after radiation failure in T1/T2 larynx cancer: outcomes following total versus conservation surgery. Otolaryngol Head Neck Surg. 2018;158(3):497–504. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Yu Z, Fang J, Chen X, Chen X, Huang Z. Salvage transoral laser microsurgery for early recurrent glottic carcinoma after primary laser treatment. Acta Otolaryngol. 2013;133(5):531–537. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 Pt 2 Suppl 111):1–13. [DOI] [PubMed] [Google Scholar]
- 23.Grover S, Swisher-McClure S, Mitra N, et al. Total laryngectomy versus larynx preservation for T4a larynx cancer: patterns of care and survival outcomes. Int J Radiat Oncol Biol Phys. 2015;92(3):594–601. [DOI] [PubMed] [Google Scholar]
- 24.Chen AY, Fedewa S, Pavluck A, Ward EM. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116 (20):4744–4752. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal DI, Mohamed AS, Weber RS, et al. Long-term outcomes after surgical or nonsurgical initial therapy for patients with T4 squamous cell carcinoma of the larynx: a 3-decade survey. Cancer. 2015;121(10):1608–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stokes WA, Jones BL, Bhatia S, et al. A comparison of overall survival for patients with T4 larynx cancer treated with surgical versus organ-preservation approaches: a National Cancer Data Base analysis. Cancer. 2017;123(4):600–608. [DOI] [PubMed] [Google Scholar]
- 27.Sessions DG, Lenox J, Spector GJ. Supraglottic laryngeal cancer: analysis of treatment results. Laryngoscope. 2005;115(8):1402–1410. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher KT, Gal TJ, Ebelhar AJ, et al. Prognostic indicators and survival in salvage surgery for laryngeal cancer. Head Neck. 2017; 39(10):2021–2026. [DOI] [PubMed] [Google Scholar]
- 29.Spector GJ, Sessions DG, Lenox J, Newland D, Simpson J, Haughey BH. Management of stage IV glottic carcinoma: therapeutic outcomes. Laryngoscope. 2004;114(8):1438–1446. [DOI] [PubMed] [Google Scholar]
- 30.Ganly I, Patel S, Matsuo J, et al. Postoperative complications of salvage total laryngectomy. Cancer. 2005;103(10):2073–2081. [DOI] [PubMed] [Google Scholar]
- 31.Patel UA, Moore BA, Wax M, et al. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1156–1162. [DOI] [PubMed] [Google Scholar]