Abstract
Objective:
New approaches are needed to interpret large amounts of physiologic data continuously recorded in the ICU. We developed and prospectively validated a versatile platform (IRIS) for real-time ICU physiologic monitoring, clinical decision making, and caretaker notification.
Methods:
IRIS was implemented in the neurointensive care unit to stream multimodal time series data, including EEG, intracranial pressure (ICP), and brain tissue oxygenation (PbtO2), from ICU monitors to an analysis server. IRIS was applied for 364 patients undergoing continuous EEG, 26 patients undergoing burst suppression monitoring, and four patients undergoing intracranial pressure and brain tissue oxygen monitoring. Custom algorithms were used to identify periods of elevated ICP, compute burst suppression ratios (BSRs), and detect faulty or disconnected EEG electrodes. Hospital staff were notified of clinically relevant events using our secure API to route alerts through a password-protected smartphone application.
Results:
Sustained increases in ICP and concordant decreases in PbtO2 were reliably detected using user-defined thresholds and alert throttling. BSR trends computed by the platform correlated highly with manual neurologist markings (r2 0.633–0.781; p<0.0001). The platform identified EEG electrodes with poor signal quality with 95% positive predictive value, and reduced latency of technician response by 93%.
Conclusion:
This study validates a flexible real-time platform for monitoring and interpreting ICU data and notifying caretakers of actionable results, with potential to reduce the manual burden of continuous monitoring services on care providers.
Significance:
This work represents an important step toward facilitating translational medical data analytics to improve patient care and reduce health care costs.
Index Terms –: Automated data analysis, Continuous data monitoring, EEG, Intensive care unit, Multimodal data
I. Introduction
PATIENT care in the intensive care unit (ICU) relies on highly complex decision making based on large volumes of monitoring data [1]–[3]. Despite increased utilization of critical care services and monitoring technologies [4]–[7], protocols for interpreting and acting on continuous data have remained largely unchanged [8]. Development of methods for inferring physiologic state and predicting patient deterioration are flourishing [9]–[12], but there has been much less progress on practical systems for data reduction and integration with caretaker workflow. Large amounts of multimodal data are continuously collected [13] but typically reviewed retrospectively (as is the case for EEG data) or in single snapshots at the bedside (for physiological telemetry data such as heart rate, blood pressure, and ICP). Reliance on manual interpretation introduces significant expense [14]–[16] and potential delays in care [17], and increases the risk of missing events, especially for telemetry data that is reviewed inconsistently. For most data streams, no systematic summary reports are generated; for others, such as continuous EEG, clinicians must rely on written reports without quantitative data analysis or trending metrics [18]. Data generated in the ICU is not just underutilized, but is often entirely wasted after patient discharge due to a lack of organized, accessible archiving [19].
New approaches for collecting and analyzing ICU data are needed to leverage the full benefit of patient datasets [9]–[11], [20]–[22]. Automated data processing and storage offers potential to improve ICU care in three key areas: 1) caretaker workflow, by reducing the burden of continuous monitoring studies on ICU staff; 2) direct patient care, by ensuring that all multimodal data streams are analyzed as they are generated with custom signal processing or machine learning algorithms [23], [24]; 3) medical research, by systematically capturing and integrating massive amounts of data for evaluating the effects of treatments and interventions. Currently available monitoring systems are inadequate, as they are generally costly, non-scalable, limited to single modalities, and restricted to a preset selection of algorithms running within the computational capacity of a local machine.
In this study, we present a data monitoring platform called IRIS (ICU Real-Time Informatics System) for use in the ICU and validate it on patients receiving neurophysiologic monitoring. IRIS streams and analyzes long-term EEG monitoring (LTM) data [25], as well as data from intracranial probes recording intracranial pressure (ICP), and brain tissue oxygen (PbtO2). It uses a central server for event detection, and delivers caretaker notifications through a custom, secure API. This API is compliant with the Health Insurance Portability and Accountability Act (HIPAA). We validate the platform’s utility by 1) detecting and reporting faulty EEG recording electrodes, 2) detecting and reporting acute changes in intracranial monitoring metrics, and 3) providing quantitative data trending through automatic calculation of burst suppression ratios. This system offers immediate clinical utility, and stands as a proof of concept for automatic, real-time analysis of continuous multimodal data in the ICU.
II. Materials and Methods
We created a platform with a modular structure to apply custom algorithms to data streams regardless of their collection hardware. The IRIS platform has three components: Data Acquisition and Management, Event Detection Analysis, and Clinical Team Notification (Fig. 1). To demonstrate the versatility of the platform structure, IRIS was implemented in two patient cohorts: 1) patients undergoing LTM monitoring (EEG only), and 2) patients undergoing EEG, intracranial pressure, and brain tissue oxygenation monitoring. This research study was carried out in parallel to regular patient care. Data collection was carried out with approval by the institutional review board of the University of Pennsylvania (“An Automated Platform for ICU EEG Monitoring and Visualizing Results”). A waiver of informed consent was obtained as these data were already collected as part of standard care with minimal patient risk, rigorous data security protocols, and minimum necessary extraction of patient health information. Platform and algorithm code is publicly available at https://github.com/sbaldassano/ICU (static release: https://zenodo.org/badge/latestdoi/186897708).
Fig. 1.
Schematic of analysis and notification platform. The three components of the pipeline are Data Acquisition and Management, Event Detection Analysis, and Clinical Team Notification.
A. Data Acquisition and Management
LTM patients:
364 sequential adult patients in the neurological ICU were monitored with 24-hour continuous EEG recording as part of standard clinical care. EEG technicians attached silver/silver-chloride electrodes to the patient scalp according to the International 10–20 System [26] (18 electrodes, with Pz omitted) using conductive paste and collodion glue. EEG signals were amplified, digitized and recorded at 250 Hz using Natus Nicolet EEG acquisition units, and impedances were measured with a goal of less than 10 kOhm at each electrode. Electrode impedance was reviewed daily and high-impedance electrodes were repaired by the technician.
Intracranial monitoring patients:
Four randomly selected adult patients in the neurological ICU were monitored with continuous EEG recordings and intracranial probes to measure ICP and brain tissue oxygenation based on standard clinical care. Intracranial monitors were inserted through multi-lumen bolts (Hemedex Quad Lumen Bolt (Waltham, MA)) placed through a burr hole in the skull by the neurosurgical team. ICP was measured using a Camino (Integra LifeSciences, Plainsboro, NJ) fiber optic sensor placed directly into white matter brain tissue. Brain oxygen was measured using a Licox (Integra) sensor, an amperometric monitor placed directly into brain tissue. EEG and intracranial signals were amplified, digitized, and recorded at 125–250 Hz using a CNS Monitor (Moberg Research, Ambler, PA). As is standard practice in our institution, EEG and intracranial probe signals were available for display at the bedside, and EEG was streamed to hospital servers for centralized monitoring by the clinical neurophysiology team. All data analysis and management through our novel platform was carried out from a central server located behind the hospital firewall to ensure that patient data was transmitted in compliance with HIPAA regulations. Active data acquisition units were automatically identified, and recorded data from these machines were extracted to the server along with patient identifying information and recording parameters. This system was unsupervised and operated continuously throughout the study period.
B. Event Detection Analysis
IRIS platform architecture and data streaming technology are scalable and customizable, allowing users to add any number of users and analysis modules. In this study, we demonstrated the utility of the platform using parallel algorithms to (1) detect electrodes with poor data quality (Matlab), (2) compute burst suppression ratios (Matlab), and (3) identify prolonged elevation in intracranial pressure (Python).
Incoming patient data streams from Natus acquisition units were converted to an open source data frame format using a custom Matlab script (publicly available at https://github.com/ieeg-portal/Nicolet-Reader). We developed a novel algorithm to identify leads with poor signal quality, usually due to poor contact with or complete detachment from the scalp. Signal amplitude, power in the 60 Hz frequency band, and line length, a measure of combined amplitude and frequency [27], were monitored in each lead. Affected leads were identified as those with outlying signal features (see Electrode Fidelity Algorithm in Appendix for details). While there exist many algorithms for identifying and removing EEG artifacts due to biologic (e.g. ocular, cardiac, or muscle) or electrical (e.g. line noise) interference [28]–[32], this algorithm is designed for the unique, practical use case of identifying leads with characteristic signal abnormalities due to mechanical, correctable factors. Baseline detection latency was assessed over a two-week period prior to platform implementation, measured as elapsed time between first change in EEG signal quality and time of identification by the EEG technician. After platform implementation, EEG technicians logged all manually identified incidents of faulty electrode signal in order to assess platform sensitivity.
We also developed a novel algorithm for detecting burst suppression patterns and trending burst suppression ratios. This algorithm continuously assesses whether the patient is currently in a state of burst suppression by monitoring the first derivative of the EEG after polynomial smoothing. If the patient enters burst suppression, EEG segments are labeled as “normal EEG” or “suppressed EEG” using k-means clustering of the EEG voltage variance. This approach accounts for the high variability in baseline signal amplitude across patients by defining clusters on aper-patientbasis. The algorithm computes mean burst suppression ratios (BSRs) at 10-minute intervals (with adjustable windowing) until burst suppression is no longer detected. The algorithm was benchmarked against expert human markings (S.W.R.). The clustering method used in this algorithm allows for semi-supervised implementation in the IRIS platform, requiring human input only to confirm the presence of burst suppression when prompted. This is in contrast to existing methods in the literature [33]–[36], in which algorithms are trained in a supervised manner using labeled burst and suppression segments. By eliminating the need for manual data labeling, this method can be easily implemented in a real-time system. See Burst Suppression Algorithm in Appendix for details.
Incoming patient data streams from the Moberg CNS Monitor were converted to an open-source data frame format using a C++ library provided by Moberg, Inc., in conjunction with custom Python scripts. This data was analyzed using a custom Python script to identify prolonged elevations in ICP, as well as concordant decreases in PbtO2 with user-defined thresholds. See ICP Monitoring Algorithm in Appendix for details.
C. Clinical Team Notification
Detected events were relayed from the analysis server to the clinical team using a custom web application. This application, Agent, was constructed for this purpose through collaboration with the Penn Medicine Center for Health Care Innovation (PCHI) and is deployed for patient care system-wide within Penn Medicine’s central hospital. Agent uses push notifications and integrates with secure messaging APIs to route electronic medical record data. The system also tracks which caregivers are responsible for first-line care for each patient to optimally target notifications. Customizable notifications, containing text and images, can be delivered to physicians, nurses, pharmacists, and technicians at their hospital email addresses or through an independent third-party password-protected smartphone application called Cureatr.
In the case of faulty lead detection, notifications include patient name, location, names of channels with poor signal quality, and an image of the last 10 minutes of EEG recording (Fig. 2). For burst suppression trending, notifications include a trend of BSR values over the past hour. For intracranial monitoring patients, the platform uses “tiered” notifications based on user-defined thresholds in ICP and PbtO2 (default settings listed in Appendix). Hospital staff subscribe to these notifications using their University of Pennsylvania Hospital System login information. This “opt-in” system ensures that patient notifications are sent only to relevant caretakers to minimize alert fatigue. Our system of notification offers opportunities for feedback from the clinical team regarding the accuracy and timing of notifications, as well as customization of the duration, intervals, and types of caretaker communication. Times of notification delivery and response were recorded to compute response latency.
Fig. 2.
Sample caretaker notification of electrodes with poor signal quality. Embedded links allow recipients to view a snapshot of the referenced EEG (inset). This format allows feedback regarding accuracy, timing, and frequency of notifications. Patient name and room number have been redacted.
D. Statistical Methods
Statistical outliers were defined in accordance with Tukey’s rule [37] as those greater than the third quartile plus 1.5 times the interquartile distance. BSR trends were compared using the Pearson correlation coefficient.
III. Results
The IRIS platform was implemented over a 6-month period during which all 364 unique patients in the neurological ICU undergoing EEG LTM on six Natus Nicolet EEG acquisition units were included. Data were captured in real time from the local EEG machines and streamed to a networked server for analysis. Clinicians were successfully notified through email and smartphone applications in the event of faulty EEG leads or burst suppression.
A. Faulty Lead Detection
We aimed to expedite detection of faulty EEG leads and improve study utility by automatically notifying caretakers of recording electrodes with poor data quality. In order to reflect the intended clinical use case, accuracy of faulty electrode detection was evaluated on the basis of whether the artifact precluded data interpretation from the affected leads by a trained epileptologist. Detections were assessed during a two-week trial period by visual inspection of the raw EEG data by two independent epileptologists (B.L., S.W.R.) (Table 1). During this period, 115 incidents of single or multiple faulty electrodes were detected across 29 unique patients with a positive predictive value of 95% (Reviewer 1: 110 true positives, 5 false positives; Reviewer 2: 109 true positives, 6 false positives; Inter-rater reliability: 98.2%). In general, false positive detections were characterized by relatively brief (< 30 seconds) intermittent high-frequency artifacts with amplitude less than twice baseline. In all false positive cases, artifacts were limited to at most two non-adjacent electrodes with clearly interpretable signal at other adjacent electrodes. All notifications took place within 5 minutes from the time of first detectable electrographic change. EEG technicians staffing the neurological ICU did not report any faulty or detached leads undetected by this system during the study period. Interviews conducted with EEG technicians at conclusion of this period indicated that this system improved workflow through remote reporting and improved study data quality by preventing extended lead disconnections.
Table 1.
IRIS platform use statistics. The positive predictive value (PPV) of faulty lead detection was measured by two independent reviewers based on 115 detections over a 2-week trial period.
| Number of patients monitored | Total recording time | Faulty Lead Detections | Number of burst suppression patients | ||
|---|---|---|---|---|---|
| Total Number | Sensitivity | PPV | |||
| 364 | 9139 patient-hours | 1149 | 100% | 94.8–95.7% | 26 |
Event response was assessed by measuring the elapsed time between the first electrographic change and EEG technician response to the delivered notification. During the trial period, EEG technicians responded within 5.9±2.8 minutes of electrode malfunction. This detection latency represents a drastic reduction relative to that accomplished by baseline technician workflow (87.8±64.7 minutes), in which electrode contacts are examined and corrected at 12-hour intervals (p<0.0001 by Wilcoxon rank sum test) (Fig. 3).
Fig. 3.
Latency of caretaker response. Response latency to malfunctioning electrodes was significantly reduced with platform notifications. Baseline latency: 87.8±64.7 minutes (mean±standard deviation), N=43, excluding 3 statistical outliers. Platform latency 5.9±2.8 minutes, N=114, excluding one statistical outlier (p<0.0001).
B. Burst Suppression Ratio Trending
We also used IRIS to automatically detect the onset and termination of burst suppression and trend BSR values. Over the 6-month trial, all 26 patients who experienced periods of burst suppression lasting longer than one hour (as determined by retrospective clinical review) were detected by the platform. The accuracy of the calculated BSR trends was assessed by comparing them to manual markings by a board certified epileptologist (S.W.R.). This comparison was carried out in a pilot sample from the first three chronological patients studied due to the time-intensive nature of manual detailed BSR marking. BSR values were marked by both IRIS and the epileptologist at 10-minute intervals over a total of 28.7 hours of data (Fig. 4). BSR trends computed by IRIS closely mimic with those derived from manual epileptologist markings (r12= 0.633, r22 = 0.781, r32 = 0.760; p<0.001 by t-statistic with N-2 degrees of freedom). This performance is comparable to the correlation reported using the bispectral index (BIS) monitor, a commercial device commonly used in the ICU to assess degree of sedation [38] (r2 = 0.6673 – 0.79) [39]–[41].
Fig. 4.
Manual and platform computed BSR trends over continuous data from three patients undergoing LTM study.
C. Intracranial Monitoring
To validate the applicability of IRIS to other types of multimodal data in the ICU, IRIS was used to analyze continuous ICP and PbtO2 signals from a group of four patients over a six-week period undergoing intracranial monitoring for acute brain injury, including intracranial hemorrhage and traumatic brain injury. Data were successfully captured from Moberg CNS Monitors and analyzed in real time. IRIS detected all ICP elevations and PbtO2 depressions meeting user-set criteria. Average length of monitoring per patient was 412.4 ± 250.1 hours (max: 721.4h, min: 111.3 h). A total of 123 alerts (1.8 alerts/day) were sent, consisting of 99 “low” alerts (mean ICP 22.4 ± 2.9 mmHg), 8 “medium” alerts (mean ICP 43.9 ± 2.4 mmHg) and 16 “high” alerts (mean ICP 25.5±4.6mmHg with mean PbtO2 13.0 ± 2.6 mmHg). An example of one such alert and corresponding clinical recordings are shown in Fig. 5.
Fig. 5.
Example intracranial monitoring alert, (a) Intracranial pressure (ICP) and brain tissue oxygenation (PbO2) shown over a 6-hour period in the clinical viewer. (b)A Cureatr alert notifying caretakers of elevated ICP. The time of the alert is synced with the red vertical line in panel (a). Patient name is redacted.
IV. Discussion
Automating ICU data analysis is essential to improve care and reduce cost, manual physician review, and notification delays associated with continuous multimodal monitoring [28]. Here, we describe and demonstrate a platform for automatically analyzing multimodal ICU data and notifying appropriate remote caretakers via secure messaging. The modular structure of IRIS supports input of any digital signal or group of signals for annotation, analysis, display, alert, or monitoring, enabling addition of customized algorithms as clinically warranted.
Using a custom signal fidelity algorithm, we detect faulty or disconnected electrodes in real time with high accuracy, improving LTM study quality. Under baseline conditions, faulty recording electrodes often go undetected for several hours before correction, compromising the utility of EEG monitoring [42]. Minimizing electrode correction latency is an important step to reduce LTM resource expenditures and decrease lengths of stay. We also discovered an unexpected benefit of using IRIS to detect EEG leads with poor signal. Typically, EEG signal quality is reviewed by technicians in the ICU using standard visualization software, which often preprocesses incoming data using bandpass and 60 Hz notch filters. During the trial period, several platform notifications initially deemed to be false positives were later confirmed to be accurate detections of high impedance electrodes once visualization filters were removed. IRIS was therefore able to recognize recording errors that otherwise were not identified by manual screening by technologists.
LTM studies provide the opportunity for collecting prolonged continuous data for computing trending metrics [43]. In this work, we present BSR as a key trending metric with direct ramifications for patient care. While manual intermittent BSR measurement is used in the ICU for drug titration in patients with refractory status epilepticus [41], caretakers lack the ability to review extended, quantitative trends over prolonged monitoring studies that may provide a clearer view of a patient’s clinical course. Furthermore, the existing review system requires physicians to be physically present at the bedside or to have access to a review workstation in order to track patient condition. We demonstrate that IRIS provides accurate, automatic trending of BSR with remote clinician notification, with potential to improve assessment of patient prognosis and increase the efficiency of drug titration. While applied here to continuous EEG data, this functionality is generalizable to other common situations in the ICU requiring closed loop therapy titration based on physiologic data streams. For example, this system could be applied for titration of continuous medication infusion to a target parameter such as blood pressure, or adjustment of other therapies such as core temperature modulation (based on continuous temperature recordings) or mechanical ventilation (based on oxygen saturation). Automatic calculation of BSR also offers other advantages compared to manual markings. Algorithmic markings incorporate larger windows of data than manual “spot checks,” producing smoother BSR trends over time. While this may slightly decrease correlation with manual markings on a point-to-point basis, it more accurately represents gradual changes in brain state (see top panel of Fig. 4). Automatic BSR calculation also has the benefit of consistency over time and across patients by eliminating inter-reviewer discrepancies.
This modular system for real-time analysis of ICU EEG has many potential clinical applications beyond those assessed in this pilot trial. For care of patients with epilepsy, these applications include detection of spikes [44] and convulsive or nonconvulsive seizures [45], [46]. More broadly, continuous ICU EEG also has utility for monitoring brain function after neurologic insults such as traumatic brain injury, hemorrhage, ischemic stroke, and hypoxia. Algorithms can be implemented to measure cortical spreading depolarizations [47]–[49], reflective of neurochemical changes that mediate secondary brain damage, and to identify areas of “silence” without electrical activity [50]. Continuous EEG has shown to aid in prediction of neurologic recovery [51]–[54], and algorithms are currently in development to automate EEG interpretation for this purpose [55], [56].
In addition to EEG analysis, we demonstrate the efficacy of this platform for analyzing multimodal intracranial monitoring data. Our system of combined threshold-based alerts for ICP and PbtO2 ensures that prolonged deviations in these metrics are detected and evaluated without the need for continuous manual data review. This application of IRIS highlights the strength of a modular data analysis platform; data can be streamed to the platform from a variety of clinical sources, and custom algorithms leveraging multimodal data can be applied to analyze them in real time. While currently limited to neuromonitoring, the clinical impact and applicability of this system will scale with incorporation of additional data streams for more generalized ICU monitoring. For instance, the same principles and platform functionality could be used to monitor treatment thresholds for any continuously or serially sampled data, such as blood pressure, cardiac output, or lung compliance. Further, the basic algorithm implementation presented here may be augmented to include more complex signal processing and machine learning methods in accordance with the user’s particular clinical goals, and to fuse outputs from many devices to give a more accurate picture of patient condition. Currently, streaming of ICU data is challenging due to reliance on devices using proprietary file formats with inconsistent networking/telemetry capabilities. It is our hope that the potential power of a unified data ecosystem to improve patient care will encourage device manufacturers to facilitate real-time data extraction from monitoring devices.
Implementing a platform with direct clinical application requires organized interaction with large numbers of hospital staff, including physicians, nursing staff, and EEG technicians. This system was dependent on caretaker participation; rapid feedback regarding notification accuracy, timing, and content was critical to inform necessary changes to algorithms or notification format to maximize the platform’s utility. Another important consideration for notification systems is alarm fatigue, an increasing problem in the ICU that can jeopardize patient safety [57]–[59]. IRIS was designed to limit alarm fatigue as much as possible, with an opt-in system to target notifications to relevant providers, individualized alarm settings (such as frequency throttling and detection thresholds), and a tiered alarm system for ICP monitoring. False positive detections are a powerful driver of alarm fatigue; while we achieved high specificity in our test cases, care should be taken in future applications to ensure accuracy of alarms prior to platform implementation. In this study, caretakers reported improved workflow, particularly as a result of remote notifications, with nearly universal adoption among EEG technicians and nursing staff.
To our knowledge, IRIS represents the first clinically-implemented system capable of real-time automated ICU/EEG data abnormality detection and delivery of targeted physician alerts. In contrast to existing proprietary solutions, IRIS automatically identifies active monitors and uses a combination of empiric thresholds and unsupervised clustering to employ custom EEG algorithms without need for manual data review or labeling. Furthermore, IRIS supports multimodal data and facilitates addition of novel analysis modules. With customizable HIPAA-compliant smartphone notifications, IRIS addresses a currently unmet need for flexible ICU monitoring and informatics tools.
A. Methodological Considerations
There are several limitations of the methodology used in this work. Sensitivity of the faulty electrode detection algorithm was based on reporting of any missed events by EEG technicians. While no missed events were reported, it is possible that electrodes with poor signal went undetected by both the algorithm and the ICU staff, or that electrode malfunctions were not properly reported. In this work, we did not perform artifact rejection for non-EEG signals (ICP, PbtO2). While we did not experience significant periods of artifact in our pilot trial, these measures are susceptible to error due to transient malfunction, misplacement, or disconnection during routine patient care [60], [61]. Additionally, the accuracy of the IRIS-computed BSR trends was assessed using only a small sample of patients, as this verification process required manually generating detailed trends with multiple measurements per hour. Despite the limited patient number, trends were computed over an average of nearly 10 hours per patient to provide a significant sample size within each patient record. This work stands as a proof of concept that potentially actionable clinical variables can be measured and reported automatically and in near real time. However, we did not directly assess action taken in response to notifications or downstream clinical outcomes such as survival, average length of stay or hospital cost per patient.
V. Conclusion
In summary, we developed a flexible platform for analyzing continuous ICU data that automatically notifies caretakers of critical events in real time. Using this platform, we designed and clinically implemented novel open-source algorithms to identify faulty EEG electrodes, track burst suppression ratios, and detect abnormalities in neuromonitoring data. We demonstrated the potential of this platform to improve ICU workflow, reduce the burden of continuous ICU data interpretation on caretakers, improve study data quality, and compute clinically important trending metrics. This work represents a framework for automatically interpreting a wide variety of ICU data streams and underscores the tremendous potential of medical data analytics to improve patient care.
Acknowledgement
This work was supported by the NIH (1T32NS091006), the Penn Medicine Center for Health Care Innovation, the Ashton Fellowship at the University of Pennsylvania, Jonathan and Bonnie Rothberg, the Mirowski Family Foundation, and Neil and Barbara Smit.
Appendix
A. Electrode Fidelity Algorithm
The electrode fidelity algorithm consists of two components. The first component is designed to detect situations in which one or several leads have poor signal. The second component is designed to detect situations in which all leads have poor signal, usually due to removal of the recording cap or amplifier malfunction. For each channel, the previous five minutes of data is segmented into 5-second windows for analysis. In each window, the signal line length is calculated for each channel as the sum of all absolute distances between N consecutive data points, x, within the window [27],
| (1) |
This value is equivalent to the absolute derivative of signal amplitude and is thereby sensitive to changes in both amplitude and frequency. In this case, x represents channel voltage referenced to the vertex (Cz) electrode. These measurements are used to determine the median line length value for each of the 18 recording channels for each window. By combining windowed line length values over all channels into a single distribution, we define a threshold value as the 75th percentile of this distribution plus twice the interquartile distance.
Channels with median line length exceeding this threshold are identified as having poor signal quality. Since this method uses comparison among channels to identify faulty leads, it is effective when only one or several leads are functioning poorly. The detection thresholds for both the single-channel and all-channel methods were determined using offline data prior to implementation for real-time monitoring. Performance metrics were computed using static, pre-tuned threshold values.
The second component of this algorithm is used to identify circumstances in which all leads are recording artifact. This “all-off” detector relies on empirically determined thresholds in amplitude and 60 Hz bandpower computed over the past five minutes of data.
B. Burst Suppression Algorithm
The burst suppression analysis algorithm consists of two components. The first component serves to monitor the EEG signal for overall suppression to detect the attainment or loss of burst suppression. The second component serves to compute a detailed trend of the burst suppression ratio after burst suppression has been achieved.
The raw EEG data is filtered using a 3rd order Savitzky-Golay filter with a window of 0.125 seconds (Fig. 6a). The past 15 minutes of data is then segmented into 2-second clips, and the average absolute value of the first derivative of the signal is computed for each clip. Extreme outliers (absolute value of mean derivative > 1000 uV per data point) and clips with a derivative of zero are removed as likely artifact. Each remaining clip is then classified as “normal EEG” or “suppressed EEG” by comparison to an empirically determined threshold. If at least 33% of the clips are classified as suppressed, the patient is considered to have entered burst suppression. Conversely, if fewer that 33% of clips are classified as suppressed in a patient that had previously been in burst suppression, that patient is considered to have ceased burst suppression. If burst suppression is detected, the user is prompted to confirm accurate detection and begin BSR trending.
Fig. 6.
Demonstration of burst suppression algorithm, (a) Data are smoothed using a polynomial filter (red), (b) Centroids (red circles) are identified for burst and suppression periods. Clustering occurs in N-dimensional space, where N is the number of channels (two dimensions shown here).
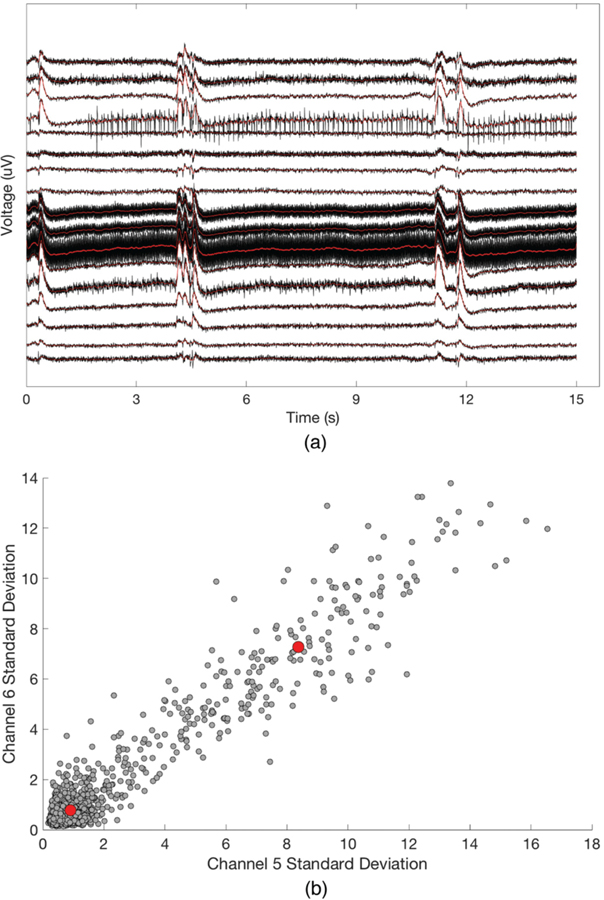
If a patient has been in burst suppression for greater than 30 minutes and BSR trending has been requested, the first 30 minutes of filtered burst suppression recording is used to train a patient-specific model for trending of BSRs. Model training on a per-patient basis is necessary due to discrepancies in signal amplitude and background noise. The training data is segmented into 0.125-second clips, and the mean signal standard deviation for each channel is computed. Each clip is thus represented by an 18-dimensional vector, with one dimension per channel. These clips are then clustered into 2 groups using k-means clustering (Fig. 6b) [62] by minimizing the objective function
| (2) |
where x are clip vectors, are centroids, and i is the cluster assignment. The cluster centroid farther from the origin is considered to be the “burst” centroid, and the centroid closer to the origin is considered to be the “suppression” centroid. Note that the vector space representation used in this algorithm assumes either global burst suppression or approximate spatial stationarity of localized bursting behavior. Once the centroids have been determined, incoming data is interpreted in 0.125-second clips and assigned to the closer centroid. At 10-minute intervals, the percentage of clips classified as suppressed is used to compute the BSR value over that time period.
C. Intracranial Pressure and Brain Tissue Oxygen Monitoring Algorithm
Incoming patient data streams from the Moberg CNS Monitor were converted to an open-source data frame format using a C++ library provided by Moberg, Inc., in conjunction with custom Python scripts. These data were used to identify elevations in ICP or drops in brain tissue oxygen (PbtO2) according to user-defined thresholds. Default settings include “low-level” alerts for ICP > 20 mmHg for more than 15 minutes, “mid-level” alerts for ICP > 40 mmHg for more than 5 minutes, and “high-level” alerts for increased ICP with a concordant drop in PbtO2 below 15 mmHg. The user interface allows for throttling of notifications, as well as assignment of low-, mid-, and high-priority notifications.
Contributor Information
Steven N. Baldassano, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA 19104, USA
Shawniqua Williams Roberson, Department of Neurology, Vanderbilt University, Nashville, TN 37232, USA.
Ramani Balu, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104, USA.
Brittany Scheid, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA 19104, USA.
John M. Bemabei, Department of Bioengineering, University of Pennsylvania, Philadelphia, PA 19104, USA
Pathmanathan Jay, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104, USA.
Brian Oommen, UPMC Pinnacle Harrisburg, Harrisburg, PA 17101, USA.
Damien Leri, Penn Medicine Center for Health Care Innovation, University of Pennsylvania, Philadelphia, PA 19104, USA.
Javier Echauz, JE Research Inc., Alpharetta, GA 30022, USA.
Michael Gelfand, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104, USA.
Paulomi Kadakia Bhalla, Department of Neurology, Stanford University, Stanford, CA 94305,USA.
Chloe E. Hill, Department of Neurology, University of Michigan, Ann Arbor, MI 48109, USA
Amanda Christini, Blackfynn, Inc., Philadelphia, PA 19107, USA.
Joost B. Wagenaar, Blackfynn, Inc., Philadelphia, PA 19107, USA
Brian Litt, Department of Neurology, University of Pennsylvania, Philadelphia, PA 19104, USA.
References
- [1].Suarez JI, “Outcome in neurocritical care: Advances in monitoring and treatment and effect of a specialized neurocritical care team,” Crit. Care Med, vol. 34, no. Suppl, pp. S232–S238, September 2006. [DOI] [PubMed] [Google Scholar]
- [2].Busl KM,Bleck TP, and Varelas PN, “Neurocritical Care Outcomes, Research, and Technology,” JAMA Neurol, January 2019. [DOI] [PubMed] [Google Scholar]
- [3].Claassen J, Taccone FS, Horn P, Holtkamp M, Stocchetti N, and Oddo M, “Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM,” Intensive Care Med, vol. 39, no. 8, pp. 1337–1351, August 2013. [DOI] [PubMed] [Google Scholar]
- [4].Okonkwo DO et al. , “Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II,” Crit. Care Med, vol. 45, no. 11, pp. 1907–1914, November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Korbakis G and Vespa PM, “Multimodal neurologic monitoring,” in Handbook of CHnical Neurology, 2017, pp. 91–105. [DOI] [PubMed] [Google Scholar]
- [6].Saugel B, Cecconi M, Wagner JY, and Reuter DA, “Noninvasive continuous cardiac output monitoring in perioperative and intensive care medicine,” Br. J. Anaesth, vol. 114, no. 4, pp. 562–575, April 2015. [DOI] [PubMed] [Google Scholar]
- [7].Green MS, Sehgal S, and Tariq R, “Near-Infrared Spectroscopy,” Semin. Cardiothorac. Vase. Anesth, vol. 20, no.3, pp. 213–224, September 2016. [DOI] [PubMed] [Google Scholar]
- [8].De Georgia MA, Kaffashi F, Jacono FJ, and Loparo KA, “Information Technology in Critical Care: Review ofMonitoring and Data Acquisition Systems for Patient Care and Research,” Sci. World J, vol. 2015, pp. 1–9, February 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nemati S, Holder A, Razmi F, Stanley MD, Clifford GD, and Buchman TG, “An Interpretable Machine Learning Model for Accurate Prediction of Sepsis in the ICU,” Crit. Care Med, vol. 46, no. 4, pp. 547–553, April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Desautels T et al. , “Prediction of Sepsis in the Intensive Care Unit With Minimal Electronic Health Record Data: A Machine Learning Approach.,” JMIR Med. informatics, vol. 4, no.3, p. e28, September 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Che Z, Purushotham S, Khemani R, and Liu Y, “Interpretable Deep Models for ICU Outcome Prediction.,” AMIA... Annu. Symp. proceedings. AMIA Symp, vol. 2016, pp. 371–380, 2016. [PMC free article] [PubMed] [Google Scholar]
- [12].Henry KE, Hager DN, Pronovost PJ, and Saria S, “A targeted real-time early warning score (TREWScore) for septic shock.,” Sci. Transl. Med, vol. 7, no. 299, p. 299ral22, August 2015. [DOI] [PubMed] [Google Scholar]
- [13].Tasneem N et al. , “Brain Multimodality Monitoring: A New Tool in Neurocritical Care of Comatose Patients,” Crit. Care Res. Pract, vol. 2017, pp. 1–8, May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yogarajah M, Powell HWR, Heaney D, Smith SJM, Duncan JS, and Sisodiya SM, “Long term monitoring in refractory epilepsy: the Gowers Unit experience.,” J. Neurol. Neurosurg. Psychiatry, vol. 80, no. 3, pp. 305–10, March 2009. [DOI] [PubMed] [Google Scholar]
- [15].Chemmanam T, Radhakrishnan A, Sarma SP, and Radhakrishnan K, “A Prospective Study on the Cost-Effective Utilization of Long-Term Inpatient Video-EEG Monitoring in a Developing Country,” J. Clin. Neurophysiol, vol. 26, no. 2, pp. 123–128, April 2009. [DOI] [PubMed] [Google Scholar]
- [16].Rowan AJ, Siegel M, and Rosenbaum DH, “Daytime intensive monitoring: comparison with prolonged intensive and ambulatory monitoring.,” Neurology, vol. 37, no.3, pp.481–4, March 1987. [DOI] [PubMed] [Google Scholar]
- [17].Herman ST et al. , “Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice.,” J. Clin. Neurophysiol, vol. 32, no. 2, pp. 96–108, April 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Foreman B and Claassen J, “Quantitative EEG for the detection of brain ischemia,” Crit. Care, vol. 16, no. 2, p. 216, March 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Anthony Celi L, Mark RG, Stone DJ, and Montgomery RA, “‘Big Data’ in the Intensive Care Unit. Closing the Data Loop,” Am. J. Respir. Crit. Care Med, vol. 187, no. 11, pp. 1157–1160, June 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pirracchio R, Petersen ML, Carone M, Rigon MR, Chevret S, and van der Laan MJ, “Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): a population-based study,” Lancet Respir. Med, vol. 3, no. 1, pp. 42–52, January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meyfroidt G, Guiza F, Ramon J, and Bruynooghe M, “Machine learning techniques to examine large patient databases,” Best Pract. Res. Clin. Anaesthesiol, vol. 23, no. 1, pp. 127–143, 2009. [DOI] [PubMed] [Google Scholar]
- [22].Johnson AEW, Ghassemi MM, Nemati S, Niehaus KE, Clifton DA, and Clifford GD, “Machine Learning and Decision Support in Critical Care.,” Proc. IEEE. Inst. Electr. Electron. Eng, vol. 104, no. 2, pp. 444–466, February 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu VX and Walkey AJ, “Machine Learning and Sepsis,” Crit. Clare Med, vol. 45, no. 11, pp. 1946–1947, November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Churpek MM, Snyder A, Sokol S, Pettit NN, and Edelson DP, “Investigating the Impact of Different Suspicion of Infection Criteria on the Accuracy of Quick Sepsis-Related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores*,” Crit. Care Med, vol.45, no. 11, pp. 1805–1812, November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lagerlund TD, Cascino GD, Cicora KM, and Sharbrough FW, “Long-Term Electroencephalographic Monitoring for Diagnosis and Management of Seizures,” Mayo Clin. Proc, vol. 71, no. 10, pp. 1000–1006, October 1996. [DOI] [PubMed] [Google Scholar]
- [26].Jasper HH, “The 10/20 international electrode system,” EEG Clin. Neurophysiol, vol. 10, pp. 371–375, 1958. [Google Scholar]
- [27].Esteller R, Echauz J, Tcheng T, Litt B, and Pless B, “Line length: an efficient feature for seizure onset detection,” in 2001 Conference Proceedings of the 23rd AnnualInternational Conference of the IEEE Engineering in Medicine and Biology Society, 2001, vol. 2, pp. 1707–1710. [Google Scholar]
- [28].Delorme A, Sejnowski T, and Makeig S, “Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis,” Neuroimage, vol. 34, no. 4, pp. 1443–1449, February 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Uriguen JA and Garcia-Zapirain B, “EEG artifact removal - State-of-the-art and guidelines,” J. NeuralEng, vol. 12, no. 3, June 2015. [DOI] [PubMed] [Google Scholar]
- [30].Nolan H, Whelan R, and Reilly RB, “FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection,” J. Neurosci. Methods, vol. 192, no. 1, pp. 152–162, September 2010. [DOI] [PubMed] [Google Scholar]
- [31].Maddirala AK and Shaik RA, “Removal of EOG Artifacts from Single Channel EEG Signals Using Combined Singular Spectrum Analysis and Adaptive Noise Canceler,” IEEE Sens. J, vol. 16, no. 23, pp. 8279–8287, December 2016. [Google Scholar]
- [32].Kilicarslan A, Grossman RG, and Contreras-Vidal JL, “A robust adaptive denoising framework for real-time artifact removal in scalp EEG measurements,” J. NeuralEng, vol. 13, no. 2, February 2016. [DOI] [PubMed] [Google Scholar]
- [33].Leistritz L et al. , “New approaches for the detection and analysis of electroencephalographic burst-suppression patterns in patients under sedation,” J. Clin. Monit. Comput, vol. 15, no. 6, pp. 357–367, 1999. [DOI] [PubMed] [Google Scholar]
- [34].Lofhede J, Lofgren N, Thordstein M, Flisberg A, Kjellmer I, and Lindecrantz K, “Classification ofburst and suppression in the neonatal electroencephalogram,” J. NeuralEng, vol. 5, no. 4, pp. 402–410, 2008. [DOI] [PubMed] [Google Scholar]
- [35].Brandon Westover M et al. , “Real-time segmentation ofburst suppression patterns in critical care EEG monitoring,” J. Neurosci. Methods, vol. 219, no. 1, pp. 131–141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sarkela M et al. , “Automatic Analysis and Monitoring of Burst Suppression in Anesthesia,” J. Clin. Monit. Comput, 17, no. 2, pp. 125–134, 2002. [DOI] [PubMed] [Google Scholar]
- [37].Tukey JW, Exploratory Data Analysis (Behavioral Science). Pearson, 1977. [Google Scholar]
- [38].Medical Advisory Secretariat, “Bispectral index monitor: an evidence-based analysis.,” Ont. Health Technol. Assess. Ser, vol. 4, no. 9, pp. 1–70, 2004. [PMC free article] [PubMed] [Google Scholar]
- [39].Arbour RB and Dissin J, “Predictive Value of the Bispectral Index for Burst Suppression on Diagnostic Electroencephalogram During Drug-Induced Coma,” J. Neurosci. Nurs, vol. 47, no. 2, pp. 113–122, April 2015. [DOI] [PubMed] [Google Scholar]
- [40].Morimoto Y, Hagihira S, Koizumi Y, Ishida K, Matsumoto M, and Sakabe T, “The relationship between bispectral index and electroencephalographic parameters during isoflurane anesthesia.,” Anesth. Analg, vol. 98, no. 5, pp. 1336–40, table of contents, May 2004. [DOI] [PubMed] [Google Scholar]
- [41].Riker RR, Fraser GL, and Wilkins ML, “Comparing the bispectral index and suppression ratio with burst suppression of the electroencephalogram during pentobarbital infusions in adult intensive care patients.,” Pharmacotherapy, vol. 23, no.9, pp. 1087–93, September 2003. [DOI] [PubMed] [Google Scholar]
- [42].Young GB, “Continuous EEG monitoring in the ICU: challenges and opportunities.,” Can. J. Neurol. Sci, vol. 36 Suppl 2, pp. S89–91, August 2009. [PubMed] [Google Scholar]
- [43].Scheuer ML and Wilson SB, “Data analysis for continuous EEG monitoring in the ICU: seeing the forest and the trees.,” J. Clin. Neurophysiol, vol. 21, no. 5, pp. 353–78. [PubMed] [Google Scholar]
- [44].Shafi MM, Westover MB, Cole AJ, Kilbride RD, Hoch DB, and Cash SS, “Absence of early epileptiform abnormalities predicts lack of seizures on continuous EEG,” Neurology, vol. 79, no. 17, pp. 1796–1801, October 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Haider HA et al. , “Sensitivity of quantitative EEG for seizure identification in the intensive care unit.,” Neurology, vol. 87, no. 9, pp. 935–44, August 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hantus S, “Monitoring for seizures in the intensive care unit,” 2019, pp. 103–107. [DOI] [PubMed] [Google Scholar]
- [47].Hartings JA et al. , “Spreading depression in continuous electroencephalography ofbrain trauma,”. Ann. Neurol, vol. 76, no. 5, pp. 681–694, November 2014. [DOI] [PubMed] [Google Scholar]
- [48].Hofmeijer J, van Kaam CR, van de Werff B, Vermeer SE, Tjepkema-Cloostermans MC, and van Putten MJAM, “Detecting cortical spreading depolarization with full band scalp electroencephalography: An illusion?,” Front. Neurol, vol. 9, no. JAN, January 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chamanzar A et al. , “An Algorithm for Automated, Noninvasive Detection of Cortical Spreading Depolarizations Based on EEG Simulations,” IEEE Trans. Biomed. Eng, vol. 66, no. 4, pp. 1115–1126, April 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chamanzar A and Grover P, “Silence Localization,” in International IEEE/EMBS Conference on Neural Engineering, NER, 2019, vol. 2019-March, pp. 1155–1158. [Google Scholar]
- [51].Selioutski O et al. , “Continuous EEG Monitoring Predicts a Clinically Meaningful Recovery Among Adult Inpatients,” J. Clin. Neurophysiol, p.1, May 2019. [DOI] [PubMed] [Google Scholar]
- [52].Fung FW, Topjian AA, Xiao R, and Abend NS, “Early EEG Features for Outcome Prediction After Cardiac Arrest in Children,” J. Clin. Neurophysiol, p.1, April 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tolonen A et al. , “Quantitative EEG Parameters for Prediction of Outcome in Severe Traumatic Brain Injury: Development Study,” Clin. EEG Neurosci, vol. 49, no. 4, pp. 248–257, July 2018. [DOI] [PubMed] [Google Scholar]
- [54].Hofmeijer J, Beemink TMJ, Bosch FH, Beishuizen A, Tjepkema-Cloostermans MC, and van Putten MJAM, “Early EEG contributes to multimodal outcome prediction of postanoxic coma.,” Neurology, vol. 85, no.2, pp. 137–43, July 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee S, Zhao X, Davis KA, Topjian AA, Litt B, and Abend NS, “Quantitative EEG predicts outcomes in children after cardiac arrest,” Neurology, vol. 92, no. 20, pp. e2329–e2338, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sanchez Fernandez I, Sansevere AJ, Gainza-Lein M, Kapur K, and Loddenkemper T, “Machine Learning for Outcome Prediction in Electroencephalograph (EEG)-Monitored Children in the Intensive Care Unit,” J. Child Neurol, vol. 33, no. 8, pp. 546–553, July 2018. [DOI] [PubMed] [Google Scholar]
- [57].Ruskin KJ and Hueske-Kraus D, “Alarm fatigue,” Curr. Opin. Anaesthesiol, vol. 28, no. 6, pp. 685–690, December 2015. [DOI] [PubMed] [Google Scholar]
- [58].Kane-Gill SL et al. “Technologic Distractions (Part 1): Summary of Approaches to Manage Alert Quantity with Intent to Reduce Alert Fatigue and Suggestions for Alert Fatigue Metrics,” Crit. Care Med, vol. 45, no. 9, pp. 1481–1488, September 2017. [DOI] [PubMed] [Google Scholar]
- [59].Rajan B and Sarnikar S, “Information Systems Design Considerations for Reducing Alarm Fatigue in ICU,” 2018 Proc, August 2018. [Google Scholar]
- [60].Megjhani M et al. , “An active learning framework for enhancing identification of non-artifactual intracranial pressure waveforms,” Physiol. Meas, vol. 40, no. 1, January 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhong J, Dujovny M, Park HK, Perez E, Perlin AR, and Diaz FG, “Advances in ICP monitoring techniques,” Neurol. Res, vol. 25, no. 4, pp.339–350,June 2003. [DOI] [PubMed] [Google Scholar]
- [62].Hartigan JA and Wong MA, “Algorithm AS 136: A K-Means Clustering Algorithm,” Appl. Stat, vol. 28, no. 1, p. 100, 1979. [Google Scholar]