Abstract
OBJECTIVE
The purpose of this article is to perform a systematic review and meta-analysis regarding the diagnostic test accuracy of MRI for detecting extramural venous invasion (EMVI) in patients with colorectal cancer.
MATERIALS AND METHODS
PubMed and EMBASE were searched up to November 9, 2018. We included diagnostic accuracy studies that used MRI for EMVI detection in patients with colorectal cancer, using pathologic analysis as the reference standard. The methodologic quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies–2 tool. Sensitivity and specificity were pooled and plotted in a hierarchic summary ROC plot. Metaregression analysis using several clinically relevant covariates was performed.
RESULTS
Fourteen studies (n = 1751 patients) were included. Study quality was moderate in general. Pooled sensitivity was 0.61 (95% CI, 0.49–0.71), and pooled specificity was 0.87 (95% CI, 0.79–0.92). There was substantial heterogeneity according to the Cochran Q test (p < 0.01) and Higgins I2 heterogeneity index (98% and 95% for sensitivity and specificity, respectively). Publication bias was present (p = 0.01). Higher rates of advanced T category, use of high-resolution MRI, and use of antispasmodic drugs were shown to significantly affect heterogeneity (p < 0.01). Location of primary tumor, preoperative treatment status, study design, definition of reference standard, magnetic field strength, and use of functional MRI were not statistically significant (p = 0.17–0.92).
CONCLUSION
MRI shows moderate sensitivity and good specificity for the detection of EMVI in colorectal cancer. The use of high-resolution MRI may improve diagnostic performance.
Keywords: colorectal cancer, extramural venous invasion, meta-analysis, MRI, systematic review
Colorectal cancer is the third most common cancer worldwide and the fourth leading cause of cancer death in the world [1]. Current treatment guidelines recommend preoperative chemoradiation therapy (CRT) followed by total mesorectal excision for patients with locally advanced rectal cancer (category T3 or higher, or node-positive disease). This approach decreases the risk of local recurrence [2], but at the expense of higher rates of treatment- related adverse effects that affect bowel and sexual functions [3]. Furthermore, up to 15% of patients are at risk of developing distant metastasis even with good local tumor control [4]. For colon cancer, although radical resection is currently the only established curative treatment, early clinical trials suggest that neoadjuvant CRT may be suitable for locally advanced disease [5]. Appropriate selection of patients who are more likely to benefit from CRT and identifying factors that carry a risk for worse prognosis are clearly warranted.
Extramural venous invasion (EMVI) is the presence of tumor cells in the vasculature beyond the muscularis propria layer in the pathologic specimen. In addition to T category, EMVI is a strong independent predictor of poor outcomes, such as local recurrence, lymph node metastasis, synchronous and metachronous distant metastases, and overall poor survival in patients with colorectal cancer [6, 7]. However, EMVI is based on pathologic assessment of the surgical specimen; thus, this information can be obtained only after surgery. If such information could be acquired before surgery, or even during initial diagnostic assessment, there is potential for enhanced decision making regarding the optimal management plan and for better prediction of outcomes.
MRI is routinely used as the standard of care for the preoperative local staging of rectal cancer [8] and is increasingly being used in the local staging of colon cancer because of its potential advantages over CT [9]. Several studies have reported that MRI may be helpful in the preoperative evaluation of EMVI. For instance, in one of the early reports, Smith et al. [10] proposed a 5-point scoring system (0–4) for assessing EMVI at MRI (Fig. 1). The presence of intermediate signal intensity (SI) within only slightly expanded vessels or definitely irregular contour or nodular expansion of vessel by tumor SI (scores 3 or 4) resulted in a sensitivity and specificity of 62% and 88%, respectively, in 142 patients with locally advanced rectal cancer. The potential predictive value of MRI-detected EMVI has been reported in many studies showing that patients with MRI-detected EMVI have increased risk of distant metastases [11], local recurrence [12], and lymph node metastasis [13]. Furthermore, recent guidelines by the European Society for Medical Oncology [14] recommend assessment of MRI-detected EMVI preoperatively for stratification of recurrence risk and for deciding whether to perform neoadjuvant therapy. In this regard, several investigators have evaluated the accuracy of MRI for detecting EMVI but have found variable diagnostic performance (sensitivity, 37–94%; specificity, 56–99%), which limits its clinical application [12, 15–17]. The purpose of our study is to systemically review the literature and perform a meta-analysis regarding the diagnostic test accuracy of MRI for detecting EMVI in patients with colorectal cancer, using surgicopathologic results as the reference standard.
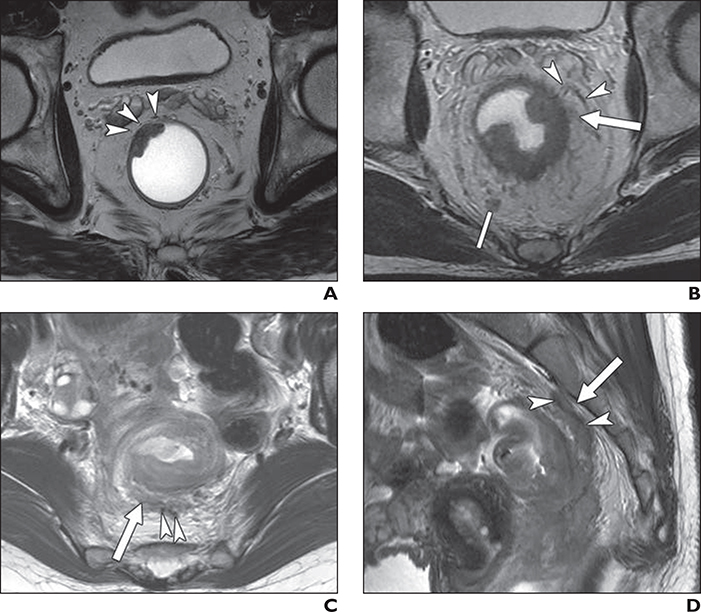
Fig. 1—
Three patients with rectal cancer, two of whom also had MRI-detected extramural venous invasion (EMVI), with corresponding pathologic results.
A, 68-year-old man with rectal cancer and MRI EMVI score of 0 (definitely absent) and negative EMVI at histopathologic examination. No definite extramural tumor extension is observed on T2-weighted oblique axial MR image. Normal-caliber perirectal vessels of low signal intensity are shown (arrowheads).
B, 61-year-old man with rectal cancer and MRI EMVI score of 2 (indeterminate) with positive EMVI at histopathologic examination. T2-weighted oblique axial MR image shows suspicious extramural tumor stranding (arrow) in vicinity of extramural vessels (arrowheads). However, tumor signal intensity does not extend to these normal-caliber perirectal vessels of low signal intensity. Note there is indeterminate perirectal lymph node (line), which was pathologically confirmed as regional lymph node metastasis.
C and D, 44-year-old woman with rectal cancer and MRI EMVI score of 4 (definitely present) with positive EMVI at histopathologic examination. T2-weighted oblique axial MR image (C) shows intermediate tumor signal with serpiginous extension (arrow) along vascular structure (arrowheads). On sagittal MR image (D), irregular tumor signal (arrow) extends cranially from primary rectal cancer along vein of low signal intensity (arrowheads).
Materials and Methods
The current study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Diagnostic Test Accuracy guidelines [18]. A research question based on the patient, index test, comparator, outcome, and study design criteria was constructed as follows: What is the diagnostic performance of MRI for the detection of EMVI in patients with colorectal cancer, compared with surgicopathologic results, in original research articles?
Literature Search
A computerized search of PubMed and EMBASE databases up to November 9, 2018, was conducted. The search query, which constituted keywords and related terms, was as follows: (colorectum OR colorectal OR colon OR colonic OR rectum OR rectal) AND (“extramural venous invasion” OR “extramural vascular invasion” OR EMVI) AND (“magnetic resonance imaging” or MRI). The reference lists were also checked to identify additionally includable studies. We did not limit the search to any particular language.
Inclusion Criteria
Studies that were relevant to our patient, index test, comparator, outcome, and study design criteria were included [18]: patients had colorectal cancer; the index test was MRI; surgicopathologic results were provided to determine EMVI status as the reference standard or comparison; the diagnostic test accuracy of MRI in determining EMVI, or raw data for calculating sensitivity and specificity, the outcome of interest, was extractable; and the publication type was original article.
Exclusion Criteria
Studies were excluded if they involved fewer than 10 patients, were publication types other than original articles, dealt with topics other than detection of EMVI using MRI, had overlapping patient populations, and did not provide enough data to calculate sensitivity and specificity. If multiple publications with an overlapping study population were identified, we included only the study with the largest patient cohort.
Two reviewers (with 1 and 3 years of experience in meta-analysis) conducted the search and study selection processes independently. A third reviewer (with 2 years of experience in meta-analysis) was consulted for disagreements between the two reviewers.
Data Extraction and Quality Assessment
Data from the included studies were extracted using a standardized form. Patient characteristics included number of patients, sex, median age and range, location of primary cancer, preoperative treatment status, and distribution of pathologic T category. Study characteristics included origin of study (authors, institution, and country), publication year, duration of patient recruitment, study design (prospective vs retrospective and whether enrollment was consecutive), multicenter study, number of MRI readers, definition of reference standard, and criteria for assessment of EMVI at MRI. Finally, MRI characteristics included magnet field strength, scanner model and manufacturer, in-plane voxel size, inclusion of contrast-enhanced (CE) MRI or DWI, and use of antispasmodic drugs. We evaluated the quality of the included studies with the Quality Assessment of Diagnostic Accuracy Studies–2 tool [19].
The above processes were conducted by the same two reviewers as mentioned previously. A third reviewer was consulted for disagreements.
Data Synthesis and Analysis
Data were formulated in 2 × 2 contingency tables to calculate sensitivity and specificity. When data from multiple independent readers were provided, the results were averaged for this meta-analysis.
Pooled sensitivity and specificity estimates were calculated with hierarchic logistic regression modeling, including bivariate modeling and hierarchic summary ROC modeling [20]. Hierarchic summary ROC curves, along with their 95% confidence and prediction regions, were used to visualize the results. Publication bias was evaluated using Deeks funnel plot and Deeks asymmetry test [21].
Heterogeneity (variation in study outcomes between the included studies) was assessed with the Cochran Q test, with p < 0.1 indicating heterogeneity, and with the Higgins I2 test, which was performed and interpreted as an inconsistency index (I2), where 0–40% indicates that heterogeneity might not be important, 30–60% indicates moderate heterogeneity, and 75–100% indicates considerable heterogeneity [22]. Finally, we searched for a threshold effect, the positive correlation between the sensitivity and false-positive rate in included studies.
To investigate the cause of heterogeneity, metaregression analysis was performed using the following variables: location of primary tumor (rectum only vs any colon), preoperative treatment status (neoadjuvant therapy received vs not received), study design (multi- vs single-center study and prospective vs retrospective), definition of reference standard (strictly EMVI vs lymphovascular invasion or unclear definition), frequency of high T category (either T3c or higher, or T4 or higher [23]; ≥ 67.3% vs < 67.3% median of all included studies), magnetic field strength (3 vs < 3 T), use of antispasmodic drugs, use of high-resolution MRI (voxel size < 0.8 mm) [24], and inclusion of CE-MRI, inclusion of DWI, and multiparametric approach (inclusion of either CE-MRI or DWI functional MRI sequence). In addition, subgroup analysis was planned for each of the criteria for assessment of EMVI at MRI.
The midas module in Stata (version 10.0, Stata-Corp) and mada package in R (version 3.5.1, R Foundation for Statistical Computing) were used for statistical analyses, with p < 0.05 indicating statistical significance.
Results
Literature Search
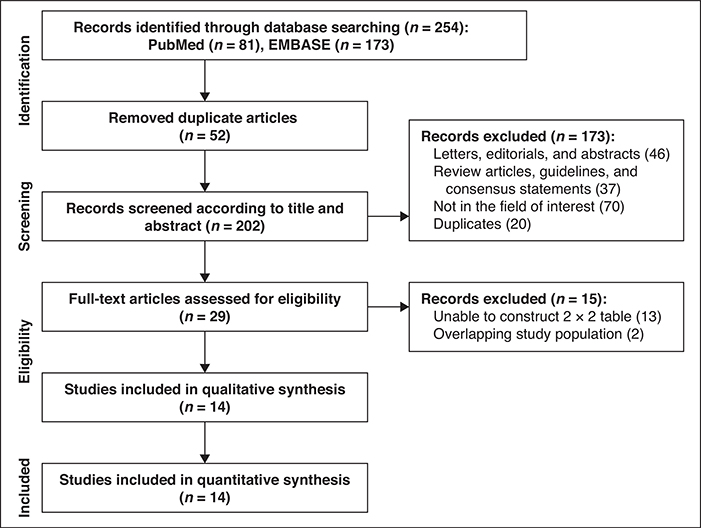
The systematic literature search initially yielded 254 articles. After removing 52 duplicates, screening of the 202 titles and abstracts yielded 29 potentially eligible studies. Full-text reviews were performed and 15 studies were excluded because they had insufficient data to reconstruct 2 × 2 tables (n = 13) or they shared study populations with other studies (n = 2). Ultimately, 14 original articles including 1751 patients assessing the diagnostic performance of MRI for detection of EMVI in patients with colorectal cancer were included [9, 10, 12, 13, 15–17, 25–31]. The detailed study selection process is shown in Figure 2.
Fig. 2—
Flow diagram showing study selection process for meta-analysis.
Characteristics of Included Studies
The patient characteristics are described in Table I. The size of the study population ranged from 28 to 489 patients, and the number of patients with pathologically confirmed EMVI ranged from four to 87. The overall frequency of patients with EMVI detected at MRI was 23.9% (418/1751). The patients had a median age of 60–74 years. Eight studies included only patients with rectal cancer [12, 13, 15, 17, 25, 28, 30, 31], whereas six studies included patients with cancer in other colon segments [9, 10, 16, 26, 27, 29]. Six studies included patients who did not receive neoadjuvant treatment [9, 13, 16, 26, 28, 29], and three included patients who did receive neoadjuvant CRT [12, 30, 31]. Another five studies included a mixed population (patients who did or did not receive neoadjuvant treatment combined) [10, 15, 17, 25, 27] and among them, two studies separately provided diagnostic performance of MRI for EMVI detection for patients who did not receive neoadjuvant treatment and, therefore, were analyzed as part of the subgroup of patients who did not receive neoadjuvant treatment in the metaregression analysis [10, 25]. Results of MRI after neoadjuvant treatment were used in the current meta-analysis, including six studies that included patients who received neoadjuvant treatment (three with only patients who received preoperative CRT [12, 30, 31] and three with mixed populations [15, 17, 27]). Eleven studies provided information regarding pathologic T category [9, 10, 12, 13, 15–17, 26–29]. The frequency of high T category ranged from 33% to 100%.
TABLE 1:
Patient Characteristics
| Study | Institution | Duration of Patient Recruitment | No. of Patients |
Age (y) |
Primary Cancer Location | Neoadjuvant Treatment Status | Tumor T Category (No. of Patients) | Frequency of High T Category (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | With Pathologic EMVI | Median | Range | |||||||
| Brown et al. [15] | The Royal Marsden Hospital | 1997–1999 | 98 | 26 | 62 | 32–88 | Rectum | Mixed (no, radiation) | T1 (6), T2 (22), T3 (59), T4 (11) | 71 |
| Smith et al. [10] | Mayday University Hospital | Jan. 2000–Dec. 2004 | 142 | 26 | NA | NA | Rectum, sigmoid colon | Mixed (no, chemoradiation)a | T0 (6), T1 (6), T2 (39), T3 (63), T4 (19) | 62 |
| Rollvén et al. [9] | Karolinska University Hospital | NA | 27 | 8 | 73 | 41–86 | Cecum, ascending colon, transverse colon, sigmoid colon | No | T2 (8), T3 (15), T4 (6) | 72 |
| Sohn et al. [25] | Severance Hospital | Jul. 2011–Dec. 2012 | 188 | 39 | 62 | 31–90 | Rectum | Mixed (no, chemoradiation, chemotherapy)a | NA | NA |
| Chand et al. [12] | The Royal Marsden Hospital | Jan. 2006–Jan. 2013 | 188 | 46 | 74 | 30–87 | Rectum | Chemoradiation | T1 (8), T2 (33), T3 (117), T4 (16) | 76 |
| Hunter et al. [26] | Croydon University Hospital, Epsom and St. Heliers University Hospital | Sep. 2010–Oct. 2012 | 54 | 19 | 69.7b | NA | Cecum, ascending colon, transverse colon, descending colon, sigmoid colon | No | T1/T2 (12), T3 (28), T4 (15) | 78 |
| Jhaveri et al. [27] | Mount Sinai Hospital and Women’s College Hospital, Toronto | Sep. 2010–Sep. 2013 | 39 | 12 | 58b | 31–81 | Rectum, sigmoid colon | Mixed (no, chemoradiation, chemotherapy, radiation) | T0–T2 (16), T3–T4 (33) | 67 |
| Liu et al. [13] | Beijing Friendship Hospital | Dec. 2011–Dec. 2014 | 183 | 47 | 57b | 30–83 | Rectum | No | T1 (15), T2 (42), T3 (108), T4 (19) | 69 |
| Yu et al. [28] | First Affiliated Hospital of Nanjing Medical University | May 2013–Mar. 2015 | 86 | 36 | 60 | 38–82 | Rectum | No | T3 (86) | 100 |
| Dam et al. [29] | Vejle Hospital | 2010–2015 | 35 | 9 | 70b | 47–83 | Sigmoid colon | No | T1–T3ab (26), T3cd–T4 (9) | 26 |
| Nerad et al. [16] | Maastricht University Medical Centre | Apr. 2014–May 2015 | 55 | 17 | 69 | 34–84 | Cecum, ascending colon, transverse colon, descending colon, sigmoid colon | No | T1 (4), T2 (15), T3ab (19), T3cd (9), T4 (8) | 31 |
| El-Kady et al. [30] | Alexandria University | NA | 50 | 4 | 50b | 20–80 | Rectum | Chemoradiation | NA | NA |
| Lee et al. [31] | National Cancer Center | Jan. 2008–Dec. 2010 | 200 | 42 | 59b | 23–87 | Rectum | Chemoradiation | NA | NA |
| Poulsen et al. [17] | Aalborg University Hospital | 2009–2013 | 454 | 87 | NA | NA | Rectum | Mixed (no, chemoradiation) | T0–T2 (157), T3 (224), T4 (17) | 61 |
Note—EMVI = extramural venous invasion, NA = not available.
Separately provided diagnostic performance of MRI for EMVI detection in patients without any neoadjuvant treatment.
Mean.
The study characteristics are shown in Table 2. The study design was prospective in two studies [15, 26] and retrospective in 12 [9, 10, 12, 13, 16, 17, 25, 27–31]. Only one study had been conducted at multiple centers [26]. The number of MRI readers ranged from one to four. Seven studies [9, 13, 16, 26, 27, 29, 31] reported the interobserver agreement for the assessment of EMVI at MRI. Six of seven studies [9, 13, 16, 27, 29, 31] reported moderate to substantial agreement, with kappa values ranging from 0.52 to 0.801, and one study [26] reported only slight agreement (κ = 0.07). Patient recruitment was consecutive in all but one study [30]. EMVI was assessed at MRI using the 5-point scoring system by Smith et al. [10] in seven studies [10, 12, 13, 25, 27, 28, 31]. Four studies simply assessed the presence or absence of a serpiginous extension of tumor signal within or invasion of a vascular structure [15, 16, 26, 29]. The remaining three studies did not provide explicit criteria [12, 17, 30]. The definition of EMVI at pathologic examination was provided in nine studies [10, 12, 13, 15, 25, 27, 28, 30, 31]; seven studies were strictly confined to tumor with venous invasion [10, 12, 13, 15, 27, 30, 31], whereas two studies encompassed not only venous but also lymphatic invasion because their pathologic reports did not provide information separately [25, 28]. The other five studies did not specify the details [9, 16, 17, 26, 29].
TABLE 2:
Study Characteristics
| Study | Publication Year | Country | Study Design | Consecutive Enrollment | Multicenter Study | No. of MRI Readers | EMVI Assessment at MRI | Definition of EMVI at Pathologic Examination |
|---|---|---|---|---|---|---|---|---|
| Brown et al. [15] | 2003 | United Kingdom | Prospective | Yes | No | 2 | Presence or absence of tumor signal within or invasion of vascular structure | Confined to venous invasion |
| Smith et al. [10] | 2008 | United Kingdom | Retrospective | Yes | No | 1 | 5-Point scoring systema | Confined to venous invasion |
| Rollvén et al. [9] | 2013 | Sweden | Retrospective | Yes | No | 2 | Not specified | Not specified |
| Sohn et al. [25] | 2015 | Korea | Retrospective | Yes | No | 2 | 5-Point scoring systema | Lymphovascular invasion |
| Chand et al. [12] | 2015 | United Kingdom | Retrospective | Yes | No | 1 | 5-Point scoring systema | Confined to venous invasion |
| Hunter et al. [26] | 2016 | United Kingdom | Prospective | Yes | Yes | 2 | Presence or absence of tumor signal within or invasion of vascular structure | Not specified |
| Jhaveri et al. [27] | 2016 | Canada | Retrospective | Yes | No | 2 | 5-Point scoring systema | Confined to venous invasion |
| Liu et al. [13] | 2016 | China | Retrospective | Yes | No | 2 | 5-Point scoring systema | Confined to venous invasion |
| Yu et al. [28] | 2016 | China | Retrospective | Yes | No | 2 | 5-Point scoring systema | Lymphovascular invasion |
| Dam et al. [29] | 2017 | Denmark | Retrospective | Yes | No | 2 | Presence or absence of tumor signal within or invasion of vascular structure | Not specified |
| Nerad et al. [16] | 2017 | The Netherlands | Retrospective | Yes | No | 2 | Presence or absence of tumor signal within or invasion of vascular structure | Not specified |
| El-Kady et al. [30] | 2018 | Egypt | Retrospective | Not specified | No | NA | Not specified | Confined to venous invasion |
| Lee et al. [31] | 2018 | Korea | Retrospective | Yes | No | 2 | 5-Point scoring systema | Confined to venous invasion |
| Poulsen et al. [17] | 2018 | Denmark | Retrospective | Yes | No | 4 | Not specified | Not specified |
Note—EMVI = extramural venous invasion, NA = not available.
Five-point scoring system (0–4) for assessing EMVI at MRI proposed by Smith et al. [10].
The MRI characteristics are described in Table 3. Three studies used 3-T scanners [13, 25, 28], nine used 1.5-T scanners [9, 10, 12, 15–17, 26, 29, 30], and two used either 1.5- or 3-T scanners [27, 31]. Regarding T2-weighted imaging, the FOV varied from 16 × 16 cm to 42 × 42 cm, and the matrix size varied from 256 × 256 to 448 × 314. In-plane voxel sizes ranged from 0.5 to 1.3 mm. A total of 10 studies solely used T2-weighted imaging for EMVI detection [9, 10, 12, 13, 15, 17, 25, 26, 28, 31], whereas four studies also used CE-MRI [27], DWI [29, 30], or both [16]. An antispasmodic agent was used in five studies [9, 16, 25–27], not used in two [13, 15], and not specified in seven [10, 12, 17, 28–31].
TABLE 3:
MRI Characteristics
| Study | Magnet Strength (T) | Vendor | Machine | T2-Weighted Imaging FOV(cm) | T2-Weighted Imaging Matrix Size | In-Plane Voxel Size (mm) | EMVI Evaluation Sequence | Antispasmodic Agent |
|---|---|---|---|---|---|---|---|---|
| Brown et al. [15] | 1.5 | GE Healthcare | Horizon Advantage | 16 × 16 | 256 × 256 | 0.6250 | T2-weighted imaging | No |
| Smith et al. [10] | 1.5 | NA | NA | 16 × 16 | 256 × 256 | 0.6250 | T2-weighted imaging | NA |
| Rollvén et al. [9] | 1.5 | Philips Healthcare | Intera | 37.5 × 26.2 | NA | 0.5–0.8 | T2-weighted imaging | Yes |
| Sohn et al. [25] | 3 | Siemens Healthcare | Magnetom TimTrio | 19.9 × 9.9–25 × 25 | 320 × 320–448 × 314 | 0.796 | T2-weighted imaging | Yes |
| Chand et al. [12] | 1.5 | NA | NA | 16 × 16 | 256 × 256 | 0.6250 | T2-weighted imaging | NA |
| Hunter et al. [26] | 1.5 | Siemens Healthcare | Magnetom Avanto | 17 × 17–42 × 42 | 256 × 256–320 × 320 | 0.66–1.3 | T2-weighted imaging | Yes |
| Jhaveri et al. [27] | 1.5 or 3 | Siemens Healthcare | Avanto, Verio | 20 × 20 | 350 × 263 | 0.6 | T2-weighted imaging + contrast-enhanced MRI | Yes |
| Liu et al. [13] | 3 | GE Healthcare | Signa Excite | 16 × 16 | 320 × 256 | 0.6 | T2-weighted imaging | No |
| Yu et al. [28] | 3 | Siemens Healthcare | Magnetom TimTrio | 22 × 22 | 384 × 296 | 0.57–0.74 | T2-weighted imaging | NA |
| Dam et al. [29] | 1.5 | Philips Healthcare | Ingenia | NA | NA | NA | T2-weighted imaging + DWI | NA |
| Nerad et al. [16] | 1.5 | Philips Healthcare | Ingenia | 39 × 39 | 392 × 392 | 1 | T2-weighted imaging + DWI + contrast-enhanced MRI | Yes |
| El-Kady et al. [30] | 1.5 | Siemens Healthcare, Philips Healthcare, GE Healthcare | Magnetom Avanto, Signa HDxt, Gyroscan Intera | NA | NA | NA | T2-weighted imaging + DWI | NA |
| Lee et al. [31] | 1.5 or 3 | Philips Healthcare, GE Healthcare | Achieva, HDx, Signa | 24 × 24 | 300 × 300 | 0.8 | T2-weighted imaging | NA |
| Poulsen et al. [17] | 1.5 | NA | NA | 16 × 16 | 256 × 256 | 0.6250 | T2-weighted imaging | NA |
Note—EMVI = extramural venous invasion, NA = not available.
Quality Assessment
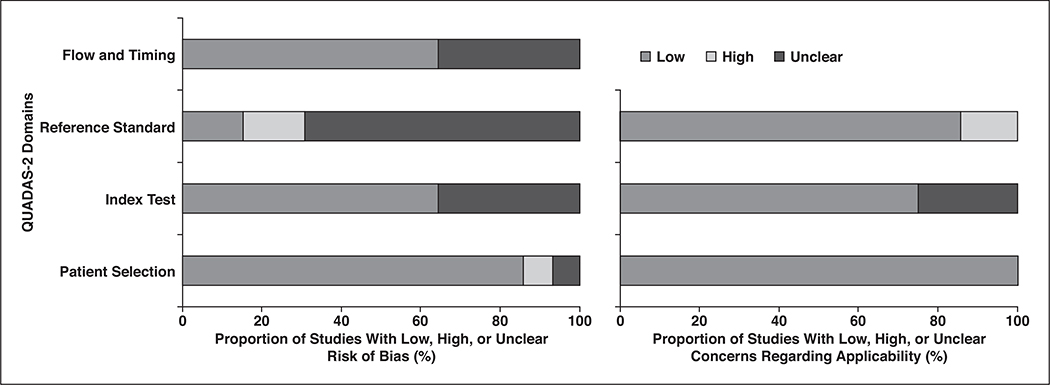
In general, the quality of the 14 studies was considered moderate, with eight satisfying more than four of the seven Quality Assessment of Diagnostic Accuracy Studies–2 domains (Fig. 3). Regarding the patient selection domain, only one study [30] was considered to have unclear risk of bias because it did not explicitly mention whether patients were consecutively enrolled. There was high risk of bias in one study [17] that excluded a number of patients for EMVI assessment without describing specific reasons. Regarding the index test domain, there was unclear risk of bias in five studies, because the criteria for MRI detection of EMVI were not explicitly described in three studies [9, 17, 30], and although the scoring system by Smith et al. [10] was used, the cutoff value for determining EMVI positivity was not provided in two studies [13, 28]. The three studies that did not provide explicit criteria were also considered to have unclear concern for applicability [9, 17, 30].
Fig. 3—
Grouped bar charts of risk of bias (left) and concerns for applicability (right) of 14 included studies using Quality Assessment of Diagnostic Accuracy Studies–2 (QUADAS-2) tool.
Regarding the reference standard domain, nine studies had unclear risk of bias, because the criteria for determining EMVI at pathologic examination were not explicitly described in three studies [9, 16, 29] and because it was unclear whether histopathologic assessment was performed blinded to MRI interpretation in eight studies [10, 13, 16, 17, 26, 28, 30, 31]. Three studies had high risk of bias, because one performed histopathologic examination without blinding to MRI findings [15], and two included both venous and lymphatic invasion for EMVI [25, 28]. The latter two also had high risk of applicability for the same reason [25, 28].
Regarding the flow and timing domain, six studies had unclear risk of bias, because the interval between MRI and histopathologic examination was not provided [10, 17, 25, 28, 30, 31]. The reported time interval in the other eight studies ranged from 1 day to within 6 weeks [9, 12, 13, 15, 16, 26, 27, 29].
Diagnostic Accuracy of MRI for Detection of Extramural Venous Invasion
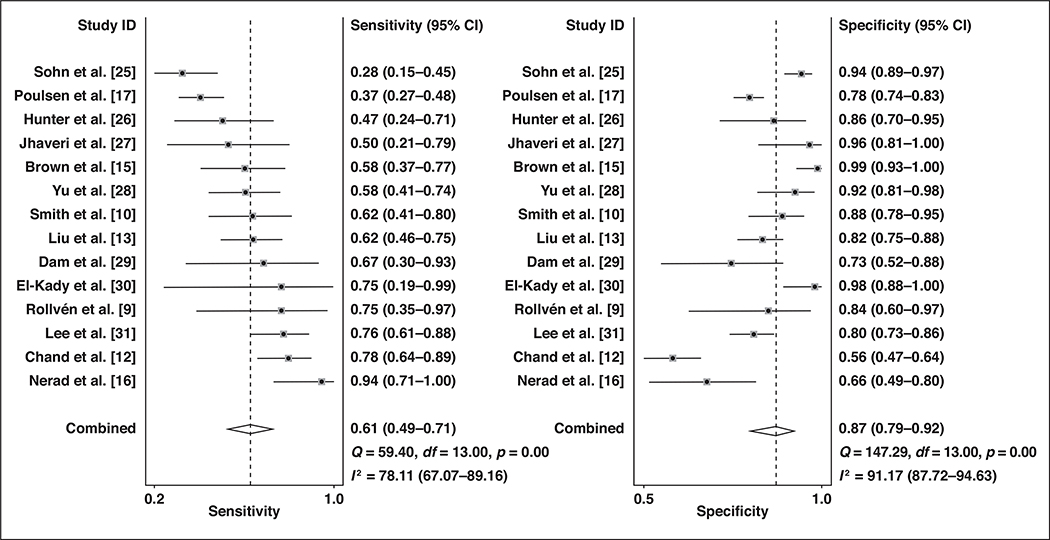
The sensitivity and specificity of the individual studies (n = 14) that provided diagnostic performance values ranged from 0.28 to 0.94 and from 0.56 to 0.99, respectively. The Cochran Q test showed that heterogeneity was present (Q = 88.443; p < 0.001). The Higgins I2 statistics showed substantial heterogeneity in terms of both the sensitivity (I2 = 98%) and specificity (I2 = 95%). A possible threshold effect was suggested on visualization of the coupled forest plot of the sensitivity and specificity (Fig. 4), albeit without a strong corresponding correlation coefficient (0.501; 95% CI, −0.040 to 0.815) between the sensitivity and the false-positive rate.
Fig. 4—
Coupled forest plots of pooled sensitivity and specificity. Numbers are pooled estimates (dots within squares) with 95% CIs (horizontal lines). Corresponding heterogeneity statistics are provided at bottom right corners. Dashed vertical lines and diamonds indicate overall meta-analytically pooled estimate. Lateral points of diamonds indicate CIs for estimate.
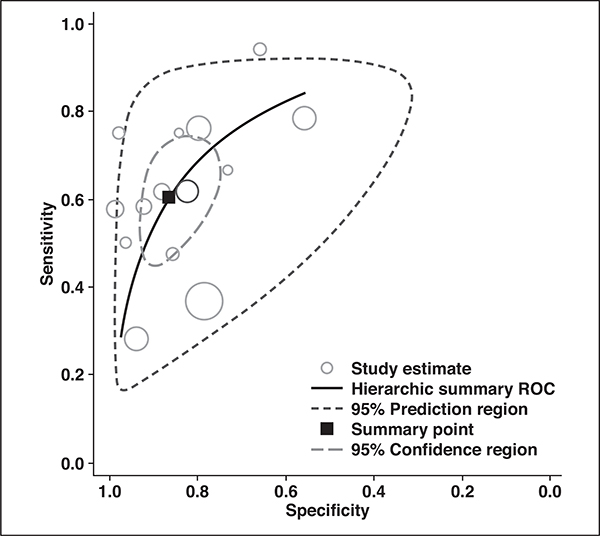
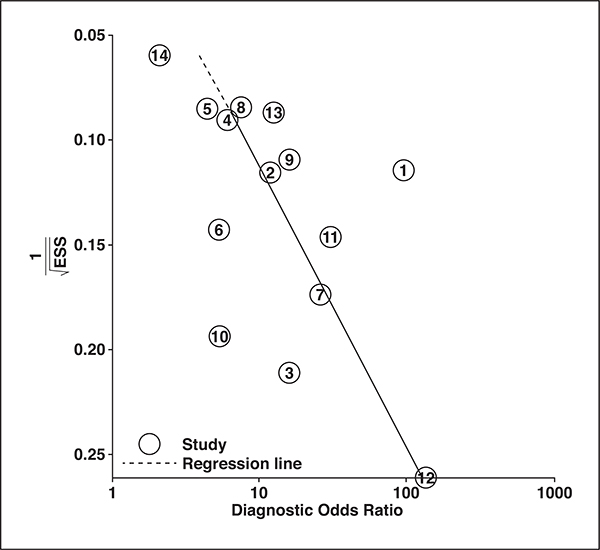
For the 14 studies combined, the pooled sensitivity was 0.61 (95% CI, 0.49–0.71) with a specificity of 0.87 (95% CI, 0.79–0.92). In the hierarchic summary ROC curve, there was a large difference between the 95% confidence and prediction regions, thereby indicating heterogeneity between the studies (Fig. 5). The hierarchic summary ROC AUC was 0.80 (95% CI, 0.77–0.84). According to the Deeks funnel plot, the likelihood of publication bias was high, with p = 0.01 for slope coefficient (Fig. 6).
Fig. 5—
Hierarchic summary ROC curve of diagnostic performance of MRI for detection of extramural venous invasion in patients with colorectal cancer.
Fig. 6—
Deeks funnel plot; p = 0.01 suggests that likelihood of publication bias is high. Numbers in circles are study number, in same order as shown in Table I. ESS = effective sample size.
Heterogeneity Exploration
The results of metaregression analysis for the 14 studies providing diagnostic test accuracy results are shown in Table 4. Among the several covariates evaluated, the frequency of high T category, use of antispasmodic agents, and high-resolution MRI were significant factors affecting heterogeneity (p < 0.01). Regarding frequency of high T category, studies with a frequency greater than or equal to 67.3% showed similar sensitivity (0.61; 95% CI, 0.49–0.74) but higher specificity (0.88; 95% CI, 0.80–0.96) compared with studies with a frequency less than 67.3% (sensitivity, 0.62; 95% CI, 0.44–0.79; specificity 0.77; 95% CI, 0.62–0.93). Studies that used antispasmodic agents showed similar sensitivity (0.58; 95% CI, 0.37–0.79) and specificity (0.88; 95% CI, 0.78–0.97) compared with those without antispasmodic agents (sensitivity, 0.59; 95% CI, 0.30–0.87; specificity, 0.91; 95% CI, 0.81–1.0). Studies with voxel sizes of less than 0.8 mm showed similar sensitivity (0.58; 95% CI, 0.44–0.72) but higher specificity (0.89, 95% CI, 0.83–0.95) compared with those using voxel sizes of greater than or equal to 0.8 mm (sensitivity, 0.63; 95% CI, 0.44–0.82; specificity, 0.78; 95% CI, 0.64–0.92). Other factors did not significantly affect the heterogeneity (p = 0.17–0.92). Although this finding was not significant, the pooled sensitivity values were substantially higher when using functional MRI sequences than not: 0.77 (95% CI, 0.54–1.00) versus 0.58 (95% CI, 0.47–0.69) for CE-MRI, 0.82 (95% CI, 0.63–1.00) versus 0.57 (95% CI, 0.46–0.67) for DWI, and 0.73 (95% CI, 0.54–0.93) versus 0.57 (95% CI, 0.46–0.69) for either. The pooled sensitivities and specificities of the subgroup of studies based on MRI criteria were as follows: 5-point scoring system by Smith et al. [10], 0.59 (95% CI, 0.46–0.72) and 0.86 (95% CI, 0.76–0.92), respectively; serpiginous extension of tumor signal within or invasion of a vascular structure, 0.69 (95% CI, 0.47–0.85) and 0.86 (95% CI, 0.65–0.95), respectively; and no explicit criteria, 0.53 (95% CI, 0.24–0.83) and 0.89 (95% CI, 0.76–1.00), respectively.
TABLE 4:
Results of Meta-Regression Analysis of MRI for Detection of Extramural Venous Invasion (EMVI) in Colorectal Cancer
| Covariate, Category | No. of Studies | Meta-Analysis Summary Estimates |
||
|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | p | ||
| Location of primary tumor | 0.61 | |||
| Rectum | 8 | 0.56 (0.43–0.70) | 0.88 (0.80–0.95) | |
| Any other colon | 6 | 0.67 (0.51–0.83) | 0.85 (0.74–0.95) | |
| Neoadjuvant treatment | 0.92 | |||
| No | 8 | 0.61 (0.47–0.75) | 0.85 (0.77–0.94) | |
| Yes or mixed | 6 | 0.59 (0.43–0.76) | 0.88 (0.79–0.97) | |
| Multi- or single-center study | 0.76 | |||
| Multicenter study | 1 | 0.47 (0.07–0.88) | 0.86 (0.63–1.00) | |
| Single-center study | 13 | 0.61 (0.51–0.72) | 0.87 (0.80–0.93) | |
| Study design | 0.34 | |||
| Prospective | 2 | 0.53 (0.26–0.81) | 0.95 (0.87–1.00) | |
| Retrospective | 12 | 0.62 (0.50–0.73) | 0.85 (0.78–0.91) | |
| Definition of reference standard | 0.22 | |||
| Strictly EMVI | 7 | 0.65 (0.52–0.77) | 0.89 (0.81–0.97) | |
| Lymphovascular invasion or unclear definition | 7 | 0.54 (0.40–0.69) | 0.84 (0.73–0.94) | |
| Frequency of high T category (%) | < 0.01 | |||
| ≥ 67.3 | 7 | 0.61 (0.49–0.74) | 0.88 (0.80–0.96) | |
| < 67.3 | 4 | 0.62 (0.44–0.79) | 0.77 (0.62–0.93) | |
| Magnet field strength (T) | 0.58 | |||
| 3 | 5 | 0.55 (0.38–0.72) | 0.90 (0.82–0.98) | |
| < 3 | 9 | 0.64 (0.50–0.77) | 0.84 (0.75–0.93) | |
| Antispasmodic agents | < 0.01 | |||
| Used | 5 | 0.58 (0.37–0.79) | 0.88 (0.78–0.97) | |
| Not used | 2 | 0.59 (0.30–0.87) | 0.91 (0.81–1.00) | |
| High-resolution MRI (voxel size < 0.8 mm) | < 0.01 | |||
| Yes | 8 | 0.58 (0.44–0.72) | 0.89 (0.83–0.0.95) | |
| No | 4 | 0.63 (0.44–0.82) | 0.78 (0.64–0.92) | |
| Contrast-enhanced MRI | 0.42 | |||
| Used | 2 | 0.77 (0.54–1.00) | 0.86 (0.67–1.00) | |
| Not used | 12 | 0.58 (0.47–0.69) | 0.87 (0.80–0.93) | |
| DWI | 0.17 | |||
| Used | 3 | 0.82 (0.63–1.00) | 0.84 (0.68–1.00) | |
| Not used | 11 | 0.57 (0.46–0.67) | 0.87 (0.81–0.94) | |
| Multiparametric approacha | 0.32 | |||
| Yes | 4 | 0.73 (0.54–0.93) | 0.88 (0.77–0.99) | |
| No | 10 | 0.57 (0.46–0.69) | 0.86 (0.79–0.94) | |
Either contrast-enhanced MRI or DWI in addition to T2-weighted imaging.
Discussion
In this meta-analysis, we evaluated the diagnostic performance of MRI for the detection of EMVI in patients with colorectal cancer. The pooled sensitivity and specificity of the included studies were 0.61 (95% CI, 0.49–0.71) and 0.87 (95% CI, 0.79–0.92), respectively. Despite moderate sensitivity, the relatively high specificity may allow MRI to provide incremental value in the preoperative assessment of patients with colorectal cancer. A recent meta-analysis based on six studies found that the detection of EMVI at MRI is also associated with an increased rate of synchronous and postoperative metachronous metastasis [11]. In addition, another study showed that patients with stage II rectal cancer with EMVI detected at MRI have 3-year disease-free survival rates similar to those for patients with stage III disease [32]. Despite such promising results, only approximately half of the physicians treating patients with rectal cancer assess EMVI at preoperative MRI [33]. If the diagnostic accuracy and prognostic value of MRI-detected EMVI are validated in future studies, it may have the possibility to be incorporated into staging systems and standardized MRI interpretation templates, and, in turn, may help individualize patient management with regard to deciding whether to perform neoadjuvant treatment and estimating postoperative prognosis.
In this study, MRI showed only moderate sensitivity of 0.61, and this may be attributed to both histopathologic- and MRI-related aspects. First, there was heterogeneity with respect to histopathologic definitions and methods used to determine EMVI in the studies included. The frequency of EMVI ranged from 8% to 42%, and there were seven studies that used a strict definition of tumor with venous invasion [10, 12, 13, 15, 27, 30, 31], whereas two studies used a broader definition in which tumors with not only venous but also lymphatic invasion were considered positive for EMVI [25, 28]. This corroborates the study by Chand et al. [34], which summarized inconsistent rates of histopathologic venous invasion in rectal cancer ranging from 9% to 61% and stressed the need to standardize the histopathologic definitions, including methods (i.e., H and E staining or elastin-based stains) and standardized reporting to achieve high-quality and consistent histopathologic results. Regarding MRI, because of the limited spatial and contrast resolution, microscopic EMVI may not be detectable with current technology [35]. Yet, in our metaregression analysis, studies that used high-resolution MRI—that is T2-weighted imaging with an in-plane voxel size smaller than 0.8 mm—showed higher specificity (0.89 vs 0.78) compared with those that did not use high-resolution MRI. This finding indicates that improved spatial resolution and an improved ability to acquire better anatomic detail may help increased diagnostic performance of detecting EMVI in patients with colorectal cancer.
A multiparametric MRI approach, consisting of two or more functional techniques such as CE-MRI or DWI in addition to conventional sequences (T2-weighted imaging), has been used in attempts to improve the diagnostic accuracy, not only in EMVI evaluation but also in rectal cancer staging evaluation. In our metaregression analysis, studies using a multiparametric approach showed substantially higher pooled sensitivity estimates than did those using T2-weighted imaging only, albeit without statistical significance. Both in rectal cancer T staging in general or evaluation of EMVI, the incremental value of CE-MRI or DWI has been a controversial issue. For instance, the addition of CE-MRI to T2-weighted imaging may improve the diagnostic performance and confidence level of readers [36], especially in equivocal cases where a filling defect seen at CE-MRI will lead to rescoring the study as definitely positive for EMVI. On the other hand, other studies also suggested that exaggerated peritumoral inflammatory reactions can mimic tumor invasion and result in overstaging and possible false-positive EMVI in rectal cancer [37, 38]. DWI has been shown to increase interobserver agreement for staging and to improve prediction of residual tumor after CRT compared with T2-weighted imaging alone [39]. On the other hand, DWI can be of nondiagnostic quality as a result of gas-induced susceptibility artifacts [40].
Other relevant MRI-related methodologic aspects, such as magnetic field strength and usage of antispasmodic agents, were assessed as potential sources of heterogeneity. Compared with studies using 1.5-T MRI, those using 3-T MRI showed comparable results for detecting EMVI. Although higher magnetic field strength allows faster image acquisition, higher spatial resolution, and higher signal-to-noise ratio, no significant improvement with respect to diagnostic accuracy has been proven in rectal cancer, possibly because of potential amplification of artifacts [41]. Regarding antispasmodic agents, although their use was a statistically significant factor affecting heterogeneity, the differences in sensitivity and specificity between studies with and without antispasmodic agents were not significant. Likewise, the benefit of routine use of antispasmodic agents has been controversial and has not reached consensus in recent guidelines [41].
Studies with higher rates of high T categories showed similar sensitivity but higher specificity compared with studies with rates less than 67.3%. It is well known that the frequency of EMVI increases as rectal cancer T category increases [32]. Although sensitivity and specificity are theoretically not related to the frequency of the disease in the population, because those are properties of the diagnostic tool, there are also many studies that argue that sensitivity and specificity may vary with disease frequency [42, 43]. The frequency and diagnostic test accuracy can hypothetically be related because of factors such as patient spectrum and reader expectation (i.e., higher disease frequency may lower reader’s intrinsic threshold and, in turn, influence accuracy) [42]. Specifically, mathematic modeling with Pearson correlation coefficient and regression framework have shown that specificity tends to increase with higher frequency [43].
Preoperative CRT had no significant effect on the diagnostic performance of MRI in our metaregression analysis. This finding is in line with those of previous studies [12, 36, 44], substantiating the ability of MRI to detect EMVI even after CRT. We speculate that this may be due to the differences in SI between viable tumor and fibrosis after CRT. Fibrosis tends to show very low T2 SI, similar to that of muscularis propria, whereas viable tumors show intermediate T2 SI. However, there are also studies with conflicting results that argue that an intermediate or high T2 SI alone does not indicate viable tumor characteristic, and other factors, such as edema, desmoplastic reaction, and inflammation, can lead to overstaging or understaging after CRT [45]. This may be another area where there is potential for CE-MRI or DWI to provide added value. Future studies are needed to resolve such controversy.
One of the major limitations of this meta-analysis was the relatively small number of included studies (n = 14). One reason for this was that several recent studies dealing with relevant issues [11, 46, 47] mainly focused on the clinical effect of MRI-detected EMVI, presuming that the diagnosis of EMVI at MRI was accurate. However, to apply MRI-detected EMVI in clinical practice, the accuracy of MRI in diagnosis of EMVI should be established first. This is the first study, to our knowledge, to systematically review and perform meta-analysis of the performance of MRI for diagnosis of EMVI in patients with colorectal cancer; therefore, it may provide insight into and justification for the application of MRI detection of EMVI. Another limitation of this meta-analysis is the presence of substantial heterogeneity among the studies, which may hinder the applicability of the results. Although we identified potential factors attributable to this heterogeneity by metaregression analysis, a possible threshold effect suggested on visualization of the coupled forest plot of the sensitivity and specificity could also be important sources of heterogeneity between the included studies [48]. We speculate that this may stem from the fact that interpretation of EMVI at MRI was based on the 5-point scoring system by Smith et al. [10] in half of the included studies. In addition, publication bias was present among the included studies. Therefore, it is possible that the diagnostic performance of MRI for detecting EMVI may be overestimated in this meta-analysis. Finally, there were six studies in our meta-analysis that dealt with primary tumor in any part of the large bowel, not confined to rectum [9, 10, 16, 26, 27, 29]. Unlike rectal cancer, which typically involves preoperative local staging with MRI as a routine workup, there is no established imaging protocol for local staging of the primary colon cancer with MRI. Although we found comparable results regarding MRI-detected EMVI diagnostic accuracy in both rectum and colon in metaregression analysis, the protocol for MRI in local staging should be established for further application of MRI-detected EMVI in primary colon cancer.
In conclusion, MRI shows moderate sensitivity and good specificity for the detection of EMVI in patients with colorectal cancer. Caution is needed for clinical application of our results because of the large degree of heterogeneity among the studies. Using high-resolution MRI has the potential to improve diagnostic performance.
Acknowledgments
This study was funded by the National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017; 66:683–691 [DOI] [PubMed] [Google Scholar]
- 2.van Gijn W, Marijnen CA, Nagtegaal ID, et al. ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011; 12:575–582 [DOI] [PubMed] [Google Scholar]
- 3.Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol 2016; 34:3300–3307 [DOI] [PubMed] [Google Scholar]
- 4.Engelen SM, Maas M, Lahaye MJ, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer 2013; 49:2311–2320 [DOI] [PubMed] [Google Scholar]
- 5.Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012; 13:1152–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betge J, Pollheimer MJ, Lindtner RA, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer 2012; 118:628–638 [DOI] [PubMed] [Google Scholar]
- 7.Roxburgh CS, McMillan DC, Richards CH, et al. The clinical utility of the combination of T stage and venous invasion to predict survival in patients undergoing surgery for colorectal cancer. Ann Surg 2014; 259:1156–1165 [DOI] [PubMed] [Google Scholar]
- 8.Benson AB 3rd, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018; 16:874–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollvén E, Holm T, Glimelius B, Lörinc E, Blomqvist L. Potentials of high resolution magnetic resonance imaging versus computed tomography for preoperative local staging of colon cancer. Acta Radiol 2013; 54:722–730 [DOI] [PubMed] [Google Scholar]
- 10.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2008; 95:229–236 [DOI] [PubMed] [Google Scholar]
- 11.Siddiqui MRS, Simillis C, Hunter C, et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br J Cancer 2017; 116:1513–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chand M, Evans J, Swift RI, et al. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg 2015; 261:473–479 [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Liu M, Yang Z, He W, Wang Z, Jin E. Correlation of MRI-detected extramural vascular invasion with regional lymph node metastasis in rectal cancer. Clin Imaging 2016; 40:456–460 [DOI] [PubMed] [Google Scholar]
- 14.Glynne-Jones R, Wyrwicz L, Tiret E, et al. ; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28(suppl_4):iv22–iv40 [DOI] [PubMed] [Google Scholar]
- 15.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 2003; 90:355–364 [DOI] [PubMed] [Google Scholar]
- 16.Nerad E, Lambregts DM, Kersten EL, et al. MRI for local staging of colon cancer: can MRI become the optimal staging modality for patients with colon cancer? Dis Colon Rectum 2017; 60:385–392 [DOI] [PubMed] [Google Scholar]
- 17.Poulsen LO, Yilmaz MK, Oddershede L, et al. Is the accuracy of preoperative MRI stage in rectal adenocarcinoma influenced by tumour height? Acta Oncol 2018; 57:728–734 [DOI] [PubMed] [Google Scholar]
- 18.McInnes MDF, Moher D, Thombs BD, et al. ; the PRISMA-DTA Group. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018; 319:388–396 [DOI] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–536 [DOI] [PubMed] [Google Scholar]
- 20.Suh CH, Park SH. Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol 2016; 17:5–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005; 58:882–893 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S, eds.; The Cochrane Collabo-ration. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Cochrane Collaboration website; handbook-5-1.cochrane.org/. Updated March 2011 Accessed March 20, 2019 [Google Scholar]
- 23.Taylor FG, Quirke P, Heald RJ, et al. ; MERCURY study group. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg 2011; 253:711–719 [DOI] [PubMed] [Google Scholar]
- 24.Kaur H, Choi H, You YN, et al. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. RadioGraphics 2012; 32:389–409 [DOI] [PubMed] [Google Scholar]
- 25.Sohn B, Lim JS, Kim H, et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol 2015; 25:1347–1355 [DOI] [PubMed] [Google Scholar]
- 26.Hunter C, Blake H, Jeyadevan N, et al. Local staging and assessment of colon cancer with 1.5-T magnetic resonance imaging. Br J Radiol 2016; 89:20160257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhaveri KS, Hosseini-Nik H, Thipphavong S, et al. MRI detection of extramural venous invasion in rectal cancer: correlation with histopathology using elastin stain. AJR 2016; 206:747–755 [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Huang DY, Xu HX, Li Y, Xu Q. Correlation between magnetic resonance imaging-based evaluation of extramural vascular invasion and prognostic parameters of T3 stage rectal cancer. J Comput Assist Tomogr 2016; 40:537–542 [DOI] [PubMed] [Google Scholar]
- 29.Dam C, Lindebjerg J, Jakobsen A, Jensen LH, Rahr H, Rafaelsen SR. Local staging of sigmoid colon cancer using MRI. Acta Radiol Open 2017; 6:2058460117720957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Kady E, Ibrahim ME, Abbas KS, El-Sedfy AS, Mohallel MA. Role of magnetic resonance imaging in loco-regional evaluation of cancer rectum, pre and post neoadjuvant therapy. Alexandria J Med 2018; 54:661–678 [Google Scholar]
- 31.Lee ES, Kim MJ, Park SC, et al. Magnetic resonance imaging-detected extramural venous invasion in rectal cancer before and after preoperative chemoradiotherapy: diagnostic performance and prognostic significance. Eur Radiol 2018; 28:496–505 [DOI] [PubMed] [Google Scholar]
- 32.Chand M, Bhangu A, Wotherspoon A, et al. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol 2014; 25:858–863 [DOI] [PubMed] [Google Scholar]
- 33.Chand M, Swift RI, Chau I, Heald RJ, Tekkis PP, Brown G. Adjuvant therapy decisions based on magnetic resonance imaging of extramural venous invasion and other prognostic factors in colorectal cancer. Ann R Coll Surg Engl 2014; 96:543–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chand M, Heald RJ, West N, Swift RI, Tekkis P, Brown G. The evolution in the detection of extramural venous invasion in rectal cancer: implications for modern-day practice. Colorectal Cancer 2014; 3:481–490 [Google Scholar]
- 35.Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR 2008; 191:1517–1522 [DOI] [PubMed] [Google Scholar]
- 36.Jhaveri KS, Hosseini-Nik H. MRI of rectal cancer: an overview and update on recent advances. AJR 2015; 205:[web]W42–W55 [DOI] [PubMed] [Google Scholar]
- 37.Gollub MJ, Lakhman Y, McGinty K, et al. Does gadolinium-based contrast material improve diagnostic accuracy of local invasion in rectal cancer MRI? A multireader study. AJR 2015; 204:[web]W160–W167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jao SY, Yang BY, Weng HH, Yeh CH, Lee LW. Evaluation of gadolinium-enhanced T1-weighted magnetic resonance imaging in the preoperative assessment of local staging in rectal cancer. Colorectal Dis 2010; 12:1139–1148 [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 2009; 253:116–125 [DOI] [PubMed] [Google Scholar]
- 40.van Griethuysen JJM, Bus EM, Hauptmann M, et al. Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T: effect of applying a micro-enema to improve image quality. Eur J Radiol 2018; 99:131–137 [DOI] [PubMed] [Google Scholar]
- 41.Gollub MJ, Arya S, Beets-Tan RG, et al. Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017. Abdom Radiol (NY) 2018; 43:2893–2902 [DOI] [PubMed] [Google Scholar]
- 42.Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 2013; 185:E537–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Fine JP. Assessing the dependence of sensitivity and specificity on prevalence in meta-analysis. Biostatistics 2011; 12:710–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel UB, Brown G, Rutten H, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol 2012; 19:2842–2852 [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Kim HM, Koom WS, et al. Profiling of rectal cancers MRI in pathological complete remission states after neoadjuvant concurrent chemoradiation therapy. Clin Radiol 2016; 71:250–257 [DOI] [PubMed] [Google Scholar]
- 46.Zhang XY, Wang S, Li XT, et al. MRI of extramural venous invasion in locally advanced rectal cancer: relationship to tumor recurrence and overall survival. Radiology 2018; 289:677–685 [DOI] [PubMed] [Google Scholar]
- 47.Schaap DP, Ogura A, Nederend J, et al. Prognostic implications of MRI-detected lateral nodal disease and extramural vascular invasion in rectal cancer. Br J Surg 2018; 105:1844–1852 [DOI] [PubMed] [Google Scholar]
- 48.Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers. Part I. General guidance and tips. Korean J Radiol 2015; 16:1175–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]