Abstract
Diabetic ketoacidosis (DKA) is the most common hyperglycemic emergency and causes the greatest risk for death in patients with diabetes mellitus. DKA more commonly occurs among those with type 1 diabetes, yet almost a third of the cases occur among those with type 2 diabetes. Although mortality rates from DKA have declined to low levels in general, it continues to be high in many developing countries. DKA is characterized by hyperglycemia, metabolic acidosis and ketosis. Proper management of DKA requires hospitalization for aggressive intravenous fluids, insulin therapy, electrolyte replacement as well as identification and treatment of the underlying precipitating event along with frequent monitoring of patient's clinical and laboratory states. The most common precipitating causes for DKA include infections, new diagnosis of diabetes and nonadherence to insulin therapy. Clinicians should be aware of the occurrence of DKA in patients prescribed sodium-glucose co-transporter 2 inhibitors. Discharge plans should include appropriate choice and dosing of insulin regimens and interventions to prevent recurrence of DKA. Future episodes of DKA can be reduced through patient education programs focusing on adherence to insulin and self-care guidelines during illness and improved access to medical providers. New approaches such as extended availability of phone services, use of telemedicine and utilization of public campaigns can provide further support for the prevention of DKA.
Keywords: Diabetes mellitus, fluid management, insulin, ketoacidosis
INTRODUCTION
Diabetic ketoacidosis (DKA) is an acute complication of uncontrolled diabetes mellitus that is associated with increased morbidity and mortality. Epidemiological studies have found that rates of DKA in new-onset type 1 diabetes vary between countries.[1] While studies from Denmark, Kuwait, Canada and Germany have found the rates of DKA in newly diagnosed type 1 diabetes to be 17.9%, 24.8%, 25.6% and 35.2%, respectively, in United Arab Emirates, its rate was 80%.[2,3,4,5,6] Saudi Arabia has one of the highest rates of DKA worldwide, with various studies showing that in newly diagnosed type 1 diabetes, these rates are 40% in the Eastern region,[7] 55% in the Northwestern region[8] and 67.2% in Riyadh city.[9]
Despite medical advances in its diagnosis and management, DKA remains an important cause of hospital admissions and mortality in children and adults, particularly in developing countries. In fact, recently, there has been a significant increase in the rates of DKA-related hospital admissions in several regions worldwide.[10,11] The most frequently reported indicators that correlate with hospitalization for DKA are poor glycemic control, lower socioeconomic status, presence of psychiatric conditions and female gender.[12]
In children and adolescents with type 1 diabetes, DKA is the most common cause of death. It is responsible for about 50% of the deaths in patients with diabetes aged <24 years.[13] The mortality rates from DKA have fallen significantly from >90% before the discovery and use of insulin in 1922 to 60% in 1923, 12% by 1945 and 3%–10% by 1974.[14,15,16,17] Current in-hospital mortality rates from DKA in developed countries are generally low, at <1% and up to 2.6% in the elderly.[11,18] However, studies from different developing countries have revealed mortality rates as high as 10%–30% for patients hospitalized for DKA.[19,20,21,22]
DKA, which was previously thought to be a unique clinical feature of type 1 diabetes, has been reported to occur in children and adult patients with type 2 diabetes. In fact, about one-third of patients who present with DKA are found to have type 2 diabetes.[23,24,25] Hospitalizations due to DKA and its treatment have been associated with a significant economic burden.[26] There are several clinical practice guidelines for the management of DKA, such as those from the American Diabetes Association, the Canadian Diabetes Association and the British Diabetes Societies. There are points of difference among these guidelines that may cause inconsistencies in the management of DKA among clinicians. In this article, we provide a narrative review of management of DKA in adults along with discussion of the various professional guidelines and propose evidence-based recommendations on the topic.
We conducted a literature search through PubMed and Google Scholar using the following search terms: “diabetic ketoacidosis,” “DKA,” “diabetic emergencies,” “diabetic crisis,” “hyperglycemic emergencies” and “hyperglycemic crises.” Original articles, consensus statements, professional guidelines, systematic and nonsystematic reviews, meta-analysis, case-control studies, cohort studies, case series and case reports published in English were selected for review for the period from 1945 to August 2019.
APPROACH TO THE PATIENT WITH DIABETIC KETOACIDOSIS
History and physical examination
The clinical presentation of DKA usually includes manifestations of hyperglycemia such as increased urination, increased thirst, weakness and weight loss. In severe cases, manifestations of acidosis such as lethargy, stupor, loss of consciousness and respiratory compromise may appear. Gastrointestinal symptoms such as abdominal pain, nausea and vomiting are common in DKA and usually resolve with treatment.[27] Several conditions can lead to the development of DKA such as infections (particularly pneumonia and urinary tract infections); new diagnosis of diabetes; poor adherence to, or inadequate doses of, insulin; myocardial infarction; stroke; acute pancreatitis; trauma; burns; surgery; medications such as glucocorticoids, beta-blockers, thiazides and atypical antipsychotics; psychological factors including depression and eating disorders and illicit substance use.[13,28] The sodium glucose co-transporter 2 (SGLT2) inhibitors, a class of oral antidiabetic agents, have been associated with the development of DKA; the occurrence of unusual symptoms such as nausea, vomiting, abdominal pain or fatigue in these patients should alert the physician to the possibility of the development of DKA.[29,30]
Physical examination should include assessment of mental status, volume status and a focused systematic examination. Patients usually present with signs of volume depletion such as tachycardia, hypotension, decreased skin turgor and dry oral mucosa. Temperature can be normal or even low in the presence of infection, mainly because of peripheral vasodilation. Other physical signs may include Kussmaul respirations (rapid and deep breathing) with acetone (fruity) breath odor, alteration in mental status, shock and coma.[13]
Laboratory findings
The initial laboratory testing should include plasma glucose, electrolytes, serum ketones (if unavailable, urine ketones can be obtained), complete blood count and initial arterial (or venous) blood gases. DKA is characterized by hyperglycemia, the presence of ketone bodies and acidosis. Plasma glucose is generally elevated to >13.9 mmol/L (250 mg/dL). However, a wide range of plasma glucose levels can be present, which is independent of the severity of DKA.[13] Normal or lower levels of glucose have been reported, a condition called “euglycemic DKA,” in about 10% of patients who present with DKA.[31] Patients who are prescribed SGLT2 inhibitor therapy may develop euglycemic DKA and the diagnosis of DKA can be missed or delayed in these cases; therefore, the physician should have a high level of suspicion when evaluating such patients.
The second feature of DKA is the presence of ketones in the urine and/or serum. There are three types of ketones: beta-hydroxybutyrate, acetoacetate and acetone. It is recommended to measure serum beta-hydroxybutyrate (normal, <0.6 mmol/L) if urine ketones are negative when the diagnosis of DKA is suspected.[32] Point-of-care capillary beta-hydroxybutyrate measurement has been found to be both sensitive and specific for DKA when compared with ketone testing using the nitroprusside method.[33]
The third diagnostic aspect in DKA is the presence of acidosis, defined as serum bicarbonate level of ≤18 mmol/L and/or arterial pH ≤7.30.[13] Measurement of venous blood gases can be used particularly in stable patients, while arterial measurement is reserved for sicker patients. Venous pH measurement provides an adequate assessment of the degree of acidosis and response to therapy as well as helps avoid the pain and possible complications that are associated with repeated arterial punctures.[34,35,36,37] Venous pH is usually 0.015–0.03 lower than arterial pH.[34,35,36] The accumulation of ketoacids results in high anion gap metabolic acidosis; the anion gap is calculated by the following formula: [Na − (Cl + HCO3)]. A normal range for anion gap depends on the laboratory's reference range and is generally 6–10 mmol/L (6–10 mEq/L) and an anion gap value of >10 mmol/L indicates the presence of high anion gap metabolic acidosis.[13]
Other tests may show elevated white blood cell count (10,000–15,000 mm3), which is common in DKA and is attributed to dehydration, stress and demargination of leukocytes.[38] Artificially low serum sodium (pseudohyponatremia) is common and results from the outflux of water from the intra-to the extracellular space induced by hyperglycemia.[39] Thus, the corrected serum sodium should be calculated to account for the level of hyperglycemia by adding 1.6 mmol/L (1.6 mEq/L) of serum sodium level for every 5.6 mmol/L (100 mg/dL) of serum glucose above 5.6 mmol/L (100 mg/dL).[13] Despite the total body deficit of potassium, serum potassium levels at the time of presentation of DKA are frequently normal or high; this occurs due to insulin deficiency and acidosis, which cause potassium movement out of the cells and can also result from reduced renal function.[40] A grading system developed by the American Diabetes Association has been proposed to assess the severity of DKA, which classifies DKA into mild, moderate or severe based on the degree of metabolic acidosis (levels of blood pH and bicarbonate) and the presence of altered mental status [Table 1].[13]
Table 1.
Classification of diabetic ketoacidosis*
| Parameter | Mild | Moderate | Severe |
|---|---|---|---|
| Serum bicarbonate (mmol/L) | 15-18 | 10-<15 | <10 |
| Arterial pH | 7.25-7.30 | 7.0-7.24 | <7.0 |
| Anion gap | >10 | >12 | >12 |
| Mental status | Alert | Alert/drowsy | Stupor/coma |
*Kitabchi et al.[13]
TREATMENT OF DIABETIC KETOACIDOSIS
DKA is a medical emergency that requires prompt management in a hospital setting. The mainstays of its management include restoring the circulatory volume, correcting electrolyte abnormalities, treating hyperglycemia and diagnosing and treating the precipitating cause.
Fluid therapy
Patients with DKA are consistently dehydrated. On average, patients with DKA have free water deficit of about 100 mL/kg of body weight.[13] Intravenous (IV) fluid therapy expands the intravascular volume, improves renal perfusion and reduces peripheral insulin resistance by reducing levels of counter-regulatory hormones; the net result will be a reduction in blood glucose levels.[40] Currently, most of the available literature regarding fluid therapy is based on consensus guidelines and expert opinions.[13,37,41]
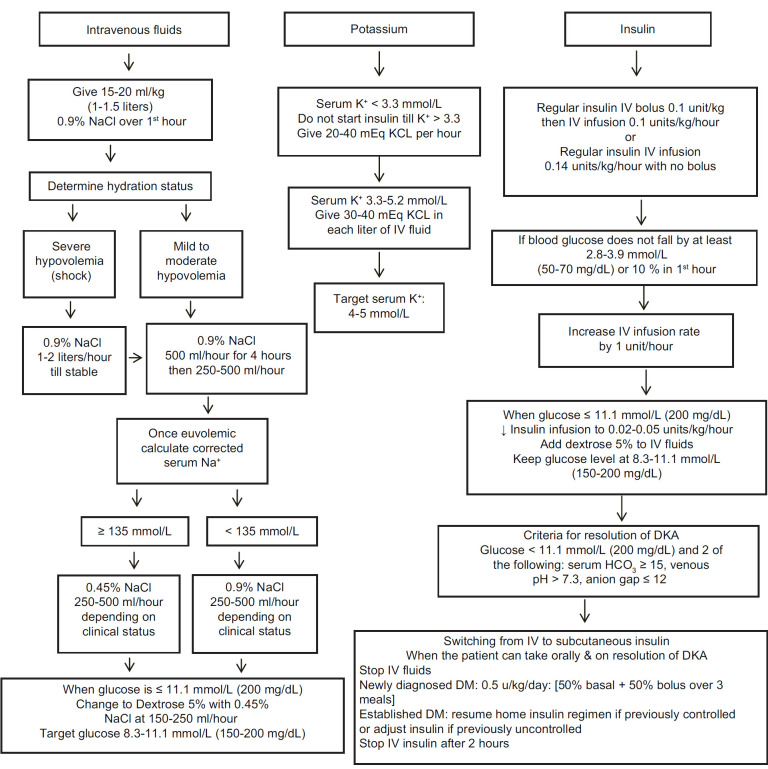
Normal saline (0.9% sodium chloride) is recommended as the initial IV fluid replacement in DKA. Initial IV fluid replacement starts with 0.9% sodium chloride at a rate of 15–20 ml/kg (about 1–1.5 L) over the 1st h. Thereafter, the rate and type of fluids is determined by assessment of the clinical condition. Patients with hypovolemic shock are continued on 0.9% sodium chloride at a rate of 1–2 L/h until their condition becomes stable, while patients with mild or moderate hypovolemia are given 0.9% sodium chloride at a rate of 500 ml/h for 4 h followed by 250–500 ml/h, depending on the clinical condition.[13,37] Once patients with severe hypovolemia become stable, the IV fluid management is changed to the same as those with mild or moderate hypovolemia. Once hypovolemia is corrected, the type of IV fluids is determined by the level of corrected serum sodium; if the level is low (<135 mmol/L), 0.9% sodium chloride is continued and if the level is normal or high (≥135 mmol/L), IV fluids should be changed to 0.45% sodium chloride.[13,37] The rate of IV fluids in both groups will be 250–500 ml/h, depending on the patient's clinical status such as weight, vital signs, urine output and the presence of comorbid conditions. Once blood glucose reaches ≤11.1 mmol/L (200 mg/dL), 5% dextrose should be added along with 0.45% sodium chloride at a rate of 150–250 ml/h to maintain blood glucose concentration at 8.3–11.1 mmol/L (150–200 mg/dL).[13]
Electrolyte therapy
DKA is associated with a significant total body deficit of serum electrolytes, particularly sodium, chloride and potassium. On average, patients with DKA have the following deficits of electrolytes per kilogram of body weight: sodium, 7–10 mEq/kg; potassium, 3–5 mEq/kg and chloride, 3–5 mmol/kg.[13,37] Replacement of sodium and chloride will follow the guidelines mentioned above.
The amount and timing of potassium replacement depend on the serum potassium concentration. No supplement is required if the serum potassium concentration is >5.2 mmol/L (5.2 mEq/L), but the levels should be monitored closely because the entry of potassium in cells would be facilitated by volume expansion, resolution of acidosis and insulin therapy, which, in turn, would result in decreased concentration of serum potassium. Once serum potassium level is ≤5.2 mmol/L, potassium replacement should be started to achieve a goal of maintaining it at 4–5 mmol/L.[13] For levels between 3.3 and 5.2 mmol/L, replacement should be started using 20–30 mEq of potassium in each liter of IV fluids. If serum potassium at presentation is <3.3 mmol/L, insulin should not be started, as it can further lower serum potassium and potassium replacement at 20–30 mEq/h should be given until serum potassium level rises to >3.3 mmol/L.
In randomized control trials, sodium bicarbonate use for treating acidosis in DKA have not shown to have an impact on the clinical outcomes.[42,43,44] Acidosis is usually corrected with the treatment of DKA, as IV fluids improve tissue perfusion and renal function, thereby increasing the excretion of organic acids, and insulin therapy stops further ketone synthesis and allows excess ketoacids to be metabolized, resulting in the regeneration of bicarbonate.[45] On the other hand, bicarbonate therapy increases the risk of hypokalemia and cerebral edema as well as slows the rate of recovery from ketosis.[45] In a systematic review, no evidence of clinical efficacy was found from the use of bicarbonate therapy in DKA and it was concluded that its use is not justified and may instead be harmful.[46] Therefore, it is recommended that bicarbonate should not be used in patients with a pH of ≥6.9.[13,40] Although no randomized studies have been carried out regarding the use of bicarbonate for managing DKA with pH values <6.9, the American Diabetes Association recommends it based on expert opinion.[13] Serum potassium should be monitored because bicarbonate therapy can lower potassium level, and thus potassium should be supplemented through IV fluids, as outline above.
Insulin therapy
Insulin therapy is a mainstay in the management of DKA because it reduces the production of hepatic glucose, increases the utilization of peripheral glucose and inhibits lipolysis, ketogenesis and glucagon secretion, resulting in lowering of plasma glucose and decreasing the production of ketoacidosis.[47] Insulin is usually given through IV route, starting with a bolus of regular insulin at a dose of 0.1 unit/kg body weight, and then, within 5 min followed by a continuous infusion of regular insulin of 0.1 unit/kg/h.[13,48] There was no difference in outcomes if IV insulin infusion is started without a bolus dose but at a higher rate of insulin at 0.14 unit/kg body weight/h.[49,50] In children, a bolus dose of insulin prior to the IV infusion is not recommended, as it does not improve clinical outcomes and may contribute to the development of cerebral edema.[45,51,52]
With insulin therapy, plasma glucose levels are expected to be reduced by about 2.8–3.9 mmol/L (50–70 mg/dL)/h or 10% from initial glucose concentration after the 1st h.[13,53] If glucose levels do not decrease by these rates, hydration status and rates of IV fluids should be evaluated and optimized if necessary. Once this is assured, and if the plasma glucose levels continue not to fall as desired, the insulin infusion rate can be increased by 1 unit/h until a steady decline in serum glucose by rates described above are achieved.[41] When plasma glucose reaches ≤11.1 mmol/L (200 mg/dL), the insulin infusion should be reduced to 0.02–0.05 units/kg/h and 5% dextrose should be added to IV fluids to allow the continuation of insulin until ketoacidosis is controlled and to avoid hypoglycemia.[13,40] The rate of IV insulin infusion and the concentration of dextrose are adjusted (10% dextrose can be used if necessary) aiming to maintain blood glucose levels at 8.3–11.1 mmol/L (150–200 mg/dL).[13]
In mild/moderate DKA patients, the use of subcutaneous rapid-acting insulin has been found to be safe and effective and can be used as an alternative to the IV infusion of regular insulin.[54,55,56] In these patients, insulin therapy should be started with an initial bolus of 0.2–0.3 units/kg followed by 0.1–0.2 units/kg every 1–2 h. The dose can then be reduced to 0.05 units/kg every 1 h or 0.01 units/kg every 2 h until resolution of DKA. One study with a small number of patients examined the use of IV rapid-acting insulin glulisine in the management of DKA and found it to be as effective as IV regular insulin.[57] Regular insulin is still recommended in the management of DKA given its well-stablished studies and lower cost compared with rapid-acting insulin. An algorithm for the management of DKA is presented in Figure 1.
Figure 1.
MANAGEMENT AFTER RESOLUTION OF DIABETIC KETOACIDOSIS
Monitoring of patient's condition
After resolution of DKA, the patient's blood pressure, pulse, hydration, fluid input, urinary output and mental status should be frequently monitored. Follow-up laboratory investigations should include measurement of blood glucose initially every hour until patient's condition is stable as well as measurement of serum electrolytes, blood urea nitrogen and creatinine every 2–4 h, depending on disease severity and the clinical response.[13] As previously mentioned, acidosis can be monitored by measurement of venous blood gases without the need to obtain arterial blood. An alternative to monitoring venous pH is to monitor serum bicarbonate concentration and serum anion gap to assess the correction of ketoacidosis.[58,59,60] Monitoring of ketones can be used, if available, by measurement of blood beta-hydroxybutyrate to assess the status of ketoacidosis.[41,61,62] Resolution of DKA is indicated by a glucose level of <11.1 mmol/L (200 mg/dL), and any two of the following: a serum bicarbonate level ≥15 mmol/L, a venous pH >7.3 and/or an anion gap ≤12 mmol/L.[13] The patient should be clinically stable and able to tolerate oral feeding. If the patient is not able to eat, it is recommended to continue the IV fluids and insulin infusion.
Subcutaneous insulin
Subcutaneous insulin should be started when DKA has resolved and the patient is able to tolerate oral feeding. It is important to continue IV insulin infusion for 2 h after starting the subcutaneous insulin regimen to ensure adequate blood insulin levels and to prevent the recurrence of hyperglycemia and ketoacidosis.[13] The decision on the doses of subcutaneous insulin will depend on the prior presence of diabetes and its control and whether there is a new diagnosis of diabetes. Patients with a previous diagnosis of diabetes can be resumed on their same insulin doses before the onset of DKA, if glucose control was previously satisfactory. If glucose control was not adequate prior to the episode of DKA, insulin regimen and doses should be revised and modified.
In patients with newly diagnosed diabetes, basal–bolus analog insulin regimens using basal (glargine, detemir or degludec) and rapid-acting insulin (lispro, aspart or glulisine) insulin analogs are recommended.[63,64] The usual initial doses are at 0.5 units/kg body weight/day with half the dose prescribed as basal insulin and the other half as bolus (meal) insulin divided between three daily meals. Basal insulin is prescribed initially once daily, usually at bedtime, while the doses of meal insulin are divided according to the size of meals, for example, 50% of total dose at the main meal and 25% with each of the other two meals. In case of issues with the availability or cost of basal–bolus analogs, an alternative regimen of conventional insulin including Neutral Protamine Hagedorn (NPH) insulin twice daily with three daily doses of regular insulin can be used; the starting dose is the same at 0.5 units/kg/day with two-third of the dose given as NPH insulin, which is further divided as two-third in the morning and one-third in the evening, while the remaining one-third is given as regular insulin and is distributed over the three main meals, as done with the meal insulin analog.
In a prospective randomized trial that included patients in whom DKA had resolved and compared the treatment regimens of basal–bolus (glargine once daily and glulisine before meals) and the split-mixed regimen of NPH plus regular insulin twice daily, no difference was found in the rates of glucose control between both regimens, but treatment with basal-bolus insulin was associated with a lower risk of hypoglycemia.[57] Therefore, the choice of subcutaneous insulin regimen should consider the patient's characteristics, risk and frequency of hypoglycemia as well as the cost and availability of medicine. There is no difference in glucose control between basal–bolus analog and conventional insulin regimens. The use of basal–bolus analog program is preferred, as it closely resembles normal physiologic insulin secretion and is associated with less hypoglycemia. In addition, rapid-acting insulin analogs have an advantage over regular insulin in that they can be injected immediately after meals. Conventional insulin regimens are generally reserved for cases when basal–bolus insulin analogs are not available or cannot be afforded.
PREVENTION OF DIABETIC KETOACIDOSIS
Prevention of future incidents of DKA and forthcoming admissions is a very important component of its management.[65] An essential factor in planning the prevention of DKA is the recognition of the precipitating cause. Poor adherence to insulin therapy was found to be a major causative factor for hospital admissions for DKA.[66,67,68,69] Omission of insulin has been found to be associated with lack of patient education, limited access to health care, economic limitations, underlying psychiatric conditions and eating disorders.[66,67,68,69,70] In addition, social and psychiatric factors such as depression, eating disorders, low socioeconomic status and sexual or physical abuse have been implicated in the occurrence of recurrent admissions for DKA.[69,71,72] Another factor associated with recurrent hospital admissions for DKA is substance use, particularly cocaine.[73,74]
Patient education is a critical part of the prevention of future hospital admissions for DKA. Educational programs should include guidelines on the management of diabetes during periods of illness (sick day management). These programs should include clear information on (1) the importance of continuing insulin, (2) early recognition of the manifestations of DKA, (3) more frequent home blood glucose and ketone (urine or blood) monitoring, (4) adjusting doses of insulin and the use of supplemental insulin, as needed and (5) instances when the health-care provider should be contacted.[13,37,75,76] Self-monitoring of blood ketones, when compared with urine ketone testing, facilitates earlier identification and treatment of ketosis, and can decrease diabetes-related emergency department visits and hospitalizations.[77,78] The frequency of recurrence of DKA can be reduced with structured patient education, behavioral intervention, providing support for patients and families, improving patients' access to medical providers, availability of extended access to telephone services and telemedicine.[79,80,81,82,83,84] In addition, public awareness campaigns focusing on education on the early signs of diabetes have been found to significantly reduce the frequency of DKA in patients with new onset diabetes.[85,86]
CONCLUSION
DKA continues to be an important cause of hospital admissions and mortality among patients with diabetes. Infections and nonadherence to insulin therapy remain the most common causes of DKA. Proper management of DKA includes prompt initiation of IV fluids, insulin therapy, electrolytes replacement and recognition and treatment of precipitating causes. Close monitoring of patient's condition by regular clinical and laboratory data and the use of management protocols help ensure better outcomes. Prevention of DKA through structured educational programs and identification of risk factors for recurrence should be part of the patient's care plan.
Peer review
This article was peer-reviewed by three independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with human development index (HDI): An updated systematic review, meta-analysis, and meta-regression. Horm Metab Res. 2018;50:209–22. doi: 10.1055/s-0044-102090. [DOI] [PubMed] [Google Scholar]
- 2.Fredheim S, Johannesen J, Johansen A, Lyngsøe L, Rida H, Andersen ML, et al. Danish Society for Diabetes in Childhood and Adolescence. Diabetic ketoacidosis at the onset of type 1 diabetes is associated with future HbA1c levels. Diabetologia. 2013;56:995–1003. doi: 10.1007/s00125-013-2850-z. [DOI] [PubMed] [Google Scholar]
- 3.Shaltout AA, Channanath AM, Thanaraj TA, Omar D, Abdulrasoul M, Zanaty N, et al. Ketoacidosis at first presentation of type 1 diabetes mellitus among children: A study from Kuwait. Sci Rep. 2016;6:27519. doi: 10.1038/srep27519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson ME, Li P, Rahme E, Simard M, Larocque I, Nakhla MM. Increasing prevalence of diabetic ketoacidosis at diabetes diagnosis among children in Quebec: A population-based retrospective cohort study. CMAJ Open. 2019;7:E300–5. doi: 10.9778/cmajo.20190047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manuwald U, Schoffer O, Hegewald J, Große J, Kugler J, Kapellen TM, et al. Ketoacidosis at onset of type 1 diabetes in children up to 14 years of age and the changes over a period of 18 years in Saxony, Eastern-Germany: A population based register study. PLoS One. 2019;14:e0218807. doi: 10.1371/journal.pone.0218807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punnose J, Agarwal MM, El Khadir A, Devadas K, Mugamer IT. Childhood and adolescent diabetes mellitus in Arabs residing in the United Arab Emirates. Diabetes Res Clin Pract. 2002;55:29–33. doi: 10.1016/s0168-8227(01)00267-4. [DOI] [PubMed] [Google Scholar]
- 7.Abduljabbar MA, Aljubeh JM, Amalraj A, Cherian MP. Incidence trends of childhood type 1 diabetes in eastern Saudi Arabia. Saudi Med J. 2010;31:413–8. [PubMed] [Google Scholar]
- 8.Habib HS. Frequency and clinical characteristics of ketoacidosis at onset of childhood type 1 diabetes mellitus in Northwest Saudi Arabia. Saudi Med J. 2005;26:1936–9. [PubMed] [Google Scholar]
- 9.Salman H, Abanamy A, Ghassan B, Khalil M. Insulin-dependent diabetes mellitus in children: Familial and clinical patterns in Riyadh. Ann Saudi Med. 1991;11:302–6. doi: 10.5144/0256-4947.1991.302. [DOI] [PubMed] [Google Scholar]
- 10.Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality — United States, 200-2014. MMWR Morb Mortal Wkly Rep. 2018;67:362–5. doi: 10.15585/mmwr.mm6712a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998-2013: A retrospective cohort study. Diabetes Care. 2018;41:1870–7. doi: 10.2337/dc17-1583. [DOI] [PubMed] [Google Scholar]
- 12.Butalia S, Johnson JA, Ghali WA, Rabi DM. Clinical and sociodemographic factors associated with diabetic ketoacidosis hospitalization in adults with type 1 diabetes. Diabet Med. 2013;30:567–73. doi: 10.1111/dme.12127. [DOI] [PubMed] [Google Scholar]
- 13.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–43. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J. 1922;12:141–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinowitch IM. Diabetic coma and diabetic mortality rates. Can Med Assoc J. 1929;21:583–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Root HF. The use of insulin and the abuse of glucose in the treatment of diabetic coma. JAMA. 1945;127:557–64. [Google Scholar]
- 17.Felig P. Diabetic ketoacidosis. N Engl J Med. 1974;290:1360–3. doi: 10.1056/NEJM197406132902405. [DOI] [PubMed] [Google Scholar]
- 18.Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia. 2016;59:2082–7. doi: 10.1007/s00125-016-4034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ndebele NFM, Naidoo M. The management of diabetic ketoacidosis at a rural regional hospital in KwaZulu-Natal. Afr J Prim Health Care Fam Med. 2018;10:e1–e6. doi: 10.4102/phcfm.v10i1.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal A, Yadav A, Gutch M, Consul S, Kumar S, Prakash V, et al. Prognostic factors in patients hospitalized with diabetic ketoacidosis. Endocrinol Metab (Seoul) 2016;31:424–32. doi: 10.3803/EnM.2016.31.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmehdawi RR, Elmagerhei HM. Profile of diabetic ketoacidosis at a teaching hospital in Benghazi, Libyan Arab Jamahiriya. East Mediterr Health J. 2010;16:292–9. [PubMed] [Google Scholar]
- 22.Mbugua PK, Otieno CF, Kayima JK, Amayo AA, McLigeyo SO. Diabetic ketoacidosis: Clinical presentation and precipitating factors at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005;82:S191–6. doi: 10.4314/eamj.v82i12.9381. [DOI] [PubMed] [Google Scholar]
- 23.Vellanki P, Umpierrez GE. Diabetic ketoacidosis: A common debut of diabetes among African Americans with type 2 diabetes. Endocr Pract. 2017;23:971–8. doi: 10.4158/EP161679.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seok H, Jung CH, Kim SW, Lee MJ, Lee WJ, Kim JH, et al. Clinical characteristics and insulin independence of Koreans with new-onset type 2 diabetes presenting with diabetic ketoacidosis. Diabetes Metab Res Rev. 2013;29:507–13. doi: 10.1002/dmrr.2421. [DOI] [PubMed] [Google Scholar]
- 25.Sapru A, Gitelman SE, Bhatia S, Dubin RF, Newman TB, Flori H. Prevalence and characteristics of type 2 diabetes mellitus in 9-18 year-old children with diabetic ketoacidosis. J Pediatr Endocrinol Metab. 2005;18:865–72. doi: 10.1515/jpem.2005.18.9.865. [DOI] [PubMed] [Google Scholar]
- 26.Dhatariya KK, Skedgel C, Fordham R. The cost of treating diabetic ketoacidosis in the UK: A national survey of hospital resource use. Diabet Med. 2017;34:1361–6. doi: 10.1111/dme.13427. [DOI] [PubMed] [Google Scholar]
- 27.Umpierrez G, Freire AX. Abdominal pain in patients with hyperglycemic crises. J Crit Care. 2002;17:63–7. doi: 10.1053/jcrc.2002.33030. [DOI] [PubMed] [Google Scholar]
- 28.Fayfman M, Pasquel FJ, Umpierrez GE. Management of hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101:587–606. doi: 10.1016/j.mcna.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YG, Jeon JY, Han SJ, Kim DJ, Lee KW, Kim HJ. Sodium-glucose co-transporter-2 inhibitors and the risk of ketoacidosis in patients with type 2 diabetes mellitus: A nationwide population-based cohort study. Diabetes Obes Metab. 2018;20:1852–8. doi: 10.1111/dom.13297. [DOI] [PubMed] [Google Scholar]
- 30.Tang H, Li D, Wang T, Zhai S, Song Y. Effect of sodium-glucose cotransporter 2 inhibitors on diabetic ketoacidosis among patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Care. 2016;39:e123–4. doi: 10.2337/dc16-0885. [DOI] [PubMed] [Google Scholar]
- 31.Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis: A review. Curr Diabetes Rev. 2017;13:315–21. doi: 10.2174/1573399812666160421121307. [DOI] [PubMed] [Google Scholar]
- 32.Kuru B, Sever M, Aksay E, Dogan T, Yalcin N, Eren ES, et al. Comparing finger-stick β-hydroxybutyrate with dipstick urine tests in the detection of ketone bodies. Turk J Emerg Med. 2014;14:47–52. doi: 10.5505/1304.7361.2014.14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooke J, Stiell M, Ojo O. Evaluation of the accuracy of capillary hydroxybutyrate measurement compared with other measurements in the diagnosis of diabetic ketoacidosis: A systematic review. Int J Environ Res Public Health. 2016;13:837. doi: 10.3390/ijerph13090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrington WG, Nye HJ, Hammersley MS, Watkinson PJ. Are arterial and venous samples clinically equivalent for the estimation of pH, serum bicarbonate and potassium concentration in critically ill patients? Diabet Med. 2012;29:32–5. doi: 10.1111/j.1464-5491.2011.03390.x. [DOI] [PubMed] [Google Scholar]
- 35.Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569–71. doi: 10.1136/emj.2007.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med. 1998;31:459–65. doi: 10.1016/s0196-0644(98)70254-9. [DOI] [PubMed] [Google Scholar]
- 37.Goguen J, Gilbert J. Hyperglycemic emergencies in adults. Diabetes Canada Clinical Practice Guidelines Expert Committee. Can J Diabetes. 2018;42:S109–14. doi: 10.1016/j.jcjd.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Wu HF, Ma SG, Bai F, Hu W, Jin Y, et al. Correlation between peripheral white blood cell counts and hyperglycemic emergencies. Int J Med Sci. 2013;10:758–65. doi: 10.7150/ijms.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases. 2014;2:488–96. doi: 10.12998/wjcc.v2.i10.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyenwe EA, Kitabchi AE. Evidence-based management of hyperglycemic emergencies in diabetes mellitus. Diabetes Res Clin Pract. 2011;94:340–51. doi: 10.1016/j.diabres.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet Med. 2011;28:508–15. doi: 10.1111/j.1464-5491.2011.03246.x. [DOI] [PubMed] [Google Scholar]
- 42.Gamba G, Oseguera J, Castrejón M, Gómez-Pérez FJ. Bicarbonate therapy in severe diabetic ketoacidosis. A double blind, randomized, placebo controlled trial. Rev Invest Clin. 1991;43:234–8. [PubMed] [Google Scholar]
- 43.Morris LR, Murphy MB, Kitabchi AE. Bicarbonate therapy in severe diabetic ketoacidosis. Ann Intern Med. 1986;105:836–40. doi: 10.7326/0003-4819-105-6-836. [DOI] [PubMed] [Google Scholar]
- 44.Hale PJ, Crase J, Nattrass M. Metabolic effects of bicarbonate in the treatment of diabetic ketoacidosis. Br Med J (Clin Res Ed) 1984;289:1035–8. doi: 10.1136/bmj.289.6451.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155–77. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 46.Chua HR, Schneider A, Bellomo R. Bicarbonate in diabetic ketoacidosis-a systematic review. Ann Intensive Care. 2011;1:23. doi: 10.1186/2110-5820-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luzi L, Barrett EJ, Groop LC, Ferrannini E, DeFronzo RA. Metabolic effects of low-dose insulin therapy on glucose metabolism in diabetic ketoacidosis. Diabetes. 1988;37:1470–7. doi: 10.2337/diab.37.11.1470. [DOI] [PubMed] [Google Scholar]
- 48.Page MM, Alberti KG, Greenwood R, Gumaa KA, Hockaday TD, Lowy C, et al. Treatment of diabetic coma with continuous low-dose infusion of insulin. Br Med J. 1974;2:687–90. doi: 10.1136/bmj.2.5921.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a priming dose of insulin necessary in a low-dose insulin protocol for the treatment of diabetic ketoacidosis? Diabetes Care. 2008;31:2081–5. doi: 10.2337/dc08-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goyal N, Miller JB, Sankey SS, Mossallam U. Utility of initial bolus insulin in the treatment of diabetic ketoacidosis. J Emerg Med. 2010;38:422–7. doi: 10.1016/j.jemermed.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 51.Hoorn EJ, Carlotti AP, Costa LA, MacMahon B, Bohn G, Zietse R, et al. Preventing a drop in effective plasma osmolality to minimize the likelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr. 2007;150:467–73. doi: 10.1016/j.jpeds.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 52.Diabetes Canada Clinical Practice Guidelines Expert Committee. Wherrett DK, Ho J, Huot C, Legault L, Nakhla M, et al. Type 1 Diabetes in Children and Adolescents. Can J Diabetes. 2018;42(Suppl 1):S234–46. doi: 10.1016/j.jcjd.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Kitabchi AE, Umpierrez GE, Fisher JN, Murphy MB, Stentz FB. Thirty years of personal experience in hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93:1541–52. doi: 10.1210/jc.2007-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umpierrez GE, Latif K, Stoever J, Cuervo R, Park L, Freire AX, et al. Efficacy of subcutaneous insulin lispro versus continuous intravenous regular insulin for the treatment of patients with diabetic ketoacidosis. Am J Med. 2004;117:291–6. doi: 10.1016/j.amjmed.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, Kitabchi AE. Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care. 2004;27:1873–8. doi: 10.2337/diacare.27.8.1873. [DOI] [PubMed] [Google Scholar]
- 56.Ersöz HO, Ukinc K, Köse M, Erem C, Gunduz A, Hacihasanoglu AB, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006;60:429–33. doi: 10.1111/j.1368-5031.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 57.Umpierrez GE, Jones S, Smiley D, Mulligan P, Keyler T, Temponi A, et al. Insulin analogs versus human insulin in the treatment of patients with diabetic ketoacidosis: A randomized controlled trial. Diabetes Care. 2009;32:1164–9. doi: 10.2337/dc09-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly AM. The case for venous rather than arterial blood gases in diabetic ketoacidosis. Emerg Med Australas. 2006;18:64–7. doi: 10.1111/j.1742-6723.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 59.Middleton P, Kelly AM, Brown J, Robertson M. Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J. 2006;23:622–4. doi: 10.1136/emj.2006.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreshak A, Chen EH. Arterial blood gas analysis: Are its values needed for the management of diabetic ketoacidosis? Ann Emerg Med. 2005;45:550–1. doi: 10.1016/j.annemergmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Klocker AA, Phelan H, Twigg SM, Craig ME. Blood ß-hydroxybutyrate vs. urine acetoacetate testing for the prevention and management of ketoacidosis in Type 1 diabetes: A systematic review. Diabetic Med. 2013;30:818–24. doi: 10.1111/dme.12136. [DOI] [PubMed] [Google Scholar]
- 62.Vanelli M, Chiari G, Capuamo C, Iovane B, Bernardini A, Giacalone T. The direct measurement of 3- beta-hydroxybutyrate enhances the management of diabetic ketoacidosis in children and reduces time and costs of treatment. Diabetes Nutr Metab. 2003;16:3126. [PubMed] [Google Scholar]
- 63.American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S98–110. doi: 10.2337/dc20-S009. [DOI] [PubMed] [Google Scholar]
- 64.Diabetes Canada Clinical Practice Guidelines Expert Committee. McGibbon A, Adams L, Ingersoll K, Kader T, Tugwell B. Glycemic management in adults with type 1 diabetes. Can J Diabetes. 2018;42(Suppl 1):S80–7. doi: 10.1016/j.jcjd.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Eledrisi MS, Alshanti MS, Shah MF, Brolosy B, Jaha N. Overview of the diagnosis and management of diabetic ketoacidosis. Am J Med Sci. 2006;331:243–51. doi: 10.1097/00000441-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Randall L, Begovic J, Hudson M, Smiley D, Peng L, Pitre N, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: Behavioral, socioeconomic, and psychosocial factors. Diabetes Care. 2011;34:1891–6. doi: 10.2337/dc11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lohiya S, Kreisberg R, Lohiya V. Recurrent diabetic ketoacidosis in two community teaching hospitals. Endocr Pract. 2013;19:829–33. doi: 10.4158/EP13057.RA. [DOI] [PubMed] [Google Scholar]
- 68.Musey VC, Lee JK, Crawford R, Klatka MA, McAdams D, Phillips LS. Diabetes in urban African-Americans I Cessation of insulin therapy is the major precipitating cause of diabetic ketoacidosis. Diabetes Care. 1995;18:483–9. doi: 10.2337/diacare.18.4.483. [DOI] [PubMed] [Google Scholar]
- 69.Skinner TC. Recurrent diabetic ketoacidosis: Causes, prevention and management. Horm Res. 2002;57(Suppl 1):78–80. doi: 10.1159/000053320. [DOI] [PubMed] [Google Scholar]
- 70.Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF. Insulin omission in women with IDDM. Diabetes Care. 1994;17:1178–85. doi: 10.2337/diacare.17.10.1178. [DOI] [PubMed] [Google Scholar]
- 71.Wagner DV, Stoeckel M, E Tudor M, Harris MA. Treating the most vulnerable and costly in diabetes. Curr Diab Rep. 2015;15:606. doi: 10.1007/s11892-015-0606-5. [DOI] [PubMed] [Google Scholar]
- 72.Everett E, Mathioudakis NN. Association of socioeconomic status and DKA readmission in adults with type 1 diabetes: Analysis of the US National Readmission Database. BMJ Open Diabetes Res Care. 2019;7:e000621. doi: 10.1136/bmjdrc-2018-000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nyenwe EA, Loganathan RS, Blum S, Ezuteh DO, Erani DM, Wan JY, et al. Active use of cocaine: An independent risk factor for recurrent diabetic ketoacidosis in a city hospital. Endocr Pract. 2007;13:22–9. doi: 10.4158/EP.13.1.22. [DOI] [PubMed] [Google Scholar]
- 74.Warner EA, Greene GS, Buchsbaum MS, Cooper DS, Robinson BE. Diabetic ketoacidosis associated with cocaine use. Arch Intern Med. 1998;158:1799–802. doi: 10.1001/archinte.158.16.1799. [DOI] [PubMed] [Google Scholar]
- 75.Brink S, Joel D, Laffel L, Lee WW, Olsen B, Phelan H, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Sick day management in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(Suppl 20):193–202. doi: 10.1111/pedi.12193. [DOI] [PubMed] [Google Scholar]
- 76.Laffel L. Sick-day management in type 1 diabetes. Endocrinol Metab Clin North Am. 2000;29:707–23. doi: 10.1016/s0889-8529(05)70160-2. [DOI] [PubMed] [Google Scholar]
- 77.Laffel LM, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: A randomized clinical trial. Diabet Med. 2006;23:278–84. doi: 10.1111/j.1464-5491.2005.01771.x. [DOI] [PubMed] [Google Scholar]
- 78.Weber C, Kocher S, Neeser K, Joshi SR. Prevention of diabetic ketoacidosis and self-monitoring of ketone bodies: An overview. Curr Med Res Opin. 2009;25:1197–207. doi: 10.1185/03007990902863105. [DOI] [PubMed] [Google Scholar]
- 79.Deeb A, Yousef H, Abdelrahman L, Tomy M, Suliman S, Attia S, et al. Implementation of a diabetes educator care model to reduce paediatric admission for diabetic ketoacidosis. J Diabetes Res. 2016;2016:3917806. doi: 10.1155/2016/3917806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellis D, Naar-King S, Templin T, Frey M, Cunningham P, Sheidow A, et al. Multisystemic therapy for adolescents with poorly controlled type 1 diabetes: Reduced diabetic ketoacidosis admissions and related costs over 24 months. Diabetes Care. 2008;31:1746–7. doi: 10.2337/dc07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drozda DJ, Dawson VA, Long DJ, Freson LS, Sperling MA. Assessment of the effect of a comprehensive diabetes management program on hospital admission rates of children with diabetes mellitus. Diabetes Educ. 1990;16:389–93. doi: 10.1177/014572179001600511. [DOI] [PubMed] [Google Scholar]
- 82.Burns K, Farrell K, Myszka R, Park K, Holmes-Walker DJ. Access to a youth-specific service for young adults with type 1 diabetes mellitus is associated with decreased hospital length of stay for diabetic ketoacidosis. Intern Med J. 2018;48:396–402. doi: 10.1111/imj.13649. [DOI] [PubMed] [Google Scholar]
- 83.Chiari G, Ghidini B, Vanelli M. Effectiveness of a toll-free telephone hotline for children and adolescents with type 1 diabetes. A 5-year study. Acta Biomed. 2003;74(Suppl 1):45–8. [PubMed] [Google Scholar]
- 84.Wagner DV, Barry S, Teplitsky L, Sheffield A, Stoeckel M, Ogden JD, et al. Texting Adolescents in Repeat DKA and Their Caregivers. J Diabetes Sci Technol. 2016;10:831–9. doi: 10.1177/1932296816639610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.King BR, Howard NJ, Verge CF, Jack MM, Govind N, Jameson K, et al. A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes. 2012;13:647–51. doi: 10.1111/j.1399-5448.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 86.Cangelosi AM, Bonacini I, Serra RP, Di Mauro D, Iovane B, Fainardi V, et al. Spontaneous dissemination in neighboring provinces of DKA prevention campaign successfully launched in nineties in Parma's Province. Acta Biomed. 2017;88:151–5. doi: 10.23750/abm.v88i2.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]