Abstract
To eliminate schistosomiasis, appropriate diagnostic tests are required to monitor its prevalence and transmission, especially in the settings with low endemicity resulting from the consecutive mass drug administration. Antibodies that react with either crude soluble schistosome egg antigens or soluble worm antigen preparations have been used to monitor infection in low-prevalence regions. However, these detection methods cannot discriminate current and past infections and are cross-reactive with other parasites because both antigens contain numerous proteins and glycans from schistosomes, and standard preparations need maintenance of the life cycle of the schistosome. To evaluate the potential utility of nine recombinant Schistosoma mansoni proteins as single defined antigens for serological diagnosis, we monitored the kinetics of antibodies to each antigen during S. mansoni infection in mice before and after the treatment with praziquantel. C57BL/6 mice were infected with 50 cercariae. The levels of immunoglobulin G (IgG) raised against five recombinant antigens (RP26, sm31, sm32, GST, and LAP1) significantly increased as early as 2–4 weeks after infection and rapidly declined by 2 weeks after the treatment, whereas those raised against crude S. mansoni egg antigens or other antigens remained elevated long after the treatment. The IgG1 raised against RP26, sm31, and serpin decreased after the treatment with praziquantel, whereas the IgE raised against serpin declined strikingly after the treatment. This study clarifies the dynamics of the serological responses to recombinant S. mansoni proteins during infection and after the treatment with praziquantel and identifies several candidate antigens with potential utility in the monitoring and surveillance of schistosomiasis toward the elimination of schistosomiasis.
Author summary
Schistosomiasis is the second most important human parasitic disease in tropical and subtropical areas: estimates show that at least 218 million people required treatment in 2015 and remains a major Neglected Tropical Disease impacting the health of the poorest populations. The global strategy for schistosomiasis control is focused on eliminating disease through periodic, large-scale population treatment with praziquantel (PZQ). With the progress towards the control and the elimination of schistosomiasis by mass drug administration (MDA) with PZQ, more sensitive diagnostics that can monitor the dynamics of schistosomiasis transmission are required to instruct MDA programs and assess reinfection. Detecting antibodies react with either crude soluble schistosome egg antigens or soluble worm antigen preparations have been used to monitor infection in low-prevalence settings. However, these detection methods cannot discriminate current and past infections and are cross-reactive with other parasites because it contains numerous proteins and glycans from schistosomes, and standard preparations need maintenance of the life cycle of the schistosome. This study clarified that the dynamics of the serological responses to defined recombinant proteins during S. mansoni infection in mice before and after the treatment with PZQ and identifies several candidate antigens with potential utility in the monitoring and surveillance of schistosomiasis toward the elimination of schistosomiasis.
Introduction
Schistosomiasis, a chronic helminthic disease, is the second most devastating tropical parasitic disease after malaria. It is a major cause of morbidity and mortality and remains a public health concern affecting the poorest populations in developing countries [1]. Epidemiological studies have estimated that more than 218 million people throughout the world are infected with schistosomes and more than 250,000 deaths per year are related to the disease and its subsequent complications [2]. Schistosoma mansoni is one of the major species of the genus Schistosoma that infect humans and is widely spread in sub-Saharan Africa, some parts of the Middle East, Central and South Americas, and some West Indian islands [3, 4]. The pathogenesis of the disease is associated with the location of the adult worms in the blood vessels of the infected host, where adult females release eggs during their long lifespan. These eggs are trapped in host tissues, such as the liver, intestine, and pelvic venous plexus, causing inflammation and pathogenicity [4, 5]. The host immune responses to these eggs lead to the formation of granulomas around the eggs and collagen deposition, with consequent liver damage [6].
The current strategy to control and eliminate schistosomiasis relies on mass drug administration (MDA) using praziquantel (PZQ) in endemic areas. However, to guide control programs and to facilitate post-elimination surveillance, novel sensitive, affordable, and user-friendly diagnostics are urgently required. The envisioned test(s) should be able to discriminate between current and previous schistosome infections in low-intensity settings and/or to detect very light infections in near-elimination settings. The Kato–Katz (KK) thick smear method is the traditional method for detecting helminthic eggs, including schistosome, in stools [7–9]. This method is simple and can differentiate Schistosoma species with high specificity, but lacks sensitivity in geographic regions with low disease prevalence and requires trained technicians, microscopy, and electricity [10]. The KK test also does not detect premature infection because it takes 4–6 weeks for schistosomula to develop into adults and start laying eggs [11–13]. To overcome the limitations of this method, the simultaneous application of different diagnostic tests has been used to monitor the transmission status of schistosomiasis. Serological methods that detect antibodies against crude antigens prepared from schistosomes have been included to improve the sensitivity of screening possibly infected individuals and to monitor the transmission status of the organism. Soluble egg antigens (SEA) and soluble worm antigen preparations (SWAP) have been studied and used for this purpose [14]. Because both SEA and SWAP are crude antigens containing numerous proteins and glycans derived from schistosomes, the detection method has the disadvantages of cross-reactivity with other parasites, its inability to discriminate between current and past infections, and the difficulty of formulating the standard preparations because the whole life cycle of schistosomes must be maintained using both its final hosts and intermediate host snails [15]. Because the eggs remain in the host after the treatment with PZQ and continue to stimulate the host’s immune responses to the egg-derived antigens, antibodies directed against the crude antigens are maintained at high levels long after the treatment, and 1–2 years may be required for them to decline to the basal levels after the treatment [16]. Currently, a point-of-care test to detect urinary circulating cathodic antigen (POC-CCA) is available for monitoring S. mansoni infections [17]. This method has many advantages, including its ability to discriminate current and past infections because it detects circulating antigens in the urine produced by the living adult worm, whereas a serological test is expected to show superior sensitivity in low-intensity and post-elimination contexts [18, 19].
Therefore, there is a critical need for novel sensitive diagnostics that can guide schistosomiasis control programs and facilitate its post-elimination surveillance. The detection of antibodies against recombinant antigens may provide an alternative method that can discriminate between current and previous schistosome infections in low-intensity settings and detect very light infections in near-elimination settings. Antibody levels have been used to detect schistosome infection and to assess the elimination of these infections in some countries [20]. The limitations of this approach are attributable to the persistence of these antibodies after cure, so identifying particular candidate antigens that induce sharp short-lived antibody responses would overcome this limitation [20]. From the various antigens of S. mansoni, a panel of nine previously reported antigens was selected to examine the dynamics of the serological responses to recombinant S. mansoni proteins [21]: RP26 (sm22.3, LGG) [22], cathepsin B (sm31) [23, 24], hemoglobinase (sm32) [24], serine protease inhibitor (serpin) [25], filamin [26–28], tropomyosin [29], glutathione S-transferase (GST) [30, 31], leucine aminopeptidase 1 (LAP1), and leucine aminopeptidase 2 (LAP2) [32]. The dynamics of immunoglobulin G4 (IgG4) and IgE raised against these recombinant antigens may provide useful information. In this study, we tested the potential utility of these S. mansoni antigens, by assessing the kinetics of specific Ig levels in sera, to monitor the status of S. mansoni infection after the treatment with praziquantel (PZQ).
Materials and methods
Ethics statement
The study protocol was approved by the Committee for Ethics on Animal Experiments (approval number 1505181226) in Nagasaki University. All of the studies were conducted under the guidelines for animal experiments, Nagasaki University and according to Japanese law for Humane Treatment and Management of Animals (Law No. 105 dated 19 October 1973 modified on 2 June 2006).
Parasite
A Puerto Rican strain of S. mansoni was used in this study. The life cycle of this parasite is maintained at the Animal Facility of Nagasaki University, with the successive passage of the parasite through Biomphalaria glabrata snails and ICR mice or jirds. ICR mice were percutaneously infected with 250 cercariae and killed 7 weeks after infection, and the adult worms were collected from their portal veins with intracardiac phosphate-buffered saline (PBS). Eggs were isolated with PBS from the livers of the infected mice, as previously described [33].
Animals, infection, and treatment
Seven-week-old female C57BL/6 mice were purchased from SLC (Shizuoka, Japan) and housed at the Animal Facility of Nagasaki University. The mice were kept under environmentally controlled, specific-pathogen-free conditions, with free access to food and water. Animal housing, handling, and feeding complied with the recommendations of Nagasaki University. All experiments were approved by the Ethical Review Committee of the Institute of Tropical Medicine (NEKKEN), Nagasaki University, and were conducted according to the Animal Facility guidelines. The mice were divided into two experimental groups: the infected untreated group and the infected treated group. Each group contained 7–8 mice, and each mouse was percutaneously infected in the inguinal area with 50 S. mansoni cercariae. In the treated group, the mice were treated at 8 weeks post-infection with PZQ (Sigma, St. Louis, MO) at a dose of 500 mg/kg body weight for two consecutive days [33]. The drug was dissolved in distilled water, and 230 μl of the solution was administered orally by gavage. The mice were killed at the end of the experiment, and their livers and intestines were analyzed for the presence of eggs. Adult worms were also collected with perfusion of the portal vein to confirm infection and were counted to measure the worm burden. The effect of treatment was assessed by the improvement in symptoms such as anemia and the mobility of the mice and the reduction in ascites. One mouse was sacrificed 1 week after treatment (9 weeks post-infection), and seven mice were sacrificed at 18 weeks post-infection to confirm the absence of adult worms in the portal vein with perfusion, to confirm the efficacy of treatment. Five uninfected untreated mice were used as negative controls.
Collection of sera from infected mice
Blood samples were collected biweekly from all mice in each group via the tail until 18 weeks post-infection. The blood samples were allowed to clot at room temperature for 2 h before centrifugation at 1,000 × g for 10 min at 4°C. The serum samples were collected into Eppendorf tubes and stored at −30°C until analysis. The sera collected from three uninfected mice were used as the negative controls.
Preparation of crude SWAP and SEA and nine recombinant proteins
SWAP
Adult worms were harvested from mice infected with S. mansoni cercariae at 7 weeks post-infection by portal vein perfusion. The worms were washed three times with PBS, homogenized with a mechanical grinder, and centrifuged at 10,000 × g for 1 h at 4°C. The supernatant was collected and filtered through a 0.22 μm mesh. The protein concentration was measured with the Bradford method (Bio-Rad Laboratories, Hercules, CA) and the protein was then stored as aliquots at −30°C.
SEA
SEA was prepared as previously reported [34]. Briefly, purified eggs from the livers of infected ICR mice were resuspended in 5 ml of cold PBS at a concentration of 100,000 eggs/ml. The suspended eggs were sonicated for 15 min on ice (to prevent heating) until more than 95% were destroyed. The homogenized eggs were centrifuged at 2,000 × g for 20 min at 4°C. The supernatant was collected in a sterile tube and then ultracentrifuged at 100,000 × g for 1 h at 4°C. The supernatant was filtered, and the protein concentration was determined as for SWAP. SEA was as stored in aliquots at −30°C until use.
Recombinant proteins
Nine recombinant S. mansoni antigens, sm31, sm32, RP26, serpin, filamin, tropomyosin, GST, LAP1, and LAP2, were prepared as described elsewhere [21].
Enzyme-linked immunosorbent assays (ELISAs) of serum antibodies
ELISAs were performed with different recombinant S. mansoni antigens as described elsewhere [21, 34], including sm31, sm32, RP26, serpin, filamin, tropomyosin, GST, LAP1, and LAP2. The SWAP and SEA crude antigens were also compared with the recombinant antigens of interest. For each recombinant antigen, Nunc MaxiSorp 96-well microtiter plates (Thermo Fisher Scientific, Waltham, MA) were coated overnight with 50 μl of antigen solution (5 μg/ml) per well (in PBS). For SWAP and SEA, the concentrations of the crude antigens were optimized to 200 ng/ml in a total volume of 50 μl. The plates were washed three times with PBS containing 0.05% Tween 20 (PBST) at room temperature and then blocked for 2 h with blocking buffer (1% bovine serum albumin [BSA; Sigma] in PBS) at room temperature. The plate wells were then washed with PBST, filled with mouse sera diluted 100 times with PBST containing 0.1% BSA, and incubated at room temperature for 2 h. After that plates had been washed as described above, they were incubated for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (R&D Systems, Minneapolis, MN) diluted 1:1000 in PBST containing 0.1% BSA. The plates were washed, and the colorimetric substrate tetramethylbenzidine (BD Pharmingen, Allschwil, Switzerland) was added. The absorbance of the wells was measured at 450 nm with a Multiskan FC Microplate Photometer (Thermo Scientific). All serum samples were assessed in duplicate. The pre-infection sera taken from the same animals were used as negative control and also assessed for reactivity against each antigen to calculate the cut-off values.
Specific IgG1 and IgE antibodies were detected with sera from the infected mice (diluted 50-fold) collected biweekly, with an HRP-conjugated rat anti-mouse-IgE antibody (AbD Serotec, Bio-Rad) or HRP-conjugated rat anti-mouse-IgG1 antibody (Invitrogen) diluted 1:1000. The remaining ELISA steps were as described for the detection of total IgG.
Parasitological examination
At the end of our experiment at 18 weeks post-infection, we killed all the mice in each group to check for the presence of adult worms, using portal vein perfusion, to assess the efficacy of the PZQ treatment.
Statistical analysis
The statistical software GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA) and Excel were used for all statistical analyses and the generation of graphs. The ELISA cut-off values for the infected mice were estimated as the mean plus three standard deviations (mean + 3SD) of the optical density (OD) of the control group sera for each antigen. OD readings equal to or less than the cut-off value were considered negative, whereas those readings greater than the cut-off value were considered positive. ANOVA and a t-test were used for normally distributed samples, and the Mann–Whitney test was used for the other non-normally distributed samples. When comparing the differences between groups, p < 0.05 was considered statistically significant.
Results
Temporal dynamics of IgG raised against recombinant antigens during S. mansoni infection
Sera were collected from naïve and infected mice and measured for the total IgG raised against SWAP, SEA, or the nine recombinant S. mansoni antigens with ELISA. Most IgG raised against the recombinant antigens was first detectable in the sera at 4 weeks post-infection, as was the IgG raised against SWAP and SEA, whereas the IgG raised against tropomyosin was detected in the sera from 2 weeks post-infection, earlier than the other antigens (Figs 1 and 2). The IgG raised against RP26 and sm31 rapidly and dramatically increased, peaking at 6 weeks post-infection (Fig 1A and 1B). The IgG raised against sm32, GST, LAP1, filamin, LAP2, and tropomyosin peaked at 10–12 weeks post-infection, and then remained unchanged or decreased (Figs 1C–1E, 2A, 2B and 2D). The IgG raised against serpin gradually and consistently increased during the infection, showing a similar pattern to the kinetics of IgG raised against both SWAP and SEA (Fig 2C, 2E and 2F). The levels of IgG raised against RP26, sm31, sm32, LAP1, and serpin were higher than those raised against GST, filamin, LAP2, and tropomyosin, and similar to those of anti-SEA IgG (Figs 1 and 2).
Fig 1. IgG responses to the S. mansoni antigens during S. mansoni infection and after the treatment.
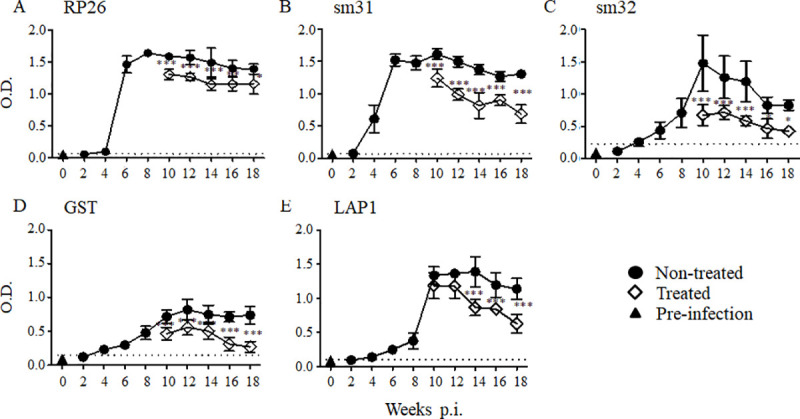
In each group, five mice were infected with 50 S. mansoni cercariae, and the sera were collected at each time point. A group of mice was orally treated twice with 500 mg/kg PZQ at 8 weeks post-infection. The experiment was repeated for 3 times and the representative data are presented. The means of the IgG level to the S. mansoni recombinant RP26 (A), sm31 (B), sm32 (C), GST (D), and LAP1 (E) are shown with the standard errors. The dashed line represents the cut-off value (mean + 3SD of the OD of the pre-infected group). The IgG levels of the treated group (open diamonds) are compared with those of the untreated group (closed circles) and with those of pre-infection samples taken at week 0 (closed triangles). *p < 0.05, **p < 0.01, and ***p < 0.001.
Fig 2. IgG responses to the S. mansoni antigens during S. mansoni infection and after the treatment.
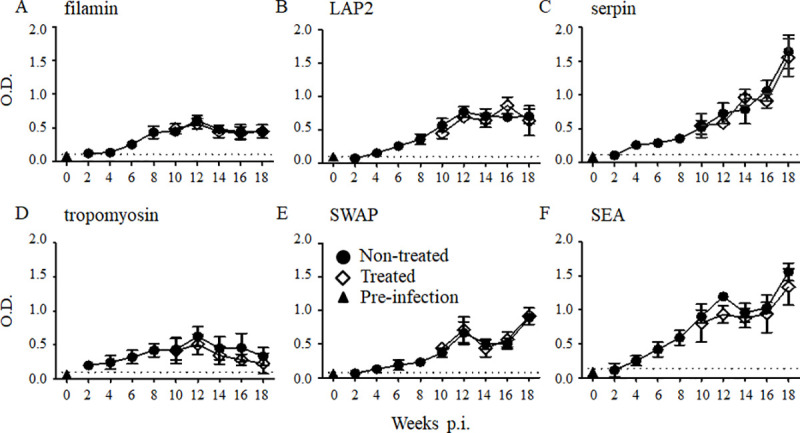
Five mice were infected with 50 S. mansoni cercariae, and the sera were collected from five mice at each time point. A group of mice was orally treated twice with 500 mg/kg PZQ at 8 weeks post-infection. The experiment was repeated for 3 times and the representative data are presented. The means of the IgG level to the S. mansoni recombinant filamin (A), LAP2 (B), serpin (C), tropomyosin (D), and crude SWAP (E) and SEA (F) are shown with the standard errors. The dashed line represents the cut-off value (mean + 3SD of the OD of the pre-infected group). Total IgG levels of the treated group (open diamonds) were compared with those of the untreated group (closed circles) and with those of pre-infection samples taken at week 0 (closed triangles).
Effects of PZQ treatment on the dynamics of IgG raised against recombinant antigens
To examine the effect of PZQ treatment on the kinetics of each IgG, mice infected with 50 S. mansoni cercariae were orally treated twice with 500 mg/kg PZQ at 8 weeks post-infection. The sera were collected every 2 weeks, and the IgG raised against each antigen was measured with ELISA. The IgG raised against RP26, sm31, sm32, and GST were significantly reduced at 2 weeks post-treatment (Fig 1A–1D), whereas it took 6 weeks for the IgG raised against LAP1 to decrease significantly after the treatment relative to those in the untreated mice (Fig 1E). For the remaining antigens, including SWAP and SEA, no significant reduction in IgG was observed after the treatment with PZQ compared with its level in the untreated group (Fig 2) at least until 10 weeks post-treatment. This result suggests that the antibody response to each antigen varies greatly during S. mansoni infection and after the treatment.
Dynamics of IgG1 subtypes that react with RP26, sm31, and serpin during S. mansoni infection and after treatment
Previous immunological and epidemiological studies of S. mansoni infection have shown that a high level of IgG4 correlates with S. mansoni infection, and IgG4 is thought to be a useful biomarker of parasitic infection. In this study, IgG raised against RP26, sm31, and serpin were dramatically induced during the infection of mice, and their excellent diagnostic value has been demonstrated previously, with significant reactivity in patient plasma [21, 33]. Because murine IgG1 is equivalent to human IgG4, we analyzed the IgG1 raised against RP26, sm31, and serpin during infection and after the treatment. The IgG1 raised against crude SWAP and SEA were induced at 4 weeks post-infection compared with the levels in uninfected mice. The IgG1 levels to both SWAP and SEA gradually increased during the infection (Fig 3D and 3E). The IgG1 raised against RP26 and sm31 were detected in the sera of mice at 4 weeks post-infection (Fig 3A–3C). The IgG1 that reacted to RP26 and sm31 increased dramatically by week 6 post-infection and plateaued, whereas those raised against serpin increased gradually during the whole period of observation, similar to the IgG that reacted to it. The IgG1 raised against RP26 and sm31 declined significantly 2 weeks after the treatment (Fig 3A–3C), whereas the IgG1 to serpin declined significantly by 10 weeks post-treatment. The IgG1 raised against crude SEA declined by 4 weeks post-treatment, too (Fig 3E). The kinetics of the IgG1 levels to the recombinant antigens during infection and after the treatment tended to be similar to those of IgG for all antigens, indicating that the detection of IgG1 and IgG4 in humans may be an alternative to the detection of total IgG.
Fig 3. IgG1 responses to the S. mansoni antigens RP26, sm31, serpin, SEA and SWAP during S. mansoni infection and after the treatment.
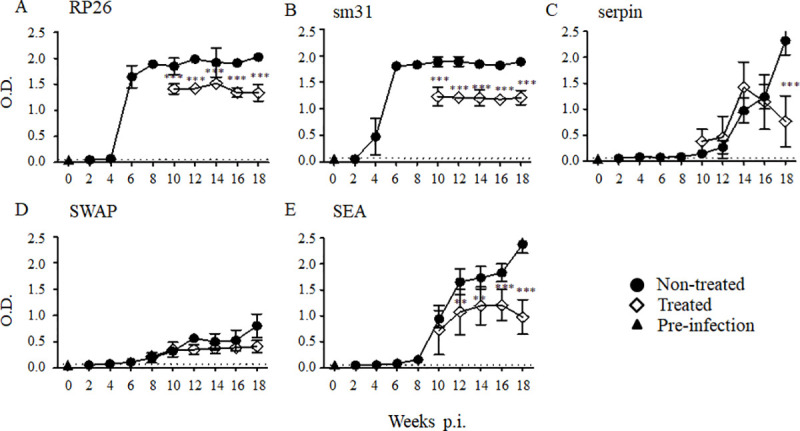
Five mice were infected with 50 S. mansoni cercariae, and the sera were collected from five mice at each time point. A group of mice was orally treated twice with 500 mg/kg PZQ at 8 weeks post-infection. The experiment was repeated for 3 times and the representative data are presented. The means of the IgG1 level to the S. mansoni recombinant RP26 (A), sm31 (B), serpin (C), and crude SWAP (D) and SEA (E) are shown with the standard errors. The dashed line represents the cut-off value (mean + 3SD of the OD of the pre-infected group). IgG1 levels of the treated group (open diamonds) were compared with those of the untreated group (closed circles) and with those of pre-infection samples taken at week 0 (closed triangles). **p < 0.01 and ***p < 0.001.
Dynamics of IgE raised against sm31, RP26, and serpin during infection and after treatment
Because IgE is known to be associated with IgG4, we next examined the reaction of IgE with recombinant proteins RP26, sm31, and serpin. The results showed that the IgE raised against sm31 and serpin were induced by 2 weeks post-infection, before the onset of egg production, which was similar to the induction of IgE to SWAP (Fig 4B–4D). The IgE raised against RP26 and sm31 remained high, even after the treatment, whereas the IgE raised against serpin declined significantly by 6 weeks post-treatment (Fig 4A–4C). There was no change in the IgE bound to SWAP or SEA after the treatment (Fig 4D and 4E). The IgE raised against each antigen remained elevated after the treatment with PZQ, except the IgE raised against serpin, which declined significantly after the administration of PZQ.
Fig 4. IgE responses to the S. mansoni antigens RP26, sm31, serpin, SEA and SWAP during S. mansoni infection and after the treatment.
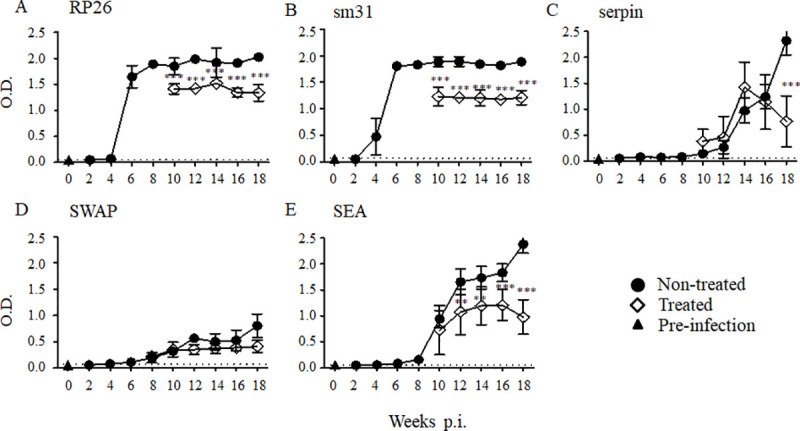
Five mice were infected with 50 S. mansoni cercariae, and the sera were collected from five mice at each time point. A group of mice was orally treated twice with 500 mg/kg PZQ at 8 weeks post-infection. The experiment was repeated for 3 times. The means of the IgE level to the S. mansoni recombinant RP26 (A), sm31 (B), serpin (C), and crude SWAP (D) and SEA (E) are shown with the standard errors. The dashed line represents the cut-off value (mean + 3SD of the OD of the pre-infected group). IgE levels of the treated group (open diamonds) were compared with those of the untreated group (closed circles) and with those of pre-infection samples taken at week 0 (closed triangles). **p < 0.01 and ***p < 0.001.
Discussion
In this study, we examined the dynamics of the serological responses to the nine recombinant S. mansoni antigens during S. mansoni infection, before and after the treatment with PZQ, and evaluated their potential as sensitive markers to monitor schistosome transmission in low-endemic settings, especially after the MDA with PZQ.
The IgG to all the tested antigens were detected within 4 weeks after the infection. The IgG raised against some of the recombinant antigens were robustly induced in the early phase of infection and maintained at high levels, whereas reportedly a longer infection period is required to induce IgG responses to most S. mansoni antigens in an endemic community [35]. The IgG to SEA and SWAP were maintained at high levels and were not affected by the treatment with PZQ, which is consistent with the observation that they persist for years after the clearance of the adult worms [35]. Among the nine selected candidate antigens, total IgG to sm31, sm32, RP26, LAP1, and GST significantly declined after the treatment with PZQ, indicating that they can be promising candidates in the study to further evaluate antigens for monitoring the transmission of schistosomiasis after MDA, facilitating the control and elimination of the disease [36, 37]. However, the levels of IgG were still higher than the baseline levels at only several weeks after the treatment. This property should be prospectively examined in human sera before and after the treatment, and it will be useful to clarify how many months are required for the IgG directed against each antigen to decline below the baseline level after the treatment with PZQ. These results suggest that the selected schistosome antigens in our study have significant potential for the monitoring and surveillance of schistosomiasis.
In this study, we analyzed the kinetics of IgG1, corresponding to IgG4 in human, and IgE directed against selected recombinant antigens. There were drastic increases in the IgG1 and IgE responses to sm31, RP26, and serpin. Interestingly, although the levels of IgG1 raised against RP26 and sm31 declined soon after the PZQ treatment to some extent with significance, IgG1 to serpin significantly declined at 10 weeks after the treatment. The kinetics of the IgG1 response to SEA in this study were similar to those reported by Matoso et al. [38]. Their study was conducted with human sera and suggested that anti-SEA IgG4 reactivity can be used as a biomarker for the serological monitoring of S. mansoni infection in endemic areas [38]. Therefore, it would be important to examine the IgG4 responses to sm31, RP26, and serpin using sera collected before and after the treatment with PZQ.
Helminthic infections, including S. mansoni, are known to induce IgE responses [39]. A study about antibody production during lymphatic filariasis suggested that the induction of IgG4 subtype and IgE antibodies was coordinately regulated [40] and that the immunoglobulins were often raised against the same antigens [41]. Previous studies have often analyzed the crude antigens of a parasite. As those preparations contain multiple allergens that are exposed to the host immune response under different conditions, it is difficult to determine which ones are responsible for the dual production of IgE and IgG4. By using recombinant proteins in this study, we were able to confirm the specific dual production of IgG1 and IgE raised against recombinant antigens during schistosome infection. In the present study, most of the antigens induced a similar pattern for IgG4 and IgE production, corroborating the report by Hussain et al. [40]. It is noteworthy that the specific IgG1 and IgE raised against the serpin antigen displayed similar patterns both before and after the treatment, insofar as they decreased simultaneously after the treatment. This combination of specific IgG1 and IgE raised against serpin could be used to monitor S. mansoni reinfection after MDA.
In summary, in this study, we have shown that the recombinant RP26, sm31, sm32, GST, LAP1, and serpin antigens can be good candidates in selecting potential markers in the study using human sera before and after the treatment for guiding programs to eliminate schistosomiasis.
Acknowledgments
This study was conducted (in part) at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University, Japan. We thank Janine Miller, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was supported by a Grants-in-Aid for International Scientific Research (A) by JSPS (17H01684 to SH). This work was conducted at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Olveda DU, Li Y, Olveda RM, Lam AK, Chau TNP, Harn DA, et al. Bilharzia: pathology, diagnosis, management and control. Trop Med Surg. 2013; 1(4): 135 10.4172/2329-9088.1000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO, Schistosomiasis: World Health Organization. http://www.who.int/schistosomiasis/en/2015
- 3.Hotez PJ, Brindley PJ, Bethony GM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008; 118(4): 1311–21. 10.1172/JCI34261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006; 368(9541): 1106–18. 10.1016/S0140-6736(06)69440-3 [DOI] [PubMed] [Google Scholar]
- 5.Warren KS, Grove DI, Pelley RP. The Schistosoma japonicum egg granuloma. II. cellular composition, granuloma size, and immunologic concomitants. Am J Trop Med Hyg, 1978; 80(2): 279–94. 10.4269/ajtmh.1978.27.271 [DOI] [PubMed] [Google Scholar]
- 6.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol, 2002; 2(7): 499–511. 10.1038/nri843 [DOI] [PubMed] [Google Scholar]
- 7.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo, 1972; 14(6): 397–400. [PubMed] [Google Scholar]
- 8.Nagi S, Chadeka EA, Sunahara T, Mutungi F, Justin YKD, Kaneko S et al. Risk factors and spatial distribution of Schistosoma mansoni infection among primary school children in Mbita district, western Kenya. PLoS Negl Trop Dis. 2014; 24; 8(7): e2991 10.1371/journal.pntd.0002991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadeka EA, Nagi S, Cheruiyot NB, Bahati F, Sunahara T, Njenga SM et al. A high-intensity cluster of Schistosoma mansoni infection around Mbita causeway, western Kenya: a confirmatory cross-sectional survey. Trop Med Health. 2019; 47: 26 10.1186/s41182-019-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D et al. Variations in helminth faecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop, 2004; 92(3): 205–12. 10.1016/j.actatropica.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 11.Soto PF, Arahuetes JG, Hernández AS, Abán JL, Santiago BV, Muro A. A loop-mediated isothermal amplification (LAMP) assay for early detection of Schistosoma mansoni in stool samples: a diagnostic approach in a murine model. PLoS Negl Trop Dis. 2014; 8(9): e3126 10.1371/journal.pntd.0003126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kongs A, Marks G, Verle P, Van der Stuyft P.The unreliability of the Kato-Katz technique limits its usefulness for evaluating S. mansoni infections. Trop Med Int Health, 2001; 6(3): 163–9. 10.1046/j.1365-3156.2001.00687.x [DOI] [PubMed] [Google Scholar]
- 13.Gray DJ, Ross AG, Li Y, McManus DP. Diagnosis and management of schistosomiasis. BMJ, 2011; 342: d2651 10.1136/bmj.d2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gool TV, Vetter H, Vervoort T, Doenhoff MJ, Wetsteyn J, Overbosch D. Serodiagnosis of imported schistosomiasis by a combination of a commercial indirect hemagglutination test with Schistosoma mansoni adult worm antigens and an enzyme-linked immunosorbent assay with S. mansoni egg antigens. J Clin Microbiol, 2002; 40(9): 3432–7. 10.1128/jcm.40.9.3432-3437.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mott KE, Dixon H. Collaborative study on antigens for immunodiagnosis of schistosomiasis. Bull World Health Organ, 1982; 60(5): 729–53. [PMC free article] [PubMed] [Google Scholar]
- 16.Bligh J, Schramm G, Chiodini PL, Doenhoff MJ. Serological analysis of the outcome of treatment of Schistosoma mansoni infections with praziquantel. Ann Trop Med Parasitol. 2010; 104(6):511–20. 10.1179/136485910X12786389891245 . [DOI] [PubMed] [Google Scholar]
- 17.Foo KT, Blackstock AJ, Ochola EA, Matete DO, Mwinzi PNM, Montgomery SP et al. Evaluation of point-of-contact circulating cathodic antigen assays for the detection of Schistosoma mansoni infection in low-, moderate-, and high-prevalence schools in western Kenya. Am J Trop Med Hyg, 2015; 92(6): 1227–32. 10.4269/ajtmh.14-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legesse M, Erko B. Field-based evaluation of a reagent strip test for diagnosis of Schistosoma mansoni by detecting circulating cathodic antigen in urine before and after chemotherapy. Trans R Soc Trop Med Hyg, 2007; 101(7): 668–73. 10.1016/j.trstmh.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 19.Lieshout VL, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop, 2000; 77(1): 69–80. 10.1016/s0001-706x(00)00115-7 [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Zhao F, Yu CX, Xiao D, Song LJ, Yin XR et al. Identification of proteins inducing short-lived antibody responses from excreted/secretory products of Schistosoma japonicum adult worms by immunoproteomic analysis. J Proteomics, 2013; 87: 53–67. 10.1016/j.jprot.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Tanigawa C, Fujii Y, Miura M, Nzou SM, Mwangi AW, Nagi S et al. Species-specific serological detection for schistosomiasis by serine protease inhibitor (SERPIN) in multiplex assay. PLoS Negl Trop Dis, 2015; 9(8): e0004021 10.1371/journal.pntd.0004021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makarova E, Goes TS, Leite MF, Goes AM. Detection of IgG binding to Schistosoma mansoni recombinant protein RP26 is a sensitive and specific method for acute schistosomiasis diagnosis. Parasitol Int, 2005; 54(1): 69–74. 10.1016/j.parint.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Sulbarán GS, Ballen DE, Bermúdez H, Lorenzo M, Noya O, Cesari IM. Detection of the Sm31 antigen in sera of Schistosoma mansoni- infected patients from a low endemic area. Parasite Immunol, 2010; 32(1): 20–8. 10.1111/j.1365-3024.2009.01152.x [DOI] [PubMed] [Google Scholar]
- 24.El-Sayed LH, Ghoneim H, Demian SR, El-Sayed MH, Tawfik NM, SakrI et al. Diagnostic significance of Schistosoma mansoni proteins Sm31 and Sm32 in human schistosomiasis in an endemic area in Egypt. Trop Med Int Health.1998; 3(9): 721–7. 10.1046/j.1365-3156.1998.00298.x [DOI] [PubMed] [Google Scholar]
- 25.Darani HY, Curtis RH, McNeice C, Price HP, Sayers JR, Doenhoff MJ. Schistosoma mansoni: anomalous immunogenic properties of a 27 kDa larval serine protease associated with protective immunity. Parasitology, 1997; 115 (3): 237–47. 10.1017/s0031182097001303 [DOI] [PubMed] [Google Scholar]
- 26.Ludolf F, Patrocínio PR, Oliveira RC, Gazzinelli A, Falcone FH, Ferreira AT et al. Serological screening of the Schistosoma mansoni adult worm proteome. PLoS Negl Trop Dis, 2014; 8(3): e2745 10.1371/journal.pntd.0002745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed MR, Shalaby KA, LoVerde PT, Abdallah NM, Karim AM. Cloning and characterization of a cDNA fragment encoding a Schistosoma mansoni actin-binding protein (Smfilamin). Parasitol Res, 2008; 102(5): 1035–42. 10.1007/s00436-007-0872-5 [DOI] [PubMed] [Google Scholar]
- 28.Cook RM, Queiroz CC, Wilding G, LoVerde PT. Nucleic acid vaccination with Schistosoma mansoni antioxidant enzyme cytosolic superoxide dismutase and the structural protein filamin confers protection against the adult worm stage. Infect Immun, 2004; 72(10): 6112–24. 10.1128/IAI.72.10.6112-6124.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H, Rekosh DM, Andrews W, Higashi GI, Nicholson L, Loverde PT. Schistosoma mansoni tropomyosin: production and purification of the recombinant protein and studies on its immunodiagnostic potential. Am J Trop Med Hyg, 1991; 45(1): 121–31. 10.4269/ajtmh.1991.45.121 [DOI] [PubMed] [Google Scholar]
- 30.Harrop R, Jennings N, Mountford AP, Coulson PS, Wilson RA. Characterization, cloning and immunogenicity of antigens released by transforming cercariae of Schistosoma mansoni. Parasitology, 2000; 121(4): 385–94. 10.1017/s003118209900640x [DOI] [PubMed] [Google Scholar]
- 31.Botros SS, Makary EA, Ahmed KM, Ibrahim AM, Nashed NN, El-Nahal HM et al. Effect of combined praziquantel and recombinant glutathione S-transferase on resistance to reinfection in murine Schistosomiasis mansoni. Int J Immunopharmacol, 2000; 22(11): 979–88. 10.1016/s0192-0561(00)00062-x [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi G, Morales ME, Alrefaei YN, Cancela M, Castillo E, Dalton JP et al. RNA interference targeting leucine aminopeptidase blocks hatching of Schistosoma mansoni eggs. Mol Biochem Parasitol, 2009; 167(2): 118–26. 10.1016/j.molbiopara.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology, 1965. 55(4): 695–700. 10.1017/s0031182000086248 [DOI] [PubMed] [Google Scholar]
- 34.Kalenda YDJ, Kato K, Goto Y, Fujii Y, Hamano S. Tandem repeat recombinant proteins as potential antigens for the sero-diagnosis of Schistosoma mansoni infection. Parasitol Int. 2015; 64(6): 503–12. 10.1016/j.parint.2015.06.012 . [DOI] [PubMed] [Google Scholar]
- 35.Van Dam GJ, Stelma FF, Gryseels B, Ferreira STF, Talla I, Niang M et al. Antibody response patterns against Schistosoma mansoni in a recently exposed community in Senegal. J Infect Dis, 1996; 173(5): 1232–41. 10.1093/infdis/173.5.1232 [DOI] [PubMed] [Google Scholar]
- 36.Alderete JF, Newton E, Dennis C, Neale KA. Antibody in sera of patients infected with Trichomonas vaginalis is to trichomonad proteinases. Genitourin Med, 1991; 67(4): 331–4. 10.1136/sti.67.4.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zang X, Atmadja AK, Gray P, Allen JE, Gray CA, Lawrence RA et al. The serpin secreted by Brugia malayi microfilariae, Bm-SPN-2, elicits strong, but short-lived, immune responses in mice and humans. J Immunol, 2000; 165(9): 5161–9. 10.4049/jimmunol.165.9.5161 [DOI] [PubMed] [Google Scholar]
- 38.Matoso LF, Prado RO, Abreu MNS, Fujiwara RT, Loverde PT, Kloos H et al. Longitudinal analysis of antigen specific response in individuals with Schistosoma mansoni infection in an endemic area of Minas Gerais, Brazil. Trans R Soc Trop Med Hyg, 2013; 107(12): 797–805. 10.1093/trstmh/trt091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pu Zhang FM. IgE: a key antibody in Schistosoma infection. Electronic Journal of Biology, 2006; 2(1): 11–14. [Google Scholar]
- 40.Hussain R, Ottesen EA. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J Immunol, 1986; 136(5): 1859–63. [PubMed] [Google Scholar]
- 41.Rihet P, Demeure CE, Bourgois A, Prata A, Dessein AJ. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol, 1991; 21(11): 2679–86. 10.1002/eji.1830211106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.


