Abstract
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is considered to be the strongest curative immunotherapy for various malignancies (primarily, but not limited to, haematologic malignancies). However, application of allo-HSCT is limited owing to its life-threatening major complications, such as graft-versus-host disease (GVHD), relapse and infections. Recent advances in large-scale DNA sequencing technology have facilitated rapid identification of the microorganisms that make up the microbiota and evaluation of their interactions with host immunity in various diseases, including cancer. This has resulted in renewed interest regarding the role of the intestinal flora in patients with haematopoietic malignancies who have received an allo-HSCT and in whether the microbiota affects clinical outcomes, including GVHD, relapse, infections and transplant-related mortality. In this Review, we discuss the potential role of intestinal microbiota in these major complications after allo-HSCT, summarize clinical trials evaluating the microbiota in patients who have received allo-HSCT and discuss how further studies of the microbiota could inform the development of strategies that improve outcomes of allo-HSCT.
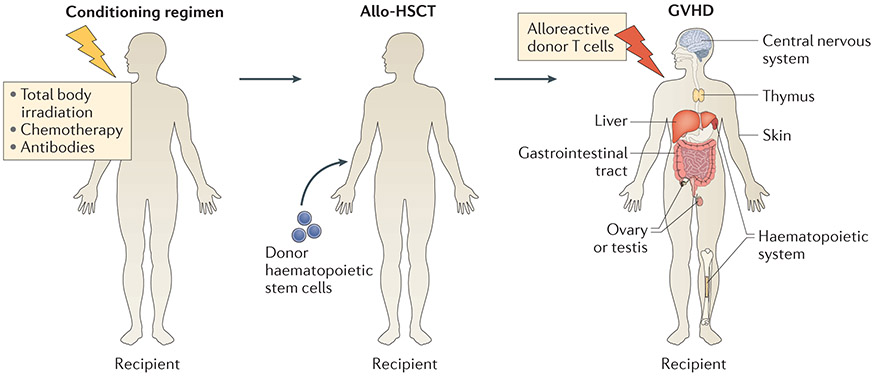
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is a combination of stem cell therapy, conventional therapy (with chemotherapy, radiation or antibodies) and immunotherapy1. Allo-HSCT consists of a conditioning regimen, including chemotherapeutic agents with or without total body irradiation and/or antibodies, that destroys cancer cells and allows the recipient immune system to then accept an immune system-reconstituting infusion of donor HSCs. In addition, allogeneic donor T cells can attack residual tumour cells, resulting in graft-versus-tumour (GVT) activity. However, these alloreactive donor T cells can also attack target organs of the host, including the skin, liver, gut, thymus, central nervous system, ovary or testis as well as the haematopoietic system1-6 (called graft-versus-host disease (GVHD)) (FIG. 1).
Figure 1 ∣. Allo-HSCT and GVHD.
Patients undergoing allogeneic haematopoietic stem cell transplantation (allo-HSCT) receive a pretransplant regimen including chemotherapeutic agents with or without total body irradiation and/or antibodies. Patients receive donor haematopoietic stem cells after this pretransplant regimen. Graft-versus-host disease (GVHD) occurs when alloreactive donor T cells attack host tissues, including the skin, liver, gastrointestinal tract, central nervous system, thymus, ovary or testis as well as the haematopoietic system.
The impact of the gut microbiota on anticancer immune responses, including chemotherapy as well as allo-HSCT, is receiving increased attention. Recent studies in mice and humans suggest important relationships between the microbiota and outcomes in allo-HSCT recipients, and several clinical trials targeting the microbiota in allo-HSCT recipients are ongoing. Associations between certain bacteria and allo-HSCT outcomes have been found repeatedly despite the use of different levels of classification7,8. Studies in the 1970s and 1980s showed that the commensal microorganisms residing in the intestinal lumen, collectively termed the gut microbiota, play an important role in the pathophysiology of GVHD9-11. Recent advances in sequencing and identification of the taxonomic composition of the host microbiota have enabled detailed evaluation of microbiota–host interactions. This Review provides an update on our current knowledge of the roles of the gut microbiota in the most common complications after allo-HSCT for cancer therapy — infections, GVHD and relapse7,12-16. We also discuss potential roles of the microbiota in immune reconstitution as well as effects of dietary changes on the microbiota and how these factors affect GVHD after allo-HSCT. Finally, we summarize various strategies to target the microbiota to achieve better outcomes in allo-HSCT. The interplay between the gut microbiota and the host immune system (including Paneth cells, intestinal epithelial cells (IECs), short-chain fatty acids (SCFAs), antimicrobial peptides (AMPs), regulatory T (Treg) cells and other immune cells) during GVHD has been discussed extensively elsewhere, so we do not cover this topic in detail. Similarly, in this Review, we do not discuss the roles of the microbiota in chemotherapy or immunotherapy in the context of antitumour activities (reviewed in REF.17).
Infectious complications of allo-HSCT
Microbial diversity predicts overall survival.
Healthy individuals have a diverse intestinal bacterial flora, which predominantly comprises members of the Firmicutes and Bacteroidetes phyla as well as populations of Proteobacteria, Actinobacteria, Verrucomicrobia and Fusobacteria18. During the course of an allo-HSCT, the diversity of the intestinal flora significantly decreases19,20. The changes can be rapid and are thought to be due to the effects of antibiotics, intestinal inflammation and changes in diet. Most patients are exposed to prophylactic and therapeutic antibiotics during neutropenia, which occurs in the first weeks after allo-HSCT21. They generally manifest loss of appetite owing to preconditioning treatment regimens, drug toxicity and GVHD22,23 (FIG. 2). In a retrospective analysis of faecal specimens collected from 80 allo-HSCT recipients at the time of neutrophil engraftment — a critical time point indicating the establishment of donor haematopoiesis — a low diversity of the faecal flora was associated with significantly increased mortality (52%) compared with a high diversity of the faecal flora (8%)24. Specifically, a greater abundance of Gammaproteobacteria (including Enterobacteriaceae) was associated with higher mortality, whereas a greater abundance of Lachnospiraceae and Actinomycetaceae was associated with a more favourable outcome.
Figure 2 ∣. Intestinal microbiota injury after allo-HSCT.
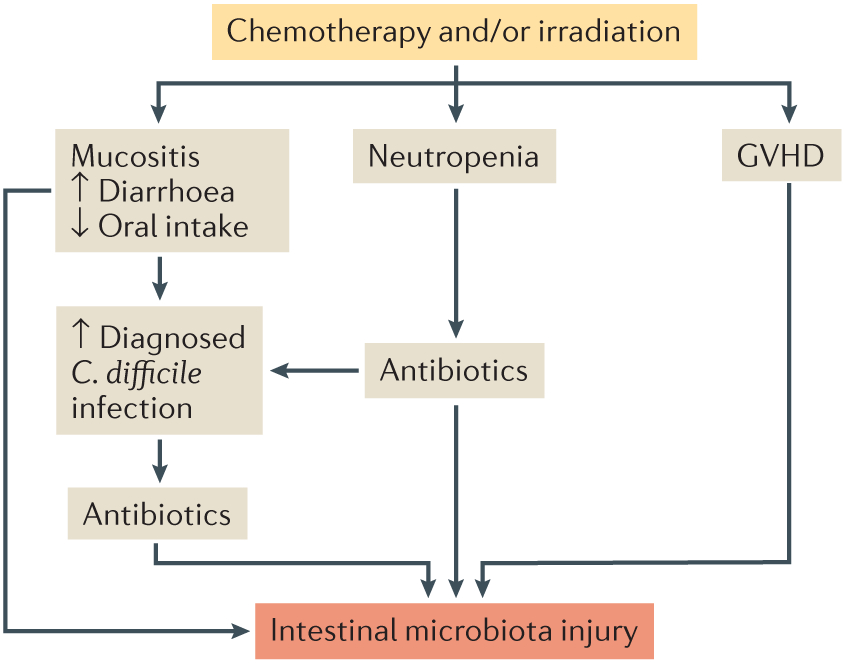
Major factors altering microbiota homeostasis after allogeneic haematopoietic stem cell transplantation (allo-HSCT) include conditional chemotherapy and/or irradiation, antibiotic therapy, graft-versus-host disease (GVHD), mucositis and infection (for example, with Clostridium difficile). Patients experience neutropenia after a conditioning regimen, and this often requires treatment with antibiotics. These factors contribute to microbiota dysbiosis, which is represented by decreased bacterial diversity, lower numbers of anaerobic commensal bacteria and expansions of pathobionts.
Infections following antibiotic treatment.
In allo-HSCT, simultaneous use of prophylactic antibiotics and antibiotic treatment of febrile neutropenia (FN) affect microbial diversity, which in turn increases susceptibility to infections (FIG. 2). Whereas a healthy individual usually carries on the order of 1,000 different species25,26, near dominance of the intestinal flora, particularly by a single species of Proteobacteria, Enterococcus or Streptococcus, is frequently observed after allo-HSCT19. In particular, exposure to the antibiotic vancomycin or metronidazole is associated with Enterococcus domination19,27. Importantly, domination of Enterococcus or Proteobacteria was correlated with the increased risk of developing bacteraemia with vancomycin-resistant Enterococcus (VRE) or Gram-negative bacteria, respectively19. By contrast, exposure to fluoroquinolone antibiotics (ciprofloxacin or levofloxacin) decreased the risk of Proteobacteria domination19. Enterobacteriaceae and other Gram-negative bacteria from the phylum Proteobacteria are associated with increased mortality24, and intestinal domination by Gammaproteobacteria was identified as a significant predictor of pulmonary complications (including infections) after allo-HSCT in a single-centre observational study irrespective of whether a patient received an antibiotic or had a pretransplant comorbidity28. These results are consistent with a hypothesis that antibiotic-induced elimination of commensal microbiota can result in expansion of pathogenic facultative bacteria in the gut consortium. Major microbiota injuries observed in studies of patients after allo-HSCT are summarized in FIG. 3.
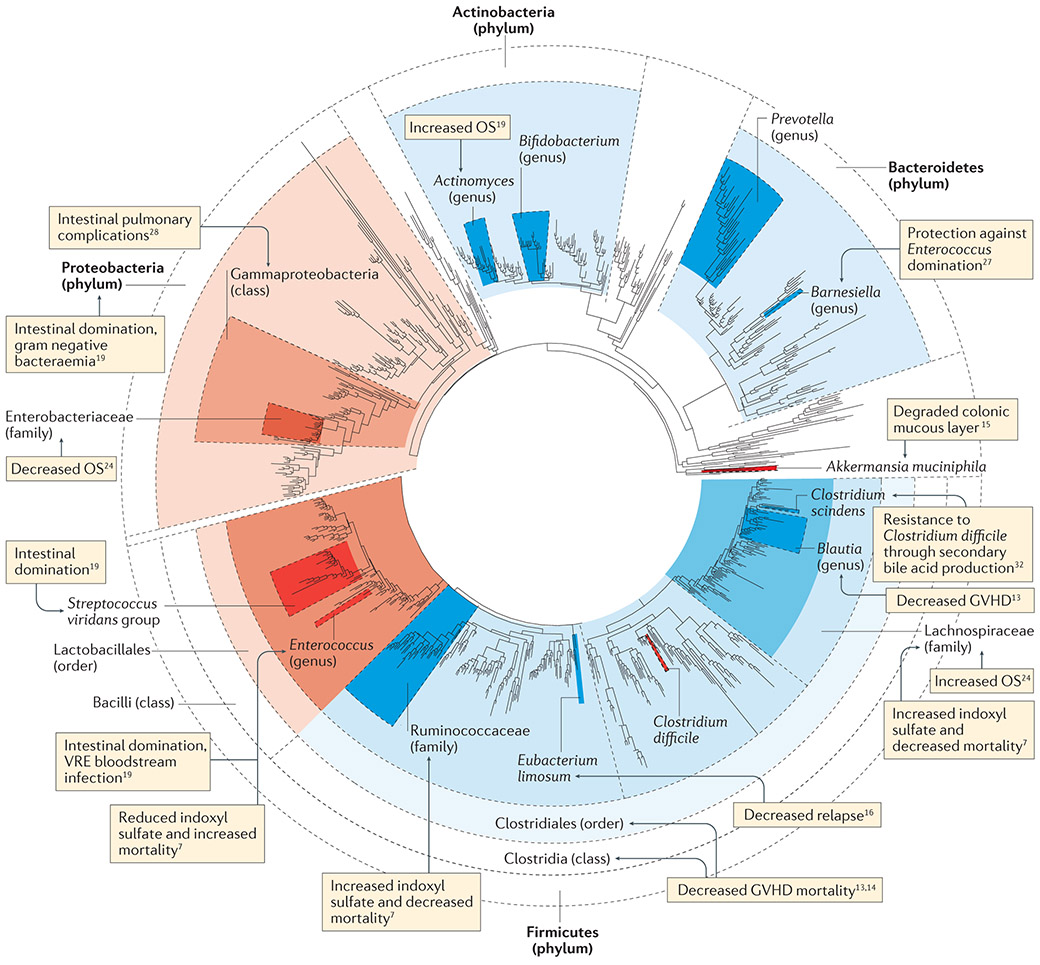
Figure 3 ∣. Studies on microbiota injuries in allo-HSCT recipients.
A phylogenetic tree of typical gut bacterial species is shown. Shaded bacterial clades represent various levels of taxonomic classification. The blue shading denotes beneficial associations, whereas the red shading denotes association with negative effects. Allo-HSCT, allogeneic haematopoietic stem cell transplantation; GVHD, graft-versus-host disease; OS, overall survival; VRE, vancomycin-resistant Enterococcus. Figure adapted with permission from REF 167, Taylor & Francis Ltd (www.tandfonline.com), and updated by A. L. Gomes and Y.S.
Antibiotic treatment can also increase susceptibility to infections with the intestinal pathogen Clostridium difficile, especially in the setting of allo-HSCT, which involves major risk factors for C. difficile infection, such as immune suppression, chemotherapy and irradiation29,30. C. difficile infection early after allo-HSCT was positively correlated with high-intensity chemotherapy but not with antibiotic administration31. By contrast Clostridium scindens can contribute to resistance to C. difficile infection through secondary bile acid synthesis32, which serves as an example of colony resistance (microbiota-mediated inhibition of specific bacteria) in allo-HSCT recipients.
Immune reconstitution after allo-HSCT is critical for the prevention of infectious complications. This could also be affected by the microbiota (BOX 1).
Box 1 ∣. Microbiota and immune reconstitution.
Given the recent findings of the role of microbiota in immune homeostasis21,49, it is possible that intestinal microbiota could affect immune reconstitution after allogeneic haematopoietic stem cell transplantation (allo-HSCT) and could be targeted as a strategy to increase post-transplant immunity. Defects in haematopoiesis have been reported in germ-free mice; specifically, the intestinal microbiota promotes steady-state myeloid cell development and increases the differentiation potential to granulocyte and/or monocyte progenitors (GMPs)147. Similarly, mice deficient in myeloid differentiation primary response protein MYD88 or nucleotide-binding oligomerization domain-containing protein 1 (NOD1) signalling (pathways involved in bacterial sensing and resulting immunomodulation) manifested reduced numbers of myeloid cells and GMPs148-150, and antibiotic-treated mice in steady-state showed multilineage repression of haematopoiesis across all progenitor subtypes in the bone marrow151. How the intestinal microbiota controls immune responses through distant sites such as the bone marrow remains to be fully revealed. A syngeneic mouse transplant model indicates the occurrence of delayed immune reconstitution in antibiotic-treated recipients (M.R.M.v.d.B. and Y.S., unpublished observations). Studies are warranted to explore the effects of microbiota changes on haematopoiesis and immune reconstitution in allo-HSCT recipients.
A study comparing the immune systems of specific pathogen-free (SPF) mice commonly used in laboratory experiments with those of feral mice (or mice from city pet shops) found that SPF mice possess fewer effector T cells in the intestinal mucosa than feral mice and that SPF mice are similar to human neonates152. Co-housing of laboratory ‘clean’ SPF mice with ‘dirty’ feral mice resulted in more effector and memory T cell populations, as typically seen in adult humans, indicating that the microbiota plays an important role in shaping the immune system. In another example, vaccine-induced murine immune responses are altered in dirty mice co-infected with chronic viruses and helminths and recapitulate the responses seen in adult humans (evaluated by gene expression changes)153, which could provide an avenue to better stimulate the human immune response to vaccination.
Clinical interventions.
Given the impact of microflora diversity loss in the gut in the course of allo-HSCT, interventions to maintain intestinal diversity might contribute to improved outcomes. One strategy could be to limit exposure to antibiotics that eliminate obligate anaerobic commensal bacteria; however, this is difficult to accomplish when patients require broad-spectrum antibiotics for FN, bacteraemia or sepsis. Another approach could be to restore microbiota diversity by faecal microbiota transplantation (FMT) with pretransplant faecal matter from the host or from a third party. Three case reports of FMT treatment for patients with refractory C. difficile-associated disease after allo-HSCT have been published showing the effectiveness and safety of this method33. A clinical trial to restore diversity and prevent C. difficile infection during allo-HSCT is currently underway34 (TABLE 1). In this study, recipients who show sufficient microbial diversity before allo-HSCT and a post-transplant loss of the protective phylum Bacteroidetes will receive their own pretransplant flora at the time of engraftment. However, there is concern for possible FMT-related sepsis in an immunocompromised host through bacterial translocation or norovirus infections, as reported in inflammatory bowel disease (IBD) or patients infected with C. difficile35,36. In addition, a recent case report in a patient with C. difficile infection demonstrated overgrowth of methane-producing bacteria contracted from the FMT donor, resulting in IBD-like symptoms without evidence of infections and gastrointestinal pathologic abnormalities37. Given these caveats, larger studies and long-term follow-up to evaluate the outcomes of FMT are required.
Table 1 ∣.
Summary of ongoing clinical trials investigating microbial interventions in allo-HSCT
| Trial title | Aims and interventions | Phase | Trial number | Refs |
|---|---|---|---|---|
| Antibiotic treatment and its impact on microbiota | ||||
| Gut decontamination in paediatric allo-HSCT | To study the effect of vancomycin–polymyxin B treatment on patient flora and on GVHD and survival | II | NCT02641236 | 101 |
| Choosing the best antibiotic to protect friendly gut bacteria during the course of stem cell transplant | To compare the effect between piperacillin–tazobactam and cefepime on patient flora (7 ± 2 days after initiation of antibiotics) | II | NCT03078010 | 89 |
| Strategies for GVHD treatment | ||||
| Medical home care for HSC transplantation | To compare the incidence of acute grade II–IV GVHD between patients receiving patient-centred medical home care and those receiving standard hospital care | II | NCT02218151 | 131 |
| Study of IL-22 IgG2-Fc (F-652) for subjects with grade II–IV lower GI acute GVHD | To examine combination treatment with corticosteroid for new-onset grade II–IV GVHD in the lower GI tract | I/II | NCT02406651 | 65 |
| Lactobacillus rhamnosus str. GG in reducing incidence of GVHD in patients who have undergone donor stem cell transplant | To study the impact of Lactobacillus rhamnosus str.GG on reducing incidence of GVHD | Pilot | NCT02144701 | 133 |
| Fructooligosaccharides in treating patients with blood cancer undergoing donor stem cell transplant | To study side effects and best dose of fructooligosaccharides for potential use for GVHD treatment | I | NCT02805075 | 123 |
| Modification of the intestinal microbiome by diet intervention to mitigate acute GVHD | To study the effect of potato-based starch on butyrate production and rates of acute GVHD | II | NCT02763033 | 124 |
| Gluten-free diet in preventing GVHD in patients undergoing donor stem cell transplant | To evaluate the impact of gluten-free diet on GVHD prophylaxis | Pilot | NCT03102060 | 168 |
| FMT trials | ||||
| Autologous FMT for prophylaxis of Clostridium difficile infection in recipients of allo-HSCT | To restore diversity and prevent Clostridium difficile infection during allo-HSCT | II | NCT02269150 | 34 |
| FMT after HSC transplantation | To assess the feasibility of empiric FMT soon after allo-HSCT; secondary outcome measures include GVHD and survival | Early I | NCT02733744 | 129 |
| Stool transplantation to reduce antibiotic-resistance transmission (START) | To investigate the outcomes of FMT aiming at eradicating gut colonization with multidrug-resistant bacteria in patients with blood disorders | NA | NCT02461199 | 169 |
| Nutrition and transplant outcome | ||||
| Randomized prospective multicentre study to compare enteral nutrition with parenteral nutrition as feeding support in patients presenting with malignant haemopathy who underwent an allogeneic HSC transplantation (NEPHA) | To compare between enteral nutrition and parenteral nutrition groups after myeloablative allo-HSCT; occurrence of transplant-related mortality, GVHD and infections will be assessed | III | NCT01955772 | 166 |
Allo-HSCT, allogeneic haematopoietic stem cell transplantation; FMT, faecal microbiota transplantation; GI, gastrointestinal; GVHD, graft-versus-host disease; HSC, haematopoietic stem cell; IL-22, interleukin-22; NA, not available.
GVHD and the microbiota
Disruption of the intestinal barrier and homeostasis.
The gut is a primary GVHD target organ and plays a major role in the development of GVHD38. Conditioning regimens for allo-HSCT disrupt the equilibrium between host and microbiota, resulting in mucositis, organ dysfunction and increased susceptibility to infections (FIG. 2). For example, bacterial lipopolysaccharide (one of the bacterial ligands) can pass through the damaged intestinal barrier during conditioning and activate the innate immune system39. The innate immune system plays an important role in the activation of alloreactive donor T cells, which are an absolute requirement for the development of GVHD. Pathways involved in the interplay between the gut microbiota and the host’s innate immune system during GVHD have been extensively described elsewhere21,40-53, and these relationships are summarized in FIG. 4. IECs maintain physical and biochemical barriers to separate intestinal tissues and luminal contents54.
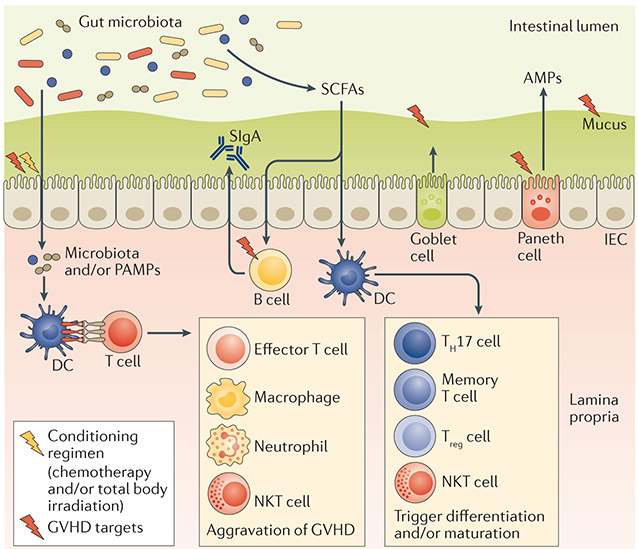
Figure 4 ∣. The interplay between gut microbiota and host physiology and immunity.
The host immune system maintains gut microbiota diversity and prevents outgrowth of pathobionts. Under steady-state conditions, the gut epithelial surface maintains an intact barrier that prevents bacterial invasion into the host tissue; this is accomplished by antimicrobial peptides (AMPs) produced by Paneth cells that create a sterility gradient, mucus produced by goblet cells that separates the microbiota from host epithelial tissue and secretory immunoglobulin A (SIgA) that neutralizes biologically active microbial antigens. Short-chain fatty acids (SCFAs) are bacterial fermentation products. They can be used as an energy source and also regulate the differentiation, recruitment and activation of immune cells. In the setting of allogeneic haematopoietic stem cell transplantation (allo-HSCT), total body irradiation and chemotherapy reduce the integrity of the intestinal surface. Intestinal bacteria and their components (pathogen-associated molecular patterns (PAMPs)) translocate into the lamina propria and are recognized by antigen-presenting cells such as dendritic cells (DCs), leading to activation of effector cells and aggravation of graft-versus-host disease (GVHD). B cells, Paneth cells and the mucous layer are known to be targets of GVHD, and damage to these creates a vicious circle exacerbating gut inflammation and bacterial translocation and resulting in worse survival after allo-HSCT. IEC, intestinal epithelial cell; NKT, natural killer T; TH17, T helper 17; Treg, regulatory T.
Not only bacteria themselves but also their metabolites can be excluded from the bases of the crypts where intestinal stem cells reside55. Paneth cells constitute a group of IECs, which reside in the intestinal crypts and secrete AMPs, leading to an AMP gradient along the intestinal crypt56. Studies in mice and humans show a marked reduction of Paneth cells during GVHD12,14,57, causing bacteraemia with impaired barrier function. Paneth cells also constitute the niche for leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5)+ intestinal stem cells and exert trophic effects through the epidermal growth factor (EGF), WNT3 and NOTCH signalling pathways58 as well as through interleukin-22 (IL-22), which increases epithelial regeneration and improves GVHD-related mortality in mouse models59. Indole-3-aldehyde is a bacterial metabolite of tryptophan that activates the aryl hydrocarbon receptor on innate lymphoid cells (ILCs). It mediates colonization resistance and induces IL-22 secretion by a specific group of ILCs60,61. The secretion of the AMP regenerating islet-derived protein 3γ (REG3γ) by Paneth cells (a biomarker of intestinal GVHD62) is regulated by IL-22, and REG3γ plays a critical role in the host defence against bacterial pathogens, possibly by maintaining flora diversity63,64. A phase II clinical trial utilizing recombinant human IL-22 in combination with standard systemic corticosteroid treatment for intestinal GVHD is ongoing65 (TABLE 1). A report in a mouse model of GVHD showed that recombinant R-spondin 1, an essential growth factor for intestinal stem cells, restored gut microflora dysbiosis by normalizing faecal levels of the AMP α-defensin and promoted the restoration of Paneth cells66. These findings indicate the importance of protecting gut microbiota to maintain the intestinal barrier and homeostasis.
Microbiota injury during GVHD.
Studies in mouse models of allo-HSCT conducted in the 1970s showed that recipient germ-free mice kept in continuous germ-free conditions throughout the course of allo-HSCT, or decontaminated with high-dose antibiotics starting 1 week before transplant manifested significantly reduced mortality from GVHD10. In this study, the protective effect of a germ-free environment was most evident when bone marrow alone was used as a stem cell source, and the effect was less when splenocytes (which include alloreactive T cells) were added to induce GVHD. This finding might indicate that GVHD is a result of the balance of innate immune system activation and the number of alloreactive T cells. Thereafter, in a clinical report of 130 patients with aplastic anaemia who were undergoing allo-HSCT from human leukocyte antigen (HLA)-identical siblings, gut decontamination and laminar-airflow isolation were shown to lower the cumulative incidence of grade II–IV GVHD11. In these early reports, no specific bacterial species were reported to be responsible for the severity of GVHD or transplant-related mortality (TRM). With recent advances in DNA sequencing and improved techniques of culturing gut-resident microorganisms, changes in the gut microbiota composition during GVHD after allo-HSCT have been studied in more detail. As mentioned, changes in intestinal microbiota after allo-HSCT can occur very rapidly19; this warrants careful longitudinal studies to better assess and define these changes in the ecology of the intestinal microbiota.
Loss of bacterial diversity affects GVHD-related outcomes after allo-HSCT. A subgroup analysis of the previously mentioned study24 revealed that loss of bacterial diversity in stool specimens collected within 7 days after neutrophil engraftment was associated with increased mortality from GVHD. Similarly, an evaluation of 64 patients after allo-HSCT demonstrated decreased bacterial diversity in stool specimens collected on day 12, which was correlated with increased GVHD-related mortality13. In another study, ileal bacterial densities in control and GVHD mice were not different; however, there was an increased abundance of Lactobacillales and a reduced abundance of Clostridiales during GVHD, which was confirmed in stool specimens of patients with GVHD14. Treatment with ampicillin, which targets Lactobacillales, worsened GVHD, and re-introduction of Lactobacillus johnsonii after ampicillin treatment improved GVHD. Other reports described an expansion of Enterococcus spp., which belong to the same order of Lactobacillales as Lactobacillus spp., during GVHD in mice67 and humans7, which may potentially contribute to increased inflammation and reduced gut integrity, given the evidence that Enterococcus spp. can compromise epithelial barrier integrity68 and stimulate tumour necrosis factor (TNF) production from macrophages69.
By contrast, Clostridiales play important anti-inflammatory homeostatic roles, including upregulation of Treg cells through the production of the SCFA butyrate70-74. Butyrate increased the recovery of IEC damage after allo-HSCT, and the re-introduction of a mixture of 17 strains of human Clostridiales isolates, which produce high levels of butyrate, prolonged the survival of mice with GVHD75. Clinical data indicate that increased abundance of the Blautia genus, which belongs to the class Clostridia, is significantly associated with less GVHD-related mortality and improved overall survival13.
Other preclinical studies have reported an association between expansion of Escherichia coli12 and Bacteroides spp. or Prevotella spp.67 and worse GVHD. Also, the Muribaculaceae family could play a role in IL-17-mediated GVHD, possibly through antagonistic and protective roles in GVHD pathology76. Recent studies showed that patients with GVHD have more Firmicutes and Proteobacteria and fewer Bacteroidetes than those without GVHD77,78 (REF. 78 is an abstract from the 57th American Society of Haematology Annual Meeting). Most of these studies report associations between these specific microorganisms and the severity of GVHD but lack mechanistic insights.
Little is known regarding the role of donor microbiota in the development of GVHD. One study in mouse models showed no difference in the severity of GVHD between recipients of an allograft from germ-free mice versus those receiving allografts from specific pathogen-free (SPF) mice79. Dietary changes after allo-HSCT may also contribute to microbiota injury and GVHD. See BOX 2 for a description of oral nutrition versus total parenteral nutrition and the impact of such diets on microbiota.
Box 2 ∣. Effect of dietary changes on GVHD.
Owing to the conditioning regimen, most allogeneic haematopoietic stem cell transplantation (allo-HSCT) recipients will incur some degree of nausea, mucositis and anorexia, resulting in a deterioration of their nutritional status in the first weeks after allo-HSCT22,23. Impaired nutritional status after allo-HSCT negatively affects patient prognosis154. Fat-restricted or carbohydrate-restricted diets result in the increase of Bacteroidetes in humans155. Other reports show that an energy restriction by 35% for 6 weeks results in increased bacterial diversity156. It is important to maintain proper oral intake of diet in allo-HSCT recipients, as changes in the microbiota induced by reduced dietary intake after allo-HSCT can be related to increased graft-versus-host disease (GVHD) and poor outcomes of allo-HSCT.
Total parenteral nutrition (TPN) is widely used to support allo-HSCT recipients, and various recommendations to improve the nutritional status of patients with GVHD after allo-HSCT have been made, including the use of TPN and a diet progression protocol for patients with gastrointestinal GVHD157-161. The detailed effects of TPN on microbiota changes have not been documented, and it is still under debate whether oral nutrition should be preferred over TPN to prevent microbiota injury and GVHD157,162,163. Oral nutrition might be more beneficial than TPN with respect to the protection of gastrointestinal digestive as well as barrier functions, and two small studies showed that TPN treatment was associated with higher acute GVHD incidence and worse survival164,165. Moreover, in one retrospective study in allo-HSCT recipients, loss of Blautia spp. was associated with receiving TPN for more than 10 days, which possibly contributed to increased GVHD mortality13. One prospective randomized multicentre study comparing enteral nutrition with TPN with regard to transplant-related mortality and GVHD is ongoing166 (TABLE 1). Nutritional assessment in patients after allo-HSCT is very challenging, as body weight is not a reliable indicator of nutrition when patients experience excessive fluid retention or loss caused by dynamic inflammatory status (including GVHD) after allo-HSCT. Establishing an accurate nutritional assessment of patients through multidisciplinary efforts involving nurses, haematologists, dieticians, nutritionists, medical assistants and nurse practitioners is necessary for future research to elucidate the impact of dietary changes on GVHD through microbial modulation.
We hypothesize that the outcome of allo-HSCT depends on the complex interactions among various bacterial species (that is, ecology), and it is important to note that perturbation of microbiota ecology by GVHD or antibiotic treatment may also result in impaired colonization resistance, resulting in the expansion of certain microbial pathogens. Data are limited regarding the composition of favourable microbiota ecologies and allo-HSCT, and it would be of great interest to explore this unique ecology in allo-HSCT.
Manipulation of the microbiota — the effect of antibiotics on GVHD.
The finding that higher bacterial diversity is associated with improved overall survival after allo-HSCT raises questions regarding the practice of bacterial decontamination in allo-HSCT recipients49. As mentioned above, initial studies in mice and humans indicated that gut decontamination with broad-spectrum antibiotics followed by treatment in sterile laminar-airflow isolation could decrease the incidence of acute GVHD in allo-HSCT recipients9-11. In addition, a more recent randomized study80 showed that the addition of metronidazole (which targets anaerobic bacteria) to ciprofloxacin as antibacterial prophylaxis resulted in reduced GVHD. However, other clinical studies did not confirm the benefit of decontamination for reducing GVHD81-83. Subsequently, the practice of laminar-airflow isolation was abandoned by most centres in the early 1990s.
A more recent retrospective study of 15 paediatric patients reported that the use of antibiotics effective against anaerobic bacteria resulted in a significant increase in GVHD with a reduction of gut anti-inflammatory Clostridia84. In this report, addition of clindamycin to levofloxacin in a mouse model of GVHD reduced Clostridia and exacerbated GVHD, recapitulating the findings from patients. In addition, a recent retrospective study of 500 patients from Canadian centres85 showed that gut decontamination before allo-HSCT resulted in higher incidence of grade II–IV acute GVHD. It is difficult to determine the causes for these different results; one possible explanation maybe the emergence of antibiotic-resistant enterococci, which makes successful gut decontamination very difficult.
Indeed, a recent retrospective study of 112 children undergoing allo-HSCT showed that failure of successful prophylactic decontamination monitored by microbiological surveillance, including stool culture, was associated with a significant increase in acute GVHD86. Colonization with antibiotic-resistant bacteria has been reported in the course of allo-HSCT19,27 and is associated with increased incidence of acute GVHD87. Meanwhile, a study of the prophylactic use of the broad-spectrum antibiotic meropenem during episodes of neutropenia (not for the purpose of prophylactic gastrointestinal decontamination) reported a favourable effect on morbidity by reducing febrile episodes without increasing the risk of infection from multidrug-resistant bacteria after allo-HSCT88. This study did not evaluate the risk of the broad-spectrum antibiotic use for the development of GVHD, and overall, no consensus has emerged regarding the ideal choice of antibiotic coverage for fever and neutropenia in allo-HSCT recipients. A recent retrospective single-centre study15 tried to address this question by analysing the effects of different-spectrum antibiotics for treating FN on the intestinal flora and transplant-related outcomes and found that the use of antibiotics with relatively more broad-spectrum activity, such as piperacillin–tazobactam, compared with the use of antibiotics with lesser broad-spectrum activity, such as cefepime, increased GVHD-related mortality. Administration of antibiotics with more broad-spectrum activity resulted in increased loss of commensal flora, including Bacteroidetes, Lactobacillus spp. and Clostridia. Further investigation in a mouse model of GVHD demonstrated that administration of antibiotics with more broad-spectrum activity could increase GVHD mortality. This was associated with similar changes in the intestinal microbiota, as seen in patients, as well as an increased abundance of Akkermansia muciniphila, which contributed to the degradation of the mucous layer in the colon. Corroborating these findings, metagenomic shotgun sequencing revealed increased levels of mucus-degrading bacterial enzymes in the stool specimens of GVHD mice treated with antibiotics with more broad-spectrum activity. These mice also showed worse GVHD of the colon, increased numbers of colon-infiltrating allogeneic CD4+ T cells and increased levels of IL-23 in the colon. A phase II randomized clinical trial is currently underway to evaluate the contribution of broad-spectrum antibiotics and microbiota injury to the development of GVHD by comparing piperacillin–tazobactam with cefepime as a treatment for FN89 (TABLE 1).
Another report confirmed the deleterious effect of antibiotics in a xenogeneic GVHD mouse model: a cocktail treatment of ampicillin, clindamycin, vancomycin and cefoperazone in the drinking water after allo-HSCT resulted in the development of more-aggressive GVHD90. Regarding the timing of systemic antibiotic administration and GVHD-related mortality, a retrospective study of 621 patients at two transplant centres showed that early exposure to antibiotics (between day −7 and day 0 of allo-HSCT) resulted in significant reduction of Clostridiales and increased GVHD-related mortality91. Taken together, these results suggest that broad-spectrum antibiotics can exacerbate colonic GVHD, and this supports the hypothesis that selecting limited-spectrum antibiotics can prevent microbiota injury and reduce colonic GVHD and GVHD-related mortality.
Although the use of fluoroquinolone prophylaxis to reduce neutropenia-related complications in patients with cancer has been disputed with the emergence of resistant organisms92-95, clinical practice guidelines96 recommend use of a fluoroquinolone for cancer patients with neutropenia to prevent febrile events and blood infections, a practice that was found in a meta-analysis to be associated with a reduction in all-cause mortality97. As an alternative to a fluoroquinolone (ciprofloxacin), a recent retrospective analysis in allo-HSCT recipients compared rifaximin (a poorly absorbed member of the rifamycin family widely utilized in IBD98,99 with less activity against Enterobacteriaceae) with ciprofloxacin plus metronidazole and found significantly reduced gut GVHD, reduced TRM and improved overall survival100.
Taken together, the above findings suggest the need for well-designed prospective studies to optimize the use of antibiotics in neutropenic allo-HSCT recipients to protect from bacteraemia or sepsis while limiting injury to the intestinal microbiota that could increase GVHD mortality. A randomized phase II clinical trial evaluating the impact of gut decontamination with vancomycin plus polymyxin B on microbial diversity after allo-HSCT is now underway101 (TABLE 1). In this paediatric population (4–23-year-old recipients of HLA-matched allo-HSCT), the effects of decontamination on immune reconstitution and GVHD are being analysed. Future studies should be performed together with detailed assessment of bacterial susceptibility to antibiotics, as the 16S ribosomal RNA sequence cannot provide this information. An example is the simultaneous use of metagenomic and metatranscriptomic sequencing in a study, which demonstrates a higher overall number and expression levels of antibiotic resistance genes in the microbiome after antibiotic treatment102.
The metabolome and GVHD.
Microbial metabolites can affect host immune responses and protect against the development of inflammatory diseases103,104. Bacterial fermentation in the colon results in the production of carbohydrate metabolites, including SCFAs, such as acetate, butyrate and propionate105. Butyrate and to a lesser extent propionate have been reported to induce Treg cells by regulating molecules that improve the homing of Treg cells to gut mucosa and increase forkhead box protein 3 (FOXP3) expression in the colon73,106. An initial report showed that acetate can ameliorate inflammation in the gut, lung and joints and that this effect was lost in mice deficient for its receptor G protein-coupled receptor 43 (GPR43; also known as FFAR2)107. Moreover, SCFAs are important energy sources for the microbiota itself as well as for IECs and are known to inhibit histone deacetylase (HDAC) activity108. A recent study analysed a possible role of SCFAs during GVHD75. The concentration of butyrate in the intestinal tissue of recipient mice was significantly decreased at day 7 after allo-HSCT, and this reduction coincided with impaired integrity of IEC junctions. When butyrate was given by intragastric gavage, integrity of IEC junctions was preserved, mitigating GVHD severity. The above-mentioned findings indicate that local changes of microbial metabolites can reduce intestinal epithelial tissue damage and mitigate GVHD severity. On the other hand, it should also be noted that butyrate may have cell type-specific effects, as it appears to inhibit the proliferation of intestinal stem cells55.
Relapse and the microbiota
The gut microbiota are potent modulators of systemic immune responses, and there is mounting evidence that microbiota can affect cancer immunosurveillance50,109-115. Several studies showed that intestinal microbiota can influence the immune response to systemic cancer chemotherapy, radiotherapy and immunotherapy17,116 and that disruption of the gut microbiota is associated with resistance to cancer therapy109,114. GVHD (especially chronic GVHD) is inversely associated with relapse1,2,117, as the allogeneic T cells causing GVHD also contribute to GVT activity. Because the development of GVHD is associated with changes in the intestinal flora as described above, studies to analyse changes in the intestinal microbiota and relapse after allo-HSCT seem warranted. A retrospective observational analysis of 541 patients undergoing allo-HSCT at a single centre identified a cluster of bacteria dominated by Eubacterium limosum that could serve as a biomarker of relapse risk: higher abundance of this cluster was associated with less relapse16. In this report, multiple stool specimens collected between day 0 and day 21 were analysed by 16S ribosomal RNA sequence. A time-weighted average abundance over the 3-week period was utilized to accommodate variegated collection time points and numbers of samples for each point. This methodological approach reduces biases that could occur when sampling only a single time point in the setting of the rapid microbiota shifts after allo-HSCT. E. limosum is a common member of the human microbiota118 and is known for its beneficial effect in an experimental model of colitis, which occurs through an increased butyrate production in the caecum119; however, no mechanism has been explored with regard to how this bacterium serves in the setting of allo-HSCT and GVHD. This finding may serve as a potential diagnostic biomarker or perhaps as a therapeutic target (for example, using a probiotic strategy discussed in the following section) to prevent relapse and improve overall survival after allo-HSCT, although these results need to be validated in multicentre observational and prospective clinical trials.
Targeting the microbiota
Various factors during allo-HSCT, including conditioning regimen, diet, infections, antibiotic use and immune reactions, result in some of the most drastic and rapid perturbations of the intestinal flora seen in any patient group19,20 (FIG. 5). Strategies targeting the microbiota to improve allo-HSCT can be summarized into four broad types: antibiotic, probiotic, prebiotic and postbiotic. In addition, the microbiota has potential for diagnostics120.
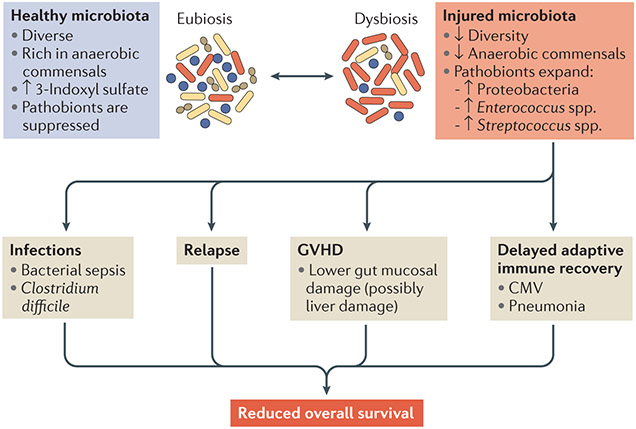
Figure 5 ∣. Microbiota injury and complications after allo-HSCT.
The top panels summarize the profiles of healthy microbiota before and after allogeneic haematopoietic stem cell transplantation (allo-HSCT) compared with those of injured microbiota after allo-HSCT. Microbiota injury results in infections, malignant relapse, graft-versus-host disease (GVHD) and possibly delayed immune reconstitution that contributes to additional infections (cytomegalovirus (CMV) infection and/or certain types of pneumonia that require adaptive immunity to eradicate). All of these can then contribute to reduced overall survival of patients after allo-HSCT.
Antibiotic strategies.
Antibiotic strategies include selecting more narrow-spectrum agents (for example, cefepime or rifaximin) that potentially spare beneficial anaerobic bacteria contributing to an anti-inflammatory milieu after allo-HSCT (for example, Blautia spp.) and minimizing the duration of antibiotic use that may result in an outgrowth of certain bacteria (for example, mucus-degrading bacteria such as A. muciniphila). Several strategies are being developed to prevent antibiotic-induced dysbiosis for purposes of preventing C. difficile-associated diarrhoea. If successful, such approaches, which include orally administered β-lactamases or activated charcoal, could be explored in the setting of GVHD121,122.
Prebiotic strategies.
Prebiotics are indigestible compounds, commonly oligosaccharides, that commensal bacteria have an advantage in metabolizing; this metabolism increases the production of metabolic products through bacterial fermentation33. Prebiotics can be administered to patients. Encouraging oral intake in general, or the administration of gastric nutritional supplementation and/or flora-targeted nutritional supplements such as inulin-type fructans and arabinoxylans (for example, arabinose and xylose), can also be considered. A phase I study administering fructooligosaccharide in patients undergoing allo-HSCT is ongoing123 (TABLE 1). Another clinical study evaluating the effect of a dietary supplement containing potato-based starch in allo-HSCT recipients is recruiting patients124 (TABLE 1) and is based on the observation that this starch can increase the production of butyrate by the microbiota in healthy subjects125.
Probiotic strategies.
A probiotic strategy consists of directly introducing one strain or selected strains of microorganisms that confer a benefit. This can be achieved by FMT (including nasoduodenal, trans-colonoscopic or enema-based applications or even oral intake of microbiota capsules).
Most patients show a decrease in bacterial diversity in the weeks after allo-HSCT, and up to one-half of bacteraemia episodes after allo-HSCT are preceded by intestinal bacterial domination of a pathobiont7,19, suggesting the potential effectiveness of administering probiotics that prevent pathobiont colonization. As mentioned above, there have been some case reports describing the effectiveness of FMT for refractory C. difficile-associated disease after allo-HSCT33, and a phase II clinical trial testing this is currently underway34 (TABLE 1). A recent clinical study indicated the safety and efficacy of FMT in patients with steroid-refractory or steroid-dependent gut GVHD126. In this small pilot study, three out of four patients receiving FMT from their spouses or relatives achieved complete responses after the treatment, which was correlated with an increase in Treg cells in the peripheral blood. Another study showed successful FMT in three patients with grade IV acute GVHD involving the lower gastrointestinal tract after the failure of established immunosuppressive therapies, including corticosteroids127. In this case report, healthy adult subjects were the faecal donor sources, and after one to six doses of FMT, all patients showed improved diversity of the gut microbiota. Treatment with oral, capsulized, frozen FMT (‘crapsules’)128 is a potential method for maintaining bacterial diversity after allo-HSCT, and a phase I study to determine the feasibility of the treatment in allo-HSCT recipients is ongoing129 (TABLE 1). Data are limited for determining the optimum faecal donor, and it is still undetermined whether it is better to use FMT from the stem cell donor or from a healthy random volunteer or sibling. Further studies are needed to prove the effectiveness of FMT in reducing GVHD and infection or in enhancing overall survival in allo-HSCT recipients.
The surrounding environment of the patients may also affect the diversity of the gut microbiota after allo-HSCT130, and an ongoing clinical study aims to compare the incidence of acute grade II—IV GVHD between patients receiving patient-centred medical home care and those receiving standard hospital care131 (TABLE 1). Administration of Lactobacillus spp. was also shown to ameliorate GVHD in murine GVHD models14,132, and a clinical trial is ongoing to analyse the benefit of oral Lactobacillus rhamnosus str. GG administration in the improvement of GVHD133 (TABLE 1). Of note, a case report describes a potential risk of probiotic use in a patient who was immunocompromised and experienced sepsis after excessive consumption of probiotic Lactobacillus acidophilus-enriched yogurt after autologous transplant134. Other probiotic bacteria, such as Bifidobacteria spp., have been associated with improved responses to cancer immunotherapy112 and resistance to E. coli infection135 in animal models. As a potential strategy for passive bacterial modification in the gut in order to improve GVHD outcome, oral immunoglobulin administration can be used to reduce pathogenic bacteria such as E. coli and increase the amount of beneficial probiotic bacteria such as Lactobacillus reuteri. In a mouse study136, immunoglobulin yolk antibodies from hens immunized with pathogenic E. coli, Clostridium perfringens and Salmonella enterica subsp. enterica serovar Typhimurium were administered starting 2 days before allo-HSCT, and this method led to reduced clinical GVHD scores and improved survival. In the near future, probiotic bacteria could likely be genetically engineered to produce certain metabolites or AMPs that mediate a benefit to the host.
Postbiotic strategies.
Postbiotic interventions involve providing bacterial metabolic products directly, bypassing the bacteria completely. This approach might be safer than probiotic strategies in allo-HSCT recipients who are severely immunocompromised. Methods include administering SCFAs (for example, butyrate) or their analogues or introducing secondary bile acids that have been shown to suppress C. difficile32,75. A pilot study of five patients with recurrent C. difficile infection indicated a potential benefit of sterile faecal filtrates — a safer method than FMT — that contain bacterial components as well as metabolites but do not contain live microorganisms to restore microbial diversity137. As a potential organic compound to modulate microbiota other than SCFAs, indole and its derivatives inhibit the growth of Gram-negative bacteria and Candida spp.61,138. In addition, various microbiota-derived aryl hydrocarbon receptor ligands have been utilized to stimulate mucosal immune cells to attenuate intestinal inflammation in a mouse colitis model139, and potentially, this could improve gut integrity and prevent gut GVHD.
Diagnostics.
Detailed studies of the alterations of the microbiota in GVHD or malignant relapse following allo-HSCT are ongoing and suggest changes in the abundance of one or more bacteria (for example, Blautia spp.13 and E. limosum16) at certain time points during allo-HSCT. These could lead to the development of bacterial diagnostic or prognostic biomarkers. Also, a clinical report suggests that genotyping of fucosyltransferase 2 (FUT2) can serve as a biomarker for GVHD and bacteraemia after allo-HSCT140. The FUT2 gene is known to regulate ABH blood group antigens in the mucous layer and is reported to play a role in shaping host microbiota141-143. The FUT2 genotype influences the risk of Crohn’s disease as well as the risk of GVHD and bacteraemia after allo-HSCT, possibly through alterations of glycosylation of intestinal surface proteins and microbiota composition of the host140,141. Furthermore, 3-indoxyl sulfate, a urinary metabolite produced by Clostridia, is another potential biomarker of gut microbiota health, and it predicts reduced gut GVHD and survival in patients7. Loss of bacterial diversity correlates with mean urinary 3-indoxyl sulfate levels, and the Firmicute families Lachnospiraceae and Ruminococcaceae (both in the Clostridia class) were identified as important contributors to the production of 3-indoxyl sulfate. These bacterial families are linked to reduced intestinal inflammation138. Given the rapid turnaround time of the assay testing for 3-indoxyl sulfate levels, it could potentially be applied to evaluate the health of microbiota in patients in clinical trials where the aim is to target restoration of injured microbiota during GVHD.
Conclusions and future directions
There has been great progress since the 1970s in discerning the role of the microbiota in allo-HSCT. Preliminary studies that may lead to novel strategies to improve outcomes after allo-HSCT include those investigating FMT, prebiotic (oligosaccharide and potato-based starch) administration, the use of different antibiotics, gut decontamination and the role of E. limosum in relapse. Given that microbiota composition can vary owing to geographic differences144,145, it is critical to carry out multicentre studies. Further studies are needed to better characterize the changes in the microbiota during allo-HSCT and GVHD in a prospective multicentre fashion, the variables that affect these changes of intestinal microbiota, and the effects of bacterial ligands146 and their metabolites on both the immune and non-immune phenotypes of the host. Novel approaches that protect the microbiota or restore it after injury need to be evaluated using multi-institutional studies; such new findings should change the current standard care routines for allo-HSCT recipients.
Alloreactive donor T cells.
T cells that have been activated after encountering antigens within the context of major histocompatibility complex (MHC) molecules other than those present during thymic selection. After allogeneic haematopoietic stem cell transplantation, T cells can be allogeneically activated by encounter with an unknown (foreign) MHC molecule (major histocompatibility mismatch) or with a known (matched) MHC molecule presenting a foreign antigen (minor histocompatibility mismatch).
Neutropenia.
A condition of abnormally low numbers of neutrophils within the blood. This is an immune system condition that may lead to infections.
Febrile neutropenia.
(FN). The development of fever, often with other signs of infections, occurring during the period of low neutrophil counts (neutropenia) after allogeneic haematopoietic stem cell transplantation or chemotherapy for cancer. Neutropenia typically occurs 8–12 days after chemotherapy and typically lasts for 10–25 days.
Bacteraemia.
The presence of bacteria in the blood (usually resulting from impaired gut barrier function owing to a conditioning regimen and/or graft-versus-host disease in allogeneic haematopoietic stem cell transplantation) that leads to sepsis.
Facultative bacteria.
A category of bacteria that can thrive under aerobic or anaerobic conditions.
Gut consortium.
A group of microbiota species or strains. Interactions among the members of a consortium may form an ecological community in which members compete with one another or in which products of one member are utilized by or are toxic to another member.
Sepsis.
The immune reaction of the host to bacteria.
Bacterial ligands.
Components of the bacterial cell surface that interact with the host cell receptor. They interact with phagocytes and stimulate subsequent evasion of innate immune responses.
Innate lymphoid cells.
(ILCs). A population of immune lymphoid cells that are morphologically similar to B cells and T cells but lack pattern-recognition receptors and rearranged antigen receptors (and thus are unable to directly mediate antigen-specific responses). They secrete a high concentration of cytokines and play key roles in effector and regulatory functions in innate immunity, metabolic homeostasis and tissue remodelling.
Germ-free mice.
Also known as gnotobiotic mice, these mice are maintained under strictly sterile conditions in which absolutely no bacteria are present in or on the animals. Individual bacterial strains or groups of strains (consortia) can be introduced in a controlled fashion (axenic conditions).
Aplastic anaemia.
A form of bone marrow failure in which the bone marrow cannot make enough new blood cells. It is characterized by peripheral pancytopenia (decrease of red blood cells, white blood cells and platelets) and bone marrow hypoplasia.
Laminar-airflow isolation.
A method that provides a low-pathogen environment and prevents exogenous infection during hospitalization of patients with neutropenia and other immunodeficient diseases. It involves filtered air moving along separate parallel flow planes to patient rooms with no or minimal crossover of air streams (or lamina).
Specific pathogen-free (SPF) mice.
Mice housed under standard laboratory conditions. These mice are free of specific pathogens but otherwise have a normal microbiota. The lists of specific pathogens tested vary by supplier and institution and typically include opportunistic organisms that do not cause illness in immunocompetent hosts but can be harmful to immunocompromised mice, for example, after allogeneic haematopoietic stem cell transplantation.
Oral nutrition.
The intake of food through the mouth and oesophagus to provide nutrients for health.
Total parenteral nutrition.
A method to feed a person intravenously, completely bypassing the usual process of eating and digestion. The nutritional formulae typically include glucose, amino acids, lipids and salts, with the supplementation of vitamins and minerals as needed.
16S ribosomal RNA sequence.
A method used for identification, classification and quantification of microorganisms (the 16S ribosomal RNA subunit is an essential, highly conserved component in the 30S ribosomal complex in prokaryotes).
Enteral nutrition.
A method of delivering a person’s calorific nutritional requirements by use of a tube (tube feeding) into the stomach, achieved by syringe, gravity or pump.
Pathobiont.
A potentially harmful (disease-causing or pathological) organism that under normal conditions lives as a component of a healthy microbiota population (symbiont).
Acknowledgements
The authors thank O.M. Smith for editing the manuscript and E. Velardi, J. U. Peled and C. Stein-Thoeringer for their scientific input and editing of the manuscript. This research was supported by US National Institutes of Health award numbers R01-HL069929 (M.R.M.v.d.B.), R01-AI101406 (M.R.M.v.d.B.), P01-CA023766 (R. J. O’Reilly), Project 4 of P01-CA023766 (M.R.M.v.d.B.), 1R01HL123340-01A1 (K. Cadwell) and R01–AI100288 (M.R.M.v.d.B.). Support was also received from The American Society for Blood and Marrow Transplantation (Y.S.), The Lymphoma Foundation, The Susan and Peter Solomon Divisional Genomics Program, Cycle for Survival and P30 CA008748 Memorial Sloan Kettering Cancer Center Support Grant/Core Grant. This research was also supported by the Parker Institute for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center.
Footnotes
Competing interests
The authors declare competing interests; see Web version for details.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jenq RR & van den Brink MR Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nature reviews. Cancer 10, 213–221 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Zeiser R & Blazar BR Acute graft-versus-host disease — biologic process, prevention, and therapy. N. Engl. J. Med 377, 2167–2179 (2017).This is a detailed review of the current advances in the pathophysiology of GVHD and its treatment.
- 3.Shono Y et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood 115, 5401–5411 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Kamble RT, Chang CC, Sanchez S & Carrum G Central nervous system graft-versus-host disease: report of two cases and literature review. Bone Marrow Transplant 39, 49–52 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Hartrampf S et al. The central nervous system is a target of acute graft versus host disease in mice. Blood 121, 1906–1910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimoji S et al. Graft-versus-host disease targets ovary and causes female infertility in mice. Blood 129, 1216–1225 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Holler B et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant 20, 640–645 (2014).This study provides serial stool specimen analyses of 31 patients after allo-HSCT and shows loss of diversity and expansion of Enterococcus spp. in patients with GVHD.
- 8.Bhatt AS et al. Sequence-based discovery of Bradyrhizobium enterica in cord colitis syndrome. N. Engl. J. Med 369, 517–528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones JM, Wilson R & Bealmear PM Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radial Res. 45, 577–588 (1971). [PubMed] [Google Scholar]
- 10.van Bekkum DW, Roodenburg J, Heidt PJ & van der Waaij D Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal micro flora. J. Natl Cancer Institute 52, 401–404 (1974).This is an early mouse study showing that germ-free conditions or gut decontamination with antibiotics reduce mortality from GVHD.
- 11.Storb R et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N. Engl. J. Med 308, 302–307 (1983). [DOI] [PubMed] [Google Scholar]
- 12.Eriguchi Y et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood 120, 223–231 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Jenq RR et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant 21, 1373–1383 (2015).A clinical study evaluating stool specimens of 64 patients (at day 12 after allo-HSCT) showing that Blautia (Clostridial species) is associated with less GVHD-related mortality.
- 14.Jenq RR et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med 209, 903–911 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shono Y et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl Med 8, 339ra71 (2016).This study shows that broad-spectrum antibiotic use is associated with increased GVHD-related mortality after allo-HSCT in both mice and humans.
- 16.Peled JU et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J. Clin. Oncol 35, 1650–1659 (2017).This is the first study showing the association between relapse and microbiota after allo-HSCT, demonstrating an inverse relationship between the abundance of Eubacterium limosum and relapse in the first 3 weeks after transplant.
- 17.Roy S & Trinchieri G Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 17, 271–285 (2017).This is a review detailing the role of the microbiota in modulating chemotherapy, radiotherapy and immunotherapy.
- 18.Eckburg PB et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taur Y et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis 55, 905–914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taur Y, Jenq RR, Ubeda C, van den Brink M & Pamer EG Role of intestinal microbiota in transplantation outcomes. Best Prcict. Res. Clin. Haematol 28, 155–161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shono Y, Docampo MD, Peled JU, Perobelli SM & Jenq RR Intestinal microbiota-related effects on graft-versus-host disease. Int. J. Hemcitol 101, 428–437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyle UG et al. Longitudinal follow-up of body composition in hematopoietic stem cell transplant patients. Bone Marrow Transplant. 35, 1171–1177 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulou A, Lloyd DR, Williams MD, Darbyshire PJ & Booth IW Gastrointestinal and nutritional sequelae of bone marrow transplantation. Arch. Dis. Childhood 75, 208–213 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taur Y et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124, 1174–1182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin J et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ubeda C et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Invest 120, 4332–4341 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris B et al. Gut microbiota predict pulmonary infiltrates after allogeneic hematopoietic cell transplantation. Am. J. Respir. Crit. Care Med 194, 450–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso CD & Man KA Clostridium difficile infection among hematopoietic stem cell transplant recipients: beyond colitis. Curr. Opin. Infect. Dis 26, 326–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willems L et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Biol. Blood Marrow Transplant 18, 1295–1301 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Kinnebrew MA et al. Early Clostridium difficile infection during allogeneic hematopoietic stem cell transplantation. PIoS ONE 9, e90158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buffie CG et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andermann TM, Rezvani A & Bhatt AS Microbiota manipulation with prebiotics and probiotics in patients undergoing stem cell transplantation. Curr. Hemcitol. Malig. Rep 11, 19–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02269150 (2017). [DOI] [PubMed]
- 35.Quera R, Espinoza R, Estay C & Rivera D Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J. Crohns Colitis 8, 252–253 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Schwartz M, Gluck M & Koon S Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am. J. Gastroenterol 108, 1367 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Chang BW & Rezaie A Irritable bowel syndrome-like symptoms following fecal microbiota transplantation: a possible donor-dependent complication. Am. J. Gastroenterol 112, 186–187 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Antin JH & Ferrara JL Cytokine dysregulation and acute graft-versus-host disease. Blood 80, 2964–2968 (1992). [PubMed] [Google Scholar]
- 39.Hill GR & Ferrara JL The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 95, 2754–2759 (2000). [PubMed] [Google Scholar]
- 40.Thaiss CA, Zmora N, Levy M & Elinav E The microbiome and innate immunity. Nature 535, 65–74 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Murphy S & Nguyen VH Role of gut microbiota in graft-versus-host disease. Leuk. Lymphoma 52, 1844–1856 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Mathewson N & Reddy P The microbiome and graft versus host disease. Curr. Stem Cell Rep 1, 39–47 (2015). [Google Scholar]
- 43.Heidegger S, van den Brink MR, Haas T & Poeck H The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation. Front. Immunol 5, 337 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Xu S, Ren Z, Jiang J & Zheng S Gut microbiota and allogeneic transplantation. J. Transl Med 13, 275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonnenberg GF & Artis D Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37, 601–610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanash AM et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Zhao Y, Cheng Q, Wu D & Liu H The role of intestinal microbiota in acute graft-versus-host disease. J. Immunol. Res 2015, 145859 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeiser R, Socie G & Blazar BR Pathogenesis of acute graft-versus-host disease: from intestinal microbiota alterations to donor T cell activation. Br. J. Haematol 175, 191–207 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Whangbo J, Ritz J & Bhatt A Antibiotic-mediated modification of the intestinal microbiome in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 52, 183–190 (2017).This is a review that extensively explores previous studies on gut decontamination for acute GVHD prevention.
- 50.Zama D et al. Gut microbiota and hematopoietic stem cell transplantation: where do we stand? Bone Marrow Transplant. 52, 7–14 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Staffas A, Burgos da Silva M & van den Brink MR The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 129, 927–933 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peled JU, Hanash AM & Jenq RR Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood 128, 2395–2402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrara JL, Smith CM, Sheets J, Reddy P & Serody JS Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis. J. Clin. Invest 127, 2441–2451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macpherson AJ, Slack E, Geuking MB & McCoy KD The mucosal firewalls against commensal intestinal microbes. Semin. Immunopathol 31, 145–149 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Kaiko GE et al. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165, 1708–1720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peterson LW & Artis D Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol 14, 141–153 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Levine JE et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood 122, 1505–1509 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato T et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindemans CA et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zelante T et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Ferrara JL et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 118, 6702–6708 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brandi K et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zenewicz LA et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol 190, 5306–5312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02406651 (2015). [DOI] [PubMed]
- 66.Hayase E et al. R-Spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J. Exp. Med 214, 3507–3518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heimesaat MM et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59, 1079–1087 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Steck N et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 141, 959–971 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Kim SO, Sheikh HI, Ha SD, Martins A & Reid G G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell. Microbiol 8, 1958–1971 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Atarashi K et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Tanoue T & Honda K Induction of Treg cells in the mouse colonic mucosa: a central mechanism to maintain host-microbiota homeostasis. Semin. Immunol 24, 50–57 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Narushima S et al. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes. 5, 333–339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arpaia N et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furusawa Y et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Mathewson ND et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol 17, 505–513 (2016).This study describes the role of butyrate in maintaining the integrity of IEC junctions that are impaired by GVHD.
- 76.Varelias A et al. Acute graft-versus-host disease is regulated by an IL-17-sensitive microbiome. Blood 129, 2172–2185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laterza L, Rizzatti G, Gaetani E, Chiusolo P & Gasbarrini A The gut microbiota and immune system relationship in human graft-versus-host disease. Mediterr. J. Hemcitol. Infect. Dis 8, e2016025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiusolo P et al. Gut microbiome changes after stem cell transplantation. Blood 126, 1953 (2015). [Google Scholar]
- 79.Tawara I et al. Influence of donor microbiota on the severity of experimental graft-versus-host-disease. Biol. Blood Marrow Transplant 19, 164–168 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beelen DW, Elmaagacli A, Muller KD, Hirche H & Schaefer UW Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood 93, 3267–3275 (1999).This is a prospective randomized trial from Germany (n = 134) indicating that the elimination of anaerobes with the addition of metronidazole to ciprofloxacin results in reduced grade II–IV GVHD.
- 81.Petersen FB et al. Infectious complications in patients undergoing marrow transplantation: a prospective randomized study of the additional effect of decontamination and laminar air flow isolation among patients receiving prophylactic systemic antibiotics. Sccind. J. Infect. Dis 19, 559–567 (1987). [DOI] [PubMed] [Google Scholar]
- 82.Passweg JR et al. Influence of protective isolation on outcome of allogeneic bone marrow transplantation for leukemia. Bone Marrow Transplant. 21, 1231–1238 (1998). [DOI] [PubMed] [Google Scholar]
- 83.Russell JA et al. Early outcomes after allogeneic stem cell transplantation for leukemia and myelodysplasia without protective isolation: a 10-year experience. Biol. Blood Marrow Transplant 6, 109–114 (2000). [DOI] [PubMed] [Google Scholar]
- 84.Simms-Waldrip TR et al. Antibiotic-induced depletion of anti-inflammatory Clostridia is associated with the development of graft-versus-host disease in pediatric stem cell transplantation patients. Biol. Blood Marrow Transplant 23, 820–829 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Routy B et al. The influence of gut-decontamination prophylactic antibiotics on acute graft-versus-host disease and survival following allogeneic hematopoietic stem cell transplantation. Oncoimmunology 6, e1258506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vossen JM et al. Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PIoS ONE 9, e105706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bilinski J et al. Impact of gut colonization by antibiotic-resistant bacteria on the outcomes of allogeneic hematopoietic stem cell transplantation: a retrospective, single-center study. Biol. Blood Marrow Transplant 22, 1087–1093 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Perez-Simon JA et al. Antibiotic prophylaxis with meropenem after allogeneic stem cell transplantation. Bone Marrow Transplant. 33, 183–187 (2004). [DOI] [PubMed] [Google Scholar]
- 89.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT03078010 (2017). [DOI] [PubMed]
- 90.Khoruts A et al. Toward revision of antimicrobial therapies in hematopoietic stem cell transplantation: target the pathogens, but protect the indigenous microbiota. Transl Res. 179, 116–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weber D et al. Microbiota disruption induced by early use of broad-spectrum antibiotics is an independent risk factor of outcome after allogeneic stem cell transplantation. Biol. Blood Marrow Transplant 23, 845–852 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bucaneve G et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N. Engl. J. Med 353, 977–987 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Cullen M et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N. Engl. J. Med 353, 988–998 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Leibovici L et al. Antibiotic prophylaxis in neutropenic patients: new evidence, practical decisions. Cancer 107, 1743–1751 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Reuter S et al. Impact of fluoroquinolone prophylaxis on reduced infection-related mortality among patients with neutropenia and hematologic malignancies. Clin. Infect. Dis 40, 1087–1093 (2005). [DOI] [PubMed] [Google Scholar]
- 96.Freifeld AG et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin. Infect. Dis 52, e56–e93 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Satlin MJ et al. Impact of prophylactic levofloxacin on rates of bloodstream infection and fever in neutropenic patients with multiple myeloma undergoing autologous hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant 21, 1808–1814 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopetuso LR, Petito V, Scaldaferri R & Gasbarrini A Gut microbiota modulation and mucosal immunity: focus on rifaximin. Mini Rev. Med. Chem 16, 179–185 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Maccaferri S et al. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J. Antimicro b. Chemother 65, 2556–2565 (2010). [DOI] [PubMed] [Google Scholar]
- 100.Weber D et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 51, 1087–1092 (2016).This retrospective study describes the preserved diversity and better allo-HSCT outcomes with rifaximin as compared with a ciprofloxacin and metronidazole decontamination regimen.
- 101.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02641236 (2016). [DOI] [PubMed]
- 102.Kaysen A et al. Integrated meta-omic analyses of the gastrointestinal tract microbiome in patients undergoing allogeneic hematopoietic stem cell transplantation. Transl Res. 186, 79–94.e1 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Wen L et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455, 1109–1113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mazmanian SK, Round JL & Kasper DL A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Rooks MG & Garrett WS Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol 16, 341–352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith PM et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maslowski KM et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wong JM, de Souza R, Kendall CW, Emam A & Jenkins DJ Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol 40, 235–243 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Iida N et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paulos CM et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8 + T cells via TLR4 signaling. J. Clin. Invest 117, 2197–2204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peuker K et al. Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat. Med 22, 506–515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sivan A et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vetizou M et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Viaud S et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zitvogel L, Ayyoub M, Routy B & Kroemer G Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Zitvogel L, Daillere R, Roberti MP, Routy B & Kroemer G Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol 15, 465–478 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Blazar BR, Murphy WJ & Abedi M Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol 12, 443–458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajilic-Stojanovic M & de Vos WM The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev 38, 996–1047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanauchi O et al. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J. Gastroenterol 12, 1071–1077 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peled JU Jenq RR Holler B & van den Brink MR Role of gut flora after bone marrow transplantation. Nat. Microbiol 1, 16036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Gunzburg J et al. Targeted adsorption of molecules in the colon with the novel adsorbent-based medicinal product, DAV132: A proof of concept study in healthy subjects. J. Clin. Pharmacol 55, 10–16 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Kaleko M et al. Development of SYN-004, an oral beta-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe 41, 58–67 (2016). [DOI] [PubMed] [Google Scholar]
- 123.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02805075 (2016). [DOI] [PubMed]
- 124.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02763033 (2016). [DOI] [PubMed]
- 125.Venkataraman A et al. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 4, 33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kakihana K et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 128, 2083–2088 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spindelboeck W et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haemcitologica 102, e210–e213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Youngster I et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312, 1772–1778 (2014). [DOI] [PubMed] [Google Scholar]
- 129.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02733744 (2017). [DOI] [PubMed]
- 130.Song SJ et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2, e00458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02218151 (2016). [DOI] [PubMed]
- 132.Gerbitz A et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood 103, 4365–4367 (2004). [DOI] [PubMed] [Google Scholar]
- 133.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02144701 (2017). [DOI] [PubMed]
- 134.Mehta A, Rangarajan S & Borate U A cautionary tale for probiotic use in hematopoietic SCT patients-Lactobacillus acidophilus sepsis in a patient with mantle cell lymphoma undergoing hematopoietic SCT. Bone Marrow Transplant. 48, 461–462 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Fukuda S et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011). [DOI] [PubMed] [Google Scholar]
- 136.Bouazzaoui A et al. Reduction of aGVHD using chicken antibodies directed against intestinal pathogens in a murine model. Blood 129, 1052–1055 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ott SJ et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 152, 799–811 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Weber D et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 126, 1723–1728 (2015). [DOI] [PubMed] [Google Scholar]
- 139.Lamas B et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nature Med. 22, 598–605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rayes A et al. A genetic modifier of the gut microbiome influences the risk of graft-versus-host disease and bacteremia after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant 22, 418–422 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Rausch P et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl Acad. Sci. USA 108, 19030–19035 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rouquier S et al. Molecular cloning of a human genomic region containing the H blood group alpha(1,2)fucosyltransferase gene and two H locus-related DNA restriction fragments. Isolation of a candidate for the human Secretor blood group locus. J. Biol. Chem 270, 4632–4639 (1995). [DOI] [PubMed] [Google Scholar]
- 143.Wacklin P et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS ONE 6, e20113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gupta VK, Paul S & Dutta C Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol 8, 1162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yatsunenko T et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kline KA, Falker S, Dahlberg S, Normark S & Henriques-Normark B Bacterial adhesins in host-microbe interactions. Cell Host Microbe 5, 580–592 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Khosravi A et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fiedler K, Kokai E, Bresch S & Brunner C MyD88 is involved in myeloid as well as lymphoid hematopoiesis independent of the presence of a pathogen. Am. J. Blood Res 3, 124–140 (2013). [PMC free article] [PubMed] [Google Scholar]
- 149.Yanez A, Goodridge HS, Gozalbo D & Gil ML TLRs control hematopoiesis during infection. Eur. J. Immunol 43, 2526–2533 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Iwamura C, Bouladoux N, Belkaid Y, Sher A & Jankovic D Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood 129, 171–176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Josefsdottir KS, Baldridge MT, Kadmon CS & King KY Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 129, 729–739 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beura LK et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reese TA et al. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe 19, 713–719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schulte C, Reinhardt W, Beelen D, Mann K & Schaefer U Low T3-syndrome and nutritional status as prognostic factors in patients undergoing bone marrow transplantation. Bone Marrow Transplant. 22, 1171–1178 (1998). [DOI] [PubMed] [Google Scholar]
- 155.Ursell LK, Metcalf JL, Parfrey LW & Knight R Defining the human microbiome. Nutr. Rev 70 (Suppl. 1), S38–44 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cotillard A et al. Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Baumgartner A et al. Revisiting nutritional support for allogeneic hematologic stem cell transplantation — a systematic review. Bone Marrow Transplant. 52, 506–513 (2017). [DOI] [PubMed] [Google Scholar]
- 158.van der Meij BS et al. Nutritional support in patients with GVHD of the digestive tract: state of the art. Bone Marrow Transplant. 48, 474–482 (2013). [DOI] [PubMed] [Google Scholar]
- 159.Martin-Salces M, de Paz R, Canales MA, Mesejo A & Hernandez-Navarro F Nutritional recommendations in hematopoietic stem cell transplantation. Nutrition 24, 769–775 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Rzepecki P, Barzal J & Oborska S Blood and marrow transplantation and nutritional support. Supportive Care Cancer 18 (Suppl. 2), S57–S65 (2010). [DOI] [PubMed] [Google Scholar]
- 161.Flowers ME et al. Long-Term Follow-Up After Hematopoietic Stem Cell Transplant. General Guidelines for Referring Physicians (Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance, Seattle, 2008). [Google Scholar]
- 162.van der Meij BS, Wierdsma NJ, Janssen JJ, Deutz NB & Visser OJ If the gut works, use it! But does the gut work in gastrointestinal GvHD? Bone Marrow Transplant. 52, 466–469 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Murray SM & Pindoria S Nutrition support for bone marrow transplant patients. Cochrane Database Syst. Rev 1, CD002920 (2009). [DOI] [PubMed] [Google Scholar]
- 164.Seguy D et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation 82, 835–839 (2006). [DOI] [PubMed] [Google Scholar]
- 165.Svahn BM et al. Case-control comparison of at-home and hospital care for allogeneic hematopoietic stem-cell transplantation: the role of oral nutrition. Transplantation 85, 1000–1007 (2008). [DOI] [PubMed] [Google Scholar]
- 166.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT01955772 (2016). [DOI] [PubMed]
- 167.Taur Y Intestinal microbiome changes and stem cell transplantation: lessons learned. Virulence 7, 930–938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT03102060 (2017). [DOI] [PubMed]
- 169.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT02461199 (2015). [DOI] [PubMed]