Abstract
In this article, we review the anatomical inputs and outputs to the mouse primary visual cortex, area V1. Our survey of data from the Allen Institute Mouse Connectivity project indicates that mouse V1 is highly interconnected with both cortical and subcortical brain areas. This pattern of innervation allows for computations that depend on the state of the animal and on behavioral goals, which contrasts with simple feedforward, hierarchical models of visual processing. Thus, to have an accurate description of the function of V1 during mouse behavior, its involvement with the rest of the brain circuitry has to be considered. Finally, it remains an open question whether the primary visual cortex of higher mammals displays the same degree of sensorimotor integration in the early visual system.
Keywords: V1 circuits, transcortical projections, corticofugal projections, corticopetal projections, sensorimotor control, behavioral state
1. INTRODUCTION
As early as 1929, Karl Lashley (1929) realized that removal of the visual cortex of rodents induced very different deficits than those resulting from retinal blindness. In the absence of a visual cortex, rodents were more severely affected on tasks that required the utilization of multiple senses. Based on the transcortical afferents of the mouse, it is apparent that primary visual cortex, area V1, is more than a duplicated retina, as large portions of the sensorimotor cortex innervate V1 (Figure 1) (Oh et al. 2014; http://connectivity.brain-map.org/). Lesions confined to the rodent visual cortex (but spanning beyond the V1 border) are known to disrupt visual behaviors such as visual acuity, pattern discrimination, motion parallax, and running toward and orienting to both stationary and moving visual stimuli (Dean 1981, Ellard et al. 1986, Ingle et al. 1979, Mlinar & Goodale 1984, Petruno et al. 2013, Schneider 1969). Lesions of V1 abolish the direction tuning of neurons in the superior colliculus (SC) (Rhoades & Chalupa 1978), thereby causing animals to undershoot the position of moving targets (Ingle 1981, Ingle et al. 1979). In mice, V1 lesions also shift the balance of bistable plaid perception, as judged by optokinetic nystagmus (OKN) (Palagina et al. 2017). Additionally, damage to cortical regions outside of V1 including the parietal, temporal, and frontal cortices produces long-term deficits in various visual tasks such as attention, recognition, and discrimination (Aggleton et al. 1997, Bussey et al. 1997, Kahn et al. 2012, Muir et al. 1996).
Figure 1.
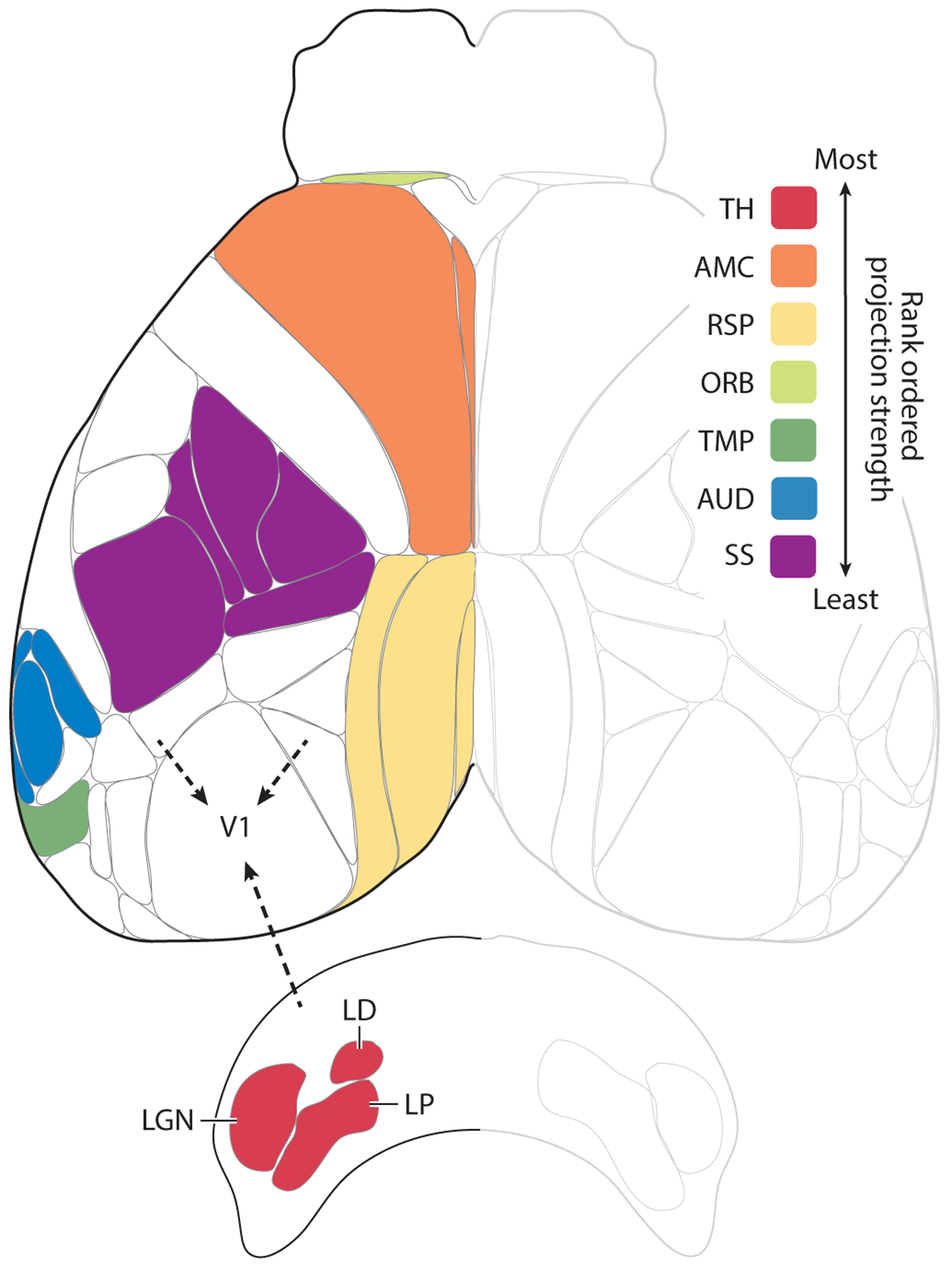
Afferent projections to V1. Shown is a top view of the mouse neocortex based on the Allen common coordinate framework v.3 plus the thalamic nuclei that project to V1. Robustness of afferent projections outside of the visual cortex to V1 is shown rank ordered according to the density of projections adjusted for injection size. The inputs from most robust to least robust were: (1) the dorsal and ventral part of the lateral geniculate nucleus (LGN), lateral posterior (LP) and lateral dorsal nuclei (LD) of the thalamus (TH); (2) the secondary motor and anterior cingulate areas (AMC); (3) the retrosplenial areas and postsubiculum (RSP); (4) the orbital areas (ORB) (note that, for visibility reasons, only the area just anterior to AMC is indicated as part of the orbital areas); (5) the temporal, ectorhinal, perirhinal, entorhinal, postrhinal, subiculum, and CA1 areas (TMP) (note that only parts of the areas are visible); (6) the auditory areas (AUD); and (7) the somatosensory areas, including the whisker representation (SS). Details regarding the injections: We examined recombinant adeno-associated virus injections into wild-type mice from the Allen dataset into visual and non-visual areas and used a combination of projection pixel density and intensity (weighted by injection volume) to develop a qualitative ranking of projection strength (robustness) between areas. For V1 afferent connectivity, injections that also infected visual areas were discarded.
The results above suggest that information exchange between V1 and other areas of the brain is important for various sensorimotor tasks that involve vision, and this involvement must be reflected in its connections with the rest of the brain. Our anatomical analysis (Figures; see Figure 1 for details) utilizing the Allen Mouse Brain Atlas (Oh et al. 2014; http://connectivity.brain-map.org/) suggests that area V1 of the mouse is highly interconnected with the neocortex, so that it has access to information regarding the sensorimotor state of an animal: multimodal, spatial, motor, and even attentional states. In addition, the corticofugal projections of V1 innervate the major oculomotor centers involved in the execution of eye movements and orienting responses to and from visual stimuli.
2. AFFERENT PROJECTIONS TO V1
When we exclude the visuo-cortical areas, the strongest input to V1 originates from the lateral geniculate nucleus (LGN) (Figure 1). LGN relays a large diversity of feature coding by mouse retinal ganglion cells to the cortex (Baden et al. 2016, Rosón et al. 2019), and for a long time, neurons in V1 were thought to linearly combine this information from the LGN. Studies of V1 have typically concentrated on neural responses to unimodal stimuli, and until recently, such experiments were often performed on anesthetized animals. It was the experimental work of Niell & Stryker (2010) that began to move the field away from this tradition. In their study, head-fixed mice were allowed to run on a spherical treadmill. Visual responses of the neurons were markedly enhanced, often doubling in frequency, and this augmentation was dependent on locomotor velocity. While Niell & Stryker found that the enhancement of activity was not evident in the LGN, subsequent work did find state-dependent modulation in the LGN (Erisken et al. 2014), indicating that some forms of state-dependent modulation do have effects upstream of V1.
Two other thalamic nuclei that project to V1 are the lateral posterior (LP) and the lateral dorsal (LD) nuclei (Figure 1). The LP nucleus is considered as the pulvinar nucleus homolog (Zhou et al. 2017a). It receives strong input from the SC and can greatly impact the activity and tuning of the cells in higher-order visual areas (Beltramo & Scanziani 2019, Tohmi et al. 2014). Projections from the LP nucleus are believed to contribute to the residual visual performance after V1 lesioning (Dean 1981), but their functional significance has not been established. Ahmadlou et al. (2018) used optogenetics to silence the SC, and they observed a reduction in V1 activity. They hypothesized that this effect was through the LP nucleus but interestingly, in these experiments, the SC modulated the activity in V1 even when the activity in the LP nucleus was suppressed, most likely through the tectogeniculate pathway. Contrary to the locomotion signals that come from LGN, axons from the LP nucleus convey information about the discrepancies between self-motion and external motion (Roth et al. 2016), and when compared to the axons from the LGN, they have distinct spatial localization within V1 (Bista et al. 2019). Based on connectivity and functional properties, the LP nucleus can be separated into three subregions and it can offer access of frontal areas to visual cortex (Bennett et al. 2019). This suggests that afferent inputs to V1 from sensorimotor areas other than visual ones may be critical for modulating the activity of the neurons during ongoing behavior. For example, mice show visual selective attention (Wang & Krauzlis 2018), and it will be interesting to test if LP could also play a role in attentional modulation as has been shown in monkeys (Zhou et al. 2016).
Indeed, after the afferent inputs from the thalamus (i.e., the LGN and LP nuclei), the most robust input to V1 of the mouse comes from the anteromedial cortex (AMC) (Figure 1), which includes the secondary motor cortex (MOs) and the anterior cingulate area (ACA). Lesions in AMC result in attentional deficits in rodents (Kahn et al. 2012, Muir et al. 1996). It has been shown that this area sends direct projections to V1 such that optogenetic activation of these fibers increases the firing of V1 neurons that are tuned to bar orientation (Zhang et al. 2014), a pathway that might act as a gain modulator during locomotion (Niell & Stryker 2010). It is well established that electrical activation of the anteromedial region of rodents evokes lateral head and body movements, as well as forward locomotion (Sinnamon & Galer 1984, Tehovnik & Yeomans 1987, Yeomans & Tehovnik 1988), and ablations of this region produce deficits in orienting to visual stimuli (Barth et al. 1982). Recordings from neurons in the frontal orienting fields in rats performing a memory-guided task show a selectivity for future-orienting movements (Erlich et al. 2011). This part of the brain in rodents might encompass a homolog of the primate medial eye field (see the sidebar titled From Primate to Rodent Brain), since electrical stimulation within this area in head-fixed mice evokes eye movements and eye and whisker movements in anesthetized, head-fixed rats (Barthas & Kwan 2017, Itokazu et al. 2018, Neafsey et al. 1986). The details of the projection systems from the AMC of the rodent to V1 remain to be dissected given that this region includes a significant proportion of the neocortex, spanning much of the frontal cortex (minus M1).
The remaining significant afferent projections to mouse V1 (in order of declining robustness as described in Figure 1) include the retrosplenial areas (RSP), the orbital areas (ORB), the temporal areas with access to the hippocampal complex (TMP), the auditory cortex (AUD), and the somatosensory cortex including the whisker representation (SS). In rodents, the RSP encompass the posterior cingulate cortex (Sugar et al. 2011) and contain neurons that are responsive to vestibular and/or visual flow-field stimuli (Alexander & Nitz 2015, Rancz et al. 2015). Through direct connections to V1, these motion signals are passed to layer 6 neurons (Vélez-Fort et al. 2014, 2018). Head-direction cells have been found in the RSP (Chen et al. 1994), and the activity of the cells correlates with running speed, location, and angular head velocity (Cho & Sharp 2001). Rodents with large lesions of the RSC are deficient at responding to large aerial visual threat objects (i.e., those subtending approximately 20° of visual angle) (Ellard & Chapman 1991), which would require information about visual flow as well as about self-orientation via the vestibular sense so as to plan the most efficient escape route. The RSC has access to visual and head-direction information from the thalamus and spatial information from the hippocampus (Mitchell et al. 2017, Sugar et al. 2011, Taube 2007). Furthermore, the RSP have been implicated in spatial memory functions (Alexander & Nitz 2015, Milczarek et al. 2018, Nelson et al. 2015), as well as being bidirectionally connected to the anteromedial cortex (Yamawaki et al. 2016).
ORB (Figure 1) show strong connections to the amygdala, have been associated with olfactory and gustatory functions, and they are where olfactory and gustatory inputs converge (Ongur 2000, Rolls & Baylis 1994). Rodents have a significant portion of the brain devoted to olfaction, as well as taste, given the prominent size of their olfactory bulbs. In mice, the ORB have been shown to be involved in decision making through interactions with the amygdala (Malvaez et al. 2019, Zimmermann et al. 2017). Moreover, distinct subpopulations of neurons exist within ORB that control feeding or social behavior (Jennings et al. 2019). The ORB are required for assessment of the quality and reward value of food items and they have been related to interoception and emotionality (Ongur 2000). Activity within the ORB has been shown to be important for allowing this information to influence attention and the decision processes (Ward et al. 2015).
TMP, which include areas of the hippocampal complex (see Figure 1 for details), have been associated with object vision and memory processing (Murray et al. 2007). TMP show extensive connectivity throughout the cortex (Agster & Burwell 2009, Burwell & Amaral 1998) and they also receive projections from many visual areas (Wang et al. 2012). This part of the rodent brain acts as an interface between the visual cortex and the hippocampal formation and provides access to the grid cells (in the entorhinal cortex) which have been implicated in specifying locations in space for the purpose of navigation (Eichenbaum & Lipton 2008, Murray et al. 2007). In rodents, damage to this region produces deficits in visual discrimination tasks involving complex visual stimuli based on more than one feature (Eacott et al. 2001).
AUD contains neurons that are responsive to a range of sound frequencies and arranged according to tonotopic maps (Brewer & Barton 2016). Like primates, rodents have ordered tonotopic maps in the auditory cortex, but rodents are responsive to much higher frequencies surpassing 30 kHz (the limit of macaques and humans) and reaching levels as high as 100 kHz, a characteristic of the mouse (Bakin & Weinberger 1996, Heffner 2004, Reynolds et al. 2010, Tsukano et al. 2017). Auditory input has been shown to modulate the responses of neurons in the early visual cortex in mice (Deneux et al. 2019, Ibrahim et al. 2016, Iurilli et al. 2012, Meijer et al. 2017, Song et al. 2017), and anatomical connections have also been found in rats and gerbils (Henschke et al. 2015, Stehberg et al. 2014). When audio-visual stimuli coincide, the responses of neurons in primary visual cortex are stronger (Deneux et al. 2019) and multisensory stimulation has been shown to affect the orientation tuning of V1 cells (Ibrahim et al. 2016, Meijer et al. 2018). This process is believed to facilitate perception.
The final region to be considered for sending projections to the V1 in the mouse is the SS. The SS contains neurons that mediate the sense of touch and proprioception for the entire body (Kaas 1993). In the rodent, this region is composed of two divisions, an anterolateral division that codes for tactile and proprioceptive sensations from the whiskers and an anteromedial division that represents tactile and proprioceptive input from the rest of the body. Much work has been done on the somatosensory cortex that mediates input from the whiskers, largely because this representation is very large in rodents (Petersen 2007). Whisker responses in the SS can be modulated by bimodal stimulation with visual stimuli (Sieben et al. 2013), and V1 neurons show contextual responses to somatosensory stimulation (Kandler et al. 2018).
Accordingly, V1 in mouse receives inputs from all of the sensory modalities, i.e., vestibular, auditory, somatosensory, gustatory, and olfactory, as coded by the neocortex. Moreover, V1 has access to spatial and navigational information via the TE, and it receives inputs about locomotor state from the AMC. To understand what motor structures V1 can influence, we describe the corticofugal projections of mouse V1.
3. SUBCORTICAL EFFERENT PROJECTIONS FROM V1
The major subcortical projections of V1 according to the Allen Mouse Brain Atlas (Oh et al. 2014; http://connectivity.brain-map.org/) are illustrated in Figure 2. These, in order of declining robustness, include innervations of the LGN, the pretectal nuclei (PT), the LP nucleus, the LD nucleus, the SC and the terminal nuclei (TN). Of these projections, two innervate sensory targets (i.e., the LGN and LP nucleus), and the remainder have been associated with various aspects of visuomotor processing. The efferent fibers to the LGN are believed to modify incoming sensory responses from the retina (Grieve & Sillito 1995, Sherman & Guillery 1998, Swadlow 1983) by enhancing the signal-to-noise ratio of V1 activity during attentional tasks (Briggs et al. 2013). In the mouse, the retinal inputs to the LGN are similar to those of other mammals (Tang et al. 2016) and have been shown to be critical during development (Murata & Colonnese 2016, Thompson et al. 2016). Feedback axons project to both LGN relay neurons and inhibitory neurons of the thalamic reticular nucleus, and in slices, there is facilitation of neuronal responses upon corticothalamic terminal stimulation (Jurgens et al. 2012). Interestingly, optogenetic suppression of input from layer 6 elicited a wide range of responses from increases to decreases of LGN activity, whereas optogenetic stimulation led to suppression (Denman & Contreras 2015). This is in contrast to the spatiotemporal enhancements in other species, but this might be due to the complexity of the circuit and the lack of more refined in vivo stimulation.
Figure 2.
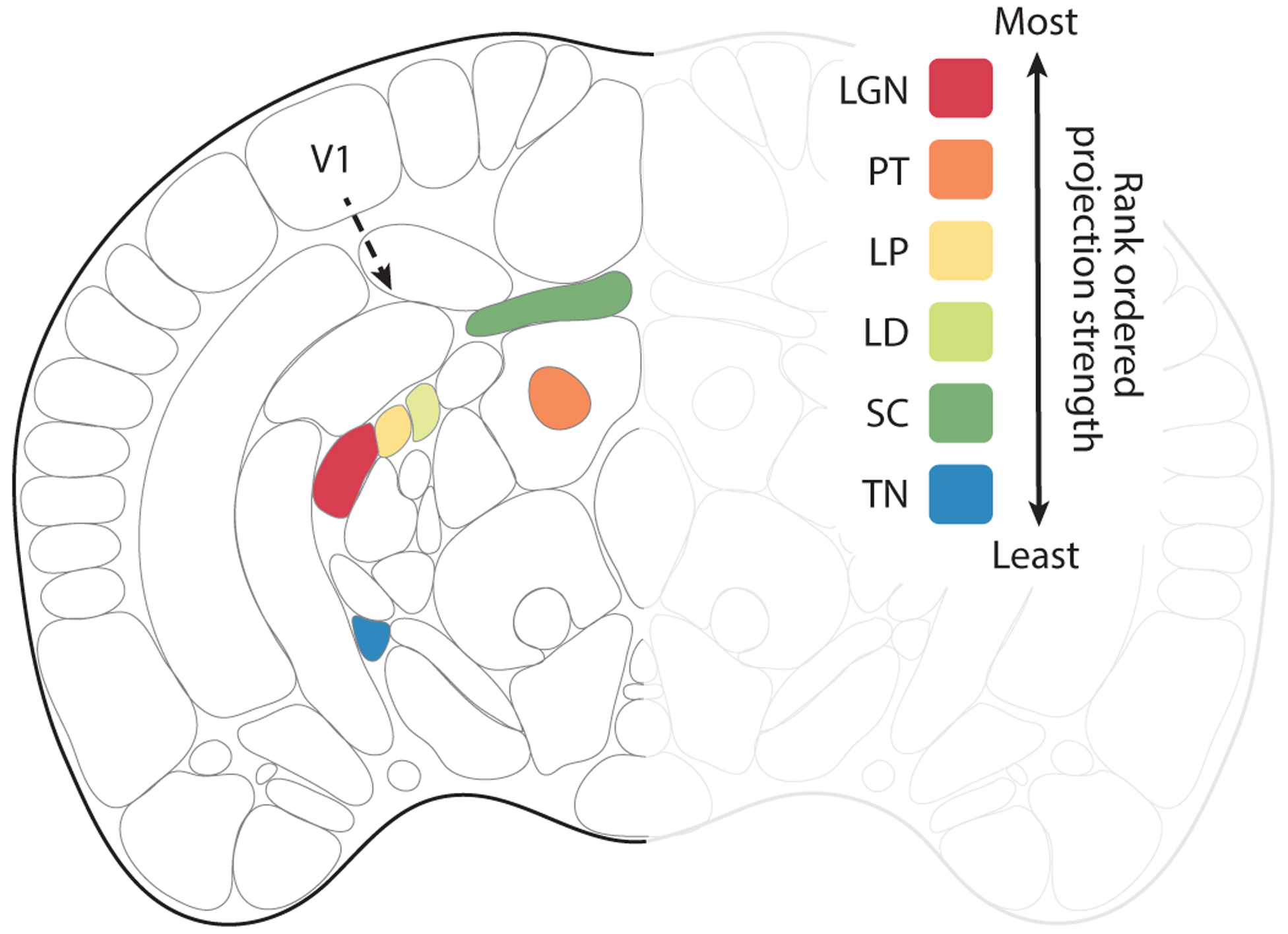
Efferent subcortical projections from V1. Coronal view of the mouse brain. Based on the Allen common coordinate framework v.3 to include the lateral dorsal nucleus of the thalamus (LD) plus efferent projection zones of V1 (area 17). Robustness of efferent projections outside of the visual cortex is shown for the wild-type mouse, rank ordered according to the density of projections adjusted for injection size. The inputs from most robust to least robust were: (1) the lateral geniculate nucleus (LGN), (2) the pretectal nuclei (PT), (3) the lateral posterior nucleus of thalamus (LP), (4) the LD, (5) the superior colliculus (SC), and (6) the terminal nuclei (TN).
The next two structures that receive input from V1 are two other thalamic nuclei: the LP nucleus and the LD nucleus. As mentioned earlier, LP is considered to be the pulvinar nucleus homolog of the rodents (Zhou et al. 2017a). Layer 5 V1 neurons provide strong input to LP (Li et al. 2003) and is believed to play a role in relaying the information through the corticothala-mocortical pathway (Sherman & Guillery 2011). In rodents, the LP nucleus is part of the pathway for processing looming stimuli (Beltramo & Scanziani 2019, Wei et al. 2015). The LP nucleus has been shown to respond to both global motion and object motion, but when V1 activity was optogenetically suppressed, responses shifted to object motion providing evidence for a cortical computation of global motion (Bennett et al. 2019). Lastly, the LP nucleus is connected to the striatum, amygdala, and AMC, which may provide V1 with access to these areas (Bennett et al. 2019, Zhou et al. 2017b). As for the V1 projections to the LD nucleus of the thalamus, much less is known about their functionality. The LD nucleus has extensive connections to the limbic system including the cingulate, the subicular, and the hippocampal formations (Spiro et al. 1980). Based on recordings of neurons located in the LD nucleus from behaving rats, it has been suggested that the LD nucleus is utilizing the visual input for spatial learning and navigation (Enkhjargal et al. 2014, Mizumori & Williams 1993).
The remaining structures receiving corticofugal projections from V1—the PT, the SC, and the TN—have all been studied within the context of visuomotor control. The PT is well known for being part of the brainstem circuitry that mediates the pupillary reflex via the pretectal olivary nucleus, which contains luminance detectors (Lucas et al. 2001, Petruno et al. 2013, Young & Lund 1994). The nucleus of the optic tract is involved in coordination of eye movements such as OKN. Projections from V1 can modulate the gain of this reflex and to an even greater extent after reduced vestibular input (Liu et al. 2016).
The SC is a well-studied structure (Basso & May 2017, Cang et al. 2018, Ito & Feldheim 2018). In rodents, it has at least two functions: approaching appetitive stimuli and avoiding visual threat objects (Cang et al. 2018, Dean et al. 1989, Ellard & Goodale 1988, Schneider 1969, Shang et al. 2019). The crossed pathway from the SC mediates orienting responses to punctate targets whereas the uncrossed pathway subserves the avoidance of large visual looming targets (Ellard & Goodale 1988). The function of the SC in mice is shifted from controlling eye movements to controlling eye and head movements (Ito & Feldheim 2018) and projections from V1 can change the gain of collicular responses to looming stimuli in awake animals (Zhao et al. 2014) and even drive freezing behavior (Zingg et al. 2017). Interestingly, cells in the SC of the mouse have similar orientation preferences that resemble the orientation columns found in V1 of large mammals (Feinberg & Meister 2015), in contrast to the presumed random distribution that is observed in V1 of rodents (Ohki et al. 2005).
The TN, which are part of the accessory optic tract system, control optokinetic nystagmus by stabilizing an image on the retina as the visual surround moves (Simpson 1984, Sun et al. 2015). These nuclei receive direct retinal input (Hayhow et al. 1960, Morin & Studholme 2014), and the neurons in TN have much larger receptive fields than the ganglion cells (van der Togt et al. 1993). They respond to movement of large visual patterns, with neurons in the medial and lateral TN being responsive to vertical motion and neurons in the dorsal TN to the horizontal motion (Hoffmann et al. 1992, Mustari & Fuchs 1989, Simpson 1984, Yonehara et al. 2009). While the connection between TN and the visuo-cortical areas has not been studied in the mouse, input from cortical areas has a modulatory effect on eye movements in monkeys (Distler & Hoffmann 2001) and could possibly carry the same function in rodents.
The discussion above suggests that mouse V1 is directly connected to subcortical structures that mediate oculomotor behaviors that can range from orienting to visual stimuli to performing OKN. Therefore, the role of V1 in modulating the behavior of the animal has to be expanded, since it can bypass the motor and premotor cortical areas. The next section discusses the connectivity of V1 and the higher-order visual areas.
4. VISUO-CORTICAL EFFERENT PROJECTIONS FROM V1
V1 of the mouse is reciprocally connected to at least nine distinct visual areas in the neocortex, which are illustrated in Figure 3 in order of progressively diminishing strength of connectivity. They are (a) the anterolateral visual area (VISal), (b) the lateromedial visual area (VISlm), (c) the rostrolateral visual area (VISrl), (d) the lateral intermediate visual area (VISli), (e) the posteromedial visual area (VISpm), (f) the posterior visual area (VISp), (g) the anteromedial visual area (VISam), (h) the postrhinal visual area (VISpor), and (i) the anterior visual area (VISa). According to Garrett et al. (2014), the first six visual areas—the VISal, the VISlm, the VISrl, the VISli, the VISpm, and the VISp—have complete visuo-topographic maps with representations out to 80° of eccentricity. The remaining areas have partial maps (i.e., the VISam and VISpor) or no reported map (i.e., the VISa designated as a parietal area).
Figure 3.
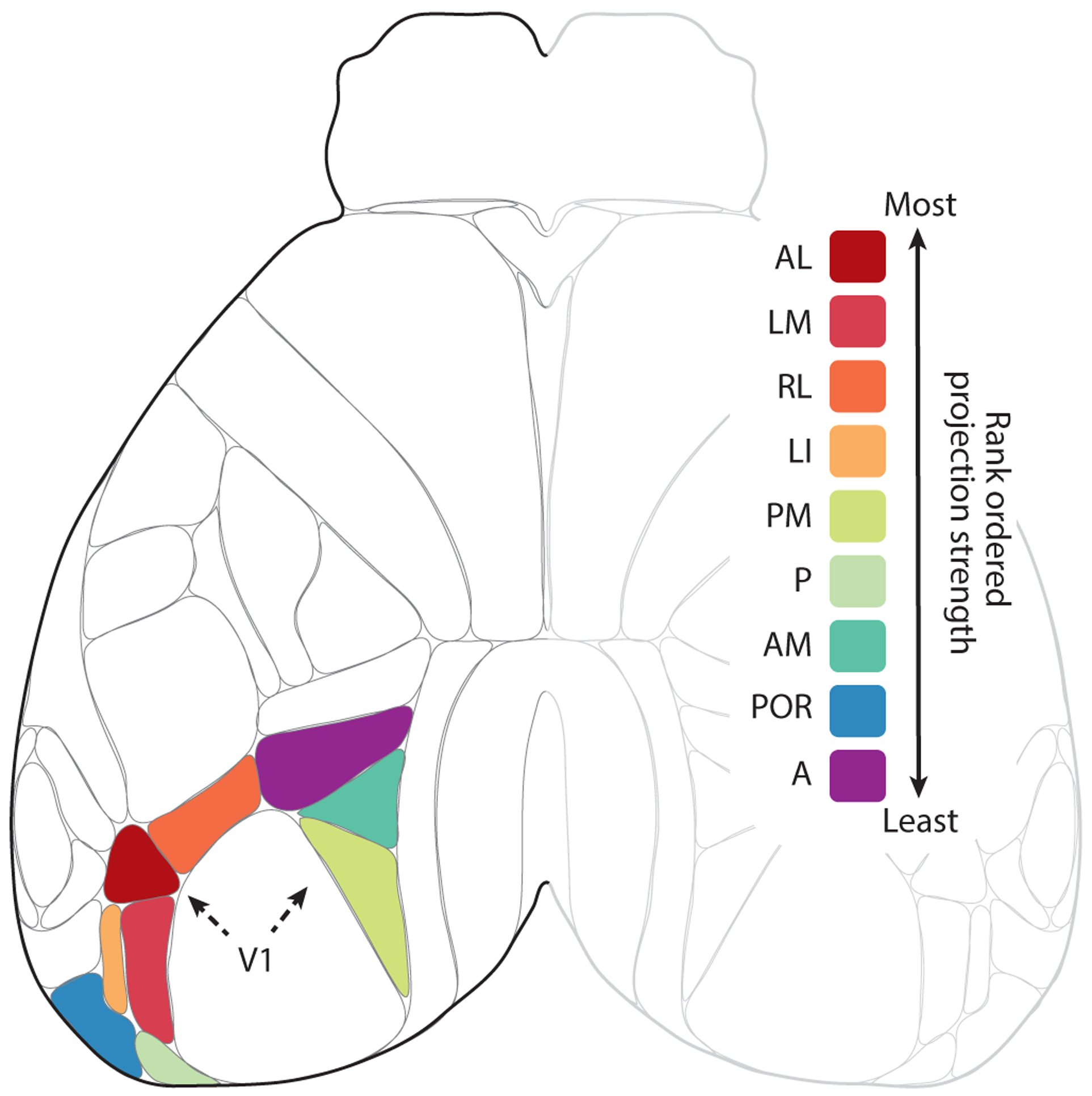
Efferent cortico-visual projections from V1. Shown is a top view of mouse neocortex based on the Allen common coordinate framework v.3. Robustness of efferent projections to and from V1 is illustrated for the wild-type mouse, rank ordered according to the density of projections of visual cortex adjusted for injection size. The inputs from most robust to least robust were: (1) the anterolateral visual area (VISal/AL), (2) the lateromedial visual area (VISlm/LM), (3) the rostrolateral visual area (VISrl/RL), (4) the lateral intermediate visual area (VISli/LI), (5) the posteromedial visual area (VISpm/PM), (6) the posterior visual area (VISp/P), (7) the anteromedial visual area (VISam/AM), (8) the postrhinal visual area (VISpor/POR), and (9) the anterior visual area (VISa/A).
Areas VISal and VISlm are strongly connected to V1 (Figure 3), and like the neurons in V1, they contain cells that are orientation tuned with a direction of motion preference (Andermann et al. 2011, Juavinett & Callaway 2015, Marshel et al. 2011). The neurons in the VISrl code for the nasal visual field, possibly for the purpose of representing the area covered by whisking (Figure 3) (Garrett et al. 2014, Olcese et al. 2013), and this area contains cells that respond to moving, textured flow-fields (Juavinett & Callaway 2015). VISli area has neurons that have a preference for high spatial frequencies (Andermann et al. 2011, Marshel et al. 2011). Their tuning properties together with the connections to areas in TMP suggest that it is part of the rodent ventral stream (Wang et al. 2012).
The VISpm, along with the VISam, which is discussed further below (Figure 3), shows strong connectivity with the RSP (Wang et al. 2012). The VISpm has large receptive fields with minimal surround suppression (Murgas et al. 2019) and a preference for high spatial frequencies (Marshel et al. 2011, Roth et al. 2012). This connectivity and functional pattern make it likely to be important for extracting visual information (such as global motion signals) that might be used for spatial navigation (Burkhalter 2016, Murgas et al. 2019, Roth et al. 2016). Areas VISp and VISpor have strong connections to areas in TMP (Wang et al. 2012). Area VISpor and target areas in TMP show similar response properties to moving objects that are distinct from V1 responses (Nishio et al. 2018). In a recent study by Beltramo & Scanziani (2019), it was found that the VISpor of the mouse is better than V1 at representing moving dots, and the motion selectivity originates from LP nucleus and not from the V1. This additional colliculocortical pathway might be important for processing moving stimuli in rodents (Beltramo & Scanziani 2019, Bennett et al. 2019, Tohmi et al. 2014).
Area VISam contains retinotopic mapping that includes only the peripheral visual field out to 80° with no representation of the central 20° (Garrett et al. 2014, Zhuang et al. 2017). The VISam and VISa areas are considered to be components of the dorsal stream of visual processing that mediate visuomotor guidance via connections with the posterior parietal cortex (PPC) but also with the AMC (Wang et al. 2012). Itokazu et al. (2018) showed AMC inputs to these areas that were involved in an eye movement task. Interestingly, there appears to be a large overlap of the anterior visual areas VISa and VISam with the PPC (Lyamzin & Benucci 2019). Anterograde tracer injections have shown that area VISa has extensive overlap with lateral PPC whereas the anterior of VISam overlaps with medial PPC (Hovde et al. 2019). PPC is believed to play an important role in the processing and accumulation of the visual information that then drives decision making (Goard et al. 2016, Licata et al. 2017, Odoemene et al. 2018).
Through these widespread visuo-cortical connections to four major output pathways, the temporal cortex (areas VISp, VISpor, and VISli), PPC (areas VISam and VISa), RSP (areas VISpm and VISam), and AMC (areas VISam, VISa, and VISpm), visual information from V1 can spread to cortical areas that take part in the behavior of the animal.
5. INTERPRETING V1 CIRCUITS
As mentioned above, the visual activity in V1 is augmented in mice that are required to walk or run (Niell & Stryker 2010). Moreover, it has been shown that the processing of natural stimuli in the visual cortex can improve during behaviorally active periods (i.e., whisking, running) (Froudarakis et al. 2014), and the activity of cells can be continuously modulated at an even faster timescale of 1–2 s (Reimer et al. 2014, Vinck et al. 2015). Consequently, an important question arises: How do anatomical connections relate to the functional properties of cells in the primary visual cortex?
5.1. Modulation by Feedback
It is believed that feedback can dynamically modulate the effective connectivity within V1, allowing neurons to change their functional properties depending on the behavioral context (Gilbert & Li 2013). That can be achieved with a differential recruitment of interneurons: feedback connections both from cortical areas VISlm and VISpm and from the LP nucleus show a balanced input to excitatory and inhibitory cells in V1, whereas feedforward connections from the LGN preferentially recruit parvalbumin (PV) interneurons (Bista et al. 2019, D’Souza et al. 2016). When activity in the area VISlm is suppressed, it has been shown to selectively decrease the responses of V1 neurons at their prefer orientation (Pafundo et al. 2016). Feedback connections reflect a similarity in the spatial frequency tuning: Layer 5 neurons from VISal that prefer low spatial frequencies target V1 neurons with a preference for low spatial frequencies. Likewise, VISpm neurons that prefer high spatial frequencies target V1 neurons with similar tuning preferences (Huh et al. 2018). Notably, when VISal or VISpm was silenced, there was a reduction in activity of neurons preferring low or high spatial frequencies respectively.
Rodent V1 can disambiguate complex visual stimuli from the surround (Ingle 1981, Ingle et al. 1979, Schnabel et al. 2018, Schneider 1969), but in general figure-ground segregation includes regions beyond V1. In a study by Schnabel et al. (2018), it was shown that figure-ground enhancement is prevalent throughout the visual cortex, as assessed with two-photon imaging. The regions exhibiting figure-ground enhancement included the majority of visual areas: V1, the VISal, the VISlm, the VISrl, the VISpm, the VISam, and the VISa (as illustrated in Figure 3), as well as the RSC (as illustrated in Figure 1). When mice performed a discrimination based on line configuration, all the visual areas noted above exhibited an augmentation of activity during the figure-ground period (i.e., 100 to 400 ms after target onset) except for the RSC [all comparisons were made with passive viewing (see Schnabel et al. 2018, figure 3d)]. It has been presumed that figure-ground potentiation in V1 is due to feedback from higher cortical areas (Lamme 1995, Lamme & Roelfsema 2000, Zipser et al. 1996). To test this idea, Schnabel et al. (2018) silenced optogenetically large cortical regions within the VISal, the VISlm, the VISpm, and the VISam while recording the activity of neurons in V1 using two-photon imaging. It was found that such manipulation reduced the figure-ground potentiation in a task difficulty–dependent manner.
Direct projections from AMC to V1 are believed to play a big part in shaping neural responses. When AMC neurons were optogenetically activated as drifting gratings were presented to the retina of mice (Zhang et al. 2014), it was found that the activity of V1 cells was enhanced by the activation only when the gratings were at their preferred orientation. Additionally, when mice were required to perform a visual discrimination, optogenetic activation improved the performance of the animals. It is noteworthy that the optogenetic stimulation employed in the experiments never evoked eye movements, but at higher magnitudes of laser intensity, eye movements were induced that exhibited saccade-like properties. Similarly, electrical stimulation of a small region of MOs in the AMC also induced eye movements (Itokazu et al. 2018), which is in keeping with the notion that the AMC is a frontal-lobe center subserving orienting responses (Yeomans & Tehovnik 1988).
To deduce the circuit responsible for the results discussed above, optogenetic activation of the AMC was performed as intracellular recordings were made of pyramidal neurons in layer 2/3 of V1 (Zhang et al. 2014). Both excitatory post synaptic potentials (EPSPs) and inhibitory post synaptic potentials (IPSPs) could be evoked in the pyramidal cells by stimulation of the axons originating from the ACA of the AMC. Since the IPSPs occurred at a longer latency than the EPSPs, and since the IPSPs could be blocked by silencing inhibitory circuits, it was presumed that the ACA has direct access to the pyramidal cells for their excitation but indirect access via a disynaptic GABAergic circuit for their inhibition. Furthermore, optogenetic activation of the axons was shown to directly excite all three major interneurons in V1—i.e., PV, somatostatin-positive (SST), and vasoactive intestinal peptide (VIP) interneurons. These interneuron types were found to be directly connected by axons originating from the ACA, with the VIP interneurons receiving the strongest connections, thereby indicating that this input could be used to potentiate the activity of pyramidal cells for optimally oriented stimuli through disinhibition. Indeed, inactivation of the VIP interneurons abolished the augmentation of the preferred orientation response induced by activation of axons from the ACA (Zhang et al. 2014).
5.2. Motor Signals in V1
It has been postulated that the feedback signals to V1 may be more coupled to the motor system than originally thought (Kaneko et al. 2017, Lee et al. 2014, Monaco et al. 2018, Zhang et al. 2014). Visuomotor experience has been shown to be important for the development of the visual system in both mice and cats (Attinger et al. 2017, Held & Hein 1963). Mice that had their self-generated motion decoupled from their visual experience had fewer neurons that responded to mismatch between self-movement and visual flow (Attinger et al. 2017). In the experiments of Lee et al. (2014), glutamatergic brainstem sites mediating locomotion (i.e., the area of pedunculo-pontine tegmental and cuneiform nuclei) were activated optogenetically in mice as visual stimuli were presented to the retina and as single-unit recordings were made from V1. All activations were done subthreshold so as not to elicit motor responses. Stimulation in the brainstem increased the activity of cells in V1 tuned to specific orientations of drifting gratings, similar to what had been reported in mice running on a spherical treadmill (Niell & Stryker 2010). To that extent, maximal brain plasticity also ensues when animals move during task performance thereby engaging multiple sensory systems to induce rapid adaption (Held & Hein 1963, Kaneko et al. 2017).
By imaging a large part of the neocortex of head-fixed yet mobile mice, Musall et al. (2019) found that, while sensory stimuli modulated distinct regions of the cortex, body movements activated massive sections of the cortex irrespective of the cognitive condition driving the behavior. When the sensory stimulus was visual, the visual cortex was activated, and when the sensory stimulus was auditory, the auditory cortex was activated. During movement execution, much of the parietal and motor cortices (primary motor cortex and MOs of the AMC) were activated. Since the realization of many behaviors requires learning, from the ability to walk to discrimination between different predators to mate selection, wide-field imaging will now be able to deduce how the neocortex is shaped to sculpt behavior.
The complete list of network structures mediating the locomotor effect is far from being finalized, given that activation of the AMC also modulates the firing of V1 cells (Zhang et al. 2014). The AMC vis-à-vis V1 has become the focus of much research on the effect of behavioral context and vision (Fiser et al. 2016, Leinweber et al. 2017, Zhang et al. 2014). Projections connecting the AMC to V1 of mice convey information not only about locomotion, but also about the visual surround as mice move through a virtual flow field (Leinweber et al. 2017). The axons from the AMC projecting to V1 of head-fixed mice were found to start firing at the onset of locomotion as visual flow fields were presented to the retina and the rate of firing varied with locomotor speed. This discharge was greatly decreased when mice were required to run in total darkness, and it was also reduced by inactivation or lesions of the AMC. The firing of the axons from the anteromedial cortex to V1 were topographically ordered such that they were activated the most in the presence of the strongest flow-fields (right or left) and activation of the axons in right V1 evoked leftward “virtual” turns and activation of the axons of left V1 evoked rightward “virtual” turns (Leinweber et al. 2017, figure 5). On this point, it was found that the AMC innervates V1 topographically such that anterior sites innervate lateral V1 (central-field representation), posterior sites innervate medial V1 (peripheral-field representation), lateral sites innervate posterior V1 (upper-field representation), and medial sites innervate anterior V1 (lower-field representation) (Leinweber et al. 2017, figure 2). Whether a similar representation exists in higher mammals is unclear (but see Lee & Tehovnik 1995).
In another study, head-fixed mice were required to locomote for reward through a virtual tunnel such that oriented gratings (45° or 135°) were presented in a sequence as recordings were made of V1 neurons using two-photon imaging to study neurons in layer 2/3 (Fiser et al. 2016). It was found that 6% of 899 neurons in V1 responded in anticipation of an upcoming grating orientation positioned in a specific location of the visual field. This occurred once the mice were exposed to repeated daily presentations of a stimulus sequence. A subset of the cells increased their response when the expected stimulus was omitted. Axons originating in the AMC and terminating in V1 similarly exhibited anticipatory activity, but they never produced an increase in response to omitted stimuli. This suggests that stimulus comparisons are registered in V1 rather than in the AMC. The topographic correspondence between V1 and the AMC may keep the two regions spatially connected for the transmission of newly learned information (Leinweber et al. 2017). The dependency of V1 on frontal lobe circuits for transferring learned information within the V1 map could be assessed in the mouse.
Vélez-Fort et al. (2018) have found that layer 6 neurons of mouse V1 receive vestibular and visuomotion input from the RSC, which is strongly connected to V1 (Figure 1). Head-fixed mice had their heads rotated passively in the horizontal dimension as extracellular or whole-cell recordings were made from all layers of left V1. The maximal rotation velocity was 80°/s, and movements were either rightward or leftward with the head marking the center of rotation. Half of the cells in layer 6 discharged best to either rightward or leftward rotation as an animal was kept in total darkness. The remaining cells responded to either direction of head motion. The responsivity of the cells (based on membrane potential changes) covaried with the angular velocity of the head. Bilateral lesions of the vestibular canals abolished the discharge. In addition to vestibular modulation, the neurons were also responsive to visual motion with some being direction selective. It was found that the vestibular and motion inputs were summed arithmetically by layer 6 neurons. Thus, vestibular information has the potential to heavily influence V1 activity.
5.3. Neuromodulatory Context: Brain State and Reward
Behavioral state in mammals is regulated by four prominent neuromodulators: norepinephrine from the locus coeruleus, acetylcholine from the nucleus basalis Meynert complex, dopamine from the ventral tegmental area/substantia nigra compacta, and serotonin from the raphe nucleus. The first two modulators—norepinephrine and acetylcholine—play a role within the contexts of wakefulness (Reimer et al. 2014, 2016) and learning (Liu et al. 2015). Dopamine is known for its role in reward, which drives operant behavior (Olds & Milner 1954, Pascoli et al. 2015, Wise & Rompre 1989), and serotonin affects sensory saliency (Jacob & Nienborh 2018). It is noteworthy that all four modulators have a weak presence in V1 according to the Allen Mouse Brain Atlas (Oh et al. 2014; http://connectivity.brain-map.org/). This paucity of innervation throughout the neocortex may be due to the fact that these modulators have few neurons to support the distribution across such a large projection area (Bigl et al. 1982, Menegas et al. 2015, Schwarz & Luo 2015). Nevertheless, the weak innervation is sufficient for control of different behavioral states, such as wakefulness, as mediated by the locus coeruleus, for example (Aston-Jones & Bloom 1981, Aston-Jones & Cohen 2005, Aston-Jones et al. 1996). How different neurotransmitter systems regulate behavioral states within the different functional divisions of the neocortex, including V1, is still not clear, but recent work has demonstrated differences between the specificity of neuromodulators: The cholinergic system can have selective projections that give rise to modality-specific effects while the noradrenergic system has more broad effects on different modalities (Kim et al. 2016, Zaborszky et al. 2015). In addition, neurotransmitter systems modulate thalamic nuclei that are reciprocally connected to V1 and that are believed to participate in executive gain control (Varela 2014).
Serotonin affects the LGN (Vertes et al. 2010) and V1 with its afferents primarily targeting the input layer 4 (Paspalas & Papadopoulos 2001). The innervation of serotonin is more homogeneous across the cortex and has dense projections in the input layer 4 of the primary sensory areas when compared to other neuromodulators (Jacob & Nienborg 2018). It can lower the gain of the V1 response in macaques and rats, thereby reducing visual saliency (Seillier et al. 2017, Waterhouse et al. 1990). Serotonin appears to be involved in the plasticity of visual circuits. In the rat, serotonin levels increase during monocular deprivation, and blockage of the serotonin release interferes with the ocular dominance shift that normally follows deprivation (Baroncelli et al. 2010). This effect of serotonin appears to be mediated through reduced inhibition and increased levels of brain-derived neurotrophic factor. However, dopaminergic terminals are much sparser in the primary visual cortex. In the rat, afferents from the ventral tegmental area project mainly in layers 5 and 6, but any effect on the neural activity is much less studied than other neuromodulatory inputs, and is hypothesized to be mediated through indirect feedback from other areas (Jacob & Nienborg 2018).
Reward is a very important aspect of behavioral state, and it has been shown that rewarded visual stimuli can lead to sharper spatial tuning in V1 (Goltstein et al. 2018). Rodents will self-stimulate when electrodes are positioned near the nucleus basalis (Olds & Milner 1954). When head-fixed, water-deprived mice were presented with a flash of light followed by a water reward (Liu et al. 2015), neurons in V1 changed their responsivity in anticipation of reward delivery after repeated exposures to a flash reward combination. Next, instead of delivering a water reward, a fictive reward signal was elicited by optogenetic activation of cholinergic axons in V1 originating from the nucleus basalis of the diagonal band, and interestingly, it was found that neurons within V1 exhibited the same responsivity profiles following repeated exposures to the fictive reward as compared to the water reward after subjecting mice to repeated flash-fictive-reward trials. In a similar manner, in a task where rats had to discriminate between visual stimuli with different temporal structure, blocking cholinergic input to the visual cortex resulted in impaired learning without affecting previously learned associations (Minces et al. 2013). In addition to promoting plastic changes, cholinergic input has also been shown to have direct effects on the processing of local information (Goard & Dan 2009, Pinto et al. 2013). When nucleus basalis was stimulated responses to natural stimuli became less correlated and more reliable.
Reimer et al. (2014) studied the responsivity of V1 neurons in head-fixed, non-locomoting mice while measuring pupil size. The pupillary response is used as an index of an animal’s state of arousal, which is believed to be mediated by the noradrenergic fibers of the locus coeruleus (Aston-Jones & Bloom 1981, Aston-Jones & Cohen 2005, Aston-Jones et al. 1996, Liu et al. 2017, McGinley et al. 2015, Shimaoka et al. 2018). It was found that, during periods of pupil dilation, the layer 2/3 direction-tuned cells of V1 (as studied with two-photon imaging) were more responsive to the presentation of the preferred direction of drifting gratings with the nonpreferred direction having no effect. A similar result was obtained when natural scenes were presented to the animals. In these experiments, trials that contained eye movements were excluded, and there is evidence to suggest that these state transitions are different from the ones during running (Larsen et al. 2018, Vinck et al. 2015). Also, during periods of immobility and pupil dilation, the VIP interneurons were depolarized, and the SOM interneurons were hyperpolarized (as assessed with whole-cell recording), which would have released pyramidal cells from inhibition. Imaging cholinergic and noradrenergic axons has shown that both neurotransmitters are active during periods of pupil dilation and running (Larsen et al. 2018, Reimer et al. 2016).
6. DISCUSSION
With the advent of two-photon imaging and optogenetic recording and activation (Boyden et al. 2005, Kerr & Denk 2008), many circuits are being studied, with most of the work concentrating on the functions of the posterior visuo-cortical areas and on how locomotion (including head displacements) and responses to moving visual stimuli are integrated by networks spanning large portions of both the posterior and the anterior neocortices (e.g. Leinweber et al. 2017, Musall et al. 2019, Shimaoka et al. 2018, Vélez-Fort et al. 2018), something that a decade ago had remained mostly within the realm of speculation due to limited methodologies (e.g. Schiller & Tehovnik 2001, 2005). The functions of the posterior visual areas have been assessed mainly with the use of oriented bars, with less work having been done using visual stimuli customized ethologically for the mouse brain (Froudarakis et al. 2014), or stimuli that are optimally driving their responses (Walker et al. 2018). Moreover, given the extensive V1 innervation by neocortical areas mediating the entire sensory space (Figure 1), there is little information on how the non-visual senses are integrated at the level of V1; yet the vestibular sense is receiving attention.
Furthermore, although investigators are determining the subcortical channels utilized by V1 for sensorimotor processing (e.g., Lee et al. 2014, Zhou et al. 2017b), much less work is being done in this regard. This is somewhat surprising given the plethora of work available on regions such as the PT, SC, and TN in lower mammals (as reviewed above). This negligence might be related to the prominence given the neocortex in systems neuroscience (e.g., Felleman & Van Essen 1991), with less regard being paid to subcortical channels, which are within the domain of oculomotor neurophysiology (Schiller & Tehovnik 2015). In addition, the preponderance of projections between V1 and the LGN and the LP and LD nuclei of thalamus highlights the importance of these projections (Sherman & Guillery 1996; Zhou et al. 2017a,b). There are many hypotheses that could be revisited on the functions of the subcortical substrates, from performing something as apparently mundane are regulating pupil size to something as important as negotiating barriers and avoiding predators (e.g. Ingle 1973, Jampel 1960, Schneider 1969, Teuber 1970, Yeomans & Frankland 1995). For example, how motion information is transmitted from the visual cortex to the SC or LP nucleus as rodents anticipate moving targets (Ingle et al. 1979, Rhoades & Chalupa 1978, Zhou et al. 2017a) has yet to be resolved at the circuit level.
In conclusion, it is clear that different behavioral contexts have significant impact on the responsivity and functionality of area V1. The most robust effects, not surprisingly, occur when animals are required to move around (Musall et al. 2019, Niell & Stryker 2010). The tight sensorimotor integration may be a universal property of all neocortical primary sensory systems, which coincides with the original thinking of Lashley that information contained within the neocortex is distributed. This may provide mammals with the most efficient exchange of multisensory information to thereby select appropriate behaviors by which to accelerate the adaptive process. Given the recent advances in technologies that allow recording from large numbers of neurons across multiple areas, precise control of the neural activity, and fine mapping of the connectivity, we might finally be able to understand the function of the primary sensory cortex in context.
FROM PRIMATE TO RODENT BRAIN.
Some of the discussion in this review uses the primate brain as a guide to how the mouse brain may be configured for visuomotor control. The visual system of the primate is somewhat different from that of the rodent, however. The primate has forward-looking eyes, and the movements of the eyes are conjugate, allowing for stereovison, and disjunctive when changing depth planes (Carpenter 1988, Schiller & Tehovnik 2015, Zhou & King 1998). In the rodent, however, the laterally placed eyes offer extended coverage of the visual field, which is important for predator avoidance (De Franceschi et al. 2016, Ellard & Chapman 1991, Meister & Cox 2013, Wallace et al. 2013, Yilmaz & Meister 2013). Their eyes, more often than not, move independently of one another, and while this can limit stereovision, it does optimize the coverage of the binocular field, which is located in front of the animal (Meister & Cox 2013, Wallace et al. 2013). Rodents show predatory activity (Hoy et al. 2016, Scholl et al. 2017, Shang et al. 2019), and neurons in V1 are tuned for binocular disparities (Scholl et al. 2013), but the dependency of depth estimation with binocular vision during these behaviors has yet to be established. Rodents lack a capacity for significant accommodation (Hughes 1977), but they can deduce depth from monocular cues, such as motion cues and motion parallax (Ellard et al. 1986, Legg & Lambert 1990). While rodents do not exhibit vergence or pursuit eye movements given that they are afoveal animals (Euler & Wässle 1995, Wallace et al. 2013), they can produce saccadic-like movements (Itokazu et al. 2018, Palagina et al. 2017, Sakatani & Isa 2007, Zhang et al. 2014). Despite the differences, rodents exhibit a vestibular ocular reflex and optokinetic nystagmus, and they have eye movements surpassing 20° in the horizontal, vertical, and torsional dimensions when the head is free; when the head is immobilized (a common condition in many of the studies evaluated in this review), eye movements become infrequent (van Alphen et al. 2010, Wallace et al. 2013). Evolutionarily, the visuomotor behavior of the rodent may be considered to be midway between those of the frog and the primate (Ingle 1973a,b; Schneider 1969; Teuber 1970).
ACKNOWLEDGMENTS
This work was supported by the Intelligence Advanced Research Projects Activity (IARPA) via Department of Interior/Interior Business Center (DoI/IBC) contract number D16PC00003. The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding copyright. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements (either expressed or implied) of IARPA, DoI/IBC, or the U.S. Government, including the National Institutes of Health. Additional funding includes: National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke under Award Number U19MH114830, R01 EY026927, DARPA #5N66001, R01 MH109556, HR0011-18-2-0025, NeuroNex NSF-1707400, NeuroNex NSF-1707359 and Big Data NSF IIS-1546273 to AST; Simons Foundation SFARI Research Award #402047 and R21 NS096640 to SMS; and support by BRASS, the Baylor College of Medicine Medical Scientist Training Program, and F30MH112312 to PGF.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Aggleton JP, Keen S, Warburton EC, Bussey TJ. 1997. Extensive cytotoxic lesions involving both the rhinal cortices and area TE impair recognition but spare spatial alternation in the rat. Brain Res. Bull 43:279–87 [DOI] [PubMed] [Google Scholar]
- Agster KL, Burwell RD. 2009. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus 19:1159–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadlou M, Zweifel LS, Heimel JA. 2018. Functional modulation of primary visual cortex by the superior colliculus in the mouse. Nat. Commun 9:3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AS, Nitz DA. 2015. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat. Neurosci 18:1143–51 [DOI] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Roumis DK, Glickfeld LL, Reid RC. 2011. Functional specialization of mouse higher visual cortical areas. Neuron 72:1025–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. 1981. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci 1:876–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci 28:403–50 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. 1996. Role of the locus coeruleus in emotional activation. Prog. Brain Res 108:379–402 [DOI] [PubMed] [Google Scholar]
- Attinger A, Wang B, Keller GB. 2017. Visuomotor coupling shapes the functional development of mouse visual cortex. Cell 169:1291–302.e14 [DOI] [PubMed] [Google Scholar]
- Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T. 2016. The functional diversity of retinal ganglion cells in the mouse. Nature 529:345–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. 1996. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. PNAS 93:11219–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli L, Sale A, Viegi A, Maya Vetencourt JF, De Pasquale R, et al. 2010. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp. Neurol 226:100–9 [DOI] [PubMed] [Google Scholar]
- Barth T, Parker S, Sinnamon H. 1982. Unilateral lesions of the anteromedial cortex in the rat impair approach to contralateral visual cues. Physiol. Behav 29:141–47 [DOI] [PubMed] [Google Scholar]
- Barthas F, Kwan AC. 2017. Secondary motor cortex: where ‘sensory’ meets ‘motor’ in the rodent frontal cortex. Trends Neurosci. 40:181–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, May PJ. 2017. Circuits for action and cognition: a view from the superior colliculus. Annu. Rev. Vis. Sci 3:197–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo R, Scanziani M. 2019. A collicular visual cortex: neocortical space for an ancient midbrain visual structure. Science 363:64–69 [DOI] [PubMed] [Google Scholar]
- Bennett C, Gale SD, Garrett ME, Newton ML, Callaway EM, et al. 2019. Higher-order thalamic circuits channel parallel streams of visual information in mice. Neuron 102:477–92.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. 1982. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res. Bull 8:727–49 [DOI] [PubMed] [Google Scholar]
- Bista P, D’Souza RD, Meier AM, Ji W, Burkhalter A. 2019. Spatial clustering of inhibition in mouse primary visual cortex. bioRxiv 608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. 2005. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 8:1263–68 [DOI] [PubMed] [Google Scholar]
- Brewer AA, Barton B. 2016. Maps of the auditory cortex. Annu. Rev. Neurosci 39:385–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Mangun GR, Usrey WM. 2013. Attention enhances synaptic efficacy and the signal-to-noise ratio in neural circuits. Nature 499:476–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A 2016. The network for intracortical communication in mouse visual cortex In Micro-, Meso- and Macro-Connectomics of the Brain, ed. Kennedy H, Van Essen DC, Christen Y, pp. 31–43. Berlin: Springer; [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. 1998. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J. Comp. Neurol 398:179–205 [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. 1997. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav. Neurosci 111:920–36 [DOI] [PubMed] [Google Scholar]
- Cang J, Savier E, Barchini J, Liu X. 2018. Visual function, organization, and development of the mouse superior colliculus. Annu. Rev. Vis. Sci 4:239–62 [DOI] [PubMed] [Google Scholar]
- Carpenter RHS. 1988. Movements of the Eyes. London: Pion Ltd; 2nd ed. [Google Scholar]
- Chen LL, Lin L-H, Barnes CA, McNaughton BL. 1994. Head-direction cells in the rat posterior cortex. Exp. Brain Res 101:24–34 [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE. 2001. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav. Neurosci 115:3–25 [DOI] [PubMed] [Google Scholar]
- De Franceschi G, Vivattanasarn T, Saleem AB, Solomon SG. 2016. Vision guides selection of freeze or flight defense strategies in mice. Curr. Biol 26:2150–54 [DOI] [PubMed] [Google Scholar]
- Dean P 1981. Grating detection and visual acuity after lesions of striate cortex in hooded rats. Exp. Brain Res 43:145–53 [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GWM. 1989. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12:137–47 [DOI] [PubMed] [Google Scholar]
- Deneux T, Harrell ER, Kempf A, Ceballo S, Filipchuk A, Bathellier B. 2019. Context-dependent signaling of coincident auditory and visual events in primary visual cortex. eLife 8:e44006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman DJ, Contreras D. 2015. Complex effects on in vivo visual responses by specific projections from mouse cortical layer 6 to dorsal lateral geniculate nucleus. J. Neurosci 35:9265–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler C, Hoffmann K-P. 2001. Cortical input to the nucleus of the optic tract and dorsal terminal nucleus (NOT-DTN) in macaques: a retrograde tracing study. Cereb. Cortex 11:572–80 [DOI] [PubMed] [Google Scholar]
- D’Souza RD, Meier AM, Bista P, Wang Q, Burkhalter A. 2016. Recruitment of inhibition and excitation across mouse visual cortex depends on the hierarchy of interconnecting areas. eLife 5:e19332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Machin PE, Gaffan EA. 2001. Elemental and configural visual discrimination learning following lesions to perirhinal cortex in the rat. Behav. Brain Res 124:55–70 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA. 2008. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus 18:1314–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard CG, Chapman DG. 1991. The effects of posterior cortical lesions on responses to visual threats in the Mongolian gerbil (Meriones unguiculatus). Behav. Brain Res 44:163–67 [DOI] [PubMed] [Google Scholar]
- Ellard CG, Goodale MA. 1988. A functional analysis of the collicular output pathways: a dissociation of deficits following lesions of the dorsal tegmental decussation and the ipsilateral collicular efferent bundle in the Mongolian gerbil. Exp. Brain Res 71:307–19 [DOI] [PubMed] [Google Scholar]
- Ellard CG, Goodale MA, Scorfield DM, Lawrence C. 1986. Visual cortical lesions abolish the use of motion parallax in the Mongolian gerbil. Exp. Brain Res 64:599–602 [DOI] [PubMed] [Google Scholar]
- Enkhjargal N, Matsumoto J, Chinzorig C, Berthoz A, Ono T, Nishijo H. 2014. Rat thalamic neurons encode complex combinations of heading and movement directions and the trajectory route during translocation with sensory conflict. Front. Behav. Neurosci 8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisken S, Vaiceliunaite A, Jurjut O, Fiorini M, Katzner S, Busse L. 2014. Effects of locomotion extend throughout the mouse early visual system. Curr. Biol 24:2899–907 [DOI] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. 2011. A cortical substrate for memory-guided orienting in the rat. Neuron 72:330–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wässle H. 1995. Immunocytochemical identification of cone bipolar cells in the rat retina. J. Comp. Neurol 361:461–78 [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Meister M. 2015. Orientation columns in the mouse superior colliculus. Nature 519:229–32 [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1:1–47 [DOI] [PubMed] [Google Scholar]
- Fiser A, Mahringer D, Oyibo HK, Petersen AV, Leinweber M, Keller GB. 2016. Experience-dependent spatial expectations in mouse visual cortex. Nat. Neurosci 19:1658–64 [DOI] [PubMed] [Google Scholar]
- Froudarakis E, Berens P, Ecker AS, Cotton RJ, Sinz FH, et al. 2014. Population code in mouse V1 facilitates readout of natural scenes through increased sparseness. Nat. Neurosci 17:851–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett ME, Nauhaus I, Marshel JH, Callaway EM. 2014. Topography and areal organization of mouse visual cortex. J. Neurosci 34:12587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W. 2013. Top-down influences on visual processing. Nat. Rev. Neurosci 14:350–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, Dan Y. 2009. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci 12:1444–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard MJ, Pho GN, Woodson J, Sur M. 2016. Distinct roles of visual, parietal, and frontal motor cortices in memory-guided sensorimotor decisions. eLife 5:e13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltstein PM, Meijer GT, Pennartz CM. 2018. Conditioning sharpens the spatial representation of rewarded stimuli in mouse primary visual cortex. eLife 7:e37683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve K, Sillito A. 1995. Differential properties of cells in the feline primary visual cortex providing the corticofugal feedback to the lateral geniculate nucleus and visual claustrum. J. Neurosci 15:4868–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhow WR, Webb C, Jervie A. 1960. The accessory optic fiber system in the rat. J. Comp. Neurol 115:187–215 [DOI] [PubMed] [Google Scholar]
- Heffner RS. 2004. Primate hearing from a mammalian perspective. Anat. Rec 281A:1111–22 [DOI] [PubMed] [Google Scholar]
- Held R, Hein A. 1963. Movement-produced stimulation in the development of visually guided behavior. J. Comp. Physiol. Psychol 56:872–76 [DOI] [PubMed] [Google Scholar]
- Henschke JU, Noesselt T, Scheich H, Budinger E. 2015. Possible anatomical pathways for short-latency multisensory integration processes in primary sensory cortices. Brain Struct. Funct 220:955–77 [DOI] [PubMed] [Google Scholar]
- Hoffmann KP, Distler C, Ilg U. 1992. Callosal and superior temporal sulcus contributions to receptive field properties in the macaque monkey’s nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract. J. Comp. Neurol 321:150–62 [DOI] [PubMed] [Google Scholar]
- Hovde K, Gianatti M, Witter MP, Whitlock JR. 2019. Architecture and organization of mouse posterior parietal cortex relative to extrastriate areas. Eur. J. Neurosci 49:1313–29 [DOI] [PubMed] [Google Scholar]
- Hoy JL, Yavorska I, Wehr M, Niell CM. 2016. Vision drives accurate approach behavior during prey capture in laboratory mice. Curr. Biol 26:3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A 1977. The refractive state of the rat eye. Vis. Res 17:927–39 [DOI] [PubMed] [Google Scholar]
- Huh CYL, Peach JP, Bennett C, Vega RM, Hestrin S. 2018. Feature-specific organization of feedback pathways in mouse visual cortex. Curr. Biol 28:114–20.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim LA, Mesik L, Ji X-Y, Fang Q, Li H-F, et al. 2016. Cross-modality sharpening of visual cortical processing through layer-1-mediated inhibition and disinhibition. Neuron 89:1031–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle D 1973a. Evolutionary perspectives on the function of the optic tectum. Brain Behav. Evol 8:211–37 [DOI] [PubMed] [Google Scholar]
- Ingle D 1973b. Two visual systems in the frog. Science 181:1053–55 [DOI] [PubMed] [Google Scholar]
- Ingle D 1981. New methods for analysis of vision in the gerbil. Behav. Brain Res 3:151–73 [DOI] [PubMed] [Google Scholar]
- Ingle D, Cheal M, Dizio P. 1979. Cine analysis of visual orientation and pursuit by the Mongolian gerbil. J. Comp. Physiol. Psychol 93:919–28 [Google Scholar]
- Ito S, Feldheim DA. 2018. The mouse superior colliculus: an emerging model for studying circuit formation and function. Front. Neural Circuits 12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokazu T, Hasegawa M, Kimura R, Osaki H, Albrecht U-R, et al. 2018. Streamlined sensory motor communication through cortical reciprocal connectivity in a visually guided eye movement task. Nat. Commun 9:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, et al. 2012. Sound-driven synaptic inhibition in primary visual cortex. Neuron 73:814–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SN, Nienborg H. 2018. Monoaminergic neuromodulation of sensory processing. Front. Neural Circuits 12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jampel RS. 1960. Convergence, divergence, pupillary reactions and accommodation of the eyes from faradic stimulation of the macaque brain. J. Comp. Neurol 115:371–99 [DOI] [PubMed] [Google Scholar]
- Jennings JH, Kim CK, Marshel JH, Raffiee M, Ye L, et al. 2019. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565:645–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juavinett AL, Callaway EM. 2015. Pattern and component motion responses in mouse visual cortical areas. Curr. Biol 25:1759–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens CWD, Bell KA, McQuiston AR, Guido W. 2012. Optogenetic stimulation of the corticothalamic pathway affects relay cells and GABAergic neurons differently in the mouse visual thalamus. PLOS ONE 7:e45717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. 1993. The functional organization of somatosensory cortex in primates. Ann. Anat 175:509–18 [DOI] [PubMed] [Google Scholar]
- Kahn JB, Ward RD, Kahn LW, Rudy NM, Kandel ER, et al. 2012. Medial prefrontal lesions in mice impair sustained attention but spare maintenance of information in working memory. Learn. Mem 19:513–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler S, Mao D, McNaughton BL, Bonin V. 2018. Encoding of tactile context in the mouse visual cortex. bioRxiv 199364 [Google Scholar]
- Kaneko M, Fu Y, Stryker MP. 2017. Locomotion induces stimulus-specific response enhancement in adult visual cortex. J. Neurosci 37:3532–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JND, Denk W. 2008. Imaging in vivo: watching the brain in action. Nat. Rev. Neurosci 9:195–205 [DOI] [PubMed] [Google Scholar]
- Kim J-H, Jung A-H, Jeong D, Choi I, Kim K, et al. 2016. Selectivity of neuromodulatory projections from the basal forebrain and locus ceruleus to primary sensory cortices. J. Neurosci 36:5314–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme V 1995. The neurophysiology of figure-ground segregation in primary visual cortex. J. Neurosci 15:1605–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VAF, Roelfsema PR. 2000. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23:571–79 [DOI] [PubMed] [Google Scholar]
- Larsen RS, Turschak E, Daigle T, Zeng H, Zhuang J, Waters J. 2018. Activation of neuromodulatory axon projections in primary visual cortex during periods of locomotion and pupil dilation. bioRxiv 502013 [Google Scholar]
- Lashley KS. 1929. Brain Mechanisms and Intelligence: A Quantitative Study of Injuries to the Brain. Chicago: Univ. Chicago Press [Google Scholar]
- Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. 2014. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83:455–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Tehovnik EJ. 1995. Topographic distribution of fixation-related units in the dorsomedial frontal cortex of the rhesus monkey. Eur. J. Neurosci 7:1005–11 [DOI] [PubMed] [Google Scholar]
- Legg CR, Lambert S. 1990. Distance estimation in the hooded rat: experimental evidence for the role of motion cues. Behav. Brain Res 41:11–20 [DOI] [PubMed] [Google Scholar]
- Leinweber M, Ward DR, Sobczak JM, Attinger A, Keller GB. 2017. A sensorimotor circuit in mouse cortex for visual flow predictions. Erratum. Neuron 96:1204. [DOI] [PubMed] [Google Scholar]
- Li J, Guido W, Bickford ME. 2003. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J. Neurophysiol 90:3429–40 [DOI] [PubMed] [Google Scholar]
- Licata AM, Kaufman MT, Raposo D, Ryan MB, Sheppard JP, Churchland AK. 2017. Posterior parietal cortex guides visual decisions in rats. J. Neurosci 37:4954–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Huberman AD, Scanziani M. 2016. Cortico-fugal output from visual cortex promotes plasticity of innate motor behaviour. Nature 538:383–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-H, Coleman JE, Davoudi H, Zhang K, Hussain Shuler MG. 2015. Selective activation of a putative reinforcement signal conditions cued interval timing in primary visual cortex. Curr. Biol 25:1551–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rodenkirch C, Moskowitz N, Schriver B, Wang Q. 2017. Dynamic lateralization of pupil dilation evoked by locus coeruleus activation results from sympathetic, not parasympathetic, contributions. Cell Rep. 20:3099–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. 2001. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat. Neurosci 4:621–26 [DOI] [PubMed] [Google Scholar]
- Lyamzin D, Benucci A. 2019. The mouse posterior parietal cortex: anatomy and functions. Neurosci. Res 140:14–22 [DOI] [PubMed] [Google Scholar]
- Malvaez M, Shieh C, Murphy MD, Greenfield VY, Wassum KM. 2019. Distinct cortical-amygdala projections drive reward value encoding and retrieval. Nat. Neurosci 22:762–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshel JH, Garrett ME, Nauhaus I, Callaway EM. 2011. Functional specialization of seven mouse visual cortical areas. Neuron 72:1040–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, et al. 2015. Waking state: Rapid variations modulate neural and behavioral responses. Neuron 87:1143–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer GT, Montijn JS, Pennartz CMA, Lansink CS. 2017. Audiovisual modulation in mouse primary visual cortex depends on cross-modal stimulus configuration and congruency. J. Neurosci 37:8783–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer GT, Pie JL, Dolman TL, Pennartz CMA, Lansink CS. 2018. Audiovisual integration enhances stimulus detection performance in mice. Front. Behav. Neurosci 12:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Cox D. 2013. Rats maintain a binocular field centered on the horizon. F1000Research 2:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegas W, Bergan JF, Ogawa SK, Isogai Y, Umadevi Venkataraju K, et al. 2015. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. eLife 4:e10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milczarek MM, Vann SD, Sengpiel F. 2018. Spatial memory engram in the mouse retrosplenial cortex. Curr. Biol 28:1975–80.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minces VH, Alexander A, Datlow M, Alfonso S, Chiba AA. 2013. The role of visual cortex acetylcholine in learning to discriminate temporally modulated visual stimuli. Front. Behav. Neurosci 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Czajkowski R, Zhang N, Jeffery K, Nelson A. 2017. Retrosplenial cortex and its role in spatial cognition. bioRxiv 190801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Williams JD. 1993. Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J. Neurosci 13:4015–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinar EJ, Goodale MA. 1984. Cortical and tectal control of visual orientation in the gerbil: evidence for parallel channels. Exp. Brain Res 55:33–48 [DOI] [PubMed] [Google Scholar]
- Monaco S, Chen Y, Menghi N, Crawford JD. 2018. Action-specific feature processing in the human visual cortex. bioRxiv 420760. [DOI] [PubMed] [Google Scholar]
- Morin LP, Studholme KM. 2014. Retinofugal projections in the mouse. J. Comp. Neurol 522:3733–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. 1996. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb. Cortex 6:470–81 [DOI] [PubMed] [Google Scholar]
- Murata Y, Colonnese MT. 2016. An excitatory cortical feedback loop gates retinal wave transmission in rodent thalamus. eLife 5:e18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgas KA, Wilson AM, Michael V, Glickfeld LL. 2019. Unique spatial integration in mouse primary visual cortex and higher visual areas. bioRxiv 643007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. 2007. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci 30:99–122 [DOI] [PubMed] [Google Scholar]
- Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK. 2019. Single-trial neural dynamics are dominated by richly varied movements. bioRxiv 308288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustari MJ, Fuchs AF. 1989. Response properties of single units in the lateral terminal nucleus of the accessory optic system in the behaving primate. J. Neurophysiol 61:1207–20 [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, et al. 1986. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 396:77–96 [DOI] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Pearce JM, Vann SD, Aggleton JP. 2015. The effect of retrosplenial cortex lesions in rats on incidental and active spatial learning. Front. Behav. Neurosci 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. 2010. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65:472–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio N, Tsukano H, Hishida R, Abe M, Nakai J, et al. 2018. Higher visual responses in the temporal cortex of mice. Sci. Rep 8:11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoemene O, Pisupati S, Nguyen H, Churchland AK. 2018. Visual evidence accumulation guides decision-making in unrestrained mice. J. Neurosci 38:10143–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, et al. 2014. A mesoscale connectome of the mouse brain. Nature 508:207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. 2005. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433:597–603 [DOI] [PubMed] [Google Scholar]
- Olcese U, Iurilli G, Medini P. 2013. Cellular and synaptic architecture of multisensory integration in the mouse neocortex. Neuron 79:579–93 [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. 1954. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol 47:419–27 [DOI] [PubMed] [Google Scholar]
- Ongur D 2000. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 10:206–19 [DOI] [PubMed] [Google Scholar]
- Pafundo DE, Nicholas MA, Zhang R, Kuhlman SJ. 2016. Top-down-mediated facilitation in the visual cortex is gated by subcortical neuromodulation. J. Neurosci 36:2904–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagina G, Meyer JF, Smirnakis SM. 2017. Complex visual motion representation in mouse area V1. J. Neurosci 37:164–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Lüscher C. 2015. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88:1054–66 [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Papadopoulos GC. 2001. Serotoninergic afferents preferentially innervate distinct subclasses of peptidergic interneurons in the rat visual cortex. Brain Res. 891:158–67 [DOI] [PubMed] [Google Scholar]
- Petersen CCH. 2007. The functional organization of the barrel cortex. Neuron 56:339–55 [DOI] [PubMed] [Google Scholar]
- Petruno SK, Clark RE, Reinagel P. 2013. Evidence that primary visual cortex is required for image, orientation, and motion discrimination by rats. PLOS ONE 8:e56543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, et al. 2013. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci 16:1857–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancz EA, Moya J, Drawitsch F, Brichta AM, Canals S, Margrie TW. 2015. Widespread vestibular activation of the rodent cortex. J. Neurosci 35:5926–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS. 2014. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84:355–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, et al. 2016. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun 7:13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RP, Kinard WL, Degraff JJ, Leverage N, Norton JN. 2010. Noise in a laboratory animal facility from the human and mouse perspectives. J. Am. Assoc. Lab. Anim. Sci 49:592–97 [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM. 1978. Functional properties of the corticotectal projection in the golden hamster. J. Comp. Neurol 180:617–33 [DOI] [PubMed] [Google Scholar]
- Rolls E, Baylis L. 1994. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J. Neurosci 14:5437–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosón MR, Bauer Y, Kotkat AH, Berens P, Euler T, Busse L. 2019. Mouse dLGN receives functional input from a diverse population of retinal ganglion cells with limited convergence. Neuron 102:462–76.e8 [DOI] [PubMed] [Google Scholar]
- Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB. 2016. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci 19:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MM, Helmchen F, Kampa BM. 2012. Distinct functional properties of primary and posteromedial visual area of mouse neocortex. J. Neurosci 32:9716–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani T, Isa T. 2007. Quantitative analysis of spontaneous saccade-like rapid eye movements in C57BL/6 mice. Neurosci. Res 58:324–31 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. 2001. Look and see: how the brain moves your eyes about. Prog. Brain Res 134:127–42 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. 2005. Neural mechanisms underlying target selection with saccadic eye movements. Prog. Brain Res 149:157–71 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. 2015. Vision and the Visual System. Oxford, UK: Oxford Univ. Press [Google Scholar]
- Schnabel UH, Kirchberger L, van Beest E, Mukherjee S, Barsegyan A, et al. 2018. Feedforward and feedback processing during figure-ground perception in mice. bioRxiv 456459 [Google Scholar]
- Schneider GE. 1969. Two visual systems. Science 163:895–902 [DOI] [PubMed] [Google Scholar]
- Scholl B, Burge J, Priebe NJ. 2013. Binocular integration and disparity selectivity in mouse primary visual cortex. J. Neurophysiol 109:3013–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl B, Pattadkal JJ, Rowe A, Priebe NJ. 2017. Functional characterization and spatial clustering of visual cortical neurons in the predatory grasshopper mouse Onychomys arenicola. J. Neurophysiol 117:910–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Luo L. 2015. Organization of the locus coeruleus-norepinephrine system. Curr. Biol 25:R1051–56 [DOI] [PubMed] [Google Scholar]
- Seillier L, Lorenz C, Kawaguchi K, Ott T, Nieder A, et al. 2017. Serotonin decreases the gain of visual responses in awake macaque V1. J. Neurosci 37:11390–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Liu A, Li D, Xie Z, Chen Z, et al. 2019. A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nat. Neurosci 22:909. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. 1996. Functional organization of thalamocortical relays. J. Neurophysiol 76:1367–95 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. 1998. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators.” PNAS 95:7121–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. 2011. Distinct functions for direct and transthalamic corticocortical connections. J. Neurophysiol 106:1068–77 [DOI] [PubMed] [Google Scholar]
- Shimaoka D, Harris KD, Carandini M. 2018. Effects of arousal on mouse sensory cortex depend on modality. Cell Rep. 22:3160–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieben K, Röder B, Hanganu-Opatz IL. 2013. Oscillatory entrainment of primary somatosensory cortex encodes visual control of tactile processing. J. Neurosci 33:5736–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J 1984. The accessory optic system. Annu. Rev. Neurosci 7:13–41 [DOI] [PubMed] [Google Scholar]
- Sinnamon H, Galer B. 1984. Head movements elicited by electrical stimulation of the anteromedial cortex of the rat. Physiol. Behav 33:185–90 [DOI] [PubMed] [Google Scholar]
- Song Y-H, Kim J-H, Jeong H-W, Choi I, Jeong D, et al. 2017. A neural circuit for auditory dominance over visual perception. Neuron 93:940–54.e6 [DOI] [PubMed] [Google Scholar]
- Spiro T, Massopust LC, Young PA. 1980. Efferent projections of the lateral dorsal nucleus in the rat. Exp. Neurol 68:171–84 [DOI] [PubMed] [Google Scholar]
- Stehberg J, Dang PT, Frostig RD. 2014. Unimodal primary sensory cortices are directly connected by long-range horizontal projections in the rat sensory cortex. Front. Neuroanat 8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien N, Cappaert N. 2011. The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat—an interactive connectome. Front. Neuroinform 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LO, Brady CM, Cahill H, Al-Khindi T, Sakuta H, et al. 2015. Functional assembly of accessory optic system circuitry critical for compensatory eye movements. Neuron 86:971–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. 1983. Efferent systems of primary visual cortex: a review of structure and function. Brain Res. Rev 6:1–24 [DOI] [PubMed] [Google Scholar]
- Tang J, Ardila Jimenez SC, Chakraborty S, Schultz SR. 2016. Visual receptive field properties of neurons in the mouse lateral geniculate nucleus. PLOS ONE 11:e0146017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. 2007. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci 30:181–207 [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Yeomans JS. 1987. Circling elicited from the anteromedial cortex and medial pons: refractory periods and summation. Brain Res. 407:240–52 [DOI] [PubMed] [Google Scholar]
- Teuber H-L. 1970. Subcortical vision: a prologue. Brain Behav. Evol 3:7–15 [DOI] [PubMed] [Google Scholar]
- Thompson AD, Picard N, Min L, Fagiolini M, Chen C. 2016. Cortical feedback regulates feedforward retino-geniculate refinement. Neuron 91:1021–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmi M, Meguro R, Tsukano H, Hishida R, Shibuki K. 2014. The extrageniculate visual pathway generates distinct response properties in the higher visual areas of mice. Curr. Biol 24:587–97 [DOI] [PubMed] [Google Scholar]
- Tsukano H, Horie M, Ohga S, Takahashi K, Kubota Y, et al. 2017. Reconsidering tonotopic maps in the auditory cortex and lemniscal auditory thalamus in mice. Front. Neural Circuits 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen B, Winkelman BHJ, Frens MA. 2010. Three-dimensional optokinetic eye movements in the C57BL/6J mouse. Investig. Ophthalmol. Vis. Sci 51:623–30 [DOI] [PubMed] [Google Scholar]
- van der Togt C, van der Want J, Schmidt M. 1993. Segregation of direction selective neurons and synaptic organization of inhibitory intranuclear connections in the medial terminal nucleus of the rat: an electro-physiological and immunoelectron microscopical study. J. Comp. Neurol 338:175–92 [DOI] [PubMed] [Google Scholar]
- Varela C 2014. Thalamic neuromodulation and its implications for executive networks. Front. Neural Circuits 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Fort M, Bracey EF, Keshavarzi S, Rousseau CV, Cossell L, et al. 2018. A circuit for integration of head-and visual-motion signals in layer 6 of mouse primary visual cortex. Neuron 98:179–91.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Fort M, Rousseau CV, Niedworok CJ, Wickersham IR, Rancz EA, et al. 2014. The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron 83:1431–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. 2010. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct. Funct 215:1–28 [DOI] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA. 2015. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86:740–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EY, Sinz FH, Froudarakis E, Fahey PG, Muhammad T, et al. 2018. Inception in visual cortex: In vivo-silico loops reveal most exciting images. bioRxiv 506956 [Google Scholar]
- Wallace DJ, Greenberg DS, Sawinski J, Rulla S, Notaro G, Kerr JND. 2013. Rats maintain an overhead binocular field at the expense of constant fusion. Nature 498:65–69 [DOI] [PubMed] [Google Scholar]
- Wang L, Krauzlis RJ. 2018. Visual selective attention in mice. Curr. Biol 28:676–85.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sporns O, Burkhalter A. 2012. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J. Neurosci 32:4386–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Winiger V, Kandel ER, Balsam PD, Simpson EH. 2015. Orbitofrontal cortex mediates the differential impact of signaled-reward probability on discrimination accuracy. Front. Neurosci 9:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Ausim Azizi S, Burne RA, Woodward DJ. 1990. Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res. 514:276–92 [DOI] [PubMed] [Google Scholar]
- Wei P, Liu N, Zhang Z, Liu X, Tang Y, et al. 2015. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun 6:6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. 1989. Brain dopamine and reward. Annu. Rev. Psychol 40:191–225 [DOI] [PubMed] [Google Scholar]
- Yamawaki N, Radulovic J, Shepherd GMG. 2016. A corticocortical circuit directly links retrosplenial cortex to M2 in the mouse. J. Neurosci 36:9365–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. 1995. The acoustic startle reflex: neurons and connections. Brain Res. Rev 21:301–14 [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Tehovnik EJ. 1988. Turning responses evoked by stimulation of visuomotor pathways. Brain Res. Rev 13:235–59 [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Meister M. 2013. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol 23:2011–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, et al. 2009. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLOS ONE 4:e4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Lund RD. 1994. The anatomical substrates subserving the pupillary light reflex in rats: origin of the consensual pupillary response. Neuroscience 62:481–96 [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, et al. 2015. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb. Cortex 25:118–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Do JPH, Chang W-C, et al. 2014. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345:660–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu M, Cang J. 2014. Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice. Neuron 84:202–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Schafer RJ, Desimone R. 2016. Pulvinar-cortex interactions in vision and attention. Neuron 89:209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Maire P, Masterson S, Bickford M. 2017a. The mouse pulvinar nucleus: organization of the tectorecipient zones. Vis. Neurosci 34:E011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Masterson SP, Damron JK, Guido W, Bickford ME. 2017b. The mouse pulvinar nucleus links the lateral extrastriate cortex, striatum, and amygdala. J. Neurosci 38:347–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, King WM. 1998. Premotor commands encode monocular eye movements. Nature 393:692–95 [DOI] [PubMed] [Google Scholar]
- Zhuang J, Ng L, Williams D, Valley M, Li Y, et al. 2017. An extended retinotopic map of mouse cortex. eLife 6:e18372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KS, Yamin JA, Rainnie DG, Ressler KJ, Gourley SL. 2017. Connections of the mouse orbitofrontal cortex and regulation of goal-directed action selection by brain-derived neurotrophic factor. Biol. Psychiatry 81:366–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Chou X-L, Zhang Z-G, Mesik L, Liang F, et al. 2017. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93:33–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser K, Lamme VAF, Schiller PH. 1996. Contextual modulation in primary visual cortex. J. Neurosci 16:7376–89 [DOI] [PMC free article] [PubMed] [Google Scholar]


