Traditional biopsy consists of the removal of cancerous or suspected cancerous tissue for diagnosis (i.e., needle biopsy, bone or marrow biopsy, etc.). Liquid biopsy has recently been presented as an alternative for earlier detection and to help inform clinical decision making. In this commentary, we detail the translational path that liquid biopsy followed to move from concept to clinical care, and highlight barriers and facilitators to its successful translation.
TISSUE VS. LIQUID BIOPSIES
Biopsies traditionally involve the removal of solid tissue for examination; done via needle biopsy, computed tomography‐guided biopsy, bone marrow biopsy, or surgical biopsy. These procedures come with inherent risks to the patient, such as infection and complications due to tumor location, as well as diagnostic risks, such as insufficient material or sampling bias due to tumor heterogeneity. 1 Liquid biopsy is the removal of blood or other bodily fluid to detect cancerous cells or cancerous DNA that arises from malignant or nonmalignant cells in the body. Compared with traditional biopsy, liquid biopsy is less invasive, offers reduced risks of complication, increased ability for longitudinal monitoring, and the possibility of quickly identifying clonal evolution and the development of resistances in cancer cells. 2
Cell‐Free and Circulating Tumor DNA Discoveries
The presence of cell‐free DNA (cfDNA) in human blood was first noted by a pair of French physicians, Mandel and Métais, in 1948 (Figure 1 ). 3 In 1977, the discovery of elevated levels of cfDNA in patients with cancer was made, opening the door for further explorations into the connection between the two. 4 Primary tumor cells undergo apoptosis, necrosis, phagocytosis, or cell detachment and, thus, release tumor‐derived cfDNA, otherwise known as circulating tumor DNA (ctDNA), into the bloodstream. As genetic sequencing technology improved with the advent of polymerase chain reaction and eventually next generation sequencing technologies, ctDNA was more thoroughly characterized. 5 Catalyzed by the new sequencing technologies, explorations into blood samples from the cancer patient populations yielded known cancer‐related mutations in the ctDNA of both patients with solid and liquid tumors, lending credence to the hypothesis that the genetic material of cancerous cells could be monitored in blood. 6
Figure 1.
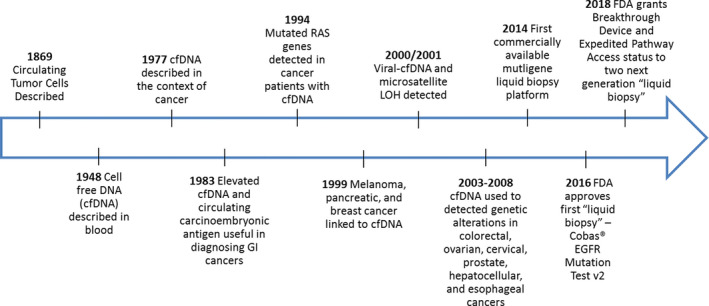
Timeline of translation for liquid biopsy technology from discovery to early clinical implementation. FDA, US Food and Drug Administration; GI, gastrointestinal; LOH, Loss of Heterozygosity.
Following the discovery of increased levels of cfDNA in patients with cancer, liquid biopsies have been explored as a potentially safer, more effective clinical detection method. Early detection of ctDNA via a simple blood draw offers the potential for population‐level screening, early detection, intervention, and progressive monitoring throughout treatment; in short, it has potential utility across the cancer care continuum. 2 Liquid biopsy’s low cost, minimal collection risks, and potential for serial or repeated testing make it an attractive alternative to traditional biopsies.
BRINGING LIQUID BIOPSY TO CLINIC: THE FIRST CASE
Roche Diagnostic’s Cobas EGFR Mutation Test version 2 is currently the only US Food and Drug Administration (FDA) approved liquid biopsy, and, thus, serves as a case study for the successful translation of this technology. The test is an adaptation of an existing tissue biopsy system for identifying specific actionable mutations in non‐small cell lung cancer (NSCLC). The tissue version of the Cobas system received premarket approval from the FDA in 2013, and full approval in 2015. Cobas was approved as a diagnostic tool for identifying patients with NSCLC with specific mutations in the EGFR gene for whom EGFR inhibitors (gefitinib and erlotinib) may be particularly effective. This approval was based on almost a decade of both laboratory and clinical research proving the effectiveness of inhibitor treatment in both primary NSCLC cell lines and patients with specific EGFR mutations. 7
The liquid Cobas test, also known as Cobas EGFR Mutation Test version 2, applies the concepts and technology from the original tissue test (Cobas version 1) with an additional innovation that allows for extraction of genomic material from blood. By utilizing an already approved clinical platform, Cobas version 2 was able to leverage the existing data used to approve Cobas version 1 for its own approval process. The pivotal trial for the liquid biopsy Cobas system was the ENSURE trial, which included comparisons of the Cobas version 2 system to other diagnostic methods, including Cobas version 1. 8 The study design included a built‐in comparison of Cobas version 2 and version 1, which was used to demonstrate noninferiority of the new technology compared with the previously approved system. The results of this trial were submitted to the FDA, which approved the Cobas version 2 system as a companion diagnostic tool for identifying patients with NSCLC eligible for inhibitor treatment on June 1, 2016. The Cobas version 2 system can also be applied once a patient’s NSCLC has mutated against frontline inhibitor treatment (erlotinib). If mutations are detected, a second‐line drug like osimertinib can be administered to prolong the effectiveness of inhibitor treatment. This additional functionality helps justify routine screening with the Cobas version 2 system even after erlotinib treatment has begun and is an example of how the diagnostic test can be repeatedly used to address clonal evolution.2
LIQUID BIOPSY: FUTURE APPLICATIONS
The approval of the Cobas EGFR Mutation Test version 2 illustrates three key lessons which could be applied for rapid and effective translation of future liquid biopsy technologies: (i) existing scientific literature linking genetic mutations with cancerous phenotypes can pre‐empt the need for extensive basic scientific investigation; (ii) liquid biopsies that seek to characterize actionable mutations have a clear outcome that can be used to establish the efficacy of the technology; and (iii) liquid biopsy tests can offer additional clinical value beyond initial diagnosis and their simplicity allows for routine monitoring without being a major imposition on the patient or care providers. Liquid biopsy platforms being explored for future use fall under two broad categories: those that target a specific cancer, and panels that screen for multiple malignancies. Targeted liquid biopsies that utilize polymerase chain reaction amplification of target sequences for cancer detection are being explored in multiple cancers in a similar vein to the Cobas system. For example, the PRODIGE‐14 trial of metastatic colorectal cancer utilized both ctDNA and circulating tumor cells to categorize patients as eligible for surgical resection of liver metastases. 9 In neuroblastoma, a cancer with limited opportunities for tissue retrieval, targeted liquid biopsy approaches offer a noninvasive alternative for monitoring disease progression and establishing prognosis. 10 Specific mutations in the oncogenes MYCN and ALK, DNA methylation profiles, chromosomal alterations, and circulating microRNAs can be targeted to better characterize a patient’s neuroblastoma. MYCN mutations are used for risk stratification of patients and represent a powerful prognostic indicator; ALK mutations represent a druggable target and ALK inhibitors are being investigated concurrently for clinical utility in neuroblastomas. The existence of an actionable target for a liquid biopsy can be a major factor in streamlining the clinical translation of such technologies.
In contrast to the targeted liquid biopsy approach, multiple versions of gene panels, some of which also incorporate protein biomarkers, are currently being explored for the diagnosis and monitoring of a broad set of cancers. Notable gene panels include FoundationONE Liquid, Guardant360, and Cancerseek, all of which and have been granted “Breakthrough Device” status by the FDA. This status facilitates interactions between manufacturers and FDA officials during the premarket approval phase, ultimately streamlining the approval process. These panels utilize a set of genes that have been shown to be associated with various cancers, some of which are the same targets utilized by more targeted liquid biopsies (e.g., EGFR in NSCLC and ALK in neuroblastoma). The intended use of these panels is early detection of cancers before metastases occur.
Although these panels have the advantage of utilizing evidence from previous studies, such as the validation of EGFR mutations, the wide array of gene targets presents a regulatory challenge. FoundationOne Liquid screens blood samples for mutations in 70 known cancer drivers and provides measures of blood tumor burden. As mentioned, some of those mutated genes, like EGFR or ALK, are druggable targets: however, other genes are strictly only useful for screening. An additional challenge for these panels is that their usage has only been approved by the Centers for Medicare and Medicaid Services for select patient populations, limiting the feasibility of this technology to be accessible to many patients. Additionally, limitations with regard to next generation sequencing capabilities in some centers compound this accessibility issue.
CONCLUSIONS AND LESSONS LEARNED
With limited side effects, low levels of invasiveness, and low cost, liquid biopsy technology could significantly improve the care of patients worldwide. As liquid biopsy becomes more common in clinical settings, it is important for the broader medical community to come to a consensus on how oversight of this technology should be handled. For example, would each gene in a panel need to be independently evaluated and approved for use, or could the panel be approved as a single unit? Would these reports be interpreted by a genetic counselor before being provided to physicians, or would physicians require specialized training to effectively use the liquid biopsy results? Although instances where an actionable target has been mutated would yield a simple report, situations in which a series of mutations in suspected oncogenes without drug targets has a less obvious response.
These questions highlight the need to define the exact role these new liquid biopsies will play on the cancer care delivery continuum moving forward. Targeted liquid biopsies show promise for establishing drug eligibility, serial monitoring of disease progression, and diagnosis when tissue samples are hard to access. Assuming further studies establish the impact of targeted liquid biopsy use on patient outcomes, they could be integrated into everyday oncology practice. Before panel‐ based liquid biopsies can become integrated, they must establish robust sensitivity and specificity parameters in large populations of healthy controls. Additionally, they must clearly define whether they are intended for screening of at‐risk populations, as companion diagnostic tools to be validated with further testing, or as a frontline diagnostic tool for use in multiple clinical settings.
Funding
This publication was made possible by Clinical and Translational Science Award (CTSA) grant number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Conflict of Interest
All authors declared no competing interests for this work.
References
- 1. Borghesi, M. et al Complications after systematic, random, and image‐guided prostate biopsy. Eur. Urol. 71, 353–365 (2017). [DOI] [PubMed] [Google Scholar]
- 2. Wan, J.C.M. et al Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017). [DOI] [PubMed] [Google Scholar]
- 3. Mandel, P. & Métais, P. Les acides nucléiques du plasma sanguin chez l'homme. C. R. Acad. Sci. Paris 142, 241–243 (1948). [PubMed] [Google Scholar]
- 4. Leon, S.A. , Shapiro, B. , Sklaroff, D.M. & Yaros, M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 37, 646–650 (1977). [PubMed] [Google Scholar]
- 5. Schwarzenbach, H. et al Cell‐free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 11, 426–437 (2011). [DOI] [PubMed] [Google Scholar]
- 6. Vasioukhin, V. et al Point mutations of the N‐ras gene in the blood plasma DNA of patients with myelodysplastic syndrome or acute myelogenous leukaemia. Br. J. Haematol. 86, 774–779 (1994). [DOI] [PubMed] [Google Scholar]
- 7. Ciardiello, F. & Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 358, 1160–1174 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Wu, Y.‐L. et al First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: analyses from the phase III, randomized, open‐label. ENSURE study. Ann. Oncol. 26, 1883–1889 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Bidard, F.‐C. et al Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic colorectal cancer: a prospective ancillary study to the Unicancer Prodige‐14 trial. Cells 8, 516 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trigg, R.M. , Shaw, J.A. & Turner, S.D. Opportunities and challenges of circulating biomarkers in neuroblastoma. Open Biol. 9, 190056 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


