Abstract
Problem:
Receptive anal intercourse (RAI) is more efficient than receptive vaginal intercourse (RVI) at transmitting HIV, but its contribution to heterosexually-acquired HIV infections among at-risk women in the US is unclear.
Method of study:
We analysed sexual behaviour data from surveys of 9,152 low-income heterosexual women living in 20 cities with high rates of HIV conducted in 2010 and 2013 as part of US National HIV Behavioral Surveillance. We estimated RAI prevalence (past-year RAI) and RAI fraction (fraction of all sex acts (RVI and RAI) at the last sexual episode that were RAI among those reporting past-year RAI) overall and by key demographic characteristics. These results and HIV incidence were used to calibrate a risk-equation model to estimate the population attributable fraction of new HIV infections due to RAI (PAFRAI) accounting for uncertainty in parameter assumptions.
Results:
RAI prevalence (overall: 32%, city range: 19-60%) and RAI fraction (overall: 27%, city-range: 18-34%) were high overall and across cities, and positively associated with exchange sex. RAI accounted for an estimated 41% (uncertainty range: 18-55%) of new infections overall (city range: 21-57%). Variability in PAFRAI estimates was most influenced by uncertainty in the estimate of the per-act increased risk of RAI relative to RVI and the number of sex acts.
Conclusions:
RAI may contribute disproportionately to new heterosexually-acquired HIV infections among at-risk low-income women in the US, meaning that tools to prevent HIV transmission during RAI are warranted. Number of RVI and RAI acts should also be collected to monitor heterosexually-acquired HIV infections.
Keywords: Anal sex, HIV, women, sexual behaviour, heterosexual, United States
Introduction
Current evidence suggests that penile-anal intercourse (receptive anal intercourse, RAI) increases the risk of HIV acquisition per sex act by up to 18-fold compared to one act of penile-vaginal intercourse (receptive vaginal intercourse, RVI)1,2. Previous modelling studies suggested that even if 5-10% of all heterosexual sex acts are RAI this may reduce the effectiveness of HIV interventions that are only efficacious for RVI, such as daily or long-lasting vaginal microbicides, by up to 50%3,4.
Recent systematic reviews suggest RAI is commonly practised by women across a variety of populations and contexts throughout their lifetime5,6. For example, 2-36% of South African women and 20-24% of sexually active women under 25 years old worldwide report ever engaging in RAI5,6. National surveys in the US and UK indicate that around 12 and 11% of women aged 18-59 years respectively engaged in RAI over the previous year7-9. RAI prevalence among women may also have increased since the 1990s8,10,11, even doubling in places8,10. Women reporting RAI often also report other practices such as exchange sex12, high numbers of sexual partners and more frequent sex acts13,14, substance use12,13,15,16, coerced sex17 and low condom use14,18 associated with increased risk of acquiring sexually transmitted infections (STI), including HIV. Nevertheless, RAI has, until recently, been sidelined from receptive partner-controlled HIV prevention and product innovation both for men who have sex with men (MSM) and women19-22. Understanding the epidemiological context of RAI among heterosexual women and its contribution to HIV and other STI, is necessary to tailor prevention messaging and product development, such as rectal microbicides23,24.
In the US, heterosexual transmission accounted for 24% of all adult and adolescent HIV infections diagnosed in 2017; whilst MSM and people who inject drugs (PWID) accounted for 70% and 6% of cases25. Black/African American (henceforth, Black) and Hispanic/Latino (henceforth, Hispanic) populations are disproportionately affected by HIV26. In 2017, the HIV diagnosis rates (per 100,000) for adult and adolescent Black and Hispanic women were respectively 15 and 3 times the rate for White women25. The Centers for Disease Control and Prevention (CDC) established the National HIV Behavioral Surveillance (NHBS), a comprehensive system for conducting behavioural surveillance among people at high risk for HIV infection in the U.S. and identifying risk factors (including RAI) associated with infection27,28.
Using NHBS data from low-income women at increased risk of HIV infection living in 20 US cities, we 1) describe RAI practices across key demographic and risk factor groups among women reporting heterosexual intercourse in the past year and 2) use these results to inform a mathematical model and estimate the annual fraction of new heterosexually-acquired HIV infections that are due to RAI among at-risk women in the NHBS sample overall and in 20 cities.
Methods
Data used
NHBS has conducted independent serial cross-sectional behavioural surveys among heterosexual women (NHBS-HET) living in high HIV prevalence metropolitan statistical areas (MSAs) every three years since 200726,27. To derive average estimates for 2010 and 2013, our analysis combines the data from 20 MSAs included in the 2010 and 2013 study cycles. The 2007 cycle was excluded from this analysis due to differences in the sampling methods. Detailed data collection procedures have already been described26,29. In short, participants were recruited through respondent driven sampling (RDS)30. Recruitment prioritised women with household income below the federal poverty guidelines31 or with no more than high school education. Individuals aged 18-60 years were eligible to participate if they lived in a participating MSA, could complete the survey in English or Spanish, provided informed consent and reported at least one episode of sexual intercourse (RVI or RAI) with an opposite-sex partner during the previous year. As our analysis focused on heterosexually-acquired HIV, we excluded the minority of women who reported injecting drugs in the previous year (2010: N=165 excluded, 3.4%; 2013: N=192 excluded, 4.0%) or who only reported oral intercourse during their most recent sexual episode (2010: N=74 excluded, 1.6%; 2013: N=76 excluded, 1.6%).
The survey was administered through face-to-face interviews (FTFI) to collect demographic information (e.g. age, race/ethnicity, marital status) and self-reported sexual behaviour over the previous 12 months (e.g. RAI, number and type of male partners, condom use, exchanging sex for money or drugs) as well as characteristics of their last sexual episode (e.g. whether participants practised RVI and/or RAI, condom use during RVI and RAI, partner type: main, casual or exchange partner, partner’s HIV status, and partner’s race - 2013 cycle only). Questions were the same across cycles apart from the question about sex work (in the 2010 cycle the definition of exchange sex included exchanging money or goods with either main or casual partners but in the 2013 cycle exchange sex included only casual partners). No information on number of sex acts per unit time or per partners was available. Health departments from participating cities obtained local institutional review board approval before initiating each cycle26,29.
Statistical analyses
In this study, the level of RAI in the population was characterised by: 1) RAI prevalence, the proportion of women reporting RAI with at least one partner in the past-year, and 2) RAI fraction, the fraction of all acts (RVI and RAI) at last sexual episode that were RAI among those reporting RAI in the past year. We calculated RAI prevalence and RAI fraction estimates stratified by key demographic and risk factors (age, race/ethnicity, exchange sex and partner type), overall and by city for the two cycles combined. We also used two binary outcome variables measuring 1) whether or not women practised RAI in the past-year (RAI and non-RAI women respectively); 2) among sex acts of RAI women at last sexual episode, whether or not the sex act was RAI. Bivariate and stratified analyses, using chi-squared and Mantel-Haenszel tests respectively,32 compared both outcomes across the levels of demographic and risk factors except for condom use during the last sexual episode across which only RAI prevalence was compared; two-sample t-test tested differences in the mean annual number of partners. Stratification controlled for city and additionally for each of the factors in turn. Among RAI women, bivariate and stratified analyses compared condom use at last vaginal sex with condom use at last anal sex. Partner’s race/ethnicity was excluded from analyses as it was only collected in one cycle. We treated the data as a convenience sample; we report unadjusted and stratified odds ratios as well as 95% confidence intervals and p-values based on normal approximation. We report estimates and confidence intervals unadjusted for network size or clustering of RDS recruitment chains because these were used to derive prior ranges for parameters in the risk equation model and in model sensitivity analyses and not to make inferences about the wider population of low-income heterosexual women at risk of HIV. These intervals are therefore likely to be narrower than if recruitment-chain clustering was accounted for. This decision was taken because in sensitivity analyses quadrupling the standard error did not affect our model predictions of the PAF.
Risk-equation model
We developed a Bernoulli risk-equation model of HIV incidence in at-risk women33. The model was stratified by demographic and risk factors (noted j with J groups) to estimate the annual cumulative risk of HIV acquisition (CIRi,j) over multiple independent sex acts per partnership among women practising and not practising RAI (noted i=1,2)(Equation 1). The model was used to estimate HIV risk overall and separately by age (J=2 groups: 18-24, 25-60 years old), race/ethnicity (J=4 groups: Hispanic, Black, White, Other), exchange sex (J=2 groups: not exchanging sex, exchanging sex), or city (J=20 groups) and finally by exchange sex within city (details in supplement tables 1&2, parameter ranges for exchange sex within city available on request). CIRi,j depends on the annual number of sexual partners (mi,j,k) of type “k” (i.e. main or casual) for women in groups ij, the probability that a male partner j’ is HIV infected (pj’), the annual number of sex acts per partnership of type k (ni,j,k,), the fraction of sex acts which are RAI (fai,j,k), the probability of using a condom during RVI (fcvi,j,k) and RAI (fcai,j,k) per partner type, condom efficacy in reducing HIV transmission during one RAI or RVI act (ec), HIV transmission probability per RVI (β), and the relative risk of HIV infection during RAI compared to RVI (RRRAI). For each factor, the overall HIV risk is the average of the cumulative incidences over the J groups weighted by relative group size and RAI status (Fi,j) (Equation in supplement A).
| Equation 1 |
Parameter assumptions and model calibration
Uniform ranges of plausible values were specified for each parameter (i.e. prior parameter range) based on the unadjusted 95% CI of the estimates from NHBS data (overall and by demographic or risk group) when available and sourced from the literature otherwise (Table 1, supplement tables 1 and 2). The HIV transmission probability per unprotected receptive vaginal intercourse (RVI) and the increase in HIV acquisition risk during RAI (RRRAI) were based on meta-analyses of observational studies2,34,35. Given the uncertainties in these estimates, we assumed wider ranges, varying RRRAI between 2-181,2,34-36 (supplement E) and the transmission probability per RVI between 0.0004-0.002. HIV prevalence of heterosexual male partners was derived from published data on male participants of NHBS-HET37. As no information on number of sex acts per partnership type (ni,j,k) was directly available from NHBS-HET surveys, we specified and independently sampled wide prior ranges for both RAI and non-RAI populations, and obtained posterior estimates of these parameters at the fitting stage (see below). Empirical estimates of overall HIV incidence rate and the incidence risk ratio for RAI vs non-RAI women were available from a cohort study (HPTN-06438,39) conducted in 2009-2010 in a comparable study population in 5 of 20 NHBS-HET sites.
Table 1.
Model parameters and their ranges for the overall model
| Parameter (for individual in RAI group i=1,2 and overall model J=1) | Parameter ranges | Source | ||||
|---|---|---|---|---|---|---|
| Symbols | Description | RAI (i=1) | Non-RAI (i=2) | |||
| Main partners (k=1) |
Casual partners (k=2) |
Main partners (k=1) |
Casual partners (k=2) |
|||
| Fi,1 | Fraction of the population | 0.31-0.33 | 0.67-0.69 | Table 2 | ||
| mi,1,k | Annual number of partners of type k | 1.3-1.5 | 7.2-10.0 | 1.1-1.1 | 2.1-2.6 | Table 3 |
| ni,1,k | Number of sex acts per year of per partner type k | 0-150 | 0-3 | 0-150 | 0-3 | 13,69 |
| fai,1,k | Fraction of sex acts that are anal among RAI women (RAI fraction) with partner of type k | 0.23-0.26 | 0.28-0.32 | 0 | 0 | Table 2 |
| fcvi,1,k | Fraction of vaginal sex acts that are protected by condoms with partner of type k | 0.06-0.08 | 0.11-0.15 | 0.12-0.14 | 0.21-0.24 | Table 3 |
| fcai,1,k | Fraction of anal sex acts that are protected by condoms with partner of type k | 0.07-0.11 | 0.04-0.08 | 0 | 0 | Table 3 |
| HIV prevalence in male partners of subgroup j’=1 | 1.3-2.7% | 37 | ||||
| β | Male to female HIV transmission probability per unprotected vaginal act | 0.0004-0.002 | 34,35 | |||
| RRRAI | Increased risk of HIV acquisition through RAI compared to RVI | 2-18 | 1,2,34,35 | |||
| ec | Condom efficacy per act | 0.8-1 | 70,71 | |||
| Fitting data | ||||||
| Annual HIV incidence | 0.14-0.74% | 39 | ||||
| Incidence risk ratio | 0.3-8.7 | 38 | ||||
| Total sex acts per year across all partners | 1-170 | 1–170 | Supplement table 3 13,69 | |||
| Ratio of total acts across all partnerships for RAI women to the total acts across all partnerships for non-RAI women | 0.5-3 | 0.5-3 | Supplement table 3 72,73 | |||
At the fitting stage, we simultaneously sampled prior parameter ranges using Latin-hypercube sampling40 to generate 10,000 parameter sets that were used to produce model predictions of the annual cumulative HIV incidence risk (CIRi,j). Predicted cumulative incidence risk estimates were converted to annual incidence rates to be comparable with data observed in 2010 from HPTN-06441 (supplement C). We retained entire parameter sets if predicted rates and risk ratio fell within the 95%CI of HPTN-064 HIV incidence rate39 and incidence risk ratio38, and if the total number of sex acts in a year across all partnerships and the ratio of sex acts reported by RAI women and non-RAI women generated by the model agreed with available data from similar US populations and other sources (prior parameter ranges in table 1; details in supplement parts A, B). Given the lack of city-specific HIV incidence data, we estimated it by applying a scaling factor, to the HPTN-064 incidence rate estimates, based on 2013 HIV diagnosis rate among adults for each city (details in supplement C). The resulting sets of fitting parameters define the baseline scenario in our modelling analysis.
Modelling analysis
For each retained parameter set, we derived two population attributable fraction (PAF) estimates measuring the fraction of heterosexually-acquired HIV infections in a year among women due to 1) RAI only (PAFRAI) and 2) RAI as well as the higher risk behaviours reported by women practising RAI (PAFRAI+Beh). PAFRAI compares the cumulative incidence between the baseline scenario where RRRAI>1 (CIRRR>1) and a counterfactual where RRRAI=1 (CIRRR=1), i.e. where per act risk during RAI is assumed to be the same as during RVI. PAFRAI+Beh compares the CIR between the baseline scenario and a second counterfactual where RRRAI=1 and where the risk behaviours of RAI women (i.e. condom use and number of sexual partners) are set to the same level as non-RAI women.
We report the median PAF and 10th-90th percentile uncertainty intervals (80%UI). We conducted an uncertainty analysis first to assess which parameter most influenced the variation across overall PAF estimates using Pearson’s correlation coefficients. Second, we conducted a more general sensitivity analysis using wider parameter ranges and fitting only on the incidence rate and incidence risk ratio (as opposed to fitting additionally to the number of acts and act ratio). The first and second analysis help determine which additional and new data to prioritise for collection in the context of the NHBS study and in settings where little data is available, respectively.
Although our analysis focused on heterosexual transmission, we also assessed the potential influence of HIV transmission by needle-sharing on PAFRAI estimates (see detailed methods in supplement D). We explored scenarios where we assumed that HIV incidence rate among PWID was the same, twice or five times larger than among women who do not inject (NIDU).
All analyses were conducted in R version 3.4.042 using R-studio version 1.0.14343.
Results
Study sample
Women had a mean age of 37 years, 24% were under 25 years old, 73% were Black, 41% reported having had only main partners in the last year, and 23% reported exchanging sex. HIV prevalence was 3% overall and similar between women who did or did not practise RAI in the past year (OR=0.99, 95%CI 0.77-1.29). Apart from race/ethnicity, other city-level demographic and risk factor patterns were broadly similar to overall patterns. In all but four cities (Denver, Los Angeles, San Diego and San Juan) the percentage of Black women was at least 60%. In these four cities, the percentage of Hispanic women was greater than 40% (Supplement figures 1a-d).
How common is RAI?
Table 2 summarises the RAI prevalence and RAI fraction of the sample participants by demographic characteristics for both study cycles combined. Overall, RAI prevalence was 32% (95%CI 31-33%). RAI prevalence was high even among 18-19 year olds (22%), but higher among older women (>31%)(Table 2). RAI prevalence was higher among Hispanics (35%) and Whites (37%) than Blacks (31%) (p=0.003) and among women reporting only casual (41%) or reporting exchange sex partners (53%) in the past year than women with only main partners (19%) or no exchange partners (26%) but consistent across marital status (Table 2).
Table 2.
Receptive anal intercourse (RAI) prevalence and RAI fraction among women by demographic characteristics and past year sexual behaviours, combined NHBS-HET study cycles 2010 and 2013.
| Sexual behaviour in the past year among all women in study population |
Last sexual episode among women who reported RAI in the past year |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RAI prevalence |
RAI women (RAI in the past year) |
Non-RAI women (No RAI in the past year) |
OR | RAI fraction |
RAI acts1 | RVI acts1 | OR | |||
| Characteristics | % (95% CI) |
N (%) | N (%) | (95%CI) | p- value |
% (95%CI ) |
Nacts (%) | Nacts (%) | (95 %CI) | p-value |
| Overall | 31.9 (30.9-32.8) | 2924 (100) | 6228 (100) | NA | 26.7 (25.3-28.1) | 1045 (100) | 2870 (100) | NA | ||
| Demographics | ||||||||||
| Age | <0.001 | 0.050 | ||||||||
| 18-19 | 22.4 (19.4-25.7) | 148 (5.1) | 512 (8.2) | 1.00 | 23.6 (18.1-30.1) | 45 (4.3) | 146 (5.1) | 1 | ||
| 20-24 | 31.7 (29.3-34.0) | 483 (16.5) | 1042 (16.7) | 1.60 (1.30-1.98) | <0.001 | 25.6 (22.3-29.1) | 162 (15.5) | 471 (16.4) | 1.12 (0.76-1.63) | 0.570 |
| 25-29 | 33.0 (30.5-35.6) | 424 (14.5) | 860 (13.8) | 1.71 (1.37-2.12) | <0.001 | 26.1 (22.7-29.9) | 147 (14.1) | 416 (14.5) | 1.14 (0.78-1.68) | 0.485 |
| 30-39 | 36.0 (33.8-38.2) | 676 (23.1) | 1203 (19.3) | 1.94 (1.58-2.39) | <0.001 | 24.0 (21.3-27.0) | 212 (20.3) | 670 (23.3) | 1.03 (0.71-1.48) | 0.889 |
| 40-60 | 31.4 (29.9-32.9) | 1193 (40.8) | 2611 (41.9) | 1.58 (1.30-1.92) | <0.001 | 29.1 (27.0-31.4) | 479 (45.8) | 1166 (40.6) | 1.33 (0.94-1.89) | 0.107 |
| Race/ethnicity | 0.003 | 0.278 | ||||||||
| Black | 31.1 (30.0-32.2) | 2079 (71.1) | 4613 (74.1) | 1.00 | 26.7 (25.1-28.4) | 741 (70.9) | 2034 (70.9) | 1 | ||
| Hispanic | 35.0 (32.8-37.3) | 607 (20.8) | 1127 (18.1) | 1.20 (1.07-1.34) | 0.002 | 27.7 (24.8-30.8) | 231 (22.1) | 603 (21.0) | 1.05 (0.88-1.25) | 0.570 |
| White | 37.2 (31.7-43.1) | 102 (3.5) | 172 (2.8) | 1.32 (1.02-1.69) | 0.033 | 19.4 (13.4-27.2) | 24 (2.3) | 100 (3.5) | 0.66 (0.42-1.04) | 0.069 |
| Other | 30.0 (25.9-34.4) | 133 (4.5) | 311 (5.0) | 0.95 (0.77-1.17) | 0.633 | 27.0 (21.0-33.9) | 48 (4.6) | 130 (4.5) | 1.01 (0.72-1.43) | 0.939 |
| Marital status | 0.187 | 0.665 | ||||||||
| Married/cohabiting | 31.0 (28.7-33.4) | 468 (16.0) | 1042 (16.7) | 1.00 | 26.3 (24.6-28.1) | 648 (62.0) | 1815 (63.2) | 1 | ||
| Never married | 31.7 (30.5-32.9) | 1,845 (63.1) | 3,982 (63.9) | 1.03 (0.91-1.17) | 0.641 | 26.6 (23.3-30.2) | 167 (16.0) | 461 (16.1) | 1.01 (0.83-1.24) | 0.886 |
| Other | 33.7 (31.5-35.9) | 611 (20.9) | 1,204 (19.3) | 1.13 (0.98-1.31) | 0.102 | 27.9 (25.0-31.1) | 230 (22.0) | 594 (20.7) | 1.08 (0.91-1.29) | 0.368 |
| Past year sexual behaviours | Last-sex3 | |||||||||
| Partner type | <0.001 | |||||||||
| Main only | 18.8 (17.6-20.1) | 707 (24.2) | 3054 (49.0) | 1.00 | 24.5 (22.8-26.3) | 577 (55.2) | 1780 (62.0) | 1 | ||
| Main and casual partners | 41.3 (39.8-42.9) | 1628 (55.7) | 2311 (37.1) | 3.04 (2.74-3.37) | NA | NA | NA | NA | ||
| Casual only | 40.6 (38.1-43.1) | 589 (20.1) | 863 (13.9) | 2.95 (2.58-3.37) | 30.0 (27.8-32.4) | 468(44.8) | 1090 (38.0) | 1.32 (1.15-1.53) | <0.001 | |
| Exchange sex2 | <0.001 | 0.001 | ||||||||
| No | 26.0 (25.0-27.0) | 1848 (63.2) | 5264 (84.5) | 1.00 | 24.9 (23.2-26.6) | 601 (57.5) | 1817 (63.3) | 1 | ||
| Yes | 52.7 (50.6-54.9) | 1075 (36.8) | 964 (15.5) | 3.18 (2.87-3.52) | <0.001 | 29.7 (27.4-32.0) | 444 (42.5) | 1052 (36.7) | 1.28 (1.10-1.47) | <0.001 |
All P-values are derived from the chi-squared statistic, 95% confidence interval using the Wilson interval and 95% confidence intervals for odds ratios are estimated using normal approximation (Wald).
NA = not available
Number of acts defined as the sum of anal or vaginal acts reported by RAI women at last their sexual episode
Definition changed across rounds: in 2010 the definition of women exchanging sex included women who had any exchange partners in the last year; in 2013 the definition of women exchanging sex included women who had only casual exchange partners in the last year.
By definition this refers to acts with main or casual sexual partners at last sexual episode.
RAI prevalence was consistently high across cities, ranging from 18% in New Orleans (95%CI 16-22%) to 60% in San Juan (95%CI 55-65%; Figure 1 and Supplement table 2). Among 18-19 year olds RAI prevalence varied substantially across cities (mean ranges: 6% in Miami to 61% in San Juan) and was lower than among 25-29 year olds in 5 cities; prevalence differed less among 25-29 year olds and older age groups; supplement figure 2a). Across cities with at least 10 participants RAI prevalence ranged between 18-50% among Blacks, 15-60% among Hispanics, 14-69% among Whites, and 20-64% among Other races/ethnicities (supplement figure 2b). In all cities, RAI prevalence was higher among women who had casual-only and main-and-casual partners over the past year than women who had main partners only (supplement figure 2c) and was higher among women exchanging sex than women who did not (supplement figure 2d).
Figure 1.
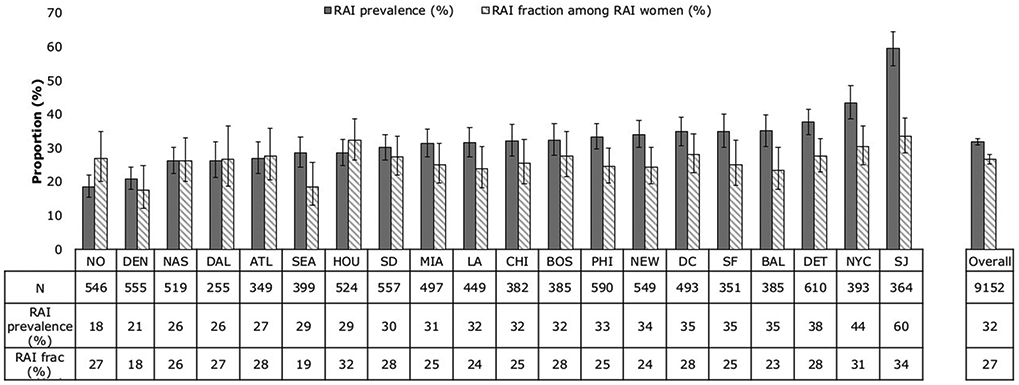
RAI prevalence and RAI fraction overall and across cities, NHBS-HET combined 2010 and 2013 cycles. City abbreviations: NO New Orleans, DEN Denver, NAS Nassau, DAL Dallas, ATL Atlanta, SEA Seattle, HOU Houston, SD San Diego, MIA Miami, LA Los Angeles, CHI Chicago, BOS Boston, PHI Philadelphia, NEW Newark, DC Washington DC, SF San Francisco, BAL Baltimore, DET Detroit, NYC New York City, SJ San Juan, Puerto Rico.
How frequently is RAI practised?
A quarter (27%, 95%CI 25-28%) of the sex acts during the most recent sexual episode of RAI women were RAI (Table 2). Similarly, a quarter of unprotected sex acts were unprotected RAI (27%, 95%CI 26-29%) (results not shown). The RAI fraction ranged between 24% among 18-19 year olds to 30% in 40-60 year olds (p=0.05), 19% among White women to 27% among Hispanic women (p=0.28) and was consistent across marital status (26-28%, p=0.67). The RAI fraction was higher in women exchanging sex (30%, OR=1.28, 95%CI 1.10-1.47, p<0.001) than women not exchanging sex (25%) (Table 2).
Across cities, the RAI fraction varied between 18% (95%CI 12-25%) in Denver and 34% (95%CI 29-39%) in San Juan. Patterns of RAI fraction by age group, race/ethnicity and exchange sex within cities resembled the overall patterns (though comparisons across cities were limited by small numbers) (data not shown).
Differences in sexual behaviours reported by women reporting/not reporting RAI in the past year
Table 3 describes the sexual behaviours of RAI and non-RAI women. RAI women reported higher numbers of sexual partners (mean difference=6.7, 95%CI 4.7-8.7), were more likely to report casual partners at last sex (OR=1.91 95%CI 1.74-2.10), report a partner with unknown/positive HIV status (OR=1.71 95%CI 1.56-1.88), but were less likely to report condoms with main or casual partners at last RVI (e.g. OR=0.44, 95%CI 0.38-0.50) and hardly changed after stratifying by city (Table 3). Stratification by age group, race/ethnicity, exchange sex produced similar results (not shown). There was only weak evidence that RAI women used condoms less often during last RAI than last RVI (mhOR=0.80, 95%CI 0.61-1.05), (Table 3).
Table 3.
Differences in sexual risk behaviours between women who reported receptive anal intercourse (RAI) and did not (non-RAI) in the past-year, combined NHBS-HET study cycles 2010 and, 2013.
| Sexual risk behaviours | RAI women N=2924 |
Non-RAI women N=6228 |
Mean difference1
(95% CI) |
p-value | ||
|---|---|---|---|---|---|---|
| Behaviours in past 12 months |
Mean (N) | 95%CI | Mean (N) | 95%CI | ||
| Number of partners | ||||||
| All partners | 10.2 | 7.7-12.7 | 3.5 | 3.0-3.9 | 6.7 (4.7-8.7) | <0.0001 |
| Main partners | 1.4 | 1.3-1.5 | 1.1 | 1.1-1.1 | 0.3 (0.1-0.4) | <0.0001 |
| Casual partners | 8.6 | 7.2-10.0 | 2.3 | 2.1-2.6 | 6.3 (4.8-7.7) | <0.0001 |
| Last-sex partner characteristics |
N | % | N | % | OR (95%CI) |
mhOR2 (95% CI) |
| Partner type | ||||||
| Main partner | 1805 | 61.8 | 4704 | 75.6 | ref | ref |
| Casual partner | 1115 | 38.2 | 1520 | 24.4 | 1.91 (1.74-2.10) | 1.84 (1.67-2.02) |
| Partner HIV status | ||||||
| Negative | 941 | 32.2 | 2792 | 44.9 | ref | ref |
| Unknown/positive | 1979 | 67.8 | 3431 | 55.1 | 1.71 (1.56-1.88) | 1.68 (1.53-1.85) |
| Condom use during last sexual episode |
N | % | N | % | OR (95%CI) |
mhOR2 (95% CI) |
| Condom use – at last RVI | ||||||
| All partners | ||||||
| UVI | 2605 | 90.8 | 5054 | 81.2 | ref | ref |
| Condom protected RVI | 264 | 9.2 | 1170 | 18.8 | 0.44 (0.38-0.50) | 0.46 (0.40-0.53) |
| Main partners | ||||||
| UVI | 1657 | 93.1 | 3984 | 84.7 | ref | ref |
| Condom protected RVI | 123 | 6.9 | 720 | 15.3 | 0.41 (0.34-0.50) | 0.43 (0.35-0.53) |
| Casual partners | ||||||
| UVI | 948 | 87.1 | 1070 | 70.4 | ref | ref |
| Condom protected RVI | 141 | 12.9 | 450 | 29.6 | 0.35 (0.29-0.44) | 0.38 (0.31-0.47) |
| Condom use - by RAI women (all partners) | - | - | ||||
| UVI | 2605 | 90.8 | - | - | ||
| Condom protected RVI | 264 | 9.2 | ref | ref | ||
| UAI | 970 | 92.8 | - | - | ||
| Condom protected AI | 75 | 7.2 | 0.76 (0.58-1.00) | 0.80 (0.61-1.05) | ||
Note. RAI, receptive anal intercourse; UVI, unprotected vaginal intercourse; UAI, unprotected anal intercourse; RVI, receptive vaginal intercourse
T-tests stratified for age, exchange sex, race/ethnicity produced similar results, not shown (with some exceptions where there was weaker evidence for a difference in main partners among Hispanic and Other racial/ethnic women and for main and casual partners among White women, p>0.05). Evidence for a difference in main partners was only observed in San Diego (p=0.02); there was evidence for a difference in casual partners in all but 4 cities (Baltimore, Denver, San Juan, Seattle).
Mantel-Haenszel OR stratified for city (adjustments for age, exchange sex, race/ethnicity produced similar results, not shown)
Predicted fraction of heterosexually acquired HIV infections due to RAI
The model suggests that RAI independently contributed two-fifths of all annual heterosexually-acquired HIV infections among this at-risk sample, i.e. PAFRAI = 41% (80%UI:18-55%) (Figure 2A). The PAFRAI+Beh reflecting the contribution of RAI and riskier sexual behaviours of RAI women was only 2percentage points higher (43%, 80%UI:17-63%) (Figure 2A).
Figure 2.
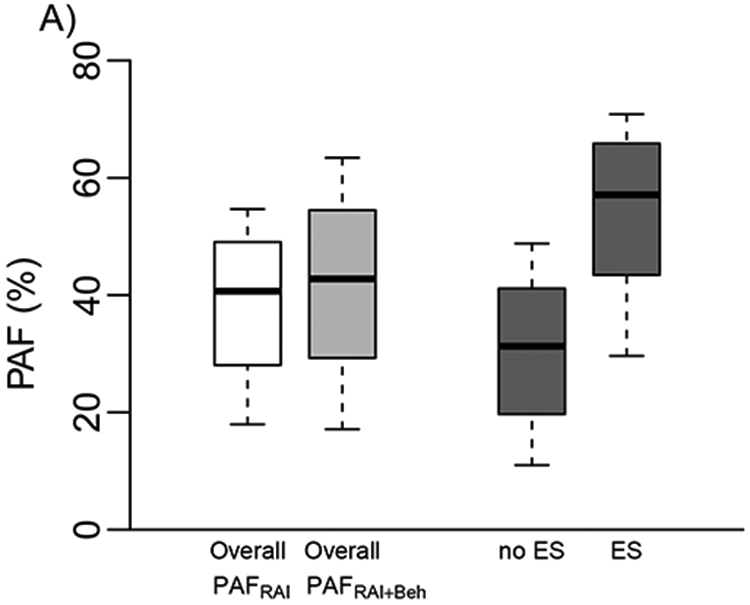
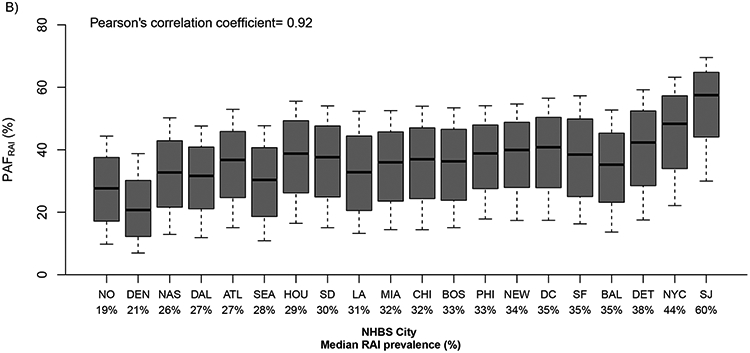
A) Model estimates of the contribution of RAI to new annual HIV infections due to RAI alone (PAFRAI) and due to RAI and riskier behaviours of RAI women (PAFRAI+Beh) overall and among those who do (ES) and do not exchange sex (No ES). b) PAFRAI in 20 US cities. Boxplots (median, and 10-90th percentiles) are shown in ascending order of city-specific median past-year RAI prevalence (Pearson’s correlation coefficient between RAI prevalence and median PAF across cities). City abbreviations are as in figure 1.
Across cities, PAFRAI ranged from 21% (80%UI:7-39%) in Denver to 57% (80%UI:30-70%) in San Juan reflecting the RAI prevalence across cities (Figure 2B). PAFRAI estimates did not differ substantially by age group, or race/ethnicity (supplement figure 3) reflecting limited differences in RAI prevalence and RAI fraction between groups. However, PAFRAI was substantially greater for women exchanging sex than women not exchanging sex overall (median: 57% vs 31%) and across all cities (ranges=exchange: 30-64%; not exchange: 15-56%) apart from Dallas (PAFRAI: exchange similar to not exchange ~30%) (Figure 2A, supplement figure 4).
Uncertainty and Sensitivity analyses
First in our uncertainty analysis, RRRAI (correlation=0.89) and the total number of RVI acts among non-RAI women (correlation=0.28) were the parameters that were most associated with the variation in PAFRAI estimates (Figure 3A). PAFRAI 80% uncertainty interval estimates increased from 20-30% to 43-58% if RRRAI increased from 5 to more than 15, respectively (Figure 3B) and decreased from 28-55% to 15-51% if number of vaginal acts increased from 50 to 150 (Figure 3C). Second, in our more general sensitivity analysis with wider parameter ranges the correlation with RRRAI was substantially lower (correlation=0.59) and RAI prevalence and RAI fraction became more influential (correlation=0.35, −0.27 respectively) followed by HIV prevalence among male partners (Figure 3D).
Figure 3.
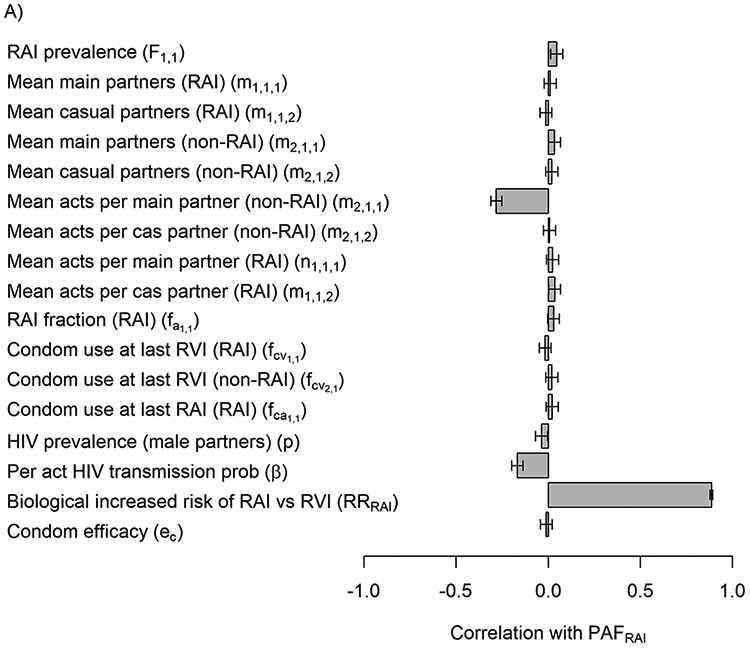
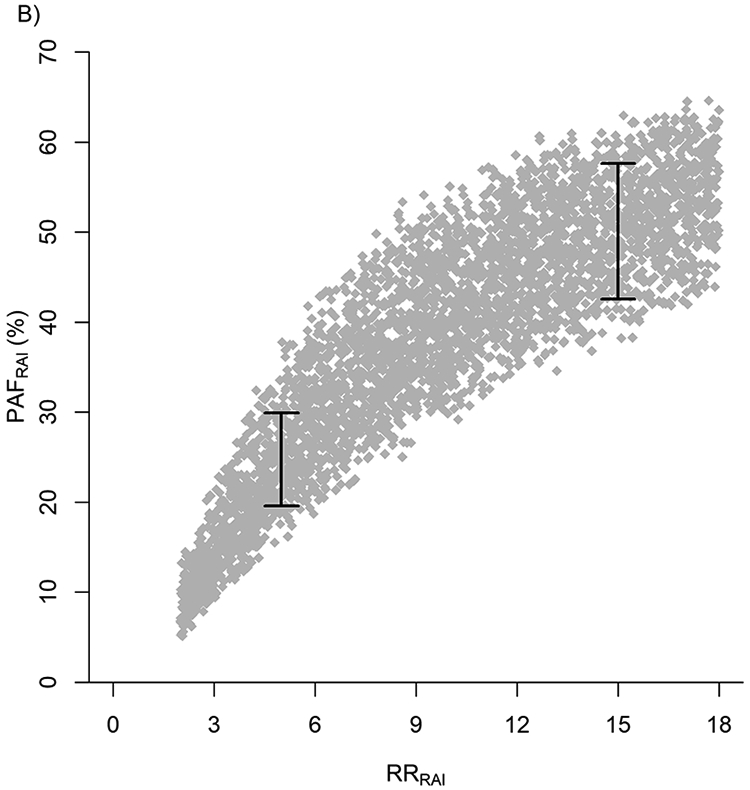
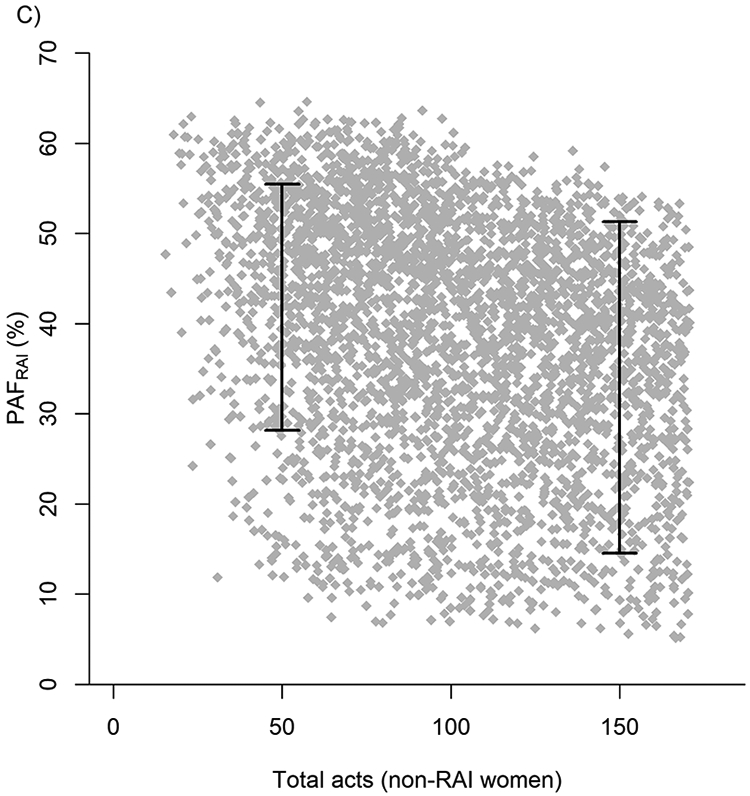
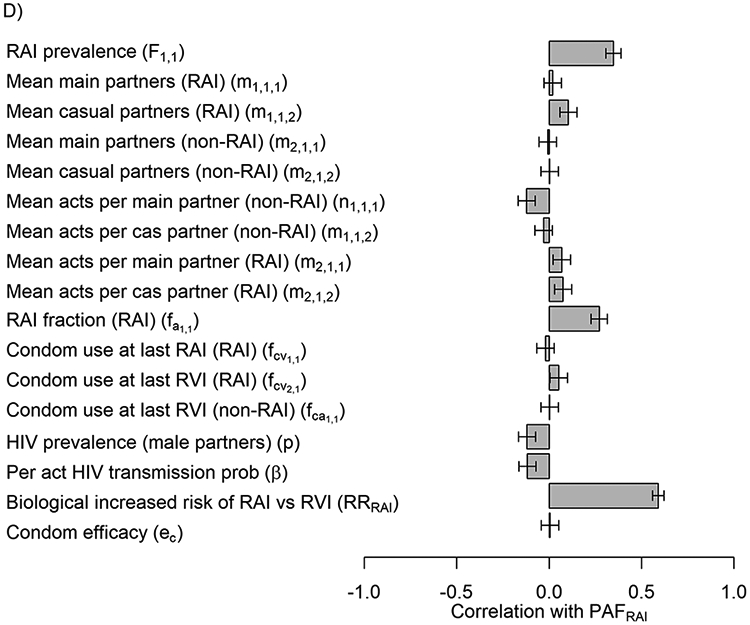
In A) Tornado plot showing the correlation between uncertainty in PAFRAI overall model (J=1) and key model parameters from NHBS-HET analysis. Input parameter ranges for each of the parameters are shown in table 1. In B&C) Scatter plots of the most influential parameters (endpoints represent 80%UI): RRRAI (in B), total number of acts among non-RAI women (in C). In D) Tornado plot showing correlation of PAFRAI variability with wider ranges of variables taken from the minimum and maximum values across cities.
Finally, we assessed the influence of transmission due to needle-sharing among PWID on our PAFRAI estimates. Assuming 100% of infections among PWID come from injecting behaviours, the PAFRAI due to RAI would range from 39% (UI:17-52%), 37% (UI:16-49%), 32% (UI:14-43%) if the HIV incidence rate among PWID was the same, twice as large, and five times larger than in NIDU.
Discussion
Our results suggest that approximately one in three low-income women at increased risk for HIV infection in the US NHBS sample practised RAI at least once a year, overall. RAI was commonly practised across cities (~1 in 2 to ~1 in 5), including by young women (18-19 years old). Women who practised RAI in the past year did so frequently (overall approximately one RAI/unprotected RAI in 4 sex acts/unprotected sex acts), which means that in the whole sample of women (reporting and not reporting RAI), about 1 in 10 of all sex acts/unprotected sex acts were RAI/unprotected RAI. Women practising RAI also reported riskier sexual behaviours than non-RAI women: more sexual partners annually and lower condom use with main and casual sexual partners, even though condom use was universally low.
Despite only 11% of all unprotected acts being unprotected RAI, RAI alone may contribute to 41% (80% UI: 18-55%; city range: 21%−57%) of heterosexually-acquired incident HIV infections annually among low-income women in the NHBS sample, due primarily to the high risk of HIV transmission during RAI. The higher sexual risk behaviour of women practising RAI had a negligible impact on the PAF. The PAFRAI was even higher among women exchanging sex (overall: ~60%, city range: 30%−64%) partly because RAI prevalence was the highest and about twice as large among women who exchanged sex (overall and across cities) than among women who did not. Even though younger and Black women tended to report lower RAI prevalence than older or White and Hispanic women, there were no major differences in PAF estimates by age or race/ethnicity given the relatively small differences in RAI prevalence and no difference in the fraction of last sex acts that were RAI across these groups.
RAI prevalence among low-income women in this study is higher overall and across all ages than general populations7, but comparable to other at-risk populations13 in the US. Our estimates of prevalence among18-19 year old women are consistent with recent review estimates among youth (20-24%)5 and adds to growing evidence of a wide (and widening) sexual repertoire among adolescents in the US44,45 and elsewhere5,46,47. Similar to our findings, other studies among at-risk and general populations also found Black women were less likely than White women to report lifetime14,48,49 or recent RAI12 and yet others observe no differences13,50. While our results do not exclude RAI as a HIV risk factor for Black women, other factors driving risk such as partner concurrency50 and the sexual network may be more influential51. Our results are consistent with findings from other studies that women who practise RAI tend to report higher numbers of sexual partners50, exchange sex12,50,52 and lower condom use during vaginal and anal sex48,50.
We know of three other mathematical models predicting the contribution of RAI to different heterosexual epidemics: two from Africa53,54 and one from the US55. Our estimates are slightly higher than O’Leary et al’s55 transmission dynamics model predictions for a nationally representative population of 13-64 year old US women over one year (PAF:28%) but are comparable to estimates for 18-34 year old women who have higher RAI prevalence (PAF:40%)55. Their model also accounts for transmission risk through injection drug use. Our estimates are more comparable when we account for HIV infection risk from needle sharing (32-39%). The remaining differences can be partly explained because the two models use different prior ranges for sensitive model parameters; O’Leary et al. use data from general populations with higher condom use, lower RAI prevalence and RAI fraction than reported by women in the NHBS survey55.
Strengths and limitations
Our analysis has several strengths and some limitations chiefly due to shortcomings in data. We report for the first time about 4 in 10 new heterosexually-acquired HIV infections among a specific population of low-income women at increased risk of HIV infection in the US may be due to RAI despite a minority of all unprotected sex acts being unprotected RAI. We benefited from detailed high quality sexual behaviour data from multiple cities, which allowed us to account for parameter uncertainties and for detailed differences in the sexual behaviour of women reporting and not reporting RAI, across different demographic and risk groups and twenty different cities. We were able to draw on a comparable study (HPTN-064) to provide estimates of HIV incidence and the relative risk of RAI to calibrate the model38,39. While, we cannot exclude the possibility of reporting biases of RAI from FTFI which can produce lower estimates for sensitive behaviours than more confidential methods56, NHBS estimates were similar to those from HPTN-064 which used more confidential methods (RAI prevalence:38%). Our analysis likely reflects the average behaviour prevailing over the 2010-2013 period rather than a specific year. Prior parameter ranges were derived from confidence intervals of estimates that were unadjusted for RDS design. However, this did not impact our modelling results since our PAF and UI estimates were very similar even after quadrupling standard errors (i.e. prior parameter ranges) in additional sensitivity analyses (results not shown). The lack of impact is because the main source of uncertainty in PAF estimates was due to uncertainty in estimates of the biological increased risk of RAI compared to RVI. Although the range for this parameter was informed by pooled estimates from systematic literature reviews, it remains uncertain because it is based on few studies1,2,34-36. We did not have data on the number of RVI and RAI acts (used to estimate the total number of sex acts and the fraction of sex acts that are RAI). Instead, we approximated the fraction of acts that were RAI using the fraction of RAI at the last sexual episode, which could be biased due to over- or under-reporting of certain practices at last sex. Our range for the RAI fraction is slightly higher but overlaps the confidence intervals of an estimate from a 1999 study among STI clinic attendees13 and is comparable with estimates from general populations55. The total number of sex acts were informed from the literature and calibrated by model fitting. Our uncertainty analysis suggested that, while this was not the most important source of uncertainty in PAF estimates, it was still influential, meaning that questions on the number of protected and unprotected RAI and RVI acts would be valuable additions to future NHBS cycles. Our past year PAF estimates may underestimate the contribution of RAI to HIV transmission at a population-level since these estimates do not account for onwards transmissions from women to their male partners, and so on. Nevertheless, our results are consistent with a transmission dynamic modelling study55.
Our extensive sensitivity analyses demonstrate the robustness of our findings and show that RAI contributes at least a third of infections even when infections from injecting drug use are accounted for. This estimate assumes all HIV infections among PWID in NHBS were due to injection practices and that the incidence rate among PWID was five times greater than the incidence rate among NIDU. These estimates are conservative because a fraction of infections among PWID are likely sexual57-59, and available studies suggest HIV incidence rates among PWID may be about twice those observed among higher-risk heterosexuals60. Even though our estimates are not representative of all low-income women at increased risk of HIV in the US, this population is historically underserved for prevention and care26,61,62 and are a priority population for HIV prevention27,28.
Public Health Implications
Our analysis highlights RAI as a key risk factor for HIV acquisition among women across a range of demographic and risk groups. This finding can usefully inform HIV prevention strategies among heterosexual women at increased risk for HIV infection in the US as well as future data collection. The consistently high prevalence of RAI in young women across cities is particularly concerning because it is often coerced17,63,64 which could further elevate the per-act HIV risk49,65-67. Although, women who engage in RAI also have higher risk practices, our results suggest that the greatest risk is due to the elevated transmission efficiency during RAI. These findings imply that vaginal microbicides or rings with no efficacy against rectal transmission may have limited utility for this population and, together with our observations of consistent low condom use, support the value of pre-exposure prophylaxis drugs with systemic activity. Finally, our larger sensitivity analysis suggested HIV studies wishing to evaluate RAI in contexts where no data exist should prioritise RAI prevalence and RAI fraction, agreeing with previous recommendations66. Despite the importance of RAI to heterosexual HIV transmission, key data to estimate its contribution to HIV epidemics such as the frequency of RAI sex acts are missing. The extent that RAI is underreported and imprecisely measured5,68 in different contexts and subgroups affects the accuracy of our estimates of RAI practices across populations and our estimates of its contribution to HIV epidemics.
Supplementary Material
Supporting file 1: Supplementary material_NHBS-HET RAI in at-risk women. Word document.
Supporting file 2: Supplementary table 2. Prior parameter ranges for cities (combined NHBS-HET 2010,2013 cycles). .
Acknowledgements
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health Award [Grant Numbers R01AI057020 and UM1 AI068619]. We thank the HPTN Modelling Centre, which is funded by the U.S. National Institutes of Health (NIH UM1 AI068617) through HPTN, for partial funding of this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest Statement: B Shacklett has received research contracts from Gilead Sciences and consulting fees from Merck, Inc.; both are unrelated to the work described in this paper. A Adimora has received personal funds from Viiv, Merck, and Gilead and received research funds from Gilead. All other authors declare no conflicts of interest
References
- 1.Baggaley RF, White RG, Boily M-C. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. International Journal of Epidemiology. 2010;39(4):1048–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggaley RF, Owen BN, Silhol R, et al. Does per-act HIV-1 transmission risk through anal sex vary by gender? An updated systematic review and meta-analysis. Am J Reprod Immunol. 2018:e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boily M-C, Dimitrov D, Abdool Karim SS, Mâsse B. The future role of rectal and vaginal microbicides to prevent HIV infection in heterosexual populations: implications for product development and prevention. Sexually Transmitted Infections. 2011;87(7):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mâsse BR, Boily M-C, Dimitrov D, Desai K. Efficacy dilution in randomized placebo-controlled vaginal microbicide trials. Emerging Themes in Epidemiology. 2009;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen B, Brock P, Butler A, et al. Prevalence and Frequency of Heterosexual Anal Intercourse Among Young People: A Systematic Review and Meta-analysis. AIDS Behav. 2015;19(7):1338–1360. [DOI] [PubMed] [Google Scholar]
- 6.Owen BN, Elmes J, Silhol R, et al. How common and frequent is heterosexual anal intercourse among South Africans? A systematic review and meta-analysis. Journal of the International AIDS Society. 2017;20(1):21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbenick D, Reece M, Schick V, Sanders SA, Dodge B, Fortenberry JD. Sexual Behavior in the United States: Results from a National Probability Sample of Men and Women Ages 14–94. The Journal of Sexual Medicine. 2010;7:255–265. [DOI] [PubMed] [Google Scholar]
- 8.Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). The Lancet. 2013;382(9907):1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbenick D, Bowling J, Fu T-C, Dodge B, Guerra-Reyes L, Sanders S. Sexual diversity in the United States: Results from a nationally representative probability sample of adult women and men. PLOS ONE. 2017;12(7):e0181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aral SO, Patel DA, Holmes KK, Foxman B. Temporal Trends in Sexual Behaviors and Sexually Transmitted Disease History Among 18- to 39-Year-Old Seattle, Washington, Residents: Results of Random Digit-Dial Surveys. Sexually Transmitted Diseases. 2005;32(11):710–717. [DOI] [PubMed] [Google Scholar]
- 11.Gindi RM, Ghanem KG, Erbelding EJ. Increases in Oral and Anal Sexual Exposure among Youth Attending STD Clinics in Baltimore, Maryland. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;42(3):307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javanbakht M, Guerry S, Gorbach PM, et al. Prevalence and Correlates of Heterosexual Anal Intercourse Among Clients Attending Public Sexually Transmitted Disease Clinics in Los Angeles County. Sexually Transmitted Diseases. 2010;37(6):369–376. [PubMed] [Google Scholar]
- 13.Tian LH, Peterman TA, Tao G, et al. Heterosexual Anal Sex Activity in the Year After an STD Clinic Visit. Sexually Transmitted Diseases. 2008;35(11):905–909. [DOI] [PubMed] [Google Scholar]
- 14.Benson LS, Martins SL, Whitaker AK. Correlates of Heterosexual Anal Intercourse among Women in the 2006–2010 National Survey of Family Growth. The Journal of Sexual Medicine. 2015;12(8):1746–1752. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds GL, Fisher DG, Rogala B. Why Women Engage in Anal Intercourse: Results from a Qualitative Study. Archives of Sexual Behavior. 2015;44(4):983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds GL, Latimore AD, Fisher DG. Heterosexual Anal Sex Among Female Drug Users: U.S. National Compared to Local Long Beach, California Data. AIDS Behav. 2008;12(5):796–805. [DOI] [PubMed] [Google Scholar]
- 17.Lescano CM, Houck CD, Brown LK, et al. Correlates of Heterosexual Anal Intercourse Among At-Risk Adolescents and Young Adults. American Journal of Public Health. 2009;99(6):1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenness SM, Begier EM, Neaigus A, Murrill CS, Wendel T, Hagan H. Unprotected Anal Intercourse and Sexually Transmitted Diseases in High-Risk Heterosexual Women. American Journal of Public Health. 2011;101(4):745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGowan I Rectal microbicides: a new focus for HIV prevention. Sexually Transmitted Infections. 2008;84(6):413–417. [DOI] [PubMed] [Google Scholar]
- 20.Halperin DT. Heterosexual anal intercourse: Prevalence, cultural factors, and HIV infection and other health risks, Part I. Aids Patient Care and Stds. 1999;13(12):717–730. [DOI] [PubMed] [Google Scholar]
- 21.McGowan I Rectal microbicides: can we make them and will people use them? AIDS Behav. 2011;15 Suppl 1:S66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MTN. https://mtnstopshiv.org/news/researchers-launch-first-ever-phase-ii-safety-study-rectal-microbicide-prevent-hiv. 2013.
- 23.Fernández-Romero JA, Deal C, Herold BC, et al. Multipurpose prevention technologies: the future of HIV and STI protection. Trends in Microbiology. 2015;23(7):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cranston RD, Lama JR, Richardson BA, et al. MTN-017: A Rectal Phase 2 Extended Safety and Acceptability Study of Tenofovir Reduced-Glycerin 1% Gel. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2017;64(5):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. HIV Surveillance Report, 2017; vol. 29 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2018. Accessed [April 2020]. [Google Scholar]
- 26.Sionean C, Le BC, Hageman K, et al. HIV Risk, prevention, and testing behaviors among heterosexuals at increased risk for HIV infection--National HIV Behavioral Surveillance System, 21 U.S. cities, 2010. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2014;63(14):1–39. [PubMed] [Google Scholar]
- 27.Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral Surveillance Among People at Risk for HIV Infection in the U.S.: The National HIV Behavioral Surveillance System. Public Health Reports. 2007;122(Suppl 1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansky A, Sullivan PS, Gallagher KM, Fleming PL. HIV Behavioral Surveillance in the U.S.: A Conceptual Framework. Public Health Reports. 2007;122(Suppl 1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC. NHBS Round 3 Model Surveillance Protocol. Behavioral Surveillance Team NCHSTP/DHAP-SE/BCSB. Available online: https://www.cdc.gov/hiv/statistics/systems/nhbs/operations.html Last accessed: Jan 2017. 2011.
- 30.Heckathorn DD. Respondent-driven sampling II: Deriving valid population estimates from chain-referral samples of hidden populations. Social Problems. 2002;49(1):11–34. [Google Scholar]
- 31.US Department for Health and Human Services. The 2009 HHS Poverty Guidelines. Available: https://aspe.hhs.gov/2009-hhs-poverty-guidelines. Federal Register. 2009;74(14):4199–4201. [Google Scholar]
- 32.Agresti A Categorical data analysis. 2nd ed. New York: Wiley-Interscience; 2002. [Google Scholar]
- 33.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39(4):1048–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boily M-C, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: a systematic review and meta-analysis of observational studies. The Lancet Infectious diseases. 2009;9(2):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28(10):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin F, Jansson J, Law M, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS. 2010;24(6):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC. HIV Infection Among Heterosexuals at Increased Risk — United States, 2010. MMWR. 2013;62(10):183–188. [PMC free article] [PubMed] [Google Scholar]
- 38.Hodder S, Jessicsa Justman, James Hughes, et al. HIV Incidence in Women at Risk for HIV Acquisition in the United States: HPTN 064 (ISIS Study) [Webinar: W-159]. 19th Conference on Retroviruses and Opportunistic Infections 2012;March 5–8 Seattle, WA. [Google Scholar]
- 39.Hodder SL, Justman J, Hughes JP, et al. HIV Acquisition Among Women From Selected Areas of the United States. Annals of internal medicine. 2013;158(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.lhs: Latin Hypercube Samples. [computer program]. Rob Carnell. R package version 0.16. https://CRAN.R-project.org/package=lhs; 2018.
- 41.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 42.R: A language and environment for statistical computing. [computer program]. R Core Team. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. 2017. [Google Scholar]
- 43.RStudio: Integrated Development for R. [computer program]. RStudio Team (2016). RStudio, Inc., Boston, MA: URL http://www.rstudio.com/.2016. [Google Scholar]
- 44.Benson L, Gilmore K, Micks e, Prager S. Heterosexual Anal Intercourse among Teenages in the United States. Journal of Pediatric and Adolescent Gynecology. 2016. [Google Scholar]
- 45.Halpern CT, Haydon AA. Sexual Timetables for Oral-Genital, Vaginal, and Anal Intercourse: Sociodemographic Comparisons in a Nationally Representative Sample of Adolescents. American Journal of Public Health. 2012;102(6):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajduković D, Štulhofer A, Baćak V. Rising popularity of anal intercourse and sexual risk taking: findings from two national probability studies of young Croatian adults. International Journal of STD & AIDS. 2012;23(11):785–791. [DOI] [PubMed] [Google Scholar]
- 47.Lewis R, Tanton C, Mercer CH, et al. Heterosexual practices among young people in Britain: Evidence from three National Surveys of Sexual Attitudes and Lifestyles. Journal of Adolescent Health. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leichliter JS, Chandra A, Liddon N, Fenton KA, Aral SO. Prevalence and correlates of heterosexual anal and oral sex in adolescents and adults in the United States. Journal of Infectious Diseases. 2007;196(12):1852–1859. [DOI] [PubMed] [Google Scholar]
- 49.Hess KL, Javanbakht M, Brown JM, Weiss RE, Hsu P, Gorbach PM. Intimate Partner Violence and Anal Intercourse In Young Adult Heterosexual Relationships. Perspectives on Sexual and Reproductive Health. 2013;45(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorbach PM, Manhart LE, Hess KL, Stoner BP, Martin DH, Holmes KK. Anal Intercourse Among Young Heterosexuals in Three Sexually Transmitted Disease Clinics in the United States. Sexually Transmitted Diseases. 2009;36(4):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adimora AA, Schoenbach VJ. Social Context, Sexual Networks, and Racial Disparities in Rates of Sexually Transmitted Infections. The Journal of Infectious Diseases. 2005;191(Supplement_1):S115–S122. [DOI] [PubMed] [Google Scholar]
- 52.Hess KL, DiNenno E, Sionean C, Ivy W, Paz-Bailey G. Prevalence and Correlates of Heterosexual Anal Intercourse Among Men and Women, 20 U.S. Cities. AIDS Behav. 2016:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maheu-Giroux M, Baral S, Vesga JF, et al. Anal Intercourse Among Female Sex Workers in Côte d’Ivoire: Prevalence, Determinants, and Model-Based Estimates of the Population-Level Impact on HIV Transmission. American Journal of Epidemiology. 2018;187(2):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silhol R, Owen B, Elmes J, Shacklett B, Van der Straten A, Dimitrov D, ... Boily M-C. The Contribution of Heterosexual Anal Intercourse (AI) to HIV Transmission in Western Cape and KwaZulu-Natal Provinces in South Africa [Oral presentation]. AIDS Research and Human Retroviruses. 2016;32(S1):301. [Google Scholar]
- 55.O’Leary A, DiNenno E, Honeycutt A, et al. Contribution of Anal Sex to HIV Prevalence Among Heterosexuals: A Modeling Analysis. AIDS Behav. 2017;21(10):2895–2903. [DOI] [PubMed] [Google Scholar]
- 56.Phillips AE, Gomez GB, Boily M-C, Garnett GP. A systematic review and meta-analysis of quantitative interviewing tools to investigate self-reported HIV and STI associated behaviours in low- and middle-income countries. International Journal of Epidemiology. 2010;39(6):1541–1555. [DOI] [PubMed] [Google Scholar]
- 57.Solomon L, Astemborski J, Warren D, et al. Differences in Risk Factors for Human Immunodeficiency Virus Type 1 Seroconversion among Male and Female Intravenous Drug Users. American Journal of Epidemiology. 1993;137(8):892–898. [DOI] [PubMed] [Google Scholar]
- 58.Strathdee SA, Galai N, Safaiean M, et al. Sex differences in risk factors for hiv seroconversion among injection drug users: A 10-year perspective. Archives of Internal Medicine. 2001;161(10):1281–1288. [DOI] [PubMed] [Google Scholar]
- 59.Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. Journal of Urban Health. 2003;80(3):iii7–iii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehta S H, Galai N, et al. HIV Incidence Among Injection Drug Users in Baltimore, Maryland (1988–2004). J Acquir Immune Defic Syndr. 2006;43(3):368–372. [DOI] [PubMed] [Google Scholar]
- 61.Aral SO, Adimora AA, Fenton KA. Understanding and responding to disparities in HIV and other sexually transmitted infections in African Americans. The Lancet.372(9635):337–340. [DOI] [PubMed] [Google Scholar]
- 62.Chandra AaS, Jonathan S, . Geography and Racial Health Disparities (February 2003). . NBER Working Paper No w9513 Available at SSRN: https://ssrncom/abstract=382444.
- 63.Marston C, Lewis R. Anal heterosex among young people and implications for health promotion: a qualitative study in the UK. BMJ Open. 2014;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roye CF, Tolman DL, Snowden F. Heterosexual Anal Intercourse among Black and Latino Adolescents and Young Adults: A Poorly Understood High-Risk Behavior. Journal of Sex Research. 2013;50(7):715–722. [DOI] [PubMed] [Google Scholar]
- 65.Maynard E, Carballo D, x e, et al. Women’s Experiences with Anal Sex: Motivations and Implications for STD Prevention. Perspectives on Sexual and Reproductive Health. 2009;41(3):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baggaley RF, Dimitrov D, Owen BN, et al. Heterosexual Anal Intercourse: A Neglected Risk Factor for HIV? American journal of reproductive immunology (New York, NY : 1989). 2013;69(0 1):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jewkes RK, Dunkle K, Nduna M, Shai N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. The Lancet. 2010;376(9734):41–48. [DOI] [PubMed] [Google Scholar]
- 68.Duby Z, Hartmann M, Mahaka I, et al. Lost in Translation: Language, Terminology, and Understanding of Penile-Anal Intercourse in an HIV Prevention Trial in South Africa, Uganda, and Zimbabwe. J Sex Res. 2016;53(9):1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adimora AA, Hughes JP, Wang J, et al. Characteristics of multiple and concurrent partnerships among women at high risk for HIV infection. J Acquir Immune Defic Syndr. 2014;65(1):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davis KR, Weller SC. The Effectiveness of Condoms in Reducing Heterosexual Transmission of HIV. Family Planning Perspectives. 1999;31(6):272–279. [PubMed] [Google Scholar]
- 71.Smith D, Md MS, Herbst J, Zhang X, Rose C. Condom Effectiveness for HIV Prevention by Consistency of Use Among Men Who Have Sex With Men in the United States. J Acquir Immune Defic Syndr. 2015;68(3):337–344. [DOI] [PubMed] [Google Scholar]
- 72.Deschamps M, Metch B, Ma MS, et al. Feasibility of Identifying a Female Sex Worker Cohort at High Risk of HIV Infection in the Caribbean for HIV Vaccine Efficacy Trials: Longitudinal Results of HVTN 907. J Acquir Immune Defic Syndr. 2016;71(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting file 1: Supplementary material_NHBS-HET RAI in at-risk women. Word document.
Supporting file 2: Supplementary table 2. Prior parameter ranges for cities (combined NHBS-HET 2010,2013 cycles). .


