Highlights
-
•
Interleukin IL-6 facilitates COVID-19 viral cell entry and replication.
-
•
Healthy IL-6 levels correlate with COVID-19 morbidity in similar matched populations.
-
•
Vitamin D administration appears to lower IL-6 levels in humans.
-
•
IL-6 modulation with Vitamin D and other means may be a useful therapeutic strategy in COVID-19.
Keywords: IL-6, COVID-19, Vitamin D, Cytokine storm, Cathepsin L, ACE2
Abstract
There is recent evidence that interleukin-6 (IL-6) levels are elevated in cases of complicated COVID-19, but it is also possible that this cytokine may have a far more important role in the pathogenesis of viral infection. IL-6 is known to be modulated by Vitamin D, and there is preliminary evidence that deficiency of this vitamin is linked to poorer outcomes. To identify whether IL-6 levels prior to infection might predict outcome, early data on COVID-19 mortality from Italy and the UK were compared with previously published results of mean IL-6 levels from these countries as well as from the USA. There was a highly significant correlation (r = 0.9883; p = 0.00025) between age-stratified mortality rates and IL-6 levels from previously published data on healthy individuals. To determine whether Vitamin D may be beneficial at lowering IL-6 levels in patients, a limited analysis of trials examining the relationship between these entities published since 2015 was undertaken. Eight out of 11 studies described a significant lowering effect of Vitamin D on IL-6. Given that IL-6 likely facilitates viral cell entry and replication, levels prior to infection may predict mortality. This provides a rationale for prophylactic and therapeutic measures directed at lowering IL-6, including Vitamin D prescription.
1. Introduction
Old age, obesity, BAME (Black, Asian, and minority ethnicity) and male gender are all associated with higher IL6 levels [1], [2], [3], [4]. Observationally, interleukin-6 (IL-6) elevation has been associated with poorer outcomes in severe covid-19 infection [5], [6]. It is possible that constitutive (premorbid) IL-6 elevation may also increase the likelihood of Covid-19 infection through the following mechanisms: COVID-19 requires cathepsin L to infect pulmonary epithelial cells [7], with cathepsin L production upregulated by IL-6 [8]. This interleukin also likely upregulates expression of the ACE2 receptor which the virus utilizes for cellular entry - see Table S2 of the article by Schouten et al. [9].
Low vitamin D levels are common in older adults, the obese and those with darkly pigmented skin [10]. These constituencies have also experienced disproportionately high morbidity and mortality from Covid-19 [11], [12].
This paper proposes that IL6 elevation lies at the nexus between low vitamin D status and higher risk of Covid-19 infection, severe morbidity and death in these vulnerable populations. It further proposes that these IL6-mediated risks can be ameliorated by vitamin D supplementation which appears to inhibit IL6 expression [13].
The primary objective of this study was to determine whether IL-6 levels in healthy individuals from a similar demographic to patients infected with COVID-19 correlate with severity of clinical manifestations. A secondary objective was to review evidence from recent clinical trials supporting the use of Vitamin D to lower IL-6 levels.
2. Materials and methods
2.1. IL-6 levels and severity of clinical manifestations
Given the range of severity of clinical manifestations of COVID-19, analysis was limited to assessment of mortality. To limit any potential confounding factors, the literature was searched for results of IL-6 determinations in healthy individuals with included age and sex data from specific geographical regions for which published mortality rates from COVID-19 were available. The largest study, in terms of numbers of subjects, by Stowe and colleagues [3], did not include detailed demographic information broken down into age groups, so analysis was performed on data from an Italian study that examined IL-6 from the Tuscany region [14]. Data on COVID-19 mortality from Italy broken down by age were sourced from statista.com [15] with a time point (to end of May 2020) chosen to correspond with the results of the study by Williamson et al. [11]. Italian gender mortality data published as raw numbers [16] were converted to death rates/million derived from accepted sources [17]. Statistical analysis on age data was carried out using the Pearson Correlation Coefficient Calculator. Data on sex, ethnic minority status and BMI were insufficient for statistical analysis, and these were assessed qualitatively. Age data were stratified slightly differently in each study, with the majority quoting in increments of 10 years from 20 years of age upwards. Where included studies stratified age groups in different formats, ages were rounded to nearest multiple of 10. For example, recently published UK data included a category of 18–40 years of age [1]; this category was rounded up to 20 to 40 in the analysis. Other studies, including that by Ferruci and colleagues [14] included a category in the format “20–39” years of age, which was rounded up to 20–40.
2.2. Vitamin D and IL-6 levels
A limited narrative review of recent clinical trials supporting the use of Vitamin D administration to lower IL-6 levels was performed by searching PubMed and Google Scholar for adult human research studies published from January 1, 2015 that included key words “vitamin D” and/or “interleukin-6” and/or “IL-6”. A total of 11 studies satisfied the inclusion criteria. As there was heterogeneity in the format of how results were published, analysis was limited to whether administration of Vitamin D resulted in a statistically significant reduction in IL-6 level.
3. Results
3.1. Premorbid IL-6 levels correlate strongly with age-stratified mortality
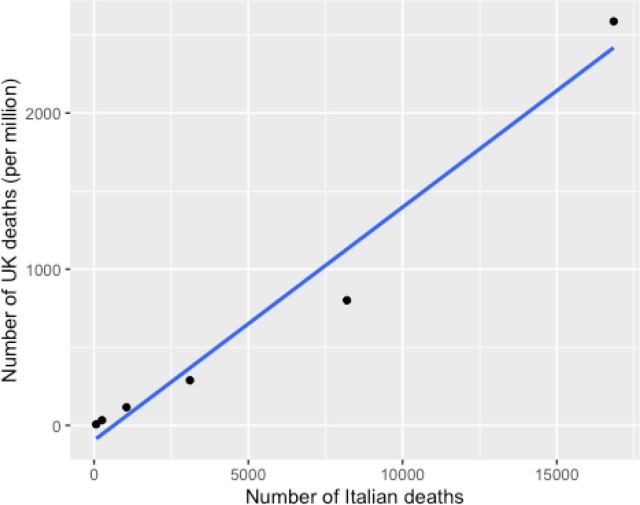
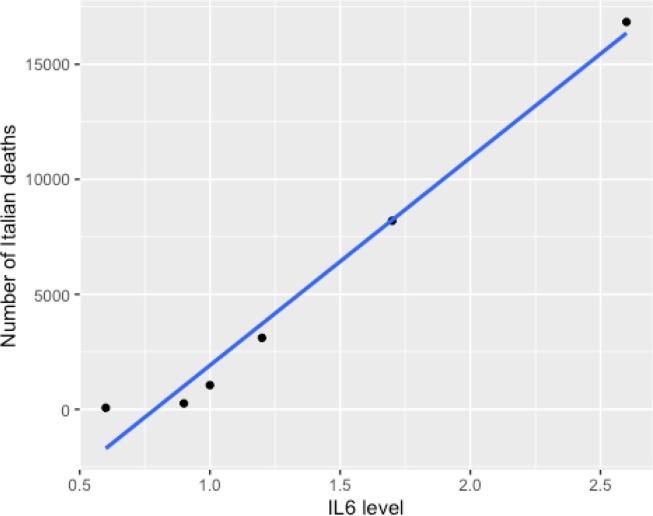
The data on Italian mortality [15] provided raw numerical deaths by population groups but did not provide information on stratified mortality rates by age group. To assess the validity of using this data, these results were compared with stratified mortality rate data in a large UK study [11] (see Table 1 ). There was an extremely strong correlation between age stratified Italian raw numerical deaths and UK mortality rates (r(6) = 0.9837; 95% CI: 0.85–1.0; p = 0.00396, see Fig. 1 ). Given the likely validity of using the Italian data, these were compared with IL-6 levels derived from a healthy Italian population [14]. There was an extremely strong correlation between age stratified Italian raw numerical deaths and IL-6 levels (r(6) = 0.9883; 95% CI: 0.89–1.0; p = 0.00025, see Table 2 and Fig. 2 ). As a broad test to confirm this, UK death rates were also compared with historic UK data published by Wei and colleagues [1] on IL-6 levels in a similar population. Mean death rates in patients over 60 were more than 10 times higher than in patients under 50; mean levels of IL-6 were higher in older patients, although only by 26%.
Table 1.
Age and death data from Italy and UK populations. Age-stratified deaths in Italy correlate strongly with UK death rates.
| Age | Italy Deaths | UK Deaths/mill |
|---|---|---|
| 20–40 | 67 | 6.7 |
| 40–50 | 257 | 32.7 |
| 50–60 | 1051 | 115.7 |
| 60–70 | 3107 | 288.1 |
| 70–80 | 8196 | 800.8 |
| 80+ | 16,842 | 2587.2 |
Fig. 1.
Age and death data from Italy and UK populations. Age-stratified deaths in Italy correlate strongly with UK death rates.
Table 2.
Mean IL-6 levels by age and death data from Italy Legend. Mean IL-6 levels correlate strongly with deaths in Italy.
| Age | IL-6 (pg/ml] | Italy Deaths |
|---|---|---|
| 20–40 | 0.6 | 67 |
| 40–50 | 0.9 | 257 |
| 50–60 | 1.0 | 1051 |
| 60–70 | 1.2 | 3107 |
| 70–80 | 1.7 | 8196 |
| 80+ | 2.6 | 16,842 |
Fig. 2.
Mean IL-6 levels by age and death data from Italy. Mean IL-6 levels correlate strongly with deaths in Italy.
3.2. Beneficial effect of Vitamin D on lowering IL-6 levels
Of the 11 studies included in the analysis, 11 identified a significant effect of Vitamin D administration on IL-6 (p < 0.05) and the remaining 3 found no effect. The results of this analysis are displayed in Table 3 .
Table 3.
Analysis of impact of Vitamin D supplementation on IL-6 levels in controlled trials published since 2015. The first column lists first author for each study. The final column details reported statistical effect of Vitamin D at lowering IL-6 level.
| Reference | Location | Condition | Age (yrs] | N | Duration (wks] | D3 Dose (IU/day) | Outcome |
|---|---|---|---|---|---|---|---|
| Bueloni-Dias [39] | Brazil | Healthy Women | 57 ± 7 | 160 | 39 | 1000 | p < 0.05 |
| Costenbader [40] | Boston | Healthy Older Adults | 65 ± 8 | 1561 | 52 | 2000 | p > 0.05 |
| Duggan [41] | Seattle | Overweight Women | 62 ± 12 | 218 | 52 | 2000 | p = 0.004 |
| Goncalves-Mendes [42] | France | Healthy Older Adults | 71 ± 6 | 38 | 13 | 6667 | p = 0.046 |
| Hashemi [43] | Iran | Multiple Sclerosis | 30 ± 9 | 75 | 8 | 7143 | p < 0.001 |
| Hashemi [44] | Iran | Multiple Sclerosis | 30 ± 10 | 75 | 8 | 7143 | p < 0.001 |
| Miroliaee [45] | Iran | Ventilator Pneumonia | 56 ± 20 | 46 | Single | 300,000 | p = 0.01 |
| Reid [46] | St Louis | Asthma | 40 ± 13 | 100 | 12 | 4000 | p > 0.05 |
| Stepanova [47] | Russia | Type 2 Diabetes | Not Stated | 62 | 24 | 5714 | p = 0.0001 |
| Tuomainen [48] | Finland | Prediabetic Adults | 66 ± 7 | 66 | 22 | 3200 | p = 0.38 |
| Żebrowska [49] | Poland | Healthy runners | 34 ± 7 | 24 | 3 | 2000 | p < 0.01 |
4. Discussion
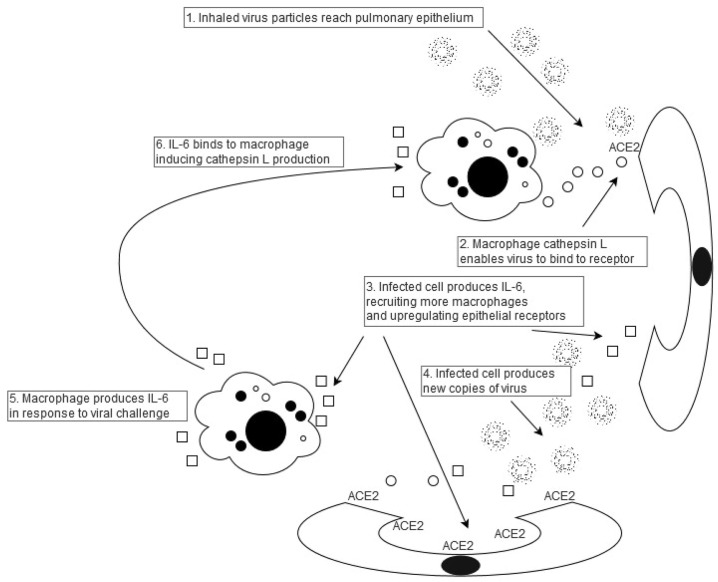
This is the first study to have demonstrated a significant correlation between IL-6 levels in matched healthy individuals and age-stratified mortality from COVID-19. The large analysis by Williamson et al. [11] also indicated other risk factors for increased mortality including sex, obesity and ethnicity. Interestingly, UK male death rates were 72% higher than amongst females [11]; this compares with data published by Wei et al. in 1992 [1] in which mean UK IL-6 levels for males were 112% greater than mean female levels. Italian death rates were 55% higher than amongst females [11]; this compares with data published by Ferruci and colleagues [14] in 2006 in which mean Italian IL-6 levels for males were 23% higher than mean female levels. Similarly, death rates amongst UK patients of black ethnicity were 40% higher than amongst white patients [11], compared with 59% greater IL-6 levels amongst black patients derived from a US study [3]. Mean UK death rates in obese patients were 48% higher than in non-obese patients [11]; this compared with IL-6 levels more than 300 times greater in obese patients from a US study [2]. These findings on sex, obesity and ethnicity, together with the age data analysis herein reported, suggest that IL-6 may have a far more important role in the pathogenesis of viral infection than previously thought. IL-6 upregulates cathepsin L production [8], which then cleaves the virus, enabling it to infect pulmonary epithelial cells [7]. IL-6 also upregulates the Angiotensin II AT1 receptor [18], and likely upregulates pulmonary epithelial cell ACE2 receptors, with a positive correlation between IL-6 and ACE2 levels in bronchioalveolar lavage (BAL) in patients with severe acute pulmonary inflammation [9]. Viral reproduction within pulmonary epithelial cells then recruits inflammatory cells which, in turn produce cytokines, as part of the “cytokine storm” phenomenon [19] including IL-6. This could be expected to set up a noxious cycle between immune cells and pulmonary epithelium which the virus utilizes to enter cells and replicate (see Fig. 3 ). The principal predisposing factor could well be IL-6, with premorbid levels correlating with stratified mortality rates. There are other manifestations of this infection that could readily be attributed to IL-6. Levels of this interleukin are generally low in children, but there is a short spurt in early childhood [20], correlating with a recently described severe COVID-19 manifestation in young children, a Kawasaki-like condition [21]. IL-6 is also thrombogenic, which may well account for this significant cause of death from the virus [22]. Intense exercise also produces large quantities of IL-6 [23], which may account for the more severe clinical manifestations of infection amongst athletes [24]. Indeed, dexamethasone, which lowers production of IL-6 by human bronchial epithelium [25], has recently been successfully trialled in COVID-19 patients [26], and there is increasing evidence that tocilizumab, an IL-6 antagonist, can result in improved outcomes amongst severely affected patients [27].
Fig. 3.
Noxious cycle induced by IL-6 interaction with macrophages and pulmonary epithelium. IL-6 enables viral binding and replication both through upregulation of ACE2 receptors (step 3] and induction of macrophage cathepsin L (step 6]. This provides a strategy for a range of therapeutic options, including Vitamin D prescription which directly lowers macrophage IL-6 production.
Can these findings be utilized to offer a rationale for Vitamin D prescription for COVID-19? The role of Vitamin D deficiency as a predictor for worse clinical outcomes remains the focus of investigation, although early results appear to support this contention [10]. What is unclear is whether Vitamin D exerts a general immune-enhancing effect, given that it appears to be protective in patients with acute respiratory tract infections [28], or whether it may have a specific role in prevention and treatment of COVID-19. Vitamin D lowers immune cell production of IL-6 in laboratory studies [13], but does it have a similar effect in human populations? Since Khoo et al. suggested that increased Vitamin D levels reduced cytokine production, including IL-6 in 2011 [29], there have been multiple studies of the relationship between Vitamin D and cytokines. Cannell and colleagues reviewed the literature in 2015, and found that Vitamin D appeared to reduce production of certain cytokines, including IL-6, in patients with inflammatory medical conditions [30]. Subsequently, Calton et al. completed a systematic review and meta-analysis of Vitamin D supplementation and IL-6 [31]. Although they could not confirm a definite impact overall, they did find a significant Vitamin D-induced reduction in the elderly [31]. In an analysis of 11 published studies since 2015, reported herein, 8 demonstrated a significant IL-6 lowering effect of Vitamin D supplementation including amongst fit and healthy individuals, as well as in patients with acute pneumonia and some chronic diseases including diabetes and multiple sclerosis. Although there is a need for additional research on the relationship between Vitamin D supplementation, IL-6 and COVID-19 outcomes, and, in particular a meta-analysis, these findings support a role for Vitamin D prescription, both prophylactic, and, potentially as a specific therapy [19]. There may well be other therapeutic strategies which could also be deployed, including nutritional supplementation with selenium [32], such as ingesting Brazil nuts [33], and administration of drugs which lower IL-6 production such as doxycycline [34] and budesonide/formoterol [35].
It should also be noted that IL-6 depletion may, potentially, have deleterious effects. Potentiation of further immunosuppression is a recognised phenomenon in patients with Rheumatoid arthritis treated with tocilizumab [36]. Hence, prolonged duration of COVID-19 prophylaxis with, for example, Vitamin D may potentially worsen mortality in some patient groups.
While the results of this work offer a rationale for the diverse clinical manifestations of COVID-19 and a range of therapeutic options for trial, this must be tempered by the limitations of this analysis. The data analysed were not strictly comparable, with premorbid data on IL-6 levels derived from different populations to those with mortality data. Further, only the age-stratified results are supported by statistical analysis as the other data on other factors including sex, ethnicity and obesity could not be assessed directly for statistical significance. While the age-stratified data comprised only 6 groups, 95% Confidence Intervals for correlation coefficient were still very high [0.89–1.0], supporting the conclusions drawn. In addition, the IL-6 analysis was tailored to correspond with mortality data published by Williamson et al. [11]; mortality rates would be expected to have fallen subsequently as communities introduced additional measures to combat viral spread, such as obligatory mask-wearing and social distancing, potentially confounding later data analysis. Nonetheless, the links drawn between the IL6 data and the Covid-19 incidence and severity data might be considered tenuous: data sets are separated by substantial time; IL6 levels presented were not taken from the same people; and there is no evidence that these IL6 levels or the individuals that they are taken from are a representative sample of the population under examination. Furthermore, no multivariate analysis was possible to adjust for other substantial confounding factors (including ethnicity, socioeconomic status, living conditions, lockdown and other social distancing measures) which could intercede in terms of causality: for instance, are the high IL-6 cohort also more obese than those with low IL6 levels? Finally, a number of cytokines are released following infection, and, as this analysis was limited to only IL-6, it is not possible to exclude other interleukins from having a role in predisposing to worse outcomes. While substantial premorbid data on IL-6 levels may never be obtainable in patients who die from COVID-19, prospective trials of therapeutic measures can and should be undertaken. However, given that Vitamin D causes little harm if administered under clinical supervision, the populations most vulnerable to this disease are likely vitamin D deficient [37], and this vitamin also likely modulates the oxidative stress occurring in severe COVID-19 [38] there is strong supports for its widespread prescription.
In conclusion, this study has demonstrated a highly significant correlation between premorbid IL-6 levels and mortality rates from COVID-19, accounting for a range of previously described clinical predictors and, potentially directing future therapeutic strategies, including in relation to Vitamin D.
Funding
No funding was obtained for this work
CRediT authorship contribution statement
Morry Silberstein: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wei J., Xu H., Davies J.L., Hemmings G.P. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 2.Khaodhiar L., Ling P.R., Blackburn G.L., Bistrian B.R. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J. Parenter. Enteral Nutr. 2004;28:410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- 3.Stowe R.P., Peek M.K., Cutchin M.P., Goodwin J.S. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:429–433. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X., Xiao Y., Zhu Q. Abstract 595: Gender difference was present in the production of cytokines in mice with PM Exposure. Art. Thromb. Vasc. Biol. 2017;37:A595. [Google Scholar]
- 5.E.A. Coomes, H. Haghbayan, Interleukin-6 in COVID-19: a systematic review and meta-analysis. medRxiv (2020). https://doi.org/10.1101/2020.03.30.20048058. [DOI] [PMC free article] [PubMed]
- 6.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang I.C., Bosch B.J., Li F. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006;281:3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber A., Welte T., Ansorge S., Bühling F. Expression of cathepsins B and L in human lung epithelial cells is regulated by cytokines. Adv. Exp. Med. Biol. 2000;477:287–292. doi: 10.1007/0-306-46826-3_31. [DOI] [PubMed] [Google Scholar]
- 9.Schouten L.R., van Kaam A.H., Kohse F. Age-dependent differences in pulmonary host responses in ARDS: a prospective observational cohort study. Ann. Intensive Care. 2019;9:55. doi: 10.1186/s13613-019-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020:1–4. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 13.Sadeghi K., Wessner B., Laggner U. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L., Corsi A., Lauretani F. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.https://www.statista.com/statistics/1105061/coronavirus-deaths-by-region-in-italy/ (mortality to 13 May2020, accessed 19 May2020).
- 16.https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths (to 21 May 2020, accessed 28 May 2020).
- 17.https://www.statista.com/statistics/786485/population-by-gender-in-italy/ (accessed 28 May 2020).
- 18.Senchenkova E.Y., Russell J., Yildirim A., Granger D.N., Gavins F.N.E. Novel role of T Cells and IL-6 (Interleukin-6) in angiotensin II-induced microvascular dysfunction. Hypertension. 2019;73:829–838. doi: 10.1161/HYPERTENSIONAHA.118.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silberstein M. Vitamin D: a simpler alternative to tocilizumab for trial in COVID-19? Med. Hypotheses. 2020:109767. doi: 10.1016/j.mehy.2020.109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sack U., Burkhardt U., Borte M., Schädlich H., Berg K., Emmrich F. Age-dependent levels of select immunological mediators in sera of healthy children. Clin. Diagn. Lab. Immunol. 1998;5:28–32. doi: 10.1128/cdli.5.1.28-32.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;(May) doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Divani A.A., Andalib S., Di Napoli M. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J. Stroke Cerebrovasc. Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen B.K., Steensberg A., Schjerling P. Exercise and interleukin-6. Curr. Opin. Hematol. 2001;8:137–141. doi: 10.1097/00062752-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Matricardi P., Dal Negro R., Nisini R. The first, comprehensive immunological model of COVID-19: implications for prevention, diagnosis, and public health measures. Preprints. 2020:2020040436. doi: 10.1111/pai.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine S.J., Larivée P., Logun C., Angus C.W., Shelhamer J.H. Corticosteroids differentially regulate secretion of IL-6, IL-8, and G-CSF by a human bronchial epithelial cell line. Am. J. Physiol. 1993;265:L360–L368. doi: 10.1152/ajplung.1993.265.4.L360. [DOI] [PubMed] [Google Scholar]
- 26.RECOVERY Collaborative Group, P. Horby, W.S. Lim, et al., Dexamethasone in hospitalized patients with Covid-19 - preliminary report, N. Engl. J. Med. (2020) 10.1056/NEJMoa2021436.
- 27.Moreno-Pérez O., Andres M., Leon-Ramirez J.M. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: a retrospective cohort study. J. Autoimmun. 2020:102523. doi: 10.1016/j.jaut.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martineau A.R., Jolliffe D.A., Hooper R.L. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoo A.L., Chai L.Y., Koenen H.J. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin. Exp. Immunol. 2011;164:72–79. doi: 10.1111/j.1365-2249.2010.04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannell J.J., Grant W.B., Holick M.F. Vitamin D and inflammation. Dermatoendocrinol. 2015;6 doi: 10.4161/19381980.2014.983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calton E.K., Keane K.N., Newsholme P., Zhao Y., Soares M.J. The impact of cholecalciferol supplementation on the systemic inflammatory profile: a systematic review and meta-analysis of high-quality randomized controlled trials. Eur. J. Clin. Nutr. 2017;71:931–943. doi: 10.1038/ejcn.2017.67. [DOI] [PubMed] [Google Scholar]
- 32.Tseng C.K., Ho C.T., Hsu H.S. Selenium is inversely associated with interleukin-6 in the elderly. J. Nutr. Health Aging. 2013;17:280–284. doi: 10.1007/s12603-012-0376-6. [DOI] [PubMed] [Google Scholar]
- 33.Thomson C.D., Chisholm A., McLachlan S.K., Campbell J.M. Brazil nuts: an effective way to improve selenium status. Am. J. Clin. Nutr. 2008;87:379–384. doi: 10.1093/ajcn/87.2.379. [DOI] [PubMed] [Google Scholar]
- 34.Conforti C., Giuffrida R., Zalaudek I., Di Meo N. Doxycycline, a widely used antibiotic in dermatology with a possible anti-inflammatory action against IL-6 in COVID-19 outbreak. Dermatol. Ther. 2020 doi: 10.1111/dth.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suda K., Tsuruta M., Eom J. Acute lung injury induces cardiovascular dysfunction: effects of IL-6 and budesonide/formoterol. Am. J. Respir. Cell Mol. Biol. 2011;45:510–516. doi: 10.1165/rcmb.2010-0169OC. [DOI] [PubMed] [Google Scholar]
- 36.Kim H., Baek S., Hong S.M. 1,25-dihydroxy Vitamin D3 and Interleukin-6 Blockade synergistically regulate Rheumatoid Arthritis by suppressing Interleukin-17 production and osteoclastogenesis. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slominski R.M., Stefan J., Athar M. COVID-19 and Vitamin D: a lesson from the skin. Exp. Dermatol. 2020 doi: 10.1111/exd.14170. [published online ahead of print, 2020 Aug 11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slominski A.T., Slominski R.M., Goepfert P.A. Reply to Jakovac and to Rocha et al.: Can vitamin D prevent or manage COVID-19 illness? Am. J. Physiol. Endocrinol. Metab. 2020;319:E455–E457. doi: 10.1152/ajpendo.00348.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bueloni-Dias F.N., Orsatti C.L., Cangussu L.M. Isolated vitamin D supplementation improves the immune-inflammatory biomarkers in younger postmenopausal women: a randomized, double-blind, placebo-controlled trial. Menopause. 2018;25:897–903. doi: 10.1097/GME.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 40.Costenbader K.H., MacFarlane L.A., Lee I.M. Effects of one year of Vitamin D and marine Omega-3 fatty acid supplementation on biomarkers of systemic inflammation in older US adults. Clin. Chem. 2019;65:1508–1521. doi: 10.1373/clinchem.2019.306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duggan C., de Dieu Tapsoba J., Mason C. Effect of Vitamin D3 supplementation in combination with weight loss on inflammatory biomarkers in postmenopausal women: a randomized controlled trial. Cancer Prev. Res. (Phila) 2015;8:628–635. doi: 10.1158/1940-6207.CAPR-14-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goncalves-Mendes N., Talvas J., Dualé C. Impact of Vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front. Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashemi R., Hosseini-Asl S.S., Arefhosseini S.R., Morshedi M. The impact of vitamin D3 intake on inflammatory markers in multiple sclerosis patients and their first-degree relatives. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0231145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashemi R., Morshedi M., Asghari Jafarabadi M., Altafi D., Saeed Hosseini-Asl S., Rafie-Arefhosseini S. Anti-inflammatory effects of dietary vitamin D3 in patients with multiple sclerosis. Neurol. Genet. 2018;4 doi: 10.1212/NXG.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miroliaee A.E., Salamzadeh J., Shokouhi S., Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J. Crit. Care. 2018;44:300–305. doi: 10.1016/j.jcrc.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 46.Reid B., Girodet P.O., Boomer J.S. Vitamin D3 treatment of vitamin D-insufficient asthmatic patients does not alter immune cell function. J. Allergy Clin. Immunol. 2016;138 doi: 10.1016/j.jaci.2015.11.030. 286–289.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stepanova A.P., Karonova T.I., Galagoudza M., Vasileva E.Y., Jude E.B. 562-P: The effect of Vitamin D supplementation on the cytokines levels in patients with type 2 diabetes mellitus and diabetic neuropathy. Diabetes. 2019;68(Suppl. 1) doi: 10.2337/db19-562-P. [DOI] [Google Scholar]
- 48.Tuomainen T.P., Virtanen J.K., Voutilainen S. Glucose metabolism effects of vitamin D in prediabetes: the VitDmet randomized placebo-controlled supplementation study. J. Diabetes Res. 2015;2015 doi: 10.1155/2015/672653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Żebrowska A., Sadowska-Krępa E., Stanula A. The effect of vitamin D supplementation on serum total 25(OH) levels and biochemical markers of skeletal muscles in runners. J. Int. Soc. Sports Nutr. 2020;17:18. doi: 10.1186/s12970-020-00347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]