Abstract
Background
SARS-CoV-2 infection has caused a global pandemic. Many of the medications identified to treat COVID-19 could be connected with QTc prolongation and its consequences.
Methods
Non-ICU hospitalized patients of the three centres involved in the study from the 19th of March to the 1st of May were included in this retrospective multicentre study. Relevant clinical data were digitally collected. The primary outcome was the incidence of QTc prolongation ≥ 500 ms, the main secondary outcomes were the Tisdale score ability to predict QTc prolongation and the incidence of ventricular arrhythmias and sudden deaths.
Results
196 patients were analysed. 20 patients (10.2%) reached a QTc ≥ 500 ms. Patients with QTc ≥ 500 ms were significantly older (66.7 ± 14.65 vs 76.6 ± 8.77 years p: 0.004), with higher Tisdale score (low 56 (31.8%) vs 0; intermediate 95 (54.0%) vs 14 (70.0%); high 25 (14.2%) vs 6 (30.0%); p: 0.007) and with higher prognostic lab values (d-dimer 1819 ± 2815 vs 11486 ± 38554 ng/ml p: 0.010; BNP 212.5 ± 288.4 vs 951.3 ± 816.7 pg/ml p < 0.001; procalcitonin 0.27 ± 0.74 vs 1.33 ± 4.04 ng/ml p: 0.003). After a multivariate analysis the Tisdale score was able to predict a QTc prolongation ≥ 500 ms (OR 1,358 95% CI 1,076–1,714p: 0,010). 27 patients died because of COVID-19 (13.7%), none experienced ventricular arrhythmias, and 2 (1.02%) patients with concomitant cardiovascular condition died of sudden death.
Conclusions
In our population, a QTc prolongation ≥ 500 ms was observed in a minority of patients, no suspected fatal arrhythmias have been observed. Tisdale score can help in predicting QTc prolongation.
Keywords: QT interval, COVID-19, Hydroxycloroquine, Azithromycin, Non ICU-patients
1. Introduction
Since December 2019, a novel coronavirus - SARS-CoV-2 - has caused a global pandemic of respiratory illness termed Covid-19. This virus has rapidly shown to be highly contagious and to cause a high number of severe complications. The scientific community has begun a rush to look for possible treatments, given the absence of known therapeutic agents for the disease. Many drugs are being tested, including antivirals (lopinavir/ritonavir, darunavir/cobicistat, remdesevir), chloroquine, hydroxychloroquine (HCQ), antibiotics (azithromycin), immune system modulators (steroids, tocilizumab, colchicine and others) and anticoagulants [1].
Among the most used drugs during this early phase of pandemic are chloroquine, HCQ, azithromycin and antivirals. HCQ and chloroquine have shown promising antiviral properties in vitro and in animal studies and in some initial reports of use in China. They could exert their effect in altering endosomal pH, the glycosylation of cell receptor of SARS-CoV-2 and through an immune system modulation. Anyway, evidence supporting their use is sparse and mainly coming from small open-label randomized trials (RCTs) [2]. Azithromycin is being used in some centres for its antibacterial and – mainly presumed – immunomodulatory property. The combination of HCQ with a second generation macrolide has shown promising results in a small open-label non-randomized clinical trial but subsequent large retrospective observational studies rose doubts on the safety and efficacy of their contemporary use [3], [4]. Many large RCTs are ongoing worldwide and hopefully will soon clarify if HCQ alone or in combination with azithromycin is safe and useful.
Finally, antivirals have shown promising pre-clinical data with mixed results in recent papers [5], [6].
Due to the lack of effective treatment, even in the absence of convincing evidences all the drugs cited above, are/have been widely used alone or in combination, even outside clinical trials. Limited data on safety are available and concerns about cardiac safety have been raised, mainly regarding the possible risk of Torsade de Pointes (TdP) due to corrected QT interval (QTc) prolongation. Some cardiology societies have therefore published practical guidelines on the argument in order to diminish the potential risk of fatal arrhythmias, despite the paucity of clinical data, mainly recommending intensive ECG monitoring and some safety thresholds of QTc prolongation mandating treatment interruption [7], [8]. However, since these drugs in the majority of cases have been used for many years (e.g. chloroquine was discovered in 1934) and their side effects are well known, why bother? Many think that in COVID-19 a perfect storm could occur: the use of many QTc-prolonging drugs together, the presence of hypokalemia due to diarrhea and sweating, and possibly the presence of fever and cytokines storm.
However, to date few small studies have investigated the risk of QTc prolongation during COVID-19 outbreak and the attributable risk of ventricular arrhythmias and sudden deaths mainly focusing on HCQ and azithromycin [9], [10], [11]. The purpose of our study is to investigate the incidence of QTc prolongation in hospital patients admitted for COVID-19 infections evaluating the potential correlation between the medication used, the QTc and their outcome with particular attention to arrhythmic events.
2. Methods
2.1. Design of the study
We performed a multicentre retrospective observational study to analyse the issue of QTc prolongation in patients with COVID-19. All the data were collected in a digital database of the coordinating centre, San Luigi Gonzaga University Hospital, and previously deidentified. The study was performed according to the Institution Review Board guidance of each participating centres and conducted in accordance with the Declaration of Helsinki and its later amendments.
All the consecutive patients with suspected COVID-19 admitted to the San Luigi Gonzaga University Hospital, Orbassano, Turin in the sub-intensive care units and low-intensity care units from the 19th of March to the 1st of May were screened to be included. From the 15th of April to the 1st of May two additional centres, the Sant’Andrea Hospital, Vercelli and the San Carlos University Hospital, Madrid, were involved in the study.
For each patient, all the variables potentially involved in QTc prolongation were collected from paper or electronic medical records, including: (i)demographic variables; (II)patient history (esp. regarding heart disease); (iii)serial ECGs; (iv)previous medications and medications used during hospitalization; (v)blood tests including high sensitivity troponin I (HsTnI – Abbot ArchitectTM) and serial measurements of electrolytes, coagulation, inflammation, kidney and liver function; (vi)Any complications occurred during hospitalizations and status of the patient at discharge.
Inclusion criteria of this study were: (i) > 18 years of age; (ii)COVID-19 confirmed by RT-PCR on nasopharyngeal swab or COVID-19 highly suspected based on history, clinical and imaging findings even in the absence of positive nasopharyngeal swab; (iii)Completeness of the data about the kind of therapy and its duration, at least two ECG examinations (one at baseline and at least one during the treatment), at least two electrolyte determinations during the treatment, performed within 48 h from each ECG recordings, and complete data about the status of the patient at the end of therapy and complications.
Patients hospitalized in the intensive care units (ICUs) were excluded from the study because they were not able to swallow HCQ. All the formulations of HCQ available in Europe are film-coated and cannot be divided so, in order to avoid potential bias in the study, we excluded all the patients not able to assume the medications properly.
2.2. Ecgs analysis
All ECGs coming from Sant’Andrea Hospital, Vercelli, and San Carlos University Hospital, Madrid were centralized to the San Luigi Gonzaga University Hospital and all the QTc were manually calculated by two expert cardiologists (PD and AL) blinded about the ongoing treatments of the patients. In case of conflicts, a third cardiologist (AP) measured QTc and the final value was established by majority.
The “Tangent” method on 12 leads ECGs was used to calculate the QT interval in lead II or in V5-V6 if T waves could not be easily measured. The RR interval and QT were used to calculate the QTc with the Bazett’s formula. In the presence of bundle branch block the Bogossian formula or the fixed QRS replacement method were used [12], [13]; in patients with paced rhythm the Bogossian formula or spline formula were used.
ECG signs of ischemia were defined as new/dynamic ST segment changes > 0.5 mm in two contiguous leads, T wave flattening or inversion in leads with predominant R wave
All patients admitted in the sub-intensive care units, but not those in low-intensity wards, were monitored with telemetry.
2.3. Treatments
The two Italian centres involved in the study adopted the Italian society of cardiology released recommendations to manage COVID-19 patients treated with medications potentially connected with QTc prolongation [7]. The Spanish centre involved adopted the European Society of Cardiology guidance [8]. Italian society of cardiology suggestions consisted in performing serial 12 leads electrocardiographic (ECG) examinations (baseline and 24 or 48 h after the beginning of medications potentially prolonging QTc), and in case of significant QTc prolongation to consult the local cardiologists for the management of QTc [7]; European Society of Cardiology has given similar recommendations, suggesting to identify and correct any modifiable risk factor for QTc prolongation before starting the treatment, to perform a baseline ECG and an ECG once on treatment, and to consider reduction or suspension of the treatment in case of QTc ≥ 500 ms or QTc prolongation ≥ 60 ms. However, no strict rules were imposed to the involved centres and physicians were left free to decide when to start and stop medications based on their clinical judgment.
HCQ was used with a loading dose of 400 mg/twice daily the first day followed by 200 mg/twice daily. Azithromycin was given at the dosage of 500 mg/daily. The length of the therapies was left to the decision of the treating physician.
2.4. Outcome measures
The primary clinical outcome of the study was to analyse the QTc prolongation in relation with pre-existing clinical conditions, clinical presentation and medications related to COVID-19 infections. The secondary outcome measures were: the incidence of TdP and sudden death during hospitalization, the COVID-19 infection outcomes (in-hospital mortality and any in-hospital adverse events) and the ability of Tisdale score to predict the risk of QTc prolongation and mortality in COVID-19 hospitalized patients [14]. Adverse in-hospital events were defined as a composite of death for COVID-19, sudden-death, TdP, AF/Flutter, ventricular fibrillation; deep vein thrombosis (DVT); pulmonary embolism (PE); myocardial infraction (MI); Takotsubo syndrome; Vascular Thrombosis.
2.5. Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median with the interquartile range (IQR) according to the normality of their distribution. Categorical variables are reported as frequencies and percentages (%). Fisher’s exact test or Chi-squared test were used to compare categorical variables. Parametric distribution of continuous variables was tested graphically and with Kolmogorov Smirnov and the appropriate analysis were used in accordance with the results. One sample t-test (if samples were normally distributed) or one sample Wilcoxon signed rank test (if samples were not normally distributed) was performed to compare each sample against a delta QTc of 0 ms (i.e. no change from baseline), and p values were adjusted using the Holm-Bonferroni method to α < 0.05. Univariate and multivariate logistic regression were performed to identify predictors of prolonged QTc (>500 ms). The univariate predictors with p < 0.05 with potential interaction with QT prolongation as well as clinical and laboratory variables with potential interaction with QT interval were selected for subsequent multivariate analysis, as allowed by our sample size. Variables included in the final models were: age, numbers of drugs prolonging QTc, antibiotics and antiarrhythmic drugs, Tisdale score, baseline QTc > 450 ms, history of cardiovascular disease (defined as previous PCI, previous myocardial infraction, history of at least moderate valvular heart disease, history of arrhythmias requiring medical intervention), ECG signs of ischemia and electrolyte abnormalities (defined as at least one value of Serum K+ < 3.5 meq/l or Serum Mg2+ <1.3 meq/l. Statistical analyses were performed using SPSS software ver. 24.0 (IBM, Armonk, NY, USA) and figures were constructed using GraphPad Prism 8.
3. Results
Out of 264 patients screened, we excluded 40 (15.5%) patients without baseline ECG and 28 (10,6%) patients without follow-up ECG. Finally, 196 patients with confirmed or highly suspected COVID-19 were analysed. Baseline characteristics are shown in Table 1 and Table 2. Two patients (1.02%) had a baseline QTc ≥ 500 ms and 25 (12.8%) had a baseline QTc ≥ 460 ms. Overall, 20 patients (10.2%) reached a QTc ≥ 500 ms. Patients who developed a QTc ≥ 500 ms were significantly older, more likely to have history of cardiovascular disease, use of antiarrhythmic drugs (amiodarone, flecainide, sotalol), use of broad-spectrum antibiotics during hospitalization, have ischemic alterations on ECG, higher Tisdale score, and exams suggestive of a more severe disease (higher D-Dimer, BNP, procalcitonin, and lower O2 saturation). Baseline characteristics of patients with QTc variation ≥ 60 ms are summarized in supplementary Table 1, Table 2.
Table 1.
Baseline characteristics, nominal variables, overall and according to QTc cut-off of 500 ms. Values are n (%).
| Overall (n 196) | QTc < 500 ms (n 176) | QTc ≥ 500 ms (n 20) | p value | |
|---|---|---|---|---|
| Female Sex | 76 (38.8%) | 71 (36.2%) | 5 (25%) | 0.182 |
Tisdale score
|
56 (28.6%) 109 (55.6%) 31 (15.8%) |
56 (31.8%) 95 (54.0%) 25 (14.2%) |
0 (0%) 14 (70.0%) 6 (30.0%) |
0.007 |
| Tisdale score Intermediate-high |
140 (71.4%) |
120 (61.2%) |
20 (100%) |
0.003 |
| SARS-CoV-2 swab positivity | 173 (88.3%) | 156 (88.6%) | 17 (85.0%) | 0.632 |
Imaging positivity:
|
7 (3.6%) 22 (11.2%) 43 (21.9%) 5 (2.6%) 119 (60.7%) |
7 (4.0%) 20 (11.4%) 41 (23.3%) 4 (2.3%) 104 (59.1%) |
0 (0%) 2 (10.0%) 2 (10.0%) 1 (5.0%) 15 (75.0%) |
0.471 |
| Asymmetric involvement | 46 (23.5%) | 41 (23.3%) | 5 (25.0%) | 0.865 |
| Fever | 161 (82.1%) | 146 (83.0%) | 15 (75.0%) | 0.379 |
| Cough | 116 (59.2%) | 108 (61.4%) | 8 (40.0%) | 0.065 |
| Dyspnea | 121 (61.7%) | 103 (58.5%) | 18 (90.0%) | 0.06 |
| Anosmia | 8 (4.1%) | 8 (4.5%) | 0 (0.0%) | 0.330 |
| Ageusia | 9 (4.6%) | 8 (4.5%) | 1 (5.0%) | 0.927 |
| Diarrhea | 19 (9.7%) | 18 (10.2%) | 1 (5.0%) | 0.454 |
| Hypertension | 117 (59.7%) | 103 (58.5%) | 14 (70.0%) | 0.321 |
| Diabetes | 43 (21.9%) | 36 (20.5%) | 7 (35.0%) | 0.136 |
| Hypercolesterolemia | 58 (29.6%) | 51 (29.0%) | 7 (35.0%) | 0.576 |
| Smoke | 11 (5.6%) | 9 (5.1%) | 2 (10.0%) | 0.368 |
| Family history of CAD | 18 (9.2%) | 17 (9.7%) | 1 (5.0%) | 0.494 |
History of cardiovascular disease:
|
144 (73.8%) 33 (16.9%) 15 (7.7%) 3 (1.5%) |
134 (76.6%) 27 (15.4%) 13 (7.4%) 1 (0.6%) |
10 (50.0%) 6 (30.0%) 2 (10.0%) 2 (10.0%) |
0.002 |
| History of lung disease | 41 (20.9%) | 36 (20.5%) | 5 (25.0%) | 0.636 |
Cancer
|
154 (78.6%) 23 (11.7%) 19 (9.7%) |
137 (77.8%) 22 (12.5%) 17 (9.7%) |
17 (85.0%) 1 (5.0%) 2 (10.0%) |
0.613 |
| Dementia | 24 (12.2%) | 21 (11.9%) | 3 (15.0%) | 0.692 |
| Liver disease | 12 (6.1%) | 9 (5.1%) | 3 (15.0%) | 0.150 |
Kidney function - eGFR:
|
74 (37.8%) 78 (39.8%) 35 (17.9%) 7 (3.6%) 2 (1.0%) |
69 (39.2%) 69 (39.2%) 30 (17.0%) 6 (3.4%) 2 (1.1%) |
5 (25.0%) 9 (45.0%) 5 (25.0%) 1 (5.0%) 0 (0.0%) |
0.717 |
| Immunosuppressive therapy | 9 (4.6%) | 8 (4.5%) | 1 (5.0%) | 0.927 |
Antiarrhythmic drugs:
|
187 (95.4%) 1 (0.5%) 7 (3.6%) 1 (0.5%) |
169 (96.0%) 1 (0.6%) 6 (3.4%) 0 (0.0%) |
18 (90.0%) 0 (0.0%) 1 (5.0%) 1 (5.0%) |
0.028 |
| Beta blocker therapy | 54 (27.6%) | 45 (25.6%) | 9 (45.0%) | 0.065 |
| Ace-inhibitor therapy | 38 (19.4%) | 32 (18.2%) | 6 (30.0%) | 0.205 |
| ARBs therapy | 20 (10.2%) | 18 (10.2%) | 2 (10.0%) | 0.975 |
| Calcium channel blockers | 32 (16.3%) | 28 (15.9%) | 5 (25.0%) | 0.303 |
| Thiazidic diuretics | 19 (9.7%) | 19 (10.8%) | 0 (0.0%) | 0.122 |
| Loop diuretics | 17 (8.7%) | 13 (7.4%) | 4 (20.0%) | 0.058 |
| Alfa-blockers | 7 (3.6%) | 7 (4.0%) | 0 (0.0%) | 0.364 |
| Antipsychotic use | 25 (12.8%) | 23 (13.1%) | 2 (10.0%) | 0.697 |
| Antihistaminic | 3 (1.5%) | 3 (1.7%) | 0 (0.0%) | 0.566 |
| Azithromycin | 37 (18.9%) | 34 (19.3%) | 3 (15.0%) | 0.640 |
| Fluoroquinolones | 10 (5.1%) | 8 (4,5%) | 2 (10.0%) | 0.293 |
| Cephalosporin | 81 (41.3%) | 74 (42.0%) | 7 (35.0%) | 0.544 |
| Piperacillin/Tazobactam | 51 (26.0%) | 42 (23.9%) | 9 (45.0%) | 0.041 |
| Meropenem | 16 (8.2%) | 11 (6.3%) | 5 (25.0%) | 0.004 |
| Vancomicin | 6 (3.0%) | 4 (2.3%) | 2 (10.0%) | 0.057 |
| Linezolid | 12 (6.1%) | 10 (5.7%) | 2 (10.0%) | 0.445 |
| Amikacine | 5 (2.5%) | 5 (2.8%) | 0 (0.0%) | 0.445 |
| Hydroxychloroquine | 170 (86.7%) | 152 (86.4%) | 18 (90.0%) | 0.650 |
| Antimycotics | 4 (2.0%) | 3 (1.7%) | 1 (5.0%) | 0.329 |
| Ritonavir/Lopinavir | 29 (14.8%) | 26 (14.8%) | 3 (15.0%) | 0.978 |
| Darunavir/Ritonavir | 59 (30.1%) | 55 (31.3%) | 4 (20.0%) | 0.299 |
| Corticosteroids | 75 (38.2%) | 67 (38.1%) | 8 (40.0%) | 0.866 |
Number of QTc prolonging medications:
|
1 (0.5%) 45 (23.0%) 88 (44.9%) 48 (23.5%) 14 (7.1%) |
1 (0.6%) 42 (23.9%) 78 (44.3%) 42 (23.9%) 13 (7.4%) |
0 (0.0%) 3 (15.0%) 10 (50.0%) 6 (30.0%) 1 (5.0%) |
0.865 |
ECG Rhythm:
|
178 (90.8%) 16 (8.2%) 2 (1.0%) |
162 (92.0%) 12 (6.8%) 2 (1.1%) |
6 (80.0%) 4 (20.0%) 0 (0.0%) |
0.114 |
Conduction disturbance:
|
168 (85.7%) 2 (1.0%) 7 (3.6%) 10 (5.1%) 9 (4.6%) |
152 (86.3%) 2 (1.1%) 5 (2.9%) 8 (4.6%) 9 (5.1%) |
16 (80%) 0 (0.0%) 2 (10.0%) 2 (10.0%) 0 (0.0%) |
0.260 |
| Ischemic alterations ECG | 14 (7.1%) | 9 (5.1%) | 5 (25.0%) | 0.001 |
| LV hypertrophy on ECG | 13 (6.6%) | 12 (6,8%) | 1 (5.0%) | 0.757 |
Table 2.
Baseline characteristics, continuous variables, overall and according to QTc cut-off of 500 ms. Values are mean ± standard deviation.
| Overall (n 196) | QTc < 500 ms | QTc ≥ 500 ms | p value | |
|---|---|---|---|---|
| Age (years) | 67.7 ± 14.46 | 66.7 ± 14.65 | 76.6 ± 8.77 | 0.004 |
| Height (m) | 1.67 ± 0.08 | 1.66 ± 0.07 | 1.66 ± 0.06 | 0.345 |
| Weight (kg) | 73.6 ± 11.93 | 73.8 ± 11.99 | 71.8 ± 11.45 | 0.478 |
| BMI (kg/m2) | 26.3 ± 3.68 | 26.4 ± 3.78 | 25.7 ± 2.68 | 0.386 |
| ATB-time (days) | 7.9 ± 8.02 | 7.5 ± 7.18 | 11.7 ± 13.02 | 0.026 |
| Serum K+ (meq/l) | 4.08 ± 0.54 | 4.06 ± 0.52 | 4.21 ± 0.67 | 0.263 |
| Serum Mg2+ (meq/l) | 2.03 ± 0.23 | 2.03 ± 0.23 | 2.06 ± 0.18 | 0.614 |
| Total Ca2+ (mmol/l) | 3.02 ± 2.40 | 3.10 ± 2.48 | 2.51 ± 1.80 | 0.413 |
| Hb (g/dl) | 12.9 ± 2.06 | 13.0 ± 2.01 | 12.5 ± 2.40 | 0.255 |
| WBC (x106/l) | 7835 ± 12905 | 7773 ± 13499 | 8381 ± 5525 | 0.842 |
| Lymphocytes (x106/l) | 1852 ± 6819 | 1917 ± 7190 | 1281 ± 838 | 0.694 |
| Platelets (x106/l) | 234280 ± 108787 | 235397 ± 108977 | 224450 ± 109374 | 0.671 |
| D-dimer (ng/ml) | 2988 ± 13644 | 1819 ± 2815 | 11486 ± 38554 | 0.010 |
| Fibrinogen (mg/dl) | 600 ± 197 | 609 ± 199 | 532 ± 170 | 0.130 |
| TnI HS (pg/l) | 28.4 ± 88.21 | 25.9 ± 85.44 | 55.3 ± 117.83 | 0.370 |
| BNP (pg/ml) | 335.6 ± 498 | 212.5 ± 288.4 | 951.3 ± 816.7 | <0.001 |
| Procalcitonin (ng/ml) | 0.39 ± 1.51 | 0.27 ± 0.74 | 1.33 ± 4.04 | 0.003 |
| O2 saturation (%) | 91.7 ± 6.6 | 92.1 ± 6.1 | 88.4 ± 9.4 | 0.017 |
| Fio2 (%) | 0.27 ± 0.14 | 0.27 ± 0.13 | 0.28 ± 0.15 | 0.622 |
| PH | 7.44 ± 0.06 | 7.44 ± 0.05 | 7.44 ± 0.04 | 0.716 |
| PO2 (mmHg) | 72.0 ± 21.58 | 71.7 ± 20.79 | 74.4 ± 28.07 | 0.598 |
| PCO2 (mmHg) | 34.9 ± 6.96 | 35.0 ± 7.21 | 34.2 ± 4.18 | 0.628 |
| P/F (mmHg/%) | 297 ± 88.72 | 298 ± 89.44 | 283 ± 83.02 | 0.479 |
| HCO3– (mmol/l) | 24.4 ± 3.11 | 24.4 ± 3.12 | 23.8 ± 3.10 | 0.412 |
| Number of ECG per patient | 2.7 ± 0.740 | 2.64 ± 0.711 | 2.80 ± 0.786 | 0.597 |
| First QTc (ms) | 427 ± 31 | 424 ± 30 | 444 ± 28 | 0.007 |
| Heart Rate (bpm) | 85 ± 19 | 85 ± 18 | 91 ± 21 | 0.621 |
| Second QTc (ms) | 438 ± 32 | 432 ± 24 | 496 ± 35 | <0.001 |
| Heart Rate (bpm) | 78 ± 18 | 78 ± 14 | 76 ± 13 | 0.416 |
| Serum K+ (meq/l) | 4.14 ± 0.56 | 4.15 ± 0.55 | 4.06 ± 0.64 | 0.471 |
| Serum Mg2+ (meq/l) | 2.05 ± 0.26 | 2.05 ± 0.26 | 2.04 ± 0.23 | 0.916 |
| P/F (mmHg/%) | 283 ± 107 | 287 ± 107 | 252 ± 102 | 0.176 |
| Third QTc (ms) | 442 ± 50 | 433 ± 47 | 493 ± 33 | <0.001 |
| Heart Rate (bpm) | 76 ± 13 | 75 ± 13 | 77 ± 14 | 0.622 |
| Serum K+ (meq/l) | 4.27 ± 0.51 | 4.30 ± 0.52 | 4.10 ± 0.40 | 0.156 |
| Serum Mg2+ (meq/l) | 2.04 ± 0.26 | 2.04 ± 0.26 | 2.04 ± 0.23 | 0.956 |
| P/F (mmHg/%) | 285 ± 120 | 293 ± 120 | 226 ± 101 | 0.071 |
CAD: coronary artery disease; Lung US: lung ultrasound; CT: computed tomography; AF: atrial fibrillation; GFR: glomerular filtration rate; ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; PM: pace maker; AV block: atrioventricular block; LBBB: left bundle branch block; RBBB: right bundle branch block; LAFB: left anterior fascicular block; LV: left ventricle; BMI: body mass index; ATB-time: antibiotic time; Hb: hemoglobin; WBC: white blood cells; TnI HS: high sensitivity troponin; BNP: brain natriuretic peptide; P/F: PaO2/FiO2;
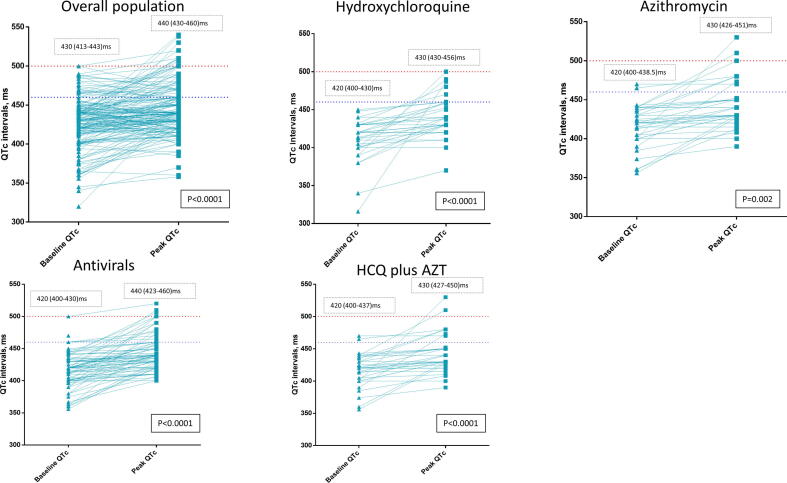
In Fig. 1, the graphic representation of QTc baseline – peak during the hospitalization shows how QTc changed during hospital stay. It is evident how QTc significantly increased during hospitalization in all the groups analysed– HCQ, azithromycin, antivirals (darunavir/ritonavir, ritonavir/lopinavir) – at a mean value of around 440 ms (IQR 430–460 ms; p < 0.05) in all groups. Moreover, the highest increase in QT values was observed in the group of patients treated with HCQ plus antiviral therapy (mean value 440 ms, IQR 422–460 ms, p < 0.0001).
Fig. 1.
QTc variation baseline – peak, overall and according to treatment. Numbers are median and Interquartile range.
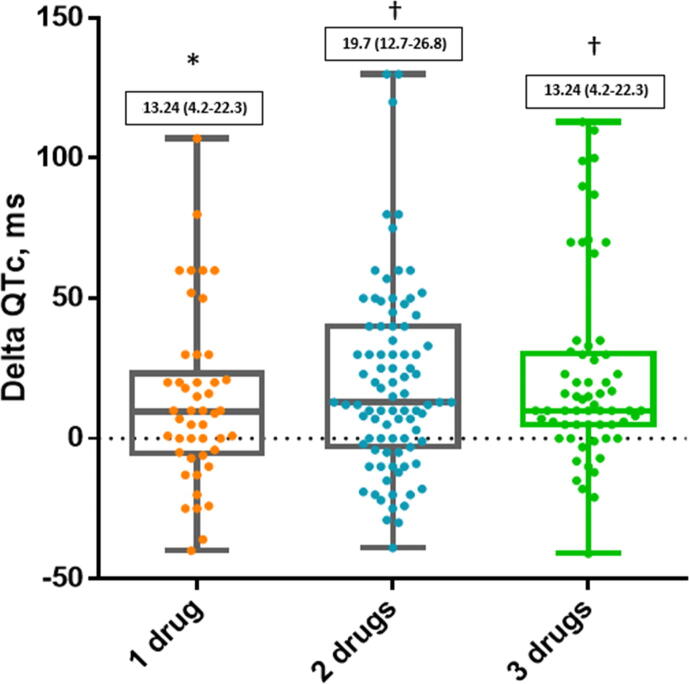
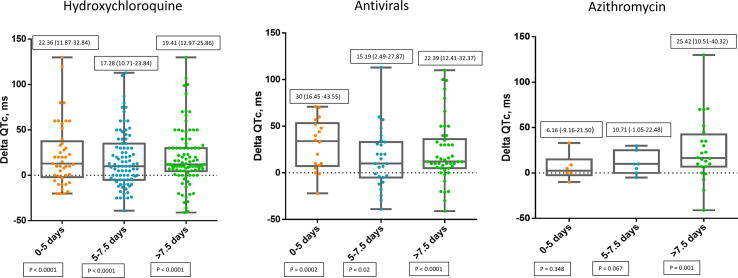
The QT variation in patients treated with COVID-19 therapies was significantly connected with the number of QT prolonging drugs they were taking as shown in Fig. 2. Moreover, the highest variation of QTc was observed at different time intervals among the drugs we investigated. In particular, the peak QTc was reached in the first five days in patients treated with HCQ and antiviral therapies and after 7.5 days for those treated with azithromycin as shown in Fig. 3.
Fig. 2.
Distribution of QTc intervals during hospitalization according to number of QTc prolonging drugs. Numbers in solid boxes are median and IQR (25%-75%). * p < 0.01; †p < 0.001.
Fig. 3.
Distribution of QTc intervals during hospitalization according to drug and days of treatment. Numbers in solid boxes are median and IQR (25%-75%) and p-values.
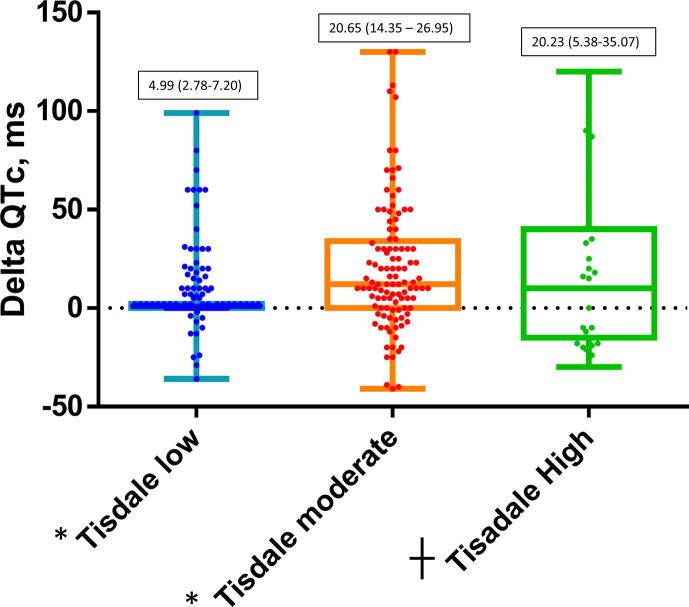
As shown in Fig. 4, patients with moderate and high Tisdale score had a higher QTc variation than those with a low Tisdale score suggesting the ability of the score to predict significant QTc variations also in COVID-19 patients.
Fig. 4.
Distribution of QTc intervals during hospitalization according to Tisdale score cathegory (low ≤ 6, moderate 7–10, high ≥ 11). Numbers in solid boxes are median and IQR (25%-75%). * p < 0.001; ┼ p < 0.01.
On a multivariate analysis (table 3) the use of meropenem (HR 5.2; 95% CI 1.2–21.2; p = 0.023) and the Tisdale Score (HR 1.4; 95% CI 1.1 – 1.7; p = 0.010) were significant predictors of QTc prolongation ≥ 500 msec.
Table 3.
Predictors of QTc prolongation ≥ 500 msec (20/196 patients; 10.2%).
| p-value | OR | 95% CI | |
|---|---|---|---|
| Age | 0.117 | 1.046 | 0.989–1.107 |
| >1 QTc prolonging drug | 0.503 | 0.770 | 0.359–1.653 |
| Meropenem | 0.023 | 5.156 | 1.252–21.243 |
| Piperacilline/Tazobactam | 0.497 | 1.578 | 0.423–5.886 |
| Tisdale Score | 0.011 | 1.361 | 1.074–1.726 |
| First QTc > 450 msec | 0.925 | 1.064 | 0.292–3.874 |
| Antiarrhytmic Drugs therapy | 0.178 | 1.501 | 0.831–2.713 |
| History of Cardiovascular Disease | 0.517 | 1.473 | 0.456–4.762 |
| ECG signs of ischemia | 0.255 | 2.430 | 0.527–11.206 |
| Electrolytes abnormalities | 0.910 | 0.193 | 4.330 |
In our population 27 patients died because of COVID-19 (13.7%), 9 experienced new-onset atrial fibrillation/flutter (4.6%), 10 patients experienced a DVT (5.10%) and 5 patients a PE (2.5%), no-one experienced documented ventricular arrhythmias, MI or Takotsubo syndrome, and only two patients (1.02%) died of sudden death: one was a patient with valvular cardiomyopathy and moderate reduction of ejection fraction, admitted to the ED for syncope while laying down, normal QTc at admission and on the day of death - the cause of death unlikely related to treatment. The other one was a patient with advanced cancer with bradyarrhythmia undergoing evaluation for pacemaker placement, QTc was normal, and he is likely to have died of asystole.
Mortality rate and adverse events resulted to be numerically higher in patients with QTc ≥ 500 ms (Table 4) and Delta QTc ≥ 60 ms (Supplementary Table 3) although without being statistically significant. Conversely, patients with a low Tisdale score presented a lower in-hospital mortality rate compared with those patients with a moderate-high Tisdale score (5.4% vs 18.6%; p = 0.019, supplementary Table 4).
Table 4.
In-hospital adverse events and outcome according to QTc value.
| Overall (n 196) | QTc < 500 ms (n 176) | QTc ≥ 500 ms (n 20) | p value | |
|---|---|---|---|---|
| In-Hospital Death | 29 (14.8%) | 24 (13.6%) | 5 (25%) | 0.175 |
| At least one adverse event* | 53 (27.0%) | 44 (25.0%) | 9 (45.0%) | 0.053 |
Cumulative incidence of: Death for COVID-19; Sudden-death; Torsade de Pointes (TdP); AF/Flutter; Ventricular fibrillation; deep vein thrombosis (DVT); pulmonary embolism (PE); MI; Takotsubo; Vascular Thrombosis.
4. Discussion
The results of our analysis on an unbiased population of confirmed or highly suspected COVID-19 patients treated in a non-ICU setting, give us various insights on the aspect of QTc monitoring and arrhythmia risk in this special population. First of all, in our cohort the number of patients with a warning QTc ≥ 500 ms was lower than reported in other papers [9], [11], despite an intensive treatment with various QTc prolonging drugs (HCQ, azithromycin and antivirals) sometimes used together. Our results are in line with those of Saleh et al. study in which the management of COVID-19 patients at risk of QT prolongation appear to be similar to ours even if they did not specify the number of patients managed in ICU [11].
Another important result is the fact that crosstables analysis show that the most important determinants of QTc prolongation could be not just the drugs themselves, but also the severity of the disease and the cardiovascular system status. Surprisingly, in fact, the variables which significantly correlated with a QTc prolongation ≥ 500 ms in this analysis were the history of cardiovascular disease, antiarrhythmic drug use, the use of powerful broad-spectrum antibiotics – meropenem and piperacillin-tazobactam, markers of concomitant/superimposed infections aggravating COVID-19 disease, ischemic alterations on ECG, and the examinations assessing the severity of COVID-19 such as D-Dimer, BNP, Procalcitonin, O2 saturation. This could be similar to what happens in QTc prolongation in the context of an acute coronary syndrome and in other cardiac injury conditions like Takotsubo syndrome [15], where myocardial injury is also responsible for QTc prolongation. Another hypothesis to explain the interaction between potent antibiotics and QT interval could be related to unpredictable drug interactions leading to pharmacodynamics alterations potentially able to explain QT interval prolongation. This hypothesis should be reassessed in future pharmacological studies.
Unluckily, a major bias in demonstrating this mechanism in our COVID-19 population was the lack of systematic echocardiographic examination. Due to the pandemic urgency and the limited availability of ultrasound machines dedicated to COVID-19 wards, it is not possible to correlate echocardiographic data with QT prolongation. The relationship among QT interval, myocardial injury and echo parameters could be a topic of interest for future studies on cardiovascular complications of COVID-19 infection. Moreover, high sensitivity troponin was not systematically measured. In fact, troponin was tested in the emergency department only and repeated just in case of clinical manifestation suggestive of ischemia based on the judgment of the treating physicians.
Concerning the 10.2% of patients with significant QTc prolongation despite a broad use of drugs against COVID-19 infection, our major hypothesis is that differently from previous studies reporting high percentages of QTc increase, in our population all people necessitating ICU stay were excluded. Patients in ICU could show a significant increase of QTc because of various reasons, the most important ones could be the severity of COVID-19 infection with a supposed higher frequency of cardiovascular involvement in a myocarditis-like fashion, and the off-label administration of crashed HCQ leading to a higher plasmatic peak and higher incidence of QT prolongation. Since in our analysis the markers of severity of COVID-19 represent the most important determinants of QTc prolongation, we expect that in the ICU population these factors could be greatly magnified. Notably, since the magnitude of QTc prolongation in COVID-19 patients not treated with any of the accused QTc prolonging drugs has never been analysed, we cannot ascertain to which extent the effect is due to the patient’s condition or the drugs themselves.
Another important result of our analysis is the absence of suspected deaths due to fatal arrhythmias associated with QTc prolongation. This is the most important finding of our study because it represents the main concern when using these drugs. Uncertainty remains to what extent it is to be attributed to a low proarrhythmic effect of the drugs or the result of the clinician’s interventions in order to carefully manage these patients (e.g. with ECG and electrolyte monitoring). Concerning the risk of turning QTc prolongation in life-threatening arrhythmias, it has to be considered that, for the same degree of QTc prolongation, different drugs could generate various clinical risk; it is the case, for example, of Sotalol and Amiodarone, both potential QTc-prolonging antiarrhythmic drugs, whose proarrhythmic risk differs for their diverse effect on the dispersion of repolarization, a phenomenon which is not visible on surface ECG and for the potent calcium channel block effect of amiodarone. Accordingly, with drugs used to treat COVID-19, we do not exactly know what is the potential proarrhythmic effect beyond the measured QTc.
Moreover, the Tisdale score, a tool developed to predict the drug induced QTc prolongation was able to predict the QTc ≥ 500 msec in our population. This observation could be of clinical relevance and the calculation of Tisdale score could be adopted to identify patients at major risk of developing a QTc prolongation even among those treated for COVID-19 infection.
Finally, during the drafting of our paper the WHO decided to suspend the HCQ arm of the SOLIDARITY trial waiting for an interim analysis of the data safety monitoring board based on the results of a large retrospective study from Mehra et al. However, an open letter from Watson J. et al published on Zenodo in May the 28th and signed by>120 researchers from all around the word has placed many doubts on methods and results of Mehra et al. retrospective study [16], in particular accusing Surgisphere, the private society owners of the data, of serious shortcomings in the management of the study. As a result, on June the 4th, Mehra et al retracted the Lancet paper and a previous study published on New England Journal of Medicine. In our opinion, all the studies which specifically evaluated the impact of QT on the safety of COVID-19 experimental drugs showed a limited number of adverse events and this should be taken into account in order to avoid further premature interruption of clinical trials potentially capable to find a disease modifying therapy.
5. Conclusions
In our population of 196 COVID-19 confirmed or highly suspected patients treated in a non-ICU setting with combinations of HCQ, azithromycin and/or antivirals, a QTc prolongation over 500 ms was observed in 10.2% of patients, and no fatal arrhythmias due to QTc prolongation has been observed. Tisdale score can help in predicting QTc prolongation. Our study, despite retrospective, suggests that current experimental therapies on COVID-19 have an acceptable cardiovascular profile. The numerous ongoing RCTs will give us a definitive answer on the safety and efficacy of COVID-19 experimental drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Therapeutic Options Under Investigation | Coronavirus Disease COVID-19. COVID-19 Treatment Guidelines https://www.covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/.
- 2.Chen Jun, L. D. & Chen Jun, L. D. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. J Zhejiang Univ (Med Sci) 49, 215–219 (2020). [DOI] [PMC free article] [PubMed]
- 3.Gautret P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Geleris J. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. New England Journal of Medicine. 2020:null. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao B. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. New England Journal of Medicine. 2020:null. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung I.F.-N. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. The Lancet. 2020;S0140673620310424 doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SIC-Covid-e-QT.pdf.
- 8.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance, https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance.
- 9.Mercuro N.J. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleh Moussa et al. The Effect of Chloroquine, Hydroxychloroquine and Azithromycin on the Corrected QT Interval in Patients with SARS-CoV-2 Infection. Circulation: Arrhythmia and Electrophysiology 0,. [DOI] [PMC free article] [PubMed]
- 11.Bessière F. Assessment of QT Intervals in a Case Series of Patients With Coronavirus Disease 2019 (COVID-19) Infection Treated With Hydroxychloroquine Alone or in Combination With Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogossian H. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm. 2014;11:2273–2277. doi: 10.1016/j.hrthm.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Yankelson L. New formula for defining ‘normal’ and ‘prolonged’ QT in patients with bundle branch block. J Electrocardiol. 2018;51:481–486. doi: 10.1016/j.jelectrocard.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 14.Tisdale J.E. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tf I. QT Prolongation and Clinical Outcomes in Patients With Takotsubo Cardiomyopathy. Pacing and clinical electrophysiology : PACE. 2016;vol. 39 doi: 10.1111/pace.12864. [DOI] [PubMed] [Google Scholar]
- 16.James Watson on the behalf of 146 signatories. An open letter to Mehra et al and The Lancet. https://zenodo.org/record/3864691#.XtV6YTozbIV (2020) doi:10.5281/zenodo.3864691.