Abstract
Background and aims
Cardiovascular disease is the main cause of death worldwide, but the collective efforts to prevent this pathological condition are directed exclusively to individuals at higher risk due to hypercholesterolemia, hypertension, obesity, diabetes. Recently, vitamin D deficiency was identified as a risk factor for cardiovascular disease in healthy people, as it predisposes to different vascular dysfunctions that can result in plaque development and fragility. In this scenario, the fundamental aim of the study was to reproduce a disease model inducing vitamin D deficiency and atheromatosis in ApoE−/- mice and then to evaluate the impact of this vitamin D status on the onset/progression of atheromatosis, focusing on plaque formation and instability.
Methods and results
In our murine disease model, vitamin D deficiency was achieved by 3 weeks of vitamin D deficient diet along with intraperitoneal paricalcitol injections, while atheromatosis by western-type diet administration. Under these experimental conditions, vitamin D deficient mice developed more unstable atheromatous plaques with reduced or absent fibrotic cap. Since calcium and phosphorus metabolism and also cholesterol and triglycerides systemic concentration were not affected by vitamin D level, our results highlighted the role of vitamin D deficiency in the formation/instability of atheromatous plaque and, although further studies are needed, suggested a possible intervention with vitamin D to prevent or delay the atheromatous disease.
Conclusions
The data obtained open the question about the potential role of the vitamins in the pharmacological treatments of cardiovascular disorders as coadjutant of the primary drugs used for these pathologies.
Keywords: Vitamin D, Inflammation, Atheromatosis, Cardiovascular disease
Graphical abstract
Highlights
-
•
The vitamin D deficiency represents a risk factor for cardiovascular disease.
-
•
The vitamin D deficiency has been correlated to atheromatosis progression.
-
•
A model of vitamin D deficiency was crucial to evaluate the role of the vitamin D the onset/progression of atheromatosis.
-
•
The vitamin D deficiency was clearly involved in an early formation of atherosclerotic lesions.
-
•
The vitamins could play a potential role in the pharmacological treatments of cardiovascular disorders.
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide [1] and in chronic kidney disease patients the risk of mortality from CVD is 15 times higher than in the general population [2].
Atheromatosis, a chronic inflammation of the arterial wall that begins with endothelial damage, is caused by several classic risk factors such as smoking, hypertension, dyslipidemia and diabetes but also by anemia and secondary hyperparathyroidism in patients with chronic renal failure [2]. In particular, in recent literature, vitamin D (VD) deficiency [25-hydroxy vitamin D (25(OH)D) serum level <15 ng/ml] is recognized as a risk factor for CV lesions in healthy people [[3], [4], [5], [6], [7]] and a predictor of CV mortality in the chronic kidney disease patients [8,9]. VD deficiency, both in the population with normal kidney function or in patients with chronic kidney disease, promotes the increase of the arterial adventitial vasa vasorum, as well as systemic inflammation, inflammatory vascular infiltration and foam cell formation that lead to plaque development and instability [[10], [11], [12]]. Therefore, correction of VD deficiency in health and nutritional/active VD restoring in chronic kidney disease could prevent the onset or slow the progression of atheromatous disease. Indeed, not only VD deficiency increases vascular calcification through the elevation of serum PTH [8] and PTH-driven mineral/skeletal abnormalities, causing ectopic calcium deposition [9], but more significantly it is associated to atheromatous disease [[10], [11], [12], [13]]. During the atheromatous process, the increase in adventitial vasa vasorum promotes endothelial dysfunction (early stage) [14,15] and plaque instability (advanced stage) [[16], [17], [18]]. It is important to note that the active form of VD was reported to suppress neo-angiogenesis and endothelial cell proliferation [19] and to counteract the increase/activation of the TNF-α Converting Enzyme [20]. In this context, by using a murine model of atheromatosis (ApoE−/− mice), the main goal of this study was to delineate the cause/effect link between systemic VD status, arterial wall thickening and plaque formation/instability in the mouse model. We hypothesize that low VD levels could negatively work in our dyslipidemic model, worsening the atheromatous disease's evolution. These studies could also contribute to identifying key therapeutic targets and optimal VD balance to prevent the onset and progression of atheromatosis.
Currently, an association has been established between VD deficiency, along with other comorbidities such as CVD, and risk of severe COVID-19 events [21], making even more important and imperative to clarify the real involvement of VD in atheromatous disease.
Methods
Animals and experimental design
Murine model of atheromatosis, endogenous murine Apolipoprotein E knockout (ApoE−/-) mice (Envigo RMS Srl., Italy), aged 5–7 weeks, with normal kidney function and weight of 20–27 g at the beginning of the study were used. The mice underwent one-week acclimatization to the animal facility before the induction of VD deficiency. The mice had free access to tap water and pellet food and were housed in standard cages with a 12 h light/dark cycle. Environmental temperature was constantly maintained at 21 °C and the mice were kept under pathogen-free conditions. All experiments were carried out according to Italians laws (Ministry of Health registration n 637/2016-PR, March 24, 2016) and complied with the Guidelines for Care and Use of Laboratory Animals at the University of Ferrara. From day one of the experiment, the mice were divided into two groups of 40 animals each: control mice and VD-deficient mice. VD deficiency was achieved by accelerating the impact of a VD deficient diet (3 weeks) by enhancing endogenous 25(OH)D degradation through intraperitoneal injections of paricalcitol (32 ng twice weekly for 2 weeks), an inducer of 25(OH)D catabolism. Calcium (2%) was supplied to attenuate secondary hyperparathyroidism [20,22]. Upon reaching VD deficiency, both control mice group and VD-deficient mice group were fed with a western-type diet (1.25% cholesterol, 16.5% fat) with water ad libitum. Only the control mice group's diet was supplemented with VD (360 ng/week) for the entire duration of the experiment. Ten mice of each group were sacrificed at 4, 8, 15 and 20 weeks to assess plaque formation, increase and composition. At sacrifice, blood was drawn, and serum was collected to perform biochemical analysis and assess systemic metabolic markers.
Serum biochemical analysis
For the biochemical analysis, blood was collected and centrifuged at 700 g for 10 min at 4 °C to obtain serum. Sera were then stored at 80 °C until analysis. Calcium (Ca2+) and phosphorus (PO4 3−) levels were evaluated by standard colorimetric analysis using the QuantiChrom™ Calcium assay kit and the QuantiChrom™ Phosphate assay kit (both from Bioassay Systems, Hayward, CA) respectively. A specific enzyme immunoassay (EIA, Immunodiagnostic Systems, The Boldons, UK) was used to determine the 25(OH)D levels. PTH serum levels were evaluated using the MicroVue Mouse PTH 1–84 ELISA kit (Quidel Corporation, Athens, USA). PTH values were expressed in pg/ml. All the kits were used according to the manufacturer's instructions.
Determination of the systemic metabolic markers: cholesterol and triglycerides levels
The amount of cholesterol (both high-density lipoprotein, HDL and low-density lipoprotein, LDL) and the triglycerides' levels were determined using specific quantitation kits (all from Sigma–Aldrich, St. Louis, MO), performing the experimental procedures according to the manufacturer's instructions. The amount of cholesterol were expressed in ng/μL while triglycerides in mg/dL.
Histological analysis
For the histological analyses, samples were fixed in formalin 10% for 24 h at 4°C and subsequently rinsed in several changes of cold 70% ethanol. The tissues were dehydrated through an alcohol series and then paraffin-embedded using a Shandon Citadel 2000 Tissue Processor (Thermo Fisher Scientific, Waltham, MA). After blocking out, 5 μm thick sections were cut and stained with Azan trichrome (Bio Optica Milano Spa, Milan, Italy) and used for the immunohistochemistry analysis after staining with the anti-α-Smooth Muscle Actin (α-SMA) antibody (Sigma–Aldrich) and the anti-mouse HRP-DAB tissue staining kit (R&D Systems, Minneapolis, MN). A negative control was obtained in each slide by carrying out the immunohistochemistry staining procedure without using the primary antibody. The images were acquired with an Aperio ScanScope® slide scanner by using the Aperio ImageScope v11.1.2.760 software (Leica Biosystems, Nussloch, Germany). Quantification was determined with the ImageJ software.
Statistical analysis
Data will be expressed as means ± standard deviation (SD). Statistical significance was calculated using one-way analysis of variance (ANOVA) and Bonferroni post-test or the unpaired t-test, followed by the Mann–Whitney correction. Statistical significance was set at p < 0.05, p < 0.01, and p < 0.001. Data analyses were performed using GraphPadPrism and Stata IC 11 softwares.
Results
Impact of VD deficiency on body weight
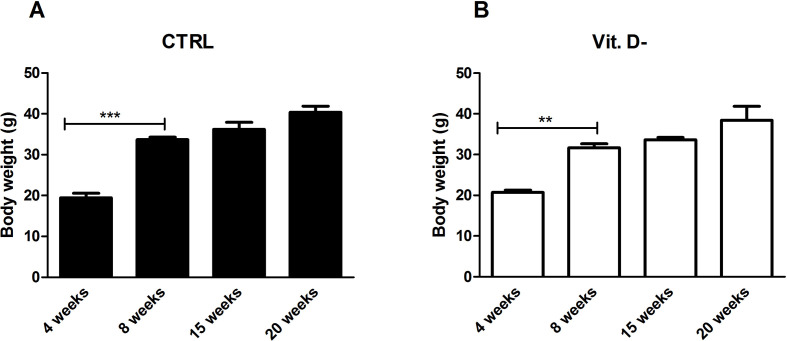
In order to make correct observations, free from any confounding factors derived from the potential heterogeneity of the study groups, the weights of all animals in this study were analyzed before the sacrifice, assuming that the initial weight of all the subjects at the beginning of the experiments was comparable and showed no significant statistical differences (Fig. 1S, Supplementary Material).
The weights of the mice in the control group compared to VD deficient group showed the same trend in both conditions and of all time points (4, 8, 15 and 20 weeks), as presented in Fig. 1S (Supplementary Material), and, as indicated in Table 1 , did not reveal any statistically significant difference for each time point that was analyzed.
Table 1.
Weights of animal model in the two groups: control (a) and VD deficient (b) mice.
| Animal weight (g) | |||
|---|---|---|---|
| GROUPS WEEKS |
Controls (a)a | Vit. D- (b)a | p value (a) vs (b) |
| 4 weeks | 19.49 ± 2.15 | 20.75 ± 1.14 | Ns |
| 8 weeks | 33.70 ± 1.26 | 31.68 ± 1.92 | Ns |
| 15 weeks | 36.23 ± 2.92 | 33.65 ± 1.12 | Ns |
| 20 weeks | 40.38 ± 3.00 | 38.43 ± 5.94 | Ns |
Data are reported as mean ± standard deviation of the weights of the ten animals of each group. Ns: not significant.
Induction of VD deficiency in ApoE−/− mice
In order to establish the 25(OH)D serum level in the mouse model, this parameter was measured at sacrifice at all time points: 4, 8, 15 and 20 weeks.
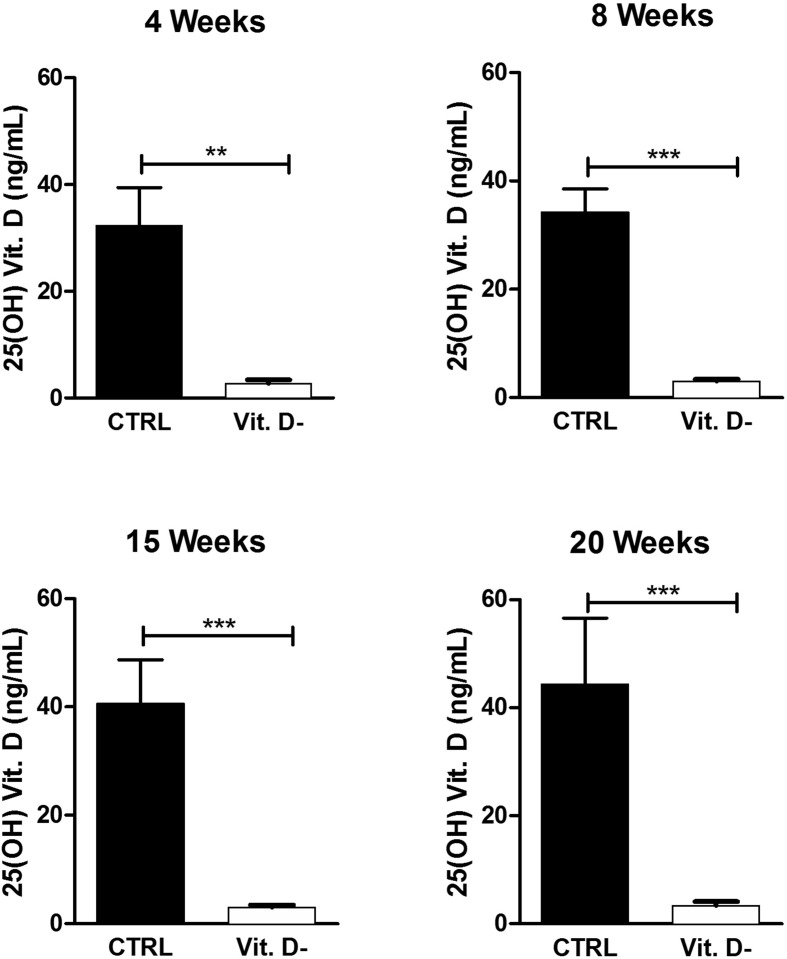
In all experimental conditions, as illustrated in Fig. 1 , the comparison between controls and VD deficient (Vit. D-) mouse samples showed a statistically significant difference.
Figure 1.
Evaluation of serum 25(OH)D levels in the control group (CTRL) and in the VD deficient mice group (Vit. D-). The data are reported as mean ± standard deviation of the 25(OH)D levels measured in duplicate. Statistical significance was evaluated using the unpaired t-test, followed by the Mann–Whitney correction. ∗∗p < 0.01 and ∗∗∗p < 0.001.
25(OH)VD serum levels in VD deficient mice were very close to the lower detection threshold and were indicative of a clear VD deficient status. Therefore, these data demonstrated that the experimental mouse model could reproduce an effective VD deficiency at all time points analyzed in the study.
Development of atheromas in VD deficient mice
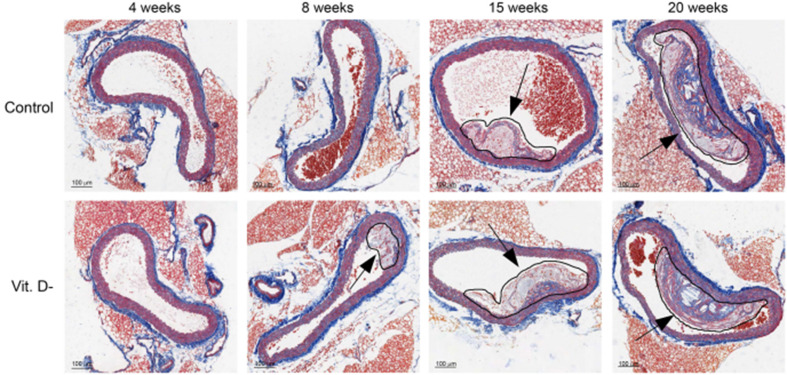
The morphological analysis achieved using the Azan trichrome staining revealed the presence of lipid plaques along the aorta arteries. As shown in Fig. 2 , lesions at various stages of development could be observed in both groups of mice, but in VD deficient animals the presence of plaques might be detectable as early as 8 weeks after ingesting high fat diet.
Figure 2.
Temporal dynamics of aorta plaques. Representative dynamic profiles of atherosclerotic plaques in Control and VD deficient (Vit. D-) mice (10× magnification). The circled regions and the arrows indicate atherosclerotic plaques.
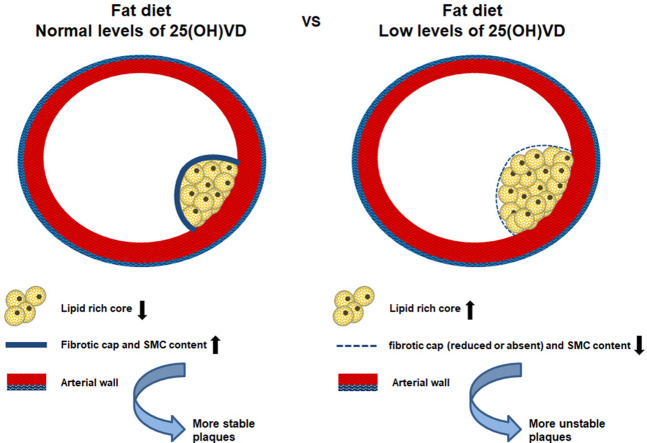
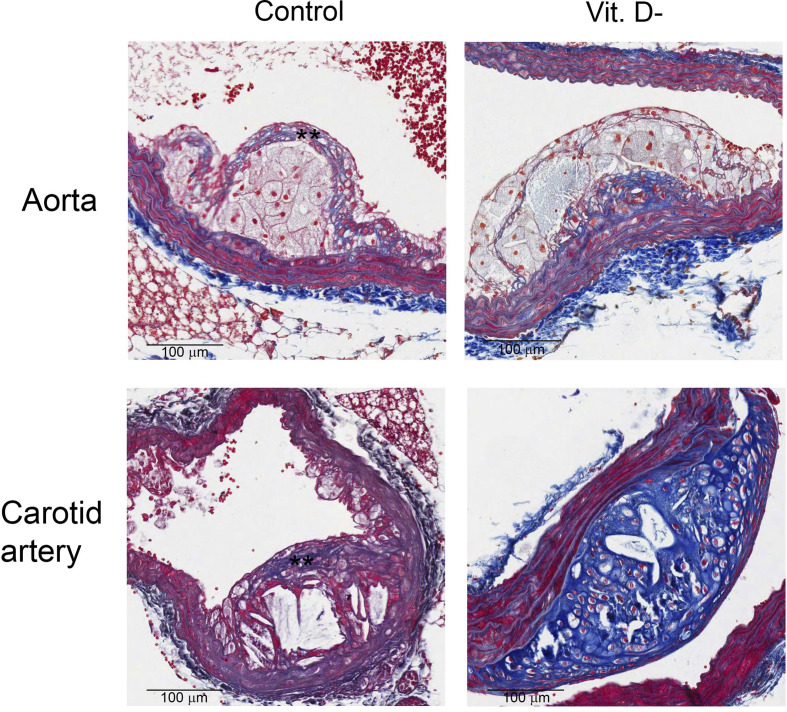
Of note, although plaques was observed in both groups at 15 and 20 weeks, only animals with normal levels of 25(OH)VD showed more stable plaques associated with the formation of a fibrotic cap (Figure 2, Figure 3 ). On the contrary, the plaques in aortas of VD deficient mice presented larger lipid-rich cores, increased collagen content and, when present, a reduced cap thickness. The same results were also obtained by analyzing carotid arteries; indeed the animals with normal levels of 25(OH)D showed a fibrotic cap that indicates a more stability of the plaque (Fig. 3). Besides, a first analysis of the carotid arteries revealed that plaques had a chondrocytic-like phenotype in VD deficient animals (Fig. 3), which is known to promote plaque calcification.
Figure 3.
Effect of VD deficiency on aortic and carotid plaques morphology. Representative images of atherosclerotic plaques in Control and VD deficient (Vit. D-) groups (20× magnification). Samples were collected 15 weeks after ingestion of fat diet. ∗∗indicates fibrotic cap.
In order to evaluate the role of the fibrogenesis in atheromatosis, the levels of α-SMA, the predominant actin of vascular smooth muscle cells (SMC), were detected by immunochemical staining.
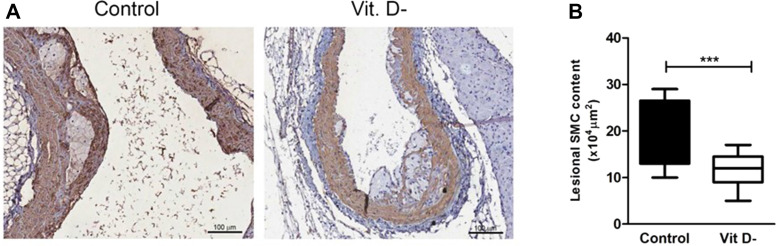
As shown in Fig. 4 A, lesional SMC content, measured as SMC positive areas, was significantly reduced in the fibrous cap of VD deficient mice leading to vulnerability and instability of the plaque, also confirmed by the presence of plaque rupture (Fig. 4B).
Figure 4.
Representative positive α-SMA immunostaining in aortic lesions. (A) α-SMA immunochemical staining of aortic tissue in Control and VD deficient (Vit. D-) groups. α-SMA positive areas indicate smooth muscle cells (10× magnification). (B) Quantification of α-SMA positive cells in aortic plaques (n = 13 per group; ∗∗∗p < 0.001).
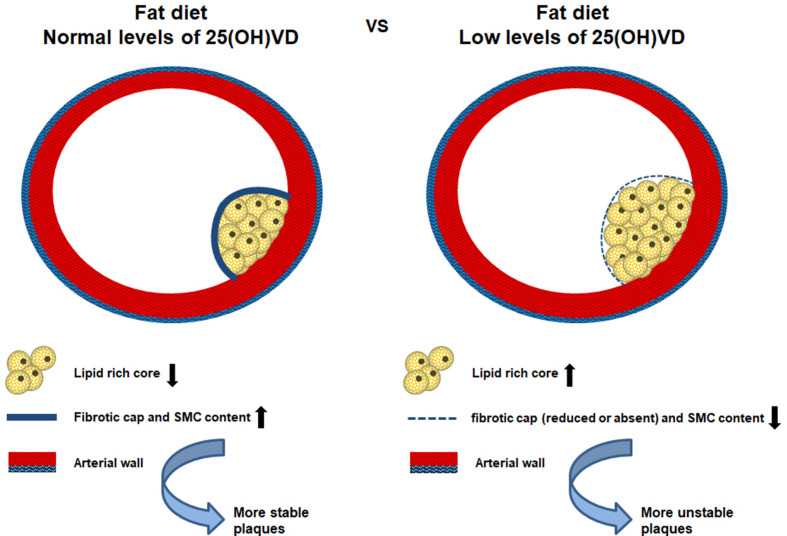
These results supported the involvement of VD in the plaque composition and stability (schematically represented in Fig. 5 ).
Figure 5.
Schematic representation of Vitamin D deficiency effects on the plaque formation and instability.
Impact of calcium and phosphorus balance on plaques formation
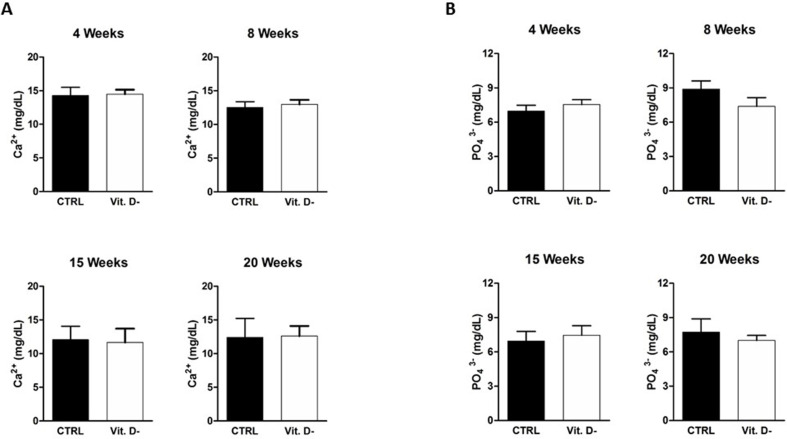
As serum 25(OH)D levels showed an homogeneous trend in the experimental design, suggesting their direct involvement in the plaque development process, it was necessary to verify if other biochemical parameters could affect the atheroma formation.
First of all, the main metabolic bone parameters, particularly the calcium (Ca2+) and the phosphorus (PO4 3−) serum levels, were measured in control and in VD deficient mice groups.
As shown in Fig. 2S (Supplementary Materials), the VD deficiency in the mouse model was not correlated with dysregulated calcium (Fig. 2S-A, Supplementary Materials) or phosphorus (Fig. 2S-B, Supplementary Materials) levels in comparison to physiological condition.
In particular, it was relevant to highlight that no statistically significant increase of phosphorus level was detected in both conditions and in all time points, although literature data showed a role of the phosphorus in the atheroma formation [23].
Evaluation of cholesterol, triglyceride and PTH serum levels in VD deficient mice
After excluding that the calcium-phosphorus balance could play a role in the atheroma formation, it was crucial to ascertain the possible involvement of the serum lipidic component (in particular triglyceride and cholesterol) in the plaque development.
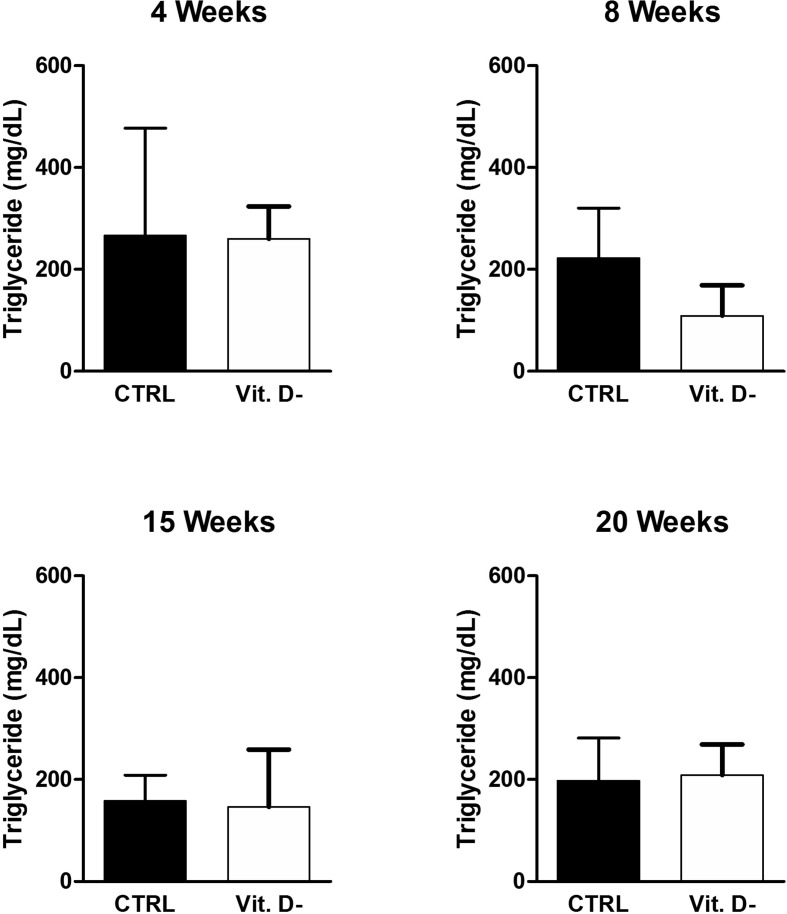
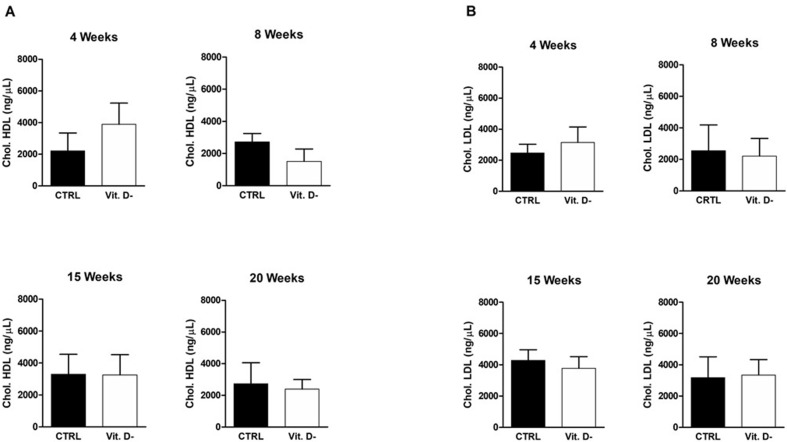
According to the literature data, no difference in triglyceride (Fig. 6 ) and cholesterol serum levels (Fig. 7 ) was observed in this animal model in control mice with respect to VD deficient group. This result was in contrast to the only article that associates low VD levels with high triglyceride levels in a Spanish population of children between 8 and 13 years old [24].
Figure 6.
Evaluation of serum triglyceride levels in the control group (CTRL) and in the VD deficient mice group (Vit. D-) at the different indicated time points. The data are reported as mean ± standard deviation of the serum triglyceride levels measured in duplicate. Statistical significance was evaluated using the unpaired t-test, followed by the Mann–Whitney correction.
Figure 7.
Evaluation of serum cholesterol levels in the control group (CTRL) and in the VD deficient mice group (Vit. D-) at the different indicated time points. The figure shows the two components of the cholesterol: in A, HDL levels are shown; in B, LDL levels are shown. The data are reported as mean ± standard deviation of the serum cholesterol levels measured in duplicate. Statistical significance was evaluated using the unpaired t-test, followed by the Mann–Whitney correction.
Similarly, the evaluation of PTH serum levels revealed no significant difference between the control group and the VD deficient one. As shown in Fig. 8 , this result was obtained both at 8 weeks after ingestion of fat diet, when was already possible to detect the plaque formation in aortas of VD deficient mice, and at 20 weeks, indicating that also at the end of the study VD deficient group did not incur in secondary hyperparathyroidism.
Figure 8.
Evaluation of PTH levels in the control group (CTRL) and in the VD deficient mice group (Vit. D-) at 8 and 20 weeks. The data are reported as mean ± standard deviation of the PTH levels measured in duplicate. Statistical significance was evaluated using the unpaired t-test, followed by the Mann–Whitney correction.
Discussion and conclusions
The pleiotropic role of VD in the pathogenesis of cardiovascular diseases has helped to consider the level of this vitamin crucial for the development of atheromatosis. The main systemic manifestation of cardiovascular disease, indeed, is represented by the atheroma (or atherosclerotic plaque) that is a thickening of the innermost layer (intimate component) of the arteries, due to the accumulation of different components, as lipid material, calcium and connective tissue [25,26].
In this context, it was of fundamental importance to reproduce in ApoE−/- mice a model of VD deficiency disease that leads to the development of atheromatosis and therefore allow us to investigate the impact of VD deficiency on the onset/progression of the disease, trying to outline and characterize the relationship between VD and atheromatosis. The adequacy of the mouse model was demonstrated by the deeply low 25(OH)D serum levels in the VD deficient mice group compared to the control animals, accelerated, in addition to the appropriate fat diet, by the treatment with paricalcitol, which improves endogenous VD degradation through the induction of 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) [[25], [26], [27], [28]].
As expected, as a consequence of the fat diet, morphological analyses of the aorta sections revealed the presence of lipid plaques in various development stages in both groups of mice, but in the VD deficient group the onset of plaques has already started from 8 weeks after ingestion of fat diet. Furthermore, the observation of the plaques found both in the aorta and in the carotid arteries showed different stability, correlated to the association with a fibrotic cap.
As described by Naghavi M. et al. [29] the instability of atheromatous plaques is defined by some criteria such as the presence of a large lipid core and a thin fibrous cap. The histological analysis revealed a marked difference of atherosclerotic plaques morphology between controls and VD deficient groups, showing that in VD deficient group the presence of a rich lipid core was associated with a thin fibrous cap. Moreover, the quantitative analyses of α-SMA, the predominant actin of vascular smooth muscle cells, indicated that the number of smooth muscle cells was significantly decreased in the fibrous cap of the VD deficient group compared to the control group. This observation was very interesting because, as reported, smooth muscle cells are fundamental constituents of the plaques involved in their stability [30]. Therefore, our results suggested that low levels of 25(OH)D are involved in an early formation of atherosclerotic lesions and in an increased plaque instability and vulnerability.
Of note, in our VD deficiency disease model, metabolism of calcium and phosphorus, which are known to increase the kinetics of atheromatosis formation, was not dysregulated compared to the physiological condition, excluding their role in the pathogenesis of plaques. It was also significant to confirm that, at the systemic level, cholesterol and triglycerides concentrations were not affected by the VD deficiency condition. The exclusion of the involvement of the main systemic metabolic markers, as calcium, phosphorus and lipid components, from the plaque development process was crucial to highlight the importance of the role played by 25(OH)D levels in this mechanism and then in the progression of the cardiovascular diseases.
The results obtained in this study agreed with other studies carried out on other animal models which underlined the fundamental role of other vitamins such as vitamin C and K in the stabilization of plaque: these vitamins help stabilize atherosclerotic plaque after angioplasty and favor vascular remodeling by increasing collagen content modulating the cholesterol levels [31,32]. This evidence in different cardiovascular disease models open questions about the potential role of the vitamins in the pharmacological treatments of cardiovascular disorders as coadjutant of the main drugs, such as statins, used for these pathologies for their well-known ability to modulate cholesterol levels.
Libby P. et al. described the statin's lipid-independent effect that contributes to the plaque stability positively altering the vascular biology [33]. The central hypothesis about this evidence is that the statins induced alterations in the function of small G proteins, contributing to an anti-inflammatory and antithrombotic effect [34]. Moreover, recent studies highlighted the importance of statins in concurring to reduce the cardiovascular events just for their ability to considerably reduce the plaque lipid-rich core and to promote the fibrous cap thickening [35,36].
A clear view and a more in-depth knowledge of the mechanism and features involved in the morphogenesis of the atheromatosis [37,38] is necessary for preclinical and clinical testing of new agents or combination of treatments expected to facilitate atherosclerosis regression and to guide the design of new therapeutic strategies aiming to modulate the plaque progression and regression by targeting its molecular mechanism.
Literature data show several evidences that chronic diseases, as cardiovascular diseases, high blood pressure and diabetes are closely related to low plasmatic level of vitamin D [25,26]. It is well known that the dominant function of VD is the elevation of plasma level of calcium and phosphate, playing a fundamental role associated to mineral homeostasis. The VD receptor is present in several cell types, thus the knowledge of the mechanism of action of the active VD, mediated by its receptor as transcription factor, could explain the multiple actions on different tissues. Indeed, vitamin D plays a role in the regulation of the innate and adaptive immune systems, shows preventive effects on cardiovascular and neurodegenerative diseases and even anti-aging effects [39].
In this scenario, our results suggest a novel potential role played by VD as a therapeutic treatment in association to the specific drugs or as an effective tool for the prevention of cardiovascular pathologies acting as a coadjutant of vascular homeostasis [40,41].
This research could be of considerable importance in a perspective of prevention and potential reduction of the cardiovascular events onset with a direct reduction in health assistance costs and with an improvement in quality of life not only for patients, as in the chronic kidney failure, but for the general population. In this respect our study could be of interest also in light of recent evidence supporting the hypothesis that VD deficiency, together with the manifestation of the comorbidities as cardiovascular disease, diabetes, metabolic syndrome, could increase the risk to develop severe COVID-19 events [42].
Funding
This study was supported by the Italian Ministry of Health (no. 43/GR-2013-02358192).
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank dr. Martina Bradaschia for the English revision of the manuscript.
Handling Editor: D. Noto
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2020.08.031.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figure 1S.
Analysis of animals' weights in the control group (CTRL) (A) and in the VD deficient group (Vit. D-) (B). The data are reported as mean ± standard deviation of the weights of the ten animals of the group. Statistical significance was evaluated using the One Way Anova test followed by the Bonferroni correction for multiple comparisons; ∗∗p < 0.01 and ∗∗∗p < 0.001.
Figure 2S.
Analysis of serum calcium (Ca2+) (A) and phosphorus (PO43−) (B) levels in the control group (CTRL) and in the VD deficient mice group (Vit. D-). The data are reported as mean ± standard deviation of the serum calcium and phosphorus levels measured in duplicate. Statistical significance was evaluated using the unpaired t-test, followed by the Mann–Whitney correction.
References
- 1.Beaglehole R., Bonina R. Global public health: a scorecard. Lancet. 2008;372:1988–1996. doi: 10.1016/S0140-6736(08)61558-5. [DOI] [PubMed] [Google Scholar]
- 2.Foley R.N., Parfrey P.S., Sarnak M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 3.Melamed M.L., Michos E.D., Post W., Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.H., O'Keefe J.H., Bell D., Hensrud D.D., Holick M.F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Andress D.L. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69(1):33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- 6.Melamed M.L., Astor B., Michos E.D., Hostetter T.H., Powe N.R., Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20:2631–2639. doi: 10.1681/ASN.2009030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf M., Shah A., Gutierrez O., Ankers E., Monroy M., Tamez H. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 8.Martinez I., Saracho R., Montenegro J., Llach F. A deficit of calcitriol synthesis may not be the initial factor in the pathogenesis of secondary hyperparathyroidism. Nephrol Dial Transplant. 1996;11(Suppl 3):22–28. doi: 10.1093/ndt/11.supp3.22. [DOI] [PubMed] [Google Scholar]
- 9.Moe S.M., Drüeke T. Improving global outcomes in mineral and bone disorders. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S127–S130. doi: 10.2215/CJN.04331206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockau L., Atkinson S.A. Vitamin D's role in health and disease: how does the present inform our understanding of the past? Int. J. Paleopathol. 2018;23:6–14. doi: 10.1016/j.ijpp.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Gluba-Brzózka A., Franczyk B., Ciałkowska-Rysz A., Olszewski R., Rysz J. Impact of vitamin D on the cardiovascular system in advanced chronic kidney disease (CKD) and dialysis patients. Nutrients. 2018;10(6):E709. doi: 10.3390/nu10060709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junarta J., Jha V., Banerjee D. Insight into the impact of vitamin D on cardiovascular outcomes in chronic kidney disease. Nephrology. 2019;24(8) doi: 10.1111/nep.13569. 7, 1-790. [DOI] [PubMed] [Google Scholar]
- 13.Ellam T., Hameed A., ul Haque R., Muthana M., Wilkie M., Francis S.E. Vitamin D deficiency and exogenous vitamin D excess similarly increase diffuse atherosclerotic calcification in apolipoprotein E knockout mice. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0088767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann J., Lerman L.O., Rodriguez-Porcel M., Holmes D.R., Jr., Richardson D.M., Ritman E.L. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 15.Moulton K.S., Heller E., Konerding M.A., Flynn E., Palinski W., Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 16.Fleiner M., Kummer M., Mirlacher M., Sauter G., Cathomas G., Krapf R. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 17.Schaar J.A., Muller J.E., Falk E., Virmani R., Fuster V., Serruys P.W. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J. 2004;25:1077–1082. doi: 10.1016/j.ehj.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Gurfinkel E., Vigliano C., Janavel J.V., Fornoni D., Caponi G., Meckert P.C. Presence of vulnerable coronary plaques in middle-aged individuals who suffered a brain death. Eur Heart J. 2009;30:2845–2853. doi: 10.1093/eurheartj/ehp303. [DOI] [PubMed] [Google Scholar]
- 19.Albert D.M., Scheef E.A., Wang S., Mehraein F., Darjatmoko S.R., Sorenson C.M. Calcitriol is a potent inhibitor of retinal neovascularization. Invest Ophthalmol Vis Sci. 2007;48:2327–2334. doi: 10.1167/iovs.06-1210. [DOI] [PubMed] [Google Scholar]
- 20.Dusso A., Arcidiacono M.V., Yang J., Tokumoto M. Vitamin D inhibition of TACE and prevention of renal osteodystrophy and cardiovascular mortality. J Steroid Biochem Mol Biol. 2010;121(1-2):193–198. doi: 10.1016/j.jsbmb.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biesalski H.K. Vitamin D deficiency and co-morbidities in COVID-19 patients- A fatal relationship? NFS Journal. 2020;20:10–21. [Google Scholar]
- 22.Stavenuiter A.W., Arcidiacono M.V., Ferrantelli E., Keuning E.D., Vila Cuenca M., ter Wee P.M. A novel rat model of vitamin D deficiency: safe and rapid induction of vitamin D and calcitriol deficiency without hyperparathyroidism. BioMed Res Int. 2015;2015:604275. doi: 10.1155/2015/604275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Moreno J.M., Herencia C., de Oca A.M., Díaz-Tocados J.M., Vergara N., Gómez-Luna M.J. High phosphate induces a pro-inflammatory response by vascular smooth muscle cells and modulation by vitamin D derivatives. Clin Sci (Lond) 2017;131(13):1449–1463. doi: 10.1042/CS20160807. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Rodríguez E., Ortega R.M., González-Rodríguez L.G., López-Sobaler A.M., UCM Research Group VALORNUT Vitamin D deficiency is an independent predictor of elevated triglycerides in Spanish school children. Eur J Nutr. 2011;50(5):373–378. doi: 10.1007/s00394-010-0145-4. 920030. [DOI] [PubMed] [Google Scholar]
- 25.Wang T.J., Pencina M.J., Booth S.L., Jacques P.F., Ingelsson E., Lanier K. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;177(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitsa A., Toutouza M., Machairas N., Mariolis A., Philippou A., Koutsilieris M. Vitamin D in cardiovascular disease. In Vivo. 2018;32(5):977–981. doi: 10.21873/invivo.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu-Wong J.R., Nakane M., Ma J. Effects of vitamin D analogs on the expression of plasminogen activator inhibitor-1 in human vascular cells. Thromb Res. 2006;118(6):709–714. doi: 10.1016/j.thromres.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Jones G., Prosser D.E., Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523(1):9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Naghavi M., Libby P., Falk E., Casscells S.W., Litovsky S., Rumberger J. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 30.Luo Z., Xu W., Ma S., Qiao H., Gao L., Zhan R. Moderate autophagy inhibits vascular smooth muscle cell senescence to stabilize progressed atherosclerotic plaque via the mTORC1/ULK1/ATG13 signal pathway. Oxid. Med. Cell. Longev. 2017;2017:3018190. doi: 10.1155/2017/3018190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orbe J., Rodríguez J.A., Arias R., Belzunce M., Nespereira B., Pérez-Ilzarbe M. Antioxidant vitamins increase the collagen content and reduce MMP-1 in a porcine model of atherosclerosis: implications for plaque stabilization. Atherosclerosis. 2003;167:45–53. doi: 10.1016/s0021-9150(02)00392-1. [DOI] [PubMed] [Google Scholar]
- 32.Libby P., Aikawa M. Vitamin C, collagen, and cracks in the plaque. Circulation. 2002;105:1396–1398. doi: 10.1161/01.cir.0000012513.58079.ea. [DOI] [PubMed] [Google Scholar]
- 33.Libby P., Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003;91:4B–8B. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- 34.Cordle A., Koenigsknecht-Talboo J., Wilkinson B., Limpert A., Landreth G. Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem. 2005;280:34202–34209. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- 35.Dave T., Ezhilan J., Vasnawala H., Somani V. Plaque regression and plaque stabilisation in cardiovascular diseases. Indian J Endocrinol Metab. 2013;17:983–989. doi: 10.4103/2230-8210.122604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kini A.S., Vengrenyuk Y., Shameer K., Maehara A., Purushothaman M., Yoshimura T. Intracoronary imaging, cholesterol efflux, and transcriptomes after intensive statin treatment: the YELLOW II study. J Am Coll Cardiol. 2017;69:628–640. doi: 10.1016/j.jacc.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Reutelingsperger C., Schurgers L. Coronary artery calcification: a janus-faced biomarker? JACC Cardiovasc Imaging. 2018;11:1324–1326. doi: 10.1016/j.jcmg.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Feig J.E. Regression of atherosclerosis: insights from animal and clinical studies. Ann Glob Health. 2014;80:13–23. doi: 10.1016/j.aogh.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gil Á., Plaza-Diaz J., Mesa M.D. Vitamin D: classic and novel actions. Ann Nutr Metab. 2018;72(2):87–95. doi: 10.1159/000486536. [DOI] [PubMed] [Google Scholar]
- 40.Carlberg C. Molecular approaches for optimizing vitamin D supplementation. Vitam Horm. 2016;100:265–272. doi: 10.1016/bs.vh.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munshi R., Hussein M.H., Toraih E.A., Elshazli R.M., Jardak C., Sultana N. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]