Membranous nephropathy (MN) is a leading cause of nephrotic syndrome in nondiabetic adults. Left untreated, two-thirds of patients with MN can develop nonprogressive chronic kidney disease or progress to end-stage renal disease in 10 years.1 The recognition of MN as an autoimmune disease has paved the way for the use of anti–B-cell therapy in its management.2 Over the last decade, rituximab has been successfully used to treat MN and was shown to be noninferior to cyclosporine in the Membranous Nephropathy Trial of Rituximab study.3 However, 40% of patients with MN do not respond to rituximab.3 Therefore, there is a need for additional treatment options for patients with MN.
Obinutuzumab (Gazyva [Genentech, South San Francisco, CA]) is a humanized and glycoengineered type II anti-CD20 monoclonal antibody that has superior in vitro B-cell cytotoxicity compared with rituximab.4 Obinutuzumab is directed at a different epitope on CD20 than that recognized by rituximab and can evoke a greater B-cell apoptotic response.4 Modification of the glycan tree structure at the Fc fragment of obinutuzumab leads to an increased affinity to FcgRIII and thereby potentiates antibody-dependent cellular cytotoxicity via natural killer cells as well as antibody-dependent cellular phagocytosis via macrophages.5 These B-cell depletional mechanisms contrast to the primarily complement-dependent cytotoxicity for rituximab. Combined with chemotherapy, obinutuzumab has been found to be more effective than rituximab in the treatment of patients with certain B-cell malignancies.6 Based on these data, the use of obinutuzumab in treatment of patients with MN is an attractive option. Herein, we present our single-center experience of treating MN with obinutuzumab.
Results
Patient Population
Ten patients with MN were treated with obinutuzumab at our center between January 2015 and December 2019. Table 1 shows baseline characteristics of these patients. The work-up for secondary causes of MN was negative except in 2 patients (1 with lupus-associated MN and 1 with de novo MN in allograft). Seven patients (70%) had received rituximab within a year before receiving obinutuzumab. Six of these patients were rituximab refractory and 1 had a response to rituximab but was switched to obinutuzumab because of an adverse reaction. Four patients received obinutuzumab after kidney transplant for recurrent MN (n = 3) or de novo MN (n = 1). The median follow-up was 18 months (interquartile range [IQR], 9–24 months).
Table 1.
Patient characteristics
| Patient no. | Age (yr) | Sex | Transplant | PLA2R/ THSD7A on Biopsy | Serum Anti-PLA2R (RU/ml) |
UPCR (g/g) | Serum albumin (g/dl) | Serum creatinine (mg/dl) | Previous treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | F | No | Negative | Negative | 14.38 | 2.9 | 1.1 | Rituximab and ARB |
| 2 | 66 | M | No | Negative | 633 | 11.3 | 1.7 | 2.1 | Rituximab and ARB |
| 3 | 41 | M | No | PLA2R+ | 39 | 10.73 | 2.7 | 0.9 | Tacrolimus, prednisone, and ARB |
| 4 | 68 | M | No | PLA2R+ | 261 | 7.8 | 2.1 | 1.3 | Rituximab and ARB |
| 5 | 49 | M | No | Negative | 4.5 | 3.9 | 0.9 | Cyclophosphamide, prednisone, and rituximab | |
| 6 | 24 | F | No | Negative | 3.65 | 2.5 | 1.1 | Cyclophosphamide, mycophenolate, prednisone, and rituximab | |
| 7 | 76 | F | Yes | PLA2R+, THSD7A+ | 79 | 5.8 | 2.8 | 1.4 | Tacrolimus, prednisone, and ARB |
| 8 | 67 | M | Yes | THSD7A+ | 57 | 8.49 | 2.8 | 1.5 | Rituximab, ARB, tacrolimus, and prednisone mycophenolate |
| 9 | 69 | F | Yes | Negative | Negative | 4.7 | 3.6 | 2.2 | Rituximab, ARB, synthetic ACTH, tacrolimus, prednisone, and mycophenolate |
| 10 | 50 | M | Yes | PLA2R+ | Negative | 4.5 | 3.6 | 1.5 | Tacrolimus, prednisone, mycophenolate, and ARB |
ACTH, adrenocorticotrophic hormone; ARB, angiotensin II receptor blocker; PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin type 1 domain-containing 7A; UPCR, urine protein-to-creatinine ratio.
Outcomes
Urine Protein-to-Creatinine Ratio
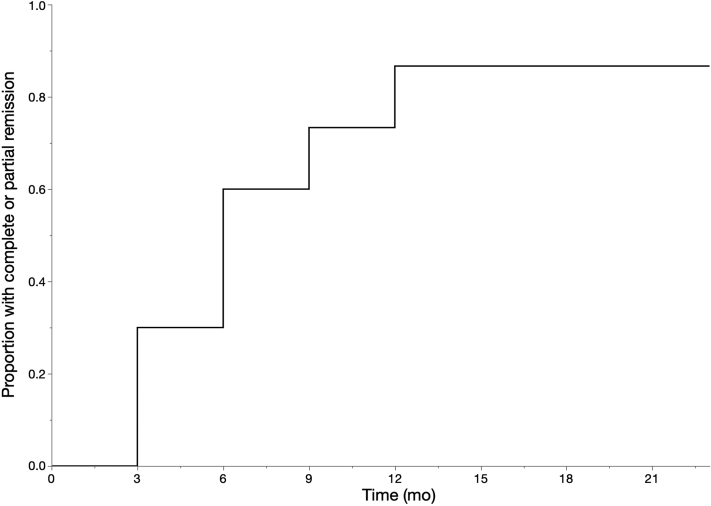
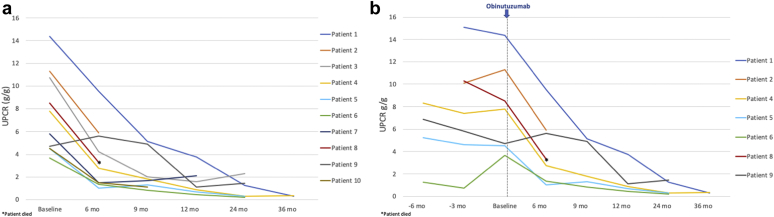
At a median follow-up of 6 months (IQR, 6–10.5 months) after obinutuzumab therapy, complete remission was achieved in 4 (40%) and partial remission was achieved in 5 (50%) of patients. The only patient who did not achieve complete or partial remission had 6 months of follow-up and had a 48% decline in proteinuria by that time. All patients who achieved complete or partial remission by 12 months and had longer follow-up (n = 5) maintained remission at 24 months. Figure 1 shows the proportion of patients who achieved complete or partial remission over time. Of the 7 patients who had received rituximab before, 4 (57.1%) achieved complete remission and 2 (28.6%) achieved partial remission. The trend of urine protein-to-creatinine ratio (UPCR) in all patients and in rituximab-experienced patients is shown in Figure 2.
Figure 1.
Kaplan–Meier curve for complete or partial remission.
Figure 2.
(a) Urine protein-to-creatinine ratio (UPCR) trend after obinutuzumab in all patients. (b) UPCR trend before and after obinutuzumab in rituximab-experienced patients.
Serum Albumin and Creatinine
All patients with a serum albumin ≤3.5 g/dl at obinutuzumab initiation (n = 7) had an improvement in serum albumin (Supplementary Figure S1). The median Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate at obinutuzumab initiation was 52.5 ml/min (IQR, 35–77.5 ml/min). Kidney function remained stable in all patients (Supplementary Figure S2).
Serum PLA2R Antibody
Five patients had elevated serum PLA2R antibodies. One of these patients (patient 4) achieved immunologic remission without improvement in proteinuria with rituximab and therefore had a normal PLA2R antibody titer at the time of obinutuzumab therapy. All 4 patients who had detectable serum PLA2R antibodies at the time of obinutuzumab administration had a decline in the titer to <14 RU/ml (negative result for our immunology laboratory; Supplementary Table S1).
Safety
One patient had mild wheezing during obinutuzumab infusion that resolved with a dose of methylprednisolone. Four patients developed leukopenia (range, 1.42–3.0 × 1000/ml) at a median of 39.5 days (IQR, 19.0–62.3 days) after the first dose. Leukopenia resolved within 2 months in 3 patients and persisted for 18 months in 1 patient. Minor infections noted in nontransplant patients included upper respiratory infections (n = 3) and urinary tract infections (n = 2). One posttransplant patient had multiple urinary tract infections and 1 episode of Clostridium difficile infection requiring hospitalization. This patient was found to have a low IgG level and was treated with i.v. immune globulin. Another 2 posttransplant patients developed cytomegalovirus viremia, 1 of whom died of glioblastoma multiforme 6 months after receiving obinutuzumab.
Discussion
The recognition of nephritogenic autoantibodies in the pathogenesis of MN has defined the key role of anti–B-cell therapy in its management.2 In this pilot study, we used obinutuzumab for treatment of 10 patients with MN and showed that 90% patients achieved either partial or complete remission with 2 doses. All patients with detectable serum PLA2R antibody achieved immunologic remission before improvement in proteinuria as seen in previous studies.3 Our patient population was largely rituximab refractory. The other conventional treatment options for these patients are calcineurin inhibitors or cyclophosphamide, which are associated with significant long- or short-term toxicity and high rates of relapse. In addition, the efficacy of these agents in patients who have failed rituximab therapy is unknown. In a recent case series of 3 patients with rituximab-refractory PLA2R MN, obinutuzumab led to complete immunologic remission and improvement in proteinuria in 2 patients.7 In our study, obinutuzumab led to complete or partial remission in 85.7% of rituximab-refractory patients.
Obinutuzumab was well-tolerated in our cohort, which included a patient who had previously experienced infusion-related anaphylaxis with rituximab. Rituximab is a chimeric monoclonal with mouse motifs in the antigen-binding region, and therefore immune responses in the recipient could inhibit or diminish efficacy or result in severe allergic reactions as seen in our patient.8 Obinutuzumab, on the contrary, is a humanized monoclonal antibody with a lower risk of immunogenicity.
The response to obinutuzumab in our study was rapid because 60% of patients achieved complete or partial remission at 6 months compared with the 35% 6-month response rate reported in the Membranous Nephropathy Trial of Rituximab study.3 In addition, obinutuzumab appeared to induce a durable response. All patients who achieved remission at 12 months and who had follow-up data available maintained remission without requiring additional doses of obinutuzumab. On the contrary, a significant proportion of patients treated with rituximab require additional doses to induce remission or treat relapse.3 This may be related to a more profound depletion of B-lymphocytes with obinutuzumab. B-lymphocyte depletion was seen in both peripheral blood and lymph nodes in a study evaluating the use of obinutuzumab for desensitization in highly sensitized kidney transplant candidates.9
The limitations of our study include a small sample size, retrospective nonprotocoled nature, and the lack of a control arm. We also did not check peripheral CD19+ or CD20+ B-cell counts. Despite these limitations, our study provides a proof of concept of the efficacy and safety of obinutuzumab in treatment of patients with MN. Obinutuzumab is an attractive alternative therapy in patients with resistance or sensitization to rituximab and could potentially be the first-line agent in the treatment of MN. Larger prospective studies are needed to confirm our findings.
Disclosure
SCJ has received grants and consulting fees from CareDx, Vitaeris, Hansa Biopharma, and CSL Behring; consulting fees from Regeneron Pharmaceuticals, Viela Bio, and Amplyx; and grants from Novartis outside the submitted work. All other authors declared no competing interests.
Footnotes
Supplementary Methods.
Figure S1. Serum albumin trend after obinutuzumab.
Figure S2. Serum creatinine trend after obinutuzumab.
Table S1. Serum PLA2R antibody titer (RU/ml).
Supplementary Material
References
- 1.Schieppati A., Mosconi L., Perna A. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 2.Beck L.H., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fervenza F.C., Appel G.B., Barbour S.J. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 4.Patz M., Isaeva P., Forcob N. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol. 2011;152:295–306. doi: 10.1111/j.1365-2141.2010.08428.x. [DOI] [PubMed] [Google Scholar]
- 5.Mössner E., Brünker P., Moser S. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goede V., Fischer K., Busch R. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 7.Klomjit N, Fervenza FC, Zand L. Successful treatment of patients with refractory PLA2R-associated membranous nephropathy with obinutuzumab: a report of 3 cases [e-pub ahead of print]. Am J Kidney Dis.https://doi.org/10.1053/j.ajkd.2020.02.444. Accessed July 9, 2020. [DOI] [PubMed]
- 8.Boyer-Suavet S., Andreani M., Lateb M. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol. 2019;10:3069. doi: 10.3389/fimmu.2019.03069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redfield R.R., Jordan S.C., Busque S. Safety, pharmacokinetics, and pharmacodynamic activity of obinutuzumab, a type 2 anti-CD20 monoclonal antibody for the desensitization of candidates for renal transplant. Am J Transplant. 2019;19:3035–3045. doi: 10.1111/ajt.15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.