Abstract
Background
Oxalate nephropathy is a potentially underestimated cause of kidney failure characterized by massive deposition of calcium oxalate crystals in the renal parenchyma. The prevalence and modes of presentation of this entity are ill-defined.
Methods
Here we report on the largest consecutive series of cases of adult oxalate nephropathy diagnosed on native kidney biopsies from January 2010 to December 2018 in the UCLouvain Kidney Disease Network.
Results
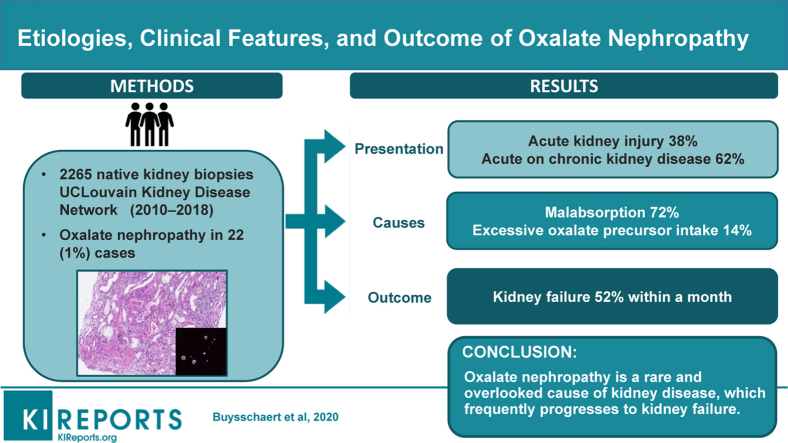
We screened 2265 native kidney biopsies and identified 22 cases (1%) of oxalate nephropathy. Patients had a mean age at diagnosis of 61 years (±20) and presented either with acute on chronic kidney disease (CKD) (62%) or with acute kidney injury (AKI) (38%). Mean serum creatinine at biopsy was 8.0 ± 4.5 mg/dl. Kidney biopsies showed abundant calcium oxalate crystal deposits, associated with acute interstitial nephritis and tubular necrosis, and variable degrees of interstitial fibrosis and tubular atrophy. Chronic pancreatitis and gastric bypass were the most common causes of oxalate nephropathy (48%). During a mean follow-up of 29 months, half of the patients (52%) progressed to kidney failure, all within the month following diagnosis. Higher serum creatinine level at presentation and interstitial fibrosis and tubular atrophy score were associated with progression to kidney failure.
Conclusion
Oxalate nephropathy is the cause of kidney disease in 1% of consecutive native kidney biopsies and typically presents as acute on CKD or AKI. The prognosis of the disease is poor, with a high rate of kidney failure within the first month after the diagnosis.
Keywords: chronic pancreatitis, fat malabsorption, gastric bypass, hyperoxaluria, steatorrhea
Graphical abstract
Oxalate nephropathy is a potentially devastating condition, characterized by deposition of calcium oxalate crystals in the renal parenchyma leading to tubular injury, interstitial fibrosis, and AKI or CKD.1 Oxalate nephropathy may occasionally result in adults from primary oxalosis, an inborn error of glyoxylate metabolism leading to overproduction of oxalate. However, it results more frequently from secondary, enteric hyperoxaluria.1, 2, 3
Causes of secondary hyperoxaluria include (i) increased dietary oxalate or oxalate precursor (such as ethylene glycol or ascorbic acid) intake, (ii) fat malabsorption from various causes (chronic pancreatitis, pancreatectomy, Roux-en-Y gastric bypass surgery, short bowel syndrome, Crohn’s disease, and use of orlistat), and (iii) decreased intestinal oxalate degradation secondary to reduced intestinal colonization with Oxalobacter formigenes.1, 2 Additionally, low estimated glomerular filtration rate (eGFR), hypovolemia, use of renin-angiotensin system inhibitors and diuretics, and older age all have been anecdotally associated with the development of oxalate nephropathy in patients with hyperoxaluria.1,2,4,5
The prevalence of oxalate nephropathy has never been studied, although it may be an under-recognized cause of kidney failure. Also, the respective importance of potential etiologies of oxalate nephropathy is unknown as no case series has been reported.2 Here we report on the etiologies, presentation, and outcome of all cases of oxalate nephropathy diagnosed during a 9-year period in the UCLouvain Kidney Disease Network.
Materials and Methods
Study Design
We retrospectively identified consecutive adult cases of oxalate nephropathy by reviewing all native kidney biopsies with 1 or more diagnostic codes including “oxalate,” “calcium,” “crystal,” “stone” and/or “interstitial nephritis of unknown etiology,” and “acute tubular necrosis of unknown etiology” analyzed in our pathology lab from January 1, 2010, to December 31, 2018. Biopsies were performed in our academic center or one of the hospitals of the UCLouvain Kidney Disease network (list provided in the Acknowledgments).
Oxalate nephropathy was defined as (i) progressive kidney disease (defined by >50% increase of serum creatinine within a year) with (ii) deposition of oxalate crystals associated with tubular injury and interstitial nephritis and (iii) exclusion of other causes of kidney disease apart from nonspecific microvascular (nephrosclerosis) and diabetic nephropathy.1,2,5 All patients had multiple calcium oxalate crystals, birefringent under polarized light, which are unlike calcium phosphate crystals.6 Two cases were previously reported, associated with orlistat use and excessive vitamin C intake, respectively.7,8
Demographics, medical history (hypertension, diabetes, CKD, nephrolithiasis, alcohol and smoking habits), and conditions known to predispose to hyperoxaluria were collected from medical charts, as were medications (renin-angiotensin system inhibitors, diuretics, vitamin C supplementation, antibiotics), clinical presentation, and biological data at the time of admission that led to the diagnosis of oxalate nephropathy. Clinical steatorrhea was defined by more than 3 loose, pale, and/or floating stools.9 eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.10 CKD stages were determined according to the KDIGO recommendations.11 AKI was defined as an increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days.12 Oxaluria was reported as urinary oxalate-to-creatinine ratio.4,13 Fecal acid steatocrit was recorded when available.14,15
Medical history, symptoms and signs of steatorrhea and malabsorption, and imaging and genetic results were carefully examined to ascertain the cause of oxalate nephropathy. The diagnosis of chronic pancreatitis was based on clinical criteria or well-defined complications, together with abnormalities in pancreas imaging, and confirmed by a senior gastroenterologist.16 Outcomes (kidney failure, death or alive without kidney failure) were analyzed at last follow-up. The Saint-Luc Hospital’s Ethical Committee approved the study (2017/26OCT/497) and the study procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Kidney Biopsy Analysis
All biopsies were reviewed by the senior pathologist (SA). They were processed according to standard techniques for light microscopy and immunofluorescence. Hematoxylin and eosin, periodic acid–Schiff, trichrome, and Jones colorations were used. Immunofluorescence was systematically performed using antibodies directed against IgA, IgG, IgM, C3, C1q, κ, λ, and fibrinogen. Calcium oxalate crystals were diagnosed based on birefringence under polarized light. Glomeruli and oxalate crystals were counted to calculate the oxalate crystals-to-glomeruli ratio. Acute interstitial nephritis, acute tubular necrosis, and interstitial fibrosis and tubular atrophy were graded on a semiquantitative scale, according to the estimated percentage of renal cortex affected, and graded as 1% to 25% (mild), 26% to 50% (moderate), and >50% (severe).1 Chronic vascular lesions and diabetic glomerulosclerosis were also evaluated using a semiquantitative scale.
Statistical Analysis
Determinants of kidney outcome (kidney failure vs. autonomous kidney function) were analyzed using the following variables: age, history of CKD, creatinine level at presentation, renal biopsy interstitial fibrosis and tubular atrophy score, acute interstitial nephritis, acute tubular necrosis, chronic vascular and diabetic glomerulosclerosis scores, and etiology of oxalate nephropathy. Data are presented as mean ±S D or median and range. Normality of distribution of continuous variables was verified using the Shapiro-Wilk test; the Student t test or the Wilcoxon test was used to assess their potential association with kidney outcome. The χ2 test for independence or the Fisher exact test was used for categorical variables.
Results
Clinical Presentation and Features
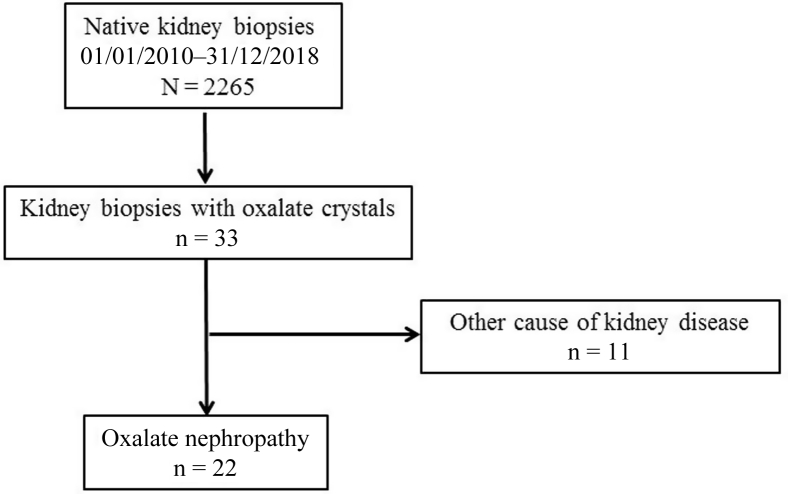
We identified 22 cases of biopsy-proven oxalate nephropathy, out of a total of 2265 (1%) native kidney biopsies performed between January 2010 and December 2018 (Figure 1). Scarce oxalate crystals were also found in 11 cases of non–oxalate nephropathy kidney diseases (Supplementary Table S1). Table 1 summarizes the characteristics of patients with oxalate nephropathy (n = 21; 1 case was excluded because of insufficient clinical and biological data). The cohort consisted of 7 women and 14 men, aged 61 ± 20 years at the time of kidney biopsy. All patients were Caucasians; 76% were hypertensive and 57% diabetic. Sixty-two percent of patients had a previous history of CKD, with an eGFR of 36 ± 7 ml/min per 1.73 m2, 14 ± 25 months before the diagnosis of oxalate nephropathy. Estimated GFR was normal in the remaining patients, 15 ± 11 months before the diagnosis of oxalate nephropathy.
Figure 1.
Study flowchart.
Table 1.
Patient characteristics on admission
| Characteristic | Value (n = 21) |
|---|---|
| Age, yr | 61 ± 20 |
| Gender, male | 14 (67) |
| Diabetes | 12 (57) |
| Type 1 / type 2 diabetes, n | 3 / 9 |
| Duration of diabetes, yr | 13 ± 8 |
| History of prior CKD | 13 (62) |
| CKD stage 3a/3b/4, n | 1 / 7 / 3 |
| Last eGFR in those with CKD history, ml/min per 1.73 m2 | 36 ± 7 |
| Time between past eGFR and biopsy, mo | 14 ± 25 |
| Hypertension | 16 (76) |
| Past urolithiasis | 3 (14) |
| Past or active alcohol abuse | 6 (29) |
| Past or active smoking | 11 (52) |
| Known conditiona predisposing to hyperoxaluria | 10 (48) |
| Gastric bypass | 5 |
| Orlistat use | 2 |
| Chronic pancreatitis | 1 |
| Pancreatectomy | 1 |
| Bowel resection (Crohn’s disease) | 1 |
| Duration of predisposing condition, yr | 6.2 ± 7.4 |
| RAS inhibitor use | 8 (38) |
| Diuretic use | 9 (43) |
| Antibiotic use within 2 mo before biopsy | 3 (14) |
| Vitamin C intake | 2 (10) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; RAS, renin-angiotensin system.
Data are mean ± SD or n (%) unless otherwise noted.
Already diagnosed before oxalate nephropathy.
Ten patients had a history of a condition known to predispose to hyperoxaluria, notably gastric bypass and orlistat (lipase inhibitor) use. Time between the predisposing condition and oxalate nephropathy diagnosis was highly variable: 1–22 years for patients with gastric bypass (n = 5) and 1 and 8 years for the 2 patients taking orlistat. Only 3 patients had a history of urinary stone(s). Twelve patients (57%) were taking a renin-angiotensin system inhibitor and/or a diuretic. There was an even distribution of cases during the 9-year period of observation (i.e., 7 cases in 2010–2012, 2013–2015, and 2016–2018, respectively).
The presentation was acute on CKD in 62% and AKI in 38% of patients, notwithstanding the variable time interval between previous blood test and presentation. In 12 patients, clinical steatorrhea was suspected at presentation (Table 2). Blood pressure averaged 128/73 mm Hg and body mass index 25 (±7) kg/m2. Serum creatinine was 8.0 ± 4.5 mg/dl, and microscopic hematuria was present in 24% of patients. Urine protein/creatinine was 1.4 ± 2.0 g/g, urine oxalate/creatinine 86 ± 58 mg/g (normal <32), and fecal steatocrit 47% ± 26% (normal <10) (Table 2). Ultrasound imaging showed mean (±SD) right and left kidney lengths of 109 mm (±15) and 110 mm (±16), respectively.
Table 2.
Clinical and biological data at presentation
| Variable | Normal range | Value | Number with data |
|---|---|---|---|
| Symptoms and clinical examination | |||
| Clinical steatorrhea | 12 (57) | 21 | |
| Blood pressure, mm Hg | 128/73 | 21 | |
| BMI | 25 ± 7 | 21 | |
| Biological data | |||
| Serum creatinine, mg/dl | <1.3 | 8.0 ± 4.5 | 21 |
| Glycated hemoglobin, % | 4.0–6.0 | 6.5 ± 1.9 | 12 |
| Microscopic hematuriaa | 5 (24) | 21 | |
| Leukocyturiab | 5 (24) | 21 | |
| Urinary oxalate-to-creatinine ratio, mg/g | <32 | 86 ± 58 | 16 |
| Urinary protein-to-creatinine ratio, g/g | <0.2 | 1.4 ± 2.0 | 15 |
| Fecal acid steatocrit, % | <10 | 47 ± 26 | 11 |
BMI, body mass index.
Data are mean ± SD or n (%) unless otherwise noted.
Defined as ≥25 red blood cells/μl.
Defined as ≥25 white blood cells/μl.
Etiologies of Oxalate Nephropathy
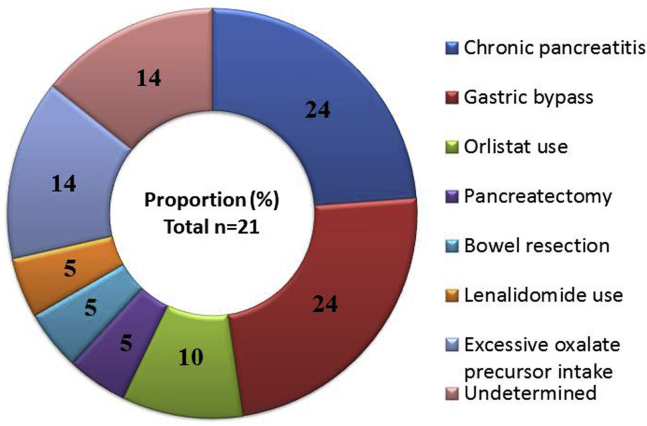
Causes of oxalate nephropathy were reviewed by the first and last authors. Oxalate nephropathy was attributed to malabsorption in 15 patients (71.5%) and excessive oxalate precursor intake in 3 patients (14%), and the cause was undetermined in the remaining 3 patients (14%) (Figure 2).
Figure 2.
Causes of oxalate nephropathy.
Malabsorptive State (n=15)
Chronic pancreatitis and gastric bypass were the main causes of oxalate nephropathy (5 patients each). Interestingly, chronic pancreatitis was diagnosed after oxalate nephropathy in 4 of 5 cases (80%), and clinical steatorrhea was present only in 3 of 5 patients at presentation. Indication for gastric bypass was gastric carcinoma and morbid obesity in 3 and 2 patients, respectively. The patients developed oxalate nephropathy a mean of 8 years (range, 1–22) after gastric bypass and only 2 had clinical steatorrhea. Two patients had been on orlistat treatment during 1 and 8 years before presenting with oxalate nephropathy. Whipple procedure was performed for pancreatic adenocarcinoma 1 year before diagnosis of oxalate nephropathy in a man who had evident clinical steatorrhea on admission although he was taking pancreatic enzyme supplementation. Oxalate nephropathy was attributed to small bowel resection in a patient with Crohn’s disease and lenalidomide-induced bile acid malabsorption in another with multiple myeloma.17 Altogether, 10 of 15 patients with hyperoxaluria secondary to malabsorption presented clinical steatorrhea at diagnosis of oxalate nephropathy.
Excessive Oxalate Precursor Intake (n=3)
One patient had ingested huge amounts of vitamin C contained in a food supplement.8 Two patients with history of alcohol abuse presented with AKI associated with ethylene glycol intoxication.
Undetermined Cause of Oxalate Nephropathy (n=3)
Among the 3 patients, 2 were men aged 59 and 61 years with chronic type 2 diabetes (14 and 15 years) and previous slowly progressive stage 3b-4 CKD (eGFR 26 and 41 ml/min per 1.73 m2 a month prior to presentation). The third patient was a 48-year-old woman with a history of opioid-managed chronic pain associated with cervical hernias. In all patients, workup for malabsorption showed absence of steatorrhea, pancreatic exocrine insufficiency, or inflammatory bowel disease and genetic testing excluded primary oxalosis.3
Pathologic Findings
Kidney biopsies showed a mean (±SD) of 15 glomeruli (±8) (Table 3; Figure 3). Oxalate crystals-to-glomerulus ratio was 2.3 ± 2.3. Acute interstitial nephritis and acute tubular necrosis lesions were mild or moderate in most patients, and interstitial fibrosis and tubular atrophy scores were highly variable. Diabetic glomerulosclerosis was present in 6 of 12 diabetic patients and mild to moderate chronic vascular lesions were found in 16 of 21 patients. No granuloma was found in any of the biopsies.
Table 3.
Kidney biopsy data
| Characteristic | Value |
|---|---|
| Number of glomeruli | 15 ± 8 |
| Number of oxalate crystals | 28 ± 27 |
| Oxalate crystals-to-glomeruli ratio | 2.3 ± 2.3 |
| Acute interstitial nephritis, absent/mild/moderate/severe | 3/10/7/1 |
| Acute tubular necrosis, absent/mild/moderate/severe | 4/7/7/3 |
| Chronic vascular lesions, absent/mild/moderate/severe | 3/5/11/2 |
| Diabetic glomerulosclerosis, absent/mild/moderate/severe | 15/3/2/1 |
| IFTA score, 0/1/2/3 | 3/7/5/6 |
IFTA, interstitial fibrosis and tubular atrophy.
Data are mean ± SD or nr.
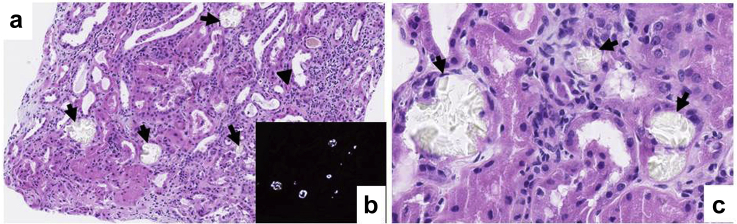
Figure 3.
Renal biopsy of a patient with oxalate nephropathy secondary to gastric bypass performed for carcinoma. On light microscopy, hematoxylin and eosin stain shows tubular acute necrosis (a, arrowhead) with numerous intratubular translucent calcium oxalate crystals (a,c, arrows). These crystals demonstrate birefringence under polarized light (b). Original magnification ×9.75 (a) and ×30 (c).
We also calculated the oxalate crystals-to-glomerulus ratio in the 11 cases of non-oxalate nephropathy containing scarce crystals and found a ratio of 0.09 ± 0.00 (Supplementary Table S1). A cut-off ratio of ≥0.25 separated patients with oxalate nephropathy from those with other kidney diseases.
Treatment and Outcome
Therapy consisted in intensive i.v. rehydration in all patients, in addition to oral calcium supplements. Patients also received counseling concerning low-fat and low-oxalate diet. Pancreatic enzyme supplementation was started or the dosage increased in the 6 patients with chronic pancreatitis or pancreatectomy. Orlistat and vitamin C were withdrawn.
During a mean follow-up of 29 months (±67), 11 of 21 (52%) patients progressed to kidney failure, all within a month of presentation (Table 4). Serum creatinine level at diagnosis (10.8 ± 3.8 vs. 4.8 ± 2.1 mg/dl, P = 0.002) and interstitial fibrosis and tubular atrophy score (2.1 ± 1.7 vs. 1.4 ± 1.0, P = 0.03) were both higher in patients who progressed to kidney failure compared with those who did not. Among the 10 remaining patients, 4 had stage 3 CKD and 5 had stage 4 or 5 CKD at last follow-up. Only 1 patient experienced complete kidney recovery after oxalate nephropathy associated with gastric bypass. One patient (with type 2 diabetes and undetermined cause of oxalate nephropathy) received a cadaveric renal transplant, without relapse of oxalate nephropathy at a 5-month follow-up. Among the patients with autonomous kidney function, 3 died of cardiovascular, cancer (pancreatic), and unknown cause.
Table 4.
Outcome
| Outcome | Value |
|---|---|
| Follow-up duration, moa | 29 ± 67 |
| Kidney failure | 11 (52) |
| Time to kidney failure, mo | 0.2 ± 0.4 |
| eGFR at follow-up (non–kidney failure), ml/min per 1.73 m2 | 32 ± 19 |
| CKD 4 or 5 at last follow-up (non–kidney failure) | 5/10 (50) |
| Death if no kidney failure | 3/10 (30) |
| Time to death, mo | 32 ± 38 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Data are mean ± SD or n (%).
Follow-up duration till kidney failure, death, or last follow-up.
Discussion
We report the first multicenter case series of all-cause oxalate nephropathy during a 9-year period. The prevalence of oxalate nephropathy was approximately 1% of native kidney biopsies. Acute on chronic kidney disease was the main mode of presentation, and chronic pancreatitis and Roux-en-Y gastric bypass, the most frequently associated etiologies of oxalate nephropathy. More than half of patients reached kidney failure within a month of presentation.
Chronic pancreatitis and Roux-en-Y gastric bypass were the causes of oxalate nephropathy in 48% of cases. A previous systematic review reported that fat malabsorption was indeed the most commonly attributed cause of secondary oxalate nephropathy.2 The diagnosis of chronic pancreatitis was made after that of oxalate nephropathy in 4 of 5 patients, highlighting the need for prompt recognition of exocrine pancreatic insufficiency. Late diagnosis of chronic pancreatitis has previously been shown in patients with oxalate nephropathy both in native and transplant kidneys.5,18 We also previously reported a high prevalence (23%) of hyperoxaluria among patients with chronic pancreatitis.4 Roux-en-Y gastric bypass was performed for weight loss in 2 of 5 patients. Of note, other less invasive bariatric surgeries such as gastric banding and sleeve gastrectomy do not induce malabsorption and hyperoxaluria.19 Hyperoxaluria was attributed to lenalidomide-induced bile acid malabsorption in one patient in our series. Lenalidomide is an immunomodulatory agent used to treat myeloma of all disease stages and was previously shown to induce bile acid malabsorption in a series of 12 patients.17 We and others previously showed a strong correlation between steatorrhea and urinary oxalate excretion in patients having malabsorptive states.4,20,21 Fecal acid steatocrit performed on a random stool sample has been shown to accurately measure steatorrhea and is easier to perform than 24-hour stool fat measurement.14,15 We were, however, unable to study the correlation between steatorrhea and urinary oxalate excretion in our cohort because of the limited number of patients with both fecal acid steatocrit and urinary oxalate measures.
It is commonly thought that fat malabsorption leads to steatorrhea, calcium binding by fatty acids, increased intestinal absorption of uncomplexed oxalate, and ileal and colonic permeability to oxalate.4,19 The resulting hyperoxaluria may then promote calcium oxalate crystal formation and deposition in the kidney. Kidney injury occurs via common molecular and cellular pathomechanisms shared by crystal-associated diseases or “crystallopathies.”22 It has also been hypothesized that dynamics of crystal deposition determines clinical presentation: (i) dehydration and acute supersaturation leads to rapid crystal formation, direct and indirect renal epithelial cytotoxicity, and inflammation-driven cell necrosis, with resulting AKI, whereas (ii) persistent mild supersaturation generates sub-acute crystal plug formation in distal tubules or collecting ducts and CKD by persistent tubule obstruction.22 We indeed found a majority of the patients presenting with acute on chronic kidney injury. Whether CKD is a predisposing factor because of reduced urinary excretion of oxalate or reflects past damage by subclinical oxalate crystal deposition or both remains unknown. We found a highly variable time frame between onset of condition predisposing to hyperoxaluria and diagnosis of oxalate nephropathy, suggesting that acute on chronic kidney injury could indeed result from hyperoxaluria combined with precipitating factors such as volume depletion secondary to diarrhea, low levels of crystallization inhibitors, or use of antibiotics affecting the intestinal microbiome.1,19,23
Altogether, prognosis was poor, with 11 of 21 (52%) patients reaching kidney failure within a month of presentation. Only 1 patient experienced complete kidney recovery after oxalate nephropathy associated with gastric bypass. In their systematic review of published cases and series of oxalate nephropathy, Lumlertgul et al also reported a high rate of patients (55%) remaining dialysis dependent.2 Not surprisingly, we found higher creatinine levels at presentation and a higher tubular atrophy and interstitial fibrosis score to be associated with kidney failure. In our series, patients who did not reach kidney failure shortly after diagnosis of oxalate nephropathy maintained autonomous kidney function during a follow-up of nearly 30 months, suggesting that treatment was of benefit. Treatment of oxalate nephropathy remains empirical, including increased fluid intake, oral calcium supplements, and correction of the hyperoxaluria-enabling condition. Early diagnosis and management may decrease intestinal oxalate absorption and deposition of calcium oxalate crystals in the kidney. Also, better understanding of the pathophysiology of oxalate nephropathy suggests that agents blocking the NLRP3 inflammasome–IL1β signaling pathway and oral administration of oxalate-degrading enzymes such as O formigenes may be attractive novel therapies.19,22
Although the reported prevalence is low (<1%), oxalate nephropathy is probably underdiagnosed. Kidney biopsy samples may not be representative of the renal parenchyma, and oxalate crystals occurring within the medulla may be missed. Also, there is no well-established definition of oxalate nephropathy. We suggest using criteria proposed by previous authors and adding an oxalate crystals-to-glomeruli ratio ≥0.25 as a prerequisite, because scarce oxalate crystals may be found in tubules in a normal kidney.1,5,22 This ratio will need to be validated in other series. We found no cases of primary oxalosis although this disease may have a late onset, in adults, such as in patients with a favorable genotype (p.Glyc170Arg variant of AGTX).24 Few patients had a history of urolithiasis, unlike in primary oxalosis. We were unable to determine the cause of oxalate nephropathy in 3 patients, despite extensive workup and genetic testing.3 Although pancreatic exocrine function may be altered in patients with type 2 diabetes, this was not the case in the 2 patients with longstanding type 2 diabetes and undetermined cause of oxalate nephropathy in our cohort.25,26
The strengths of our study are the inclusion of all cases of biopsy-proven oxalate nephropathy diagnosed during a 9-year period, the centralized biopsy reading and the multicenter nature of the study. Clinical data and etiology of oxalate nephropathy were reviewed by the first and last authors for all cases. Limitations that need to be acknowledged are the limited number of cases, the retrospective nature of the study, and the absence of systemic genetic testing in all patients.
In conclusion, oxalate nephropathy is the cause of kidney disease in 1% of consecutive native kidney biopsies and typically presents as acute on CKD or AKI. The prognosis of the disease is poor, with a high rate of kidney failure within the first month after the diagnosis. Prompt diagnosis and management may help to decrease intestinal oxalate absorption and deposition of calcium oxalate crystals in the kidney.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank all the collaborators of the UCLouvain Kidney Disease Network: Nicolas Cecere (Grand Hopital de Charleroi, Gilly, Belgium), Miguel Guillen (EpiCURA, Hornu, Belgium), Christine Hurtgen (CHR Sambre et Meuse, Namur, Belgium), Philippe Leroy (CHR Mons-Hainaut, Mons, Belgium), Eleonore Ponlot (Grand Hopital de Charleroi, Gilly, Belgium), Francois Reginster (Cliniques de l’Europe, Brussels, Belgium), Michel Tintillier (Clinique et Maternité Sainte-Elisabeth, Namur, Belgium), and Charlotte Van Ende (Clinique et Maternité Sainte-Elisabeth, Namur, Belgium).
We also thank Lieven Desmet (Institut de Statistique, Biostatistique et Sciences Actuarielles, Université catholique de Louvain, Louvain-La-Neuve, Belgium) for statistical assistance.
Footnotes
Table S1. Details of kidney biopsies with at least 1 oxalate crystal.
Supplementary Material
References
- 1.Nasr S.H., D’Agati V.D., Said S.M. Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3:1676–1683. doi: 10.2215/CJN.02940608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumlertgul N., Siribamrungwong M., Jaber B.L. Secondary oxalate nephropathy: a systematic review. Kidney Int Rep. 2018;3:1363–1372. doi: 10.1016/j.ekir.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cochat P., Rumsby G. Primary hyperoxaluria. N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 4.Demoulin N., Issa Z., Crott R. Enteric hyperoxaluria in chronic pancreatitis. Medicine. 2017;96:e6758. doi: 10.1097/MD.0000000000006758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartery C., Faguer S., Karras A. Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol. 2011;6:1895–1902. doi: 10.2215/CJN.00010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demoulin N., Jadoul M., Cosyns J.P. An easily overlooked iatrogenic cause of renal failure. Clin Nephrol. 2008;70:176–177. doi: 10.5414/cnp70176. [DOI] [PubMed] [Google Scholar]
- 7.Buysschaert B., Aydin S., Morelle N. Weight loss at a high cost: orlistat-induced late-onset severe kidney disease. Diabetes Metab. 2016;42:62–64. doi: 10.1016/j.diabet.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Borceux P., Aydin S., Demoulin N. Acute renal failure and a “rejuvenating powder. Kidney Int. 2020;97:219–220. doi: 10.1016/j.kint.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Dumasy V., Delhaye M., Cotton F. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol. 2004;99:1350–1354. doi: 10.1111/j.1572-0241.2004.30661.x. [DOI] [PubMed] [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 12.Ostermann M., Bellomo R., Burdmann E.A. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020;98:294–309. doi: 10.1016/j.kint.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habbig S., Beck B.B., Hoppe B. Nephrocalcinosis and urolithiasis in children. Kidney Int. 2011;80:1278–1291. doi: 10.1038/ki.2011.336. [DOI] [PubMed] [Google Scholar]
- 14.Amann S.T., Josephson S.A., Toskes P.P. Acid steatocrit: a simple, rapid gravimetric method to determine steatorrhea. Am J Gastroenterol. 1997;92:2280–2284. [PubMed] [Google Scholar]
- 15.Van den Neucker A.M., Kerkvliet E.M., Theunissen P.M. Acid steatocrit: a reliable screening tool for steatorrhoea. Acta Paediatr. 2001;90:873–875. [PubMed] [Google Scholar]
- 16.Buchler M.W., Martignoni M.E., Freiss H. A proposal for a new clinical classification of chronic pancreatitis. BMC Gastroenterol. 2009;9:93. doi: 10.1186/1471-230X-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlyn C., Khan M.S., Muls A. Lenalidomide-induced diarrhea in patients with myeloma is caused by bile acid malabsorption that responds to treatment. Blood. 2014;124:2467–2468. doi: 10.1182/blood-2014-06-583302. [DOI] [PubMed] [Google Scholar]
- 18.Cuvelier C., Goffin E., Cosyns J.P. Enteric hyperoxaluria: a hidden cause of early renal graft failure in two successive transplants: spontaneous late graft recovery. Am J Kidney Dis. 2002;40:E3.1–E3.6. doi: 10.1053/ajkd.2002.33934. [DOI] [PubMed] [Google Scholar]
- 19.Nazzal L., Puri S., Goldfarb D.S. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol Dial Transplant. 2016;31:375–382. doi: 10.1093/ndt/gfv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stauffer J.Q. Hyperoxaluria and intestinal disease: the role of steatorrhea and dietary calcium in regulating intestinal oxalate absorption. Am J Dig Dis. 1977;22:921–928. doi: 10.1007/BF01076170. [DOI] [PubMed] [Google Scholar]
- 21.McDonald G.B., Earnest D.L., Admirand W.H. Hyperoxaluria correlates with fat malabsorption in patients with sprue. Gut. 1977;18:561–566. doi: 10.1136/gut.18.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulay S.R., Anders H.J. Crystal nephropathies: mechanisms of crystal-induced kidney injury. Nat Rev Nephrol. 2017;13:226–240. doi: 10.1038/nrneph.2017.10. [DOI] [PubMed] [Google Scholar]
- 23.Mole D.R., Tomson C.R., Mortensen N. Renal complications of jejuno-ileal bypass for obesity. QJM. 2001;94:69–77. doi: 10.1093/qjmed/94.2.69. [DOI] [PubMed] [Google Scholar]
- 24.van der Hoeven S.M., van Woerden C.S., Groothoff J.W. Primary hyperoxaluria type 1, a too often missed diagnosis and potentially treatable cause of end-stage renal disease in adults: results of the Dutch cohort. Nephrol Dial Transplant. 2012;27:3855–3862. doi: 10.1093/ndt/gfs320. [DOI] [PubMed] [Google Scholar]
- 25.Larger E., Philippe M.F., Barbot-Trystram L. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29:1047–1054. doi: 10.1111/j.1464-5491.2012.03597.x. [DOI] [PubMed] [Google Scholar]
- 26.Hardt P.D., Ewald N. Exocrine pancreatic insufficiency in diabetes mellitus: a complication of diabetic neuropathy or a different type of diabetes? Exp Diabetes Res. 2011;2011:761950. doi: 10.1155/2011/761950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.