Abstract
Introduction
The majority of primary membranous nephropathy (MN) cases are no longer considered idiopathic with the discovery of the podocytic autoantigens: phospholipase A2 receptor (PLA2R) and thrombospondin type 1 domain–containing 7A (THSD7A). Limited data on PLA2R-related MN in Indians exist in literature, and THSD7A-related MN remains undocumented in this population. We aimed to characterize the baseline PLA2R and THSD7A profile of adult and pediatric membranous nephropathy (MN) in a large Indian single-institution cohort.
Methods
A retrospective analysis of all cases of MN (primary and secondary) between 2014 and 2017 was performed with PLA2R direct immunofluorescence and THSD7A immunohistochemistry on the biopsies and anti-PLA2R enzyme-linked immunosorbent assay (ELISA) on baseline sera.
Results
MN constituted 10% of kidney biopsies received in the study period. A total of 216 cases with adequate tissue underwent PLA2R direct immunofluorescence, and 110 of them had available sera for PLA2R ELISA. Combining both testing methods, the prevalence of PLA2R-related primary MN was 72.8%, with moderate concordance between the 2 methods (kappa 0.61). PLA2R was also detected in 16.7% cases of secondary MN, most commonly lupus MN. THSD7A immunohistochemistry performed on 176 cases showed a prevalence of 3.4% in primary MN. One case of lupus MN was also positive for THSD7A. Dual positivity (PLA2R and THSD7A) was noted in 2 cases. The large pediatric cohort tested showed a prevalence of 44% of PLA2R based on tissue testing, whereas 1 case demonstrated THSD7A positivity.
Conclusion
This study in a large cohort of Indian patients demonstrates prevalence rates of PLA2R- and THSD7A-related MN similar to world literature, including the substantial cohort of pediatric MN. It also confirms variation in MN in the form of outliers within PLA2R (related to tissue and serum testing), dual positivity for PLA2R and THSD7A, and PLA2R/THSD7A-positive secondary MN.
Keywords: membranous nephropathy, PLA2R, THSD7A, thrombospondin, ELISA
Graphical abstract
Membranous nephropathy (MN), defined by the presence of subepithelial immune deposits that induce a spectrum of changes in the glomerular basement membrane, is considered to be primary in 80% of cases. In the rest, it is secondary to a systemic autoimmune disorder, infection, malignancy, or drugs.1 This distinction is necessary because the mode of clinical management is different for both the categories. Identification of PLA2R as the major target autoantigen causing autoantibody production in primary MN by Beck et al. in 2009 explained approximately 70% of the primary MN cases.2 PLA2R is a 185-kD transmembrane glycoprotein, normally expressed on the surface of podocytes. It exists in 2 different configurations, bent and open. It is the open configuration in which the N-terminal CysR domain points away from the cell membrane, which is antigenic. The shedding and accumulation of PLA2R antigens with altered configuration in the subepithelial space combined with anti-PLA2R antibodies leads to the characteristic pathologic alterations in glomerular basement membrane. Extensive work has shown that serum anti-PLA2R antibodies correlate with disease activity and help in predicting the disease course and response to treatment in a given patient, thereby serving as a good biomarker of disease activity.3
In 2014, another putative antigen, THSD7A, was identified in 2% to 5% of cases of primary MN.4 THSD7A, also a transmembrane glycoprotein, is similar to PLA2R and is expressed in the foot process of podocytes near the slit diaphragm. It has many thrombospondin type 1 repeats that bind to the extracellular matrix, playing a physiological role in cell adhesion. Recently, the major epitope of THSD7A has been discovered: the T28mer present in the N-terminal region. The T28mer has been found to have sequence homology to the antigenic PLA2R motif, the P28mer, and cross-reactivity of anti-PLA2R and anti-THSD7A antibodies to this common motif has also been demonstrated. Hence, it was proposed that this common motif could be the common dominant epitope and B cell–triggering factor in PMN.5,6
Though the worldwide prevalence of PLA2R- and THSD7A-associated MN has been well established, data regarding Indian population are sparse and lack information, in particular, on THSD7A-associated primary MN. Therefore, in this cross-sectional study, we aimed to comprehensively evaluate patients with MN at baseline to determine their PLA2R and THSD7A profile with tissue and serum studies. Primary MN patients who are negative for both PLA2R and THSD7A would be ideal candidates for further testing in view of the newly discovered autoantibodies in MN.7,8
Materials and Methods
Cases of MN diagnosed over a period of 4 years (from January 2014 to December 2017) were retrieved from the archives of the Department of Pathology and included in the study if adequate tissue and relevant clinical details were available. Baseline serum (collected at the time of the biopsy) wherever available was retrieved from the biologic repository maintained by the Department of Nephrology. The study protocol was approved by the institute ethics committee (IEC-PG-502/2017, IEC-NP-259/2013).
Classification into Primary and Secondary MN
Before being labeled as primary MN, every patient was screened with the following investigations to rule out common causes of secondary MN: HBsAg ELISA, anti–hepatitis C virus serology, syphilis serology; workup for autoimmune diseases—serum ANA, anti-dsDNA, serum complement levels, serum ANCA; relevant investigations to rule out malignancies—chest radiograph, abdominal ultrasonogram, urine for atypical cells, stool for occult blood, mammogram (female cases), prostate-specific antigen levels (male cases), and history of intake of drugs that are known to cause MN. American College of Rheumatology criteria were used for the diagnosis of systemic lupus erythematosus. All relevant clinical details (including any prior immunosuppressive therapy) were recorded along with baseline investigations including urine routine microscopy, spot urine protein-to-creatinine ratio for 24-hour protein estimation, serum albumin, and serum creatinine.
PLA2R Direct Immunofluorescence
Tissue staining for PLA2R antigen was done by a paraffin immunofluorescence protocol, using the anti-PLA2R antibody (HPA01265, 1:50; Sigma-Aldrich, St Louis, MO). The validated protocol in literature was used after standardizing the procedure for our laboratory conditions.9 Briefly, 3-μm-thick sections were cut, slides were deparaffinized and treated with proteinase K enzyme, followed by incubation with primary antibody for 30 minutes. The slides were then incubated with biotin-conjugated universal secondary antibody, followed by streptavidin-conjugated phycoerythrin. Glycerol-mounted slides were viewed using a fluorescence microscope under the appropriate filter. Granular capillary wall staining was considered positive. Faint dotlike cytoplasmic staining in podocytes served as the internal control. Positive and negative external controls were run with all batches. Whenever staining was equivocal or was discrepant with serum anti-PLA2R results, tissue staining was repeated.
Serum Anti-PLA2R ELISA
Anti-PLA2R antibody estimation was performed on the corresponding baseline sera. Quantitative serum antibody estimation was done by ELISA using commercial kit according to manufacturer’s protocol (Euroimmune, Lubeck, Germany). The manufacturer-recommended cutoff values of >20 RU/ml for positive and 14 to 20 RU/ml for borderline anti-PLA2R antibody titer10 were considered.
THSD7A Immunohistochemistry
Tissue staining for THSD7A antigen was performed by immunohistochemistry using the anti-THSD7A antibodies (HPA000923, 1:800; Sigma-Aldrich). The validated protocol available in literature was followed after standardizing for our laboratory conditions.11 Briefly, the slides were deparaffinized, followed by heat-induced antigen retrieval in pH 9 buffer and endogenous peroxidase blocking. Overnight incubation with primary antibody was done, after which the slides were incubated with secondary antibody. 3,3′-Diaminobenzidine chromogen was added and the reaction was stopped at optimal staining, and hematoxylin counterstaining was done. Diffuse granular capillary wall staining was considered positive. The paranuclear (cytoplasmic) dotlike staining in podocytes and luminal surface staining in proximal tubules served as internal control.
Statistical Analysis
Data were analyzed using the IBM-SPSS statistics software, and appropriate parametric and nonparametric tests were employed that include 2-sample Wilcoxon rank-sum (Mann-Whitney) test, paired t test, Fisher exact test, and kappa agreement test according to the studied variable. P < 0.05 was considered statistically significant.
Results
In the study period of 4 years, 3935 kidney biopsies were received in our laboratory, of which 405 cases were diagnosed as MN, constituting 10.3% of cases. Of these 405 cases, 216 had adequate tissue and clinical data and they were enrolled in the study. They included 184 adult and 32 pediatric (<18 years) patients with a median age of 34 years and an age range of 6 to 84 years. The male-to-female ratio was 1.44. The duration of illness ranged from 0 to 240 months, with a mean of 26 months.
Clinical Parameters
All patients had some degree of proteinuria, with the most common clinical presentation being nephrotic syndrome, which was present in 50% of the cases. Nephrotic range proteinuria without overt nephrotic syndrome was seen in 25% of the patients and subnephrotic proteinuria, in the remaining 25% patients. Oliguria was seen in 4.3% of the patients, whereas gross hematuria was seen in 1.9% cases.
Of the 216 patients, 164 (76%) were primary and 52 (24%) were secondary MN based on the relevant investigations performed. In both the adult and pediatric cohort, primary MN was more common than secondary MN. The most frequent underlying cause of secondary MN was systemic lupus erythematosus (79%), followed by hepatitis B (10%) and hepatitis C (2%). Secondary MN was significantly more common in female cases, consistent with the higher incidence of autoimmune diseases in them. Overt nephrotic syndrome and higher degree of proteinuria was more frequent in primary MN (69%) than secondary MN (48%) (P value = 0.03). In 67 (36.4%) adult patients, a history of immunosuppressive therapy was recorded, the rest were treatment naive. All pediatric patients had received a trial of steroids before the biopsy.
Histologic Parameters
In addition to the diffuse and global capillary wall thickening characteristic of MN, mesangial expansion with or without hypercellularity was observed in 18.4% of the cases. Secondary segmental sclerosis was present in 13.8% and crescents in 0.9% of the cases. Moderate to severe interstitial fibrosis and tubular atrophy (>25% of the biopsied cortex) was seen in 8.8% of the cases. Presence of mesangial expansion with or without mesangial hypercellularity correlated significantly with secondary MN (P < 0.05). On direct immunofluorescence for Igs and complement, no significant differences were observed between primary and secondary MN.
Tissue PLA2R Direct Immunofluorescence
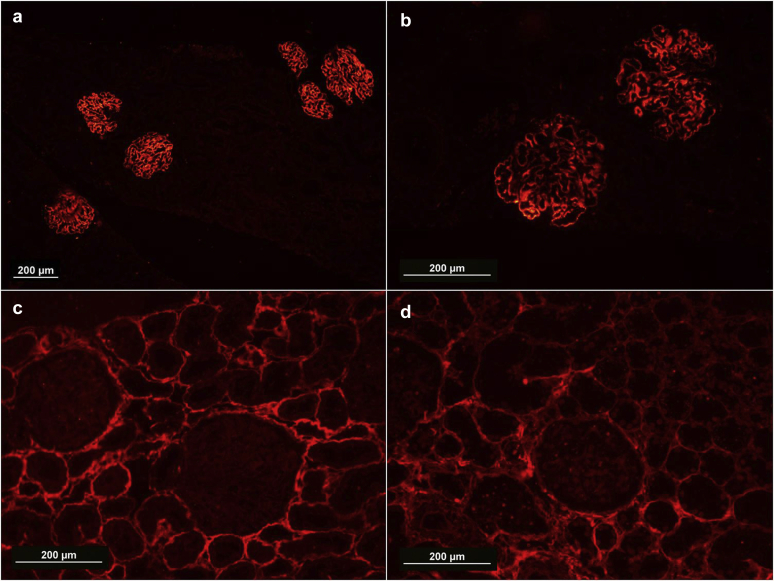
Glomerular capillary wall staining for PLA2R was seen in 52% cases of primary MN (86 of 164), compared to 11.5% of secondary MN cases (P < 0.05). PLA2R-positive secondary MN cases included 5 cases of lupus MN and 1 case of hepatitis B–associated MN. The sensitivity and specificity of tissue PLA2R staining for primary MN was 52.4% and 88.5%, respectively (Figure 1).
Figure 1.
Immunofluorescence for phospholipase A2 receptor demonstrating strong and diffuse capillary wall granular staining in a case of membranous nephropathy (a, original magnification ×10; b, original magnification ×20) and negative staining in a case of membranous nephropathy (c, original magnification ×20). Negative control; a case of minimal change disease also demonstrates lack of capillary wall granular staining (d, original magnification ×20).
Serum Anti-PLA2R ELISA
Serum for PLA2R study was available in 110 patients (92 primary MN and 18 secondary MN). Significant serum anti-PLA2R antibodies were found in 59.7% (55 of 92) of primary MN cases and 11.1% (2 of 18) of secondary MN cases (P < 0.05). This included 2 cases with borderline titers, both of whom had no prior history of immunosuppression. Overall, the titers ranged from 14.77 to 4191.9 RU/ml, with a mean of 240 RU/ml and a median of 97.89 RU/ml. In the adult cohort, the average PLA2R titer was 247.36 RU/ml. In the pediatric cohort, lower titers were noted, with a mean of 138.61 RU/ml and a median of 52.39 RU/ml. Overall, the sensitivity and specificity of serum anti-PLA2R ELISA for primary MN was 61.1% and 88.9%, respectively.
Moderate concordance between tissue PLA2R and serum PLA2R was observed with kappa coefficient of 0.61. On combining both testing methods in the 110 patients, the prevalence of PLA2R-related MN in our cohort rose to 63.6% (72.8% for primary MN and 16.7% for secondary MN)
As shown in Table 1, a total of 22 cases showed discrepancy between tissue staining and serum assay of which 10 cases were negative for tissue PLA2R but showed positive serum anti-PLA2R antibodies. In the baseline sample, 12 cases positive for tissue PLA2R did not show anti-PLA2R antibodies.
Table 1.
Tissue immunofluorescence for PLA2R and serum PLA2R ELISA results in 110 cases of adult and pediatric membranous nephropathy
| Tissue PLA2R | Serum PLA2R | n (%) |
|---|---|---|
| + | + | 49 (45) |
| + | – | 12 (11) |
| – | + | 10 (9) |
| – | – | 39 (35) |
ELISA, enzyme-linked immunosorbent assay; PLA2R, phospholipase A2 receptor.
Tissue THSD7A Staining
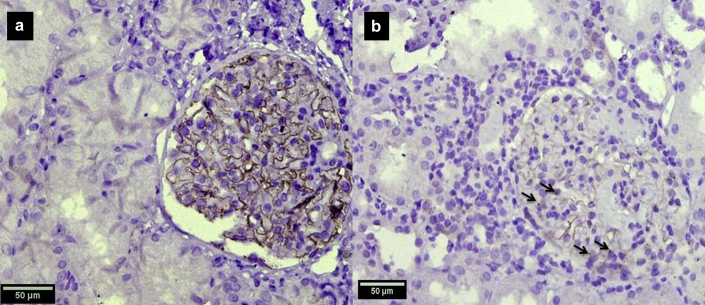
Tissue was available for THSD7A immunohistochemistry in 176 of the 216 cases including 146 cases of primary MN and 30 cases of secondary MN. Five cases (3.4%) of primary MN including 4 adults and 1 pediatric case were positive for THSD7A (Figure 2). One case of adult membranous lupus nephritis was also positive as shown in Table 2. None of the cases had a history of malignancy or a positive malignancy screen at baseline.
Figure 2.
Immunohistochemistry for thrombospondin type 1 domain–containing 7A (THSD7A) demonstrates capillary wall granular staining in a case of THSD7A-related membranous nephropathy (a, original magnification ×40). In a negative case, no capillary wall staining was identified though a dotlike podocytic cytoplasmic staining is noted (b, original magnification ×40).
Table 2.
Clinicopathologic features of THSD7A-immunopositive cases with PLA2R results
| Patient no. | Age/sex | Type of membranous nephropathy | Tissue PLA2R immunofluorescence | Serum anti-PLA2R ELISA |
|---|---|---|---|---|
| 1 | 32/M | Primary | 0 | Negative (12.5 RU/ml) |
| 2 | 50/M | Primary | Positive | Not available |
| 3 | 37/M | Primary | 0 | Positive (111.9 RU/ml) |
| 4 | 49/M | Primary | 0 | Not available |
| 5 | 18/F | Secondary (lupus) | 0 | Negative (not detectable) |
| 6 | 17/F | Primary | 0 | Not available |
ELISA, enzyme-linked immunosorbent assay; F, female; M, male; PLA2R, phospholipase A2 receptor; THSD7A, thrombospondin type 1 domain–containing 7A.
Two cases were dual positive for both PLA2R and THSD7A—one was PLA2R positive by tissue IF and the other by serum ELISA for PLA2R (Table 2).
Comparison of Clinical and Histologic Features Between PLA2R Positive and Negative Groups
Comparison of clinical features between PLA2R positive and negative groups did not show any significant difference, except for degree of proteinuria, which was higher in the PLA2R-positive group (P < 0.01). The quantitative serum anti-PLA2R titer showed good correlation with 24-hour proteinuria, with Spearman correlation coefficient of 0.6. Both the cases which showed crescents histologically showed positive tissue PLA2R staining. PLA2R-negative cases had more significant interstitial fibrosis and tubular atrophy (>25% cortical area) than the PLA2R-positive cases (P = 0.001).
Pediatric MN
This cohort included 32 patients ranging from 6 to 17 years of which clinically 25 were primary and 7 were secondary. The underlying condition in secondary MN included 4 lupus MN, 2 hepatitis B–related MN and 1 hepatitis C virus–related MN. Eleven of the 25 primary MN (44%) were positive for PLA2R on tissue staining, whereas none of the secondary MN were positive. Serum was available for testing in 5 cases of primary MN, 4 of which were positive (which were also positive on tissue staining). THSD7A was tested for in 29 cases with sufficient tissue and was found to be positive in a single case—a 17-year-old with primary MN.
Secondary MN
A total of 7 secondary MN cases (13.5%; 6 cases of lupus MN and 1 case of hepatitis B–related MN) of the total of 52 showed PLA2R positivity. A case of lupus MN showed THSD7A positivity (Table 3).
Table 3.
Clinicopathologic features of PLA2R- and THSD7A-positive secondary membranous nephropathy
| Patient no. | Age/sex | Etiology | Tissue PLA2R | Serum anti-PLA2R | Tissue THSD7A |
|---|---|---|---|---|---|
| 1 | 35/F | SLE | + | NA | — |
| 2 | 34/F | SLE | + | NA | NA |
| 3 | 40/M | SLE | + | NA | NA |
| 4 | 42/F | SLE | + | — | — |
| 5 | 32/M | SLE | + | — | — |
| 6 | 31/F | SLE | — | + | — |
| 7 | 33/M | Hepatitis B | + | + | NA |
| 8 | 18/F | SLE | — | — | + |
F, female; M, male; NA, not available; PLA2R, phospholipase A2 receptor; SLE, systemic lupus erythematosus; THSD7A, thrombospondin type 1 domain–containing 7A.
Discussion
A large number of studies worldwide have shown that 70% to 80% of primary MN are PLA2R related12,13 though variation has been observed in patients of different ethnicities. In the Indian population, the 5 studies14, 15, 16, 17, 18, 19 available so far demonstrate a prevalence of PLA2R of 55% to 88% in primary MN and 0 to 3.7% in secondary MN using serum- and/or tissue-testing techniques. Only 1 study used multiple methods of testing, including serum ELISA, serum indirect immunofluorescence, and tissue immunohistochemistry, with a combined prevalence of 82.45% in the 114 cases of primary MN included; however, it did not assess secondary MN.15 No study from India has investigated the second autoantigen THSD7A. This study from a large cohort in North India has shown a PLA2R prevalence of approximately 72.8% in primary MN using both tissue immunofluorescence testing and serum ELISA studies and 52% on a larger cohort tested with only tissue PLA2R immunofluorescence. This study has also for the first time documented THSD7A-positive MN in Indian patients, and a prevalence rate of 3.4% in primary MN similar to studies in other cohorts.
Our PLA2R-positive MN were significantly more proteinuric than the PLA2R-negative cases with good concordance between titers and degree of proteinuria. The PLA2R-negative MN had lesser degree of proteinuria and higher IF-TA scores, suggesting delay in presentation. Moderate concordance (kappa coefficient 0.61) was observed between tissue PLA2R and serum anti-PLA2R testing, with a small percentage of outliers. In 12 patients, 6 of whom had received immunosuppression prior to the biopsy, tissue PLA2R positivity was observed with negative serum baseline PLA2R. This may be the result of therapy-induced antibody clearance or the well-described “sink phenomenon.”20 In 10 patients, positive serum testing with repeatedly negative tissue PLA2R was noted. It is possible that the antigenic epitopes recognized by the commercial antibody may be masked by the bound autoantibodies, thus evading detection.20 Thus, in our experience, a combined approach appears to be most beneficial to determine the presence of a PLA2R-related MN.
THSD7A testing by immunohistochemistry demonstrated a prevalence of 3.4% in primary MN. In addition, 1 patient of lupus MN also demonstrated positivity. THSD7A is highly specific for primary MN, with studies in secondary MN largely showing negative results, except for 2 patients in literature who showed positive serum anti-THSD7A antibodies.21 One patient had lupus MN and the other had advanced prostatic carcinoma. Tissue staining was not performed in either patient. Though studies have shown association between THSD7A-associated MN and underlying malignancy, with malignant tumors being detected in 6% to 20% of patients with THSD7A-related MN22,23 in our study, all THSD7A-positive cases had a negative malignancy screen.
Interestingly, 2 of our cases showed dual positivity for PLA2R and THSD7A. Though PLA2R and THSD7A were previously believed to be mutually exclusive, rare cases of dual positivity have been described. Six such cases have been described in the literature so far (all being PMN).24,25 The possible explanation is that the common antigenic motif present in the N-terminal region of both PLA2R and THSD7A activates B cells to produce antibodies, which may be directed against both or predominantly 1 of the 2 antigens.
The large cohort studied included 32 cases of pediatric MN, 25 of which were primary MN and 7 secondary MN. Only a few studies have studied the prevalence of PLA2R staining in pediatric primary MN and have shown a lower prevalence in children compared to adults: 45% by Cossey et al.—the largest study so far, with 22 pediatric cases26—and 50% by Dettmar et al.27 An Indian study involving 5 pediatric primary MN cases had shown PLA2R positivity in all 5 cases studied.28 This study also pointed out that the anti-PLA2R titer was significantly lower than the titer seen in adults. Though only few pediatric patients had baseline serum for testing in our study, the mean serum anti-PLA2R titer in children was lower than that of adults. This is likely related to the steroid trial given to all pediatric patients before biopsy.
Though PLA2R was thought to be specific to primary MN, recent studies have shown PLA2R positivity in secondary MN (ranging from 0 to 64%) with underlying hepatitis, sarcoidosis, and malignancies.9 We observed a PLA2R prevalence of 11.5% (6 of 52) in secondary MN, most of which were lupus membranous. PLA2R positivity in lupus MN was not recognized in literature until recently Garcia et al. described 7 PLA2R-positive cases among 37 lupus MN cases studied (19%).29 Whether PLA2R positivity in secondary MN is due to a coincidental presence of primary MN in patients with an unrelated systemic disease is not clearly known. IgG subclass staining might help resolve the correct diagnosis in these cases, predominant IgG4 pattern favoring coexistent disease with PLA2R-related PMN.
Conclusion
In a large cohort of Indian patients comprehensively evaluated at baseline, this study demonstrates prevalence rates of PLA2R- and THSD7A-related MN similar to world literature, including our substantial cohort of pediatric MN. In addition, this study also shows the variation in MN in the form of outliers within PLA2R (related to tissue and serum testing), dual positivity for PLA2R and THSD7A, and PLA2R/THSD7A-positive secondary MN.
Disclosure
All the authors declared no competing interests.
Acknowledgements
This work was presented in part in the 3rd International Renal Pathology Conference (Meeting of the Renal Pathology Society) held in New Delhi, India, February 10–12, 2017.
References
- 1.Jennette J.C., Olson J.L., Silva F.G., D’Agati V., editors. Heptinstall’s Pathology of the Kidney. 7th ed. Wolters Kluwer; Philadelphia, PA: 2015. [Google Scholar]
- 2.Beck L.H., Bonegio R.G.B., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vriese A.S., Glassock R.J., Nath K.A. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomas N.M., Beck L.H., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck L.H. PLA2R and THSD7A: disparate paths to the same disease? J Am Soc Nephrol. 2017;28:2579–2589. doi: 10.1681/ASN.2017020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fresquet M., Rhoden S.J., Jowitt T.A. Autoantigens PLA2R and THSD7A in membranous nephropathy share a common epitope motif in the N-terminal domain. J Autoimmun. 2019;106:102308. doi: 10.1016/j.jaut.2019.102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi S, Debiec H, Madden B, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 97:163–174. [DOI] [PubMed]
- 8.Sethi S., Madden B.J., Debiec H. Exostosin 1/exostosin 2–associated membranous nephropathy. J Am Soc Nephrol. 2019;30:1123–1136. doi: 10.1681/ASN.2018080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen C.P., Messias N.C., Silva F.G. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 10.Dähnrich C., Komorowski L., Probst C. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013;421:213–218. doi: 10.1016/j.cca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Larsen C.P., Cossey L.N., Beck L.H. THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol. 2016;29:421–426. doi: 10.1038/modpathol.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. Erratum in: Correction. Clin J Am Soc Nephrol. 2017;12:1528. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai H., Zhang H., He Y. Diagnostic accuracy of PLA2R autoantibodies and glomerular staining for the differentiation of idiopathic and secondary membranous nephropathy: an updated meta-analysis. Sci Rep. 2015;5:8803. doi: 10.1038/srep08803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopalakrishnan N., Abeesh P., Dineshkumar T. Prevalence of serum anti M-type phospholipase A2 receptor antibody in primary membranous nephropathy: a single center experience. Indian J Nephrol. 2016;26:257–261. doi: 10.4103/0971-4065.160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran R., Kumar V., Kumar A. PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians. Nephrol Dial Transplant. 2016;31:1486–1493. doi: 10.1093/ndt/gfv399. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran R., Yadav A.K., Kumar V. Temporal association between PLA2R antibodies and clinical outcomes in primary membranous nephropathy. Kidney Int Rep. 2017;3:142–147. doi: 10.1016/j.ekir.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S., Korula A., Basu G. Immunohistochemical glomerular expression of phospholipase A2 receptor in primary and secondary membranous nephropathy: a retrospective study in an Indian cohort with clinicopathological correlations. Nephron Extra. 2017;7:1–9. doi: 10.1159/000453675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudipati A., Uppin M.S., Kalidindi R.K. Immunohistochemical analysis of anti-phospholipase A2 receptor antibody on renal biopsies: a single tertiary care center study. Indian J Nephrol. 2017;27:353–358. doi: 10.4103/ijn.IJN_79_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yachha M., Sharma R.K., Mehrotra S. Anti-phospholipase A2 receptor antibody in membranous nephropathy; an Indian experience. J Renal Inj Prev. 2018;7:16–21. [Google Scholar]
- 20.Francis J.M., Beck L.H., Salant D.J. Membranous nephropathy: a journey from bench to bedside. Am J Kidney Dis. 2016;68:138–147. doi: 10.1053/j.ajkd.2016.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren S., Wu C., Zhang Y. An update on clinical significance of use of THSD7A in diagnosing idiopathic membranous nephropathy: a systematic review and meta-analysis of THSD7A in IMN. Ren Fail. 2018;40:306–313. doi: 10.1080/0886022X.2018.1456457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoxha E., Beck L.H., Wiech T. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain–containing 7A–specific antibodies in membranous nephropathy. J Am Soc Nephrol. 2017;28:520–531. doi: 10.1681/ASN.2016010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S.G., Larsen C.P. Tissue staining for THSD7A in glomeruli correlates with serum antibodies in primary membranous nephropathy: a clinicopathological study. Mod Pathol. 2018;31:616–622. doi: 10.1038/modpathol.2017.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwakura T., Ohashi N., Kato A. Prevalence of enhanced granular expression of thrombospondin type-1 domain-containing 7A in the glomeruli of Japanese patients with idiopathic membranous nephropathy. PLoS One. 2015;10:e0138841. doi: 10.1371/journal.pone.0138841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen C.P., Cossey L.N., Beck L.H. THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol. 2016;29:421–426. doi: 10.1038/modpathol.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cossey L.N., Walker P.D., Larsen C.P. Phospholipase A2 receptor staining in pediatric idiopathic membranous glomerulopathy. Pediatr Nephrol Berl Ger. 2013;28:2307–2311. doi: 10.1007/s00467-013-2574-9. [DOI] [PubMed] [Google Scholar]
- 27.Dettmar A.K., Wiech T., Kemper M.J. Pediatric MN Study Group, Immunohistochemical and serological characterization of membranous nephropathy in children and adolescents. Pediatr Nephrol. 2018;33:463–472. doi: 10.1007/s00467-017-3817-y. [DOI] [PubMed] [Google Scholar]
- 28.Kumar V., Ramachandran R., Kumar A. Antibodies to m-type phospholipase A2 receptor in children with idiopathic membranous nephropathy: PLA2R antibodies in paediatric IMN. Nephrology. 2015;20:572–575. doi: 10.1111/nep.12478. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Vives E., Solé C., Moliné T. Antibodies to M-type phospholipase A2 receptor (PLA 2 R) in membranous lupus nephritis. Lupus. 2019;28:396–405. doi: 10.1177/0961203319828521. [DOI] [PubMed] [Google Scholar]