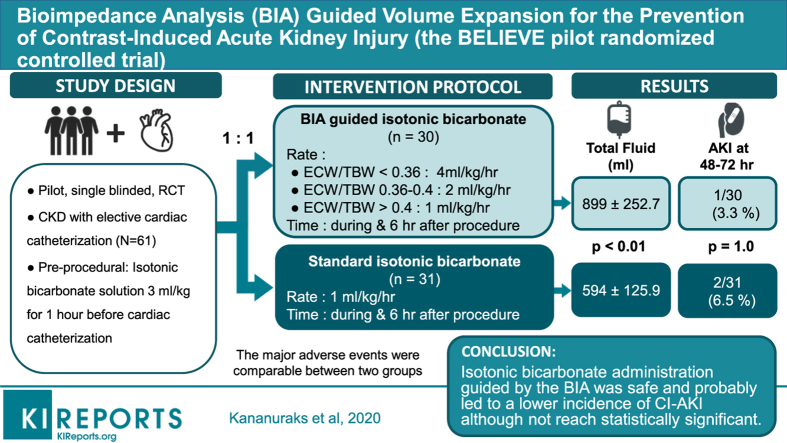
Abstract
Introduction
Peri-procedural i.v. fluid administration is important for the prevention of contrast-induced acute kidney injury (CI-AKI). However, standardized fluid management protocols may not be suitable for all patients. We therefore wished to determine whether an individualized fluid administration protocol guided by measuring extracellular water (ECW) using bioimpedance analysis (BIA) would be safe and would reduce the incidence CI-AKI compared to a standardized fluid administration prescription.
Methods
In this pilot, randomized, parallel-group, single-blind, controlled trial, we compared the effect of BIA-guided isotonic bicarbonate administration according to the ratio of ECW to total body water (ECW/TBW) to our standard isotonic bicarbonate protocol in regard to the safety and efficacy of preventing CI-AKI in chronic kidney disease patients undergoing elective cardiac angiography. Our primary outcome was the incidence of CI-AKI, which was defined as a ≥0.3 mg/dl or 150% increase in serum creatinine concentration within 48 to 72 hours after cardiac angiography.
Results
We studied 61 patients, 30 in the bioimpedance group and 31 in the control group. Age was similar (72.5 ± 7 vs. 71.4 ± 7.9 years), as were body mass index (25.5 vs. 25.8 kg/m2) and baseline serum creatinine (1.3 ± 0.3 vs. 1.4 ± 0.4 mg/dl). The peri-procedural fluid volume administered was significantly greater in the BIA-guided hydration group (899.0 ± 252.7 ml vs. 594.4 ± 125.9 ml, P < .01). The incidence of CI-AKI was 3.3% in BIA-guided hydration group and 6.5% in the control group (relative risk = 0.52, 95% confidence interval = 0.05−5.40, P = 1.00). Adverse events reported were comparable between groups (6.7% vs. 6.5%, P = 1.00).
Conclusions
The overall incidence of CI-AKI after cardiac angiography in our patients with mild-to-moderate renal insufficiency was lower than anticipated. Isotonic bicarbonate administration guided by bioimpedance measurements was safe, and probably led to a lower incidence of CI-AKI, although this not reach statistical significance.
Keywords: bioimpedance analysis, cardiac catheterization, contrast-induced acute kidney injury, i.v. fluid
Graphical abstract
Contrast-induced acute kidney injury (CI-AKI) is the most common complication after cardiac angiography, especially in high-risk populations such as patients with chronic kidney disease, those with diabetes mellitus, elderly patients, and patients with heart failure,1, 2, 3 resulting in increased patient morbidity and mortality, increased health care costs, and prolonged hospitalization.4,5 Iodinated contrast media can induce renal vasoconstriction and can have a direct cytotoxic effect leading to acute tubular injury. Once AKI occurs, there is no specific treatment to reduce tubular injury, so studies have concentrated on prevention.6, 7, 8 Many preventive strategies have been evaluated, including periprocedural i.v. volume expansion, use of renal vasodilatory and antioxidant drugs, use of low- or iso-osmolar iodinated contrast agents, hemodialysis, or hemodiafiltration have been evaluated. Although hydration with sodium-containing fluids is currently the most effective strategy in reducing CI-AKI, study protocols differ in the rate of administration and in the amount of fluid given before, during, and after angiography.8, 9, 10, 11, 12
Recently, several clinical trials have shown the benefit of individualized i.v. fluid administration according to fluid status assessed by left ventricular end-diastolic pressure (LVEDP) using the RenalGuard Therapy system (RenalGuard Solutions, Inc., Milford, MA) as well as the benefit of invasively measuring central venous pressure on the reduction of the incidence of CI-AKI.13, 14, 15, 16 However, these all require either invasive measurements or specialized equipment, limiting their application to patients attending for routine day case angiography. On the othe hand, BIA is a rapid, noninvasive, inexpensive, accurate tool for determining ECW, and is used clinically to aid volume assessment in the management of patients with chronic kidney disease, those needing dialysis, and those with heart failure.17, 18, 19, 20 An earlier observational study reported that patients with a lower ECW as measured by bioimpedance had a greater risk of CI-AKI,21 and that administering fluid at a faster rate reduced the subsequent risk of CI-AKI in patients with stable coronary artery disease who had lower ECW as measured by bioimpedance at the time of hospital admission.22
We therefore wished to determine whether bioimpedance analysis−guided volume expansion could be safe and effective in preventing contrast-induced acute kidney injury, by comparing the incidence of CI-AKI in patients with chronic kideney disases attending for routine cardiac angiography who had fluid admistration individualized according to their bioimpedance measurements as compared to our standard fluid administration protocol.
Materials and Methods
Study Population
Between May 1, 2015 and January 8, 2016, we screened all consecutive patients referred for cardiac angiography to the Ramathibodi Hospital of Mahidol University, Bangkok, Thailand. The inclusion criteria for our study included age 18 to 90 years and estimated glomerular filtration rate (eGFR) of ≤60 ml/min per 1.73 m2 who were attending for elective cardiac catheterization. We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Exclusion criteria included emergency cardiac catheterization (e.g., primary percutaneous coronary intervention for ST-segment elevation myocardial infarction), administration of radiographic contrast within the previous 14 days, congestive heart failure (New York Heart Association class III and IV), ascites, pleuro-pericardial effusion, severe valvular heart disease, left ventricular ejection fraction of <40%, history of kidney transplantation, renal replacement therapy, allergy to radiographic contrast agent, unstable renal function (change in serum creatinine concentration of ≥0.5 mg/dl or 25% within the previous 14 days), liver cirrhosis Child class B or C, change in dosage of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker within the previous 14 days, and inability to provide informed consent.
Study Protocol
Eligible patients were randomly assigned in a 1:1 ratio to either the BIA-guided hydration group or the standard fluid administration group (control group). After eligible patients had been enrolled, they were assigned to their group using sealed opaque envelopes that had a randomization number of block sizes of 4. The study was single blind, and patients were not told to which group they had been allocated. Laboratory personnel likewise did not know about the patients’ group assignments. The investigating physicians were not blinded, whereas cardiologists were blinded.
The ECW and TBW were measured in all patients using multifrequency BIA (InBody 720, Seoul, South Korea), and then all patients received a bolus infusion of isotonic sodium bicarbonate at 3 ml/kg for 1 hour before cardiac catheterization. After this bolus infusion, patients in the BIA-guided group were given additional i.v. isotonic bicarbonate for the duration of procedure and then 6 hours after the procedure, adjusted according to the ratio of ECW/TBW as follows: 4 ml/kg per hour if ECW/TBW was <0.36; 2 ml/kg per hour if ECW/TBW was 0.36 to 0.4; and 1 ml/kg per hour if ECW/TBW was >0.4 respectively. The control group was hydrated with isotonic bicarbonate at 1 ml/kg/h for the duration of procedure and for 6 hours postprocedure. All study participants received intra-arterial low-osmolar (non-ionic monomer) contrast media. The LVEDP was measured at the time of cardiac catheterization.
We measured baseline serum creatinine and cystatin C concentrations on the day of cardiac catheterization before the procedure and fluid administration. Serum creatinine and serum cystatin C level were reassessed again at 48 to 72 hours after cardiac catheterization. All patients had been instructed to discontinue taking metformin, diuretics, and nonsteroidal anti-inflammatory drugs 24 hours before and then after angiography. All patients were followed up for 14 days after their cardiac catheterization.
Endpoints
The primary endpoint was the incidence of CI-AKI, defined as an increase in serum creatinine concentration of ≥0.3 mg/dl or 150% from the baseline value within 48 to 72 hours after cardiac catheterization. The secondary endpoints were an increased serum cystatin C concentration of 10% or more above the baseline value within 48 to 72 hours after cardiac catheterization, composite of an increase in serum creatinine concentration or an increase in serum cystatin C concentration and requirement of renal replacement therapy within 14 days. We recorded safety endpoints including complications of i.v. fluid administration and cardiac catheterization such as pulmonary edema, ventricular arrhythmias, and bleeding.
Statistical Analysis
Data were checked for normality; continuous data are presented as mean ± SD or as median and interquartile range. Continuous variables were compared with the Student t test or Mann–Whitney rank sum test, respectively. Categorical data are reported as absolute values and percentages. Categorical variables were compared using Fisher exact tests. Pearson univariate correlation coefficients were calculated comparing LVEDP to ECW/TBW. Relative risk and 95% confidence interval for the primary CI-AKI endpoint were calculated.
We conducted a pilot and feasibility study because of the unavailability of safety and efficacy data from different protocols of peri-procedural isotonic bicarbonate use. However, we determined a priori a sample size of 60 participants in which randomization into 2 groups would be enable us to observe the safety and efficacy of the intervention.
Analyses were done with Stata version 14.0 (StataCorp, College Station, TX). All tests were 2-sided, and significant differences were reported when P values were < 0.05.
Ethics
This study was approved by the Internal Review Board of Ramathibodi Hospital (ID 03-58-28), and all patients provided written informed consent, following the principles of the Declaration of Helsinki. The trial was registered with ClinicalTrials.gov (number NCT 02449317).
Results
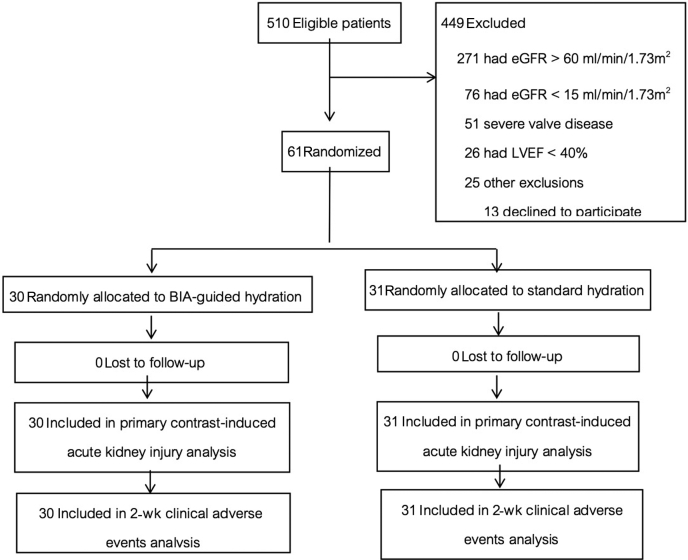
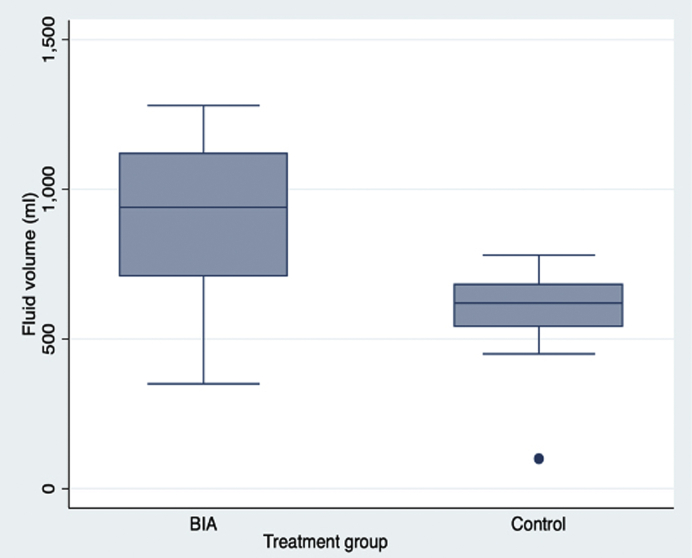
We enrolled 61 of a potential pool of 510 patients, who were randomly assigned to the BIA-guided hydration group (n = 30) and the standard hydration group (n = 31) (Figure 1). Baseline characteristics were not significantly different between groups (Table 1). The Mehran risk score3 for CI-AKI was 6.1 ± 2.6. The total mean (SD) volume of isotonic bicarbonate administered in 7 hours was greater in the BIA-guided hydration group compared to the control group (Figure 2).
Figure 1.
Study Consolidated Standards of Reporting Trials (CONSORT) diagram. Number of patients who were recruited to the study, assigned to a study group, and completed the protocol. BIA, bioimpedance analysis; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction.
Table 1.
Baseline characteristics of the study population
| Parameters | BIA-guided hydration group (n = 30) | Control group (n = 31) |
|---|---|---|
| Age, yr | 72.5 ± 7.0 | 71.4 ± 7.9 |
| Female sex, n (%) | 12 (40.0) | 13 (41.9) |
| Weight, kg | 66.9 ± 13.0 | 65.6 ± 15.9 |
| Height, cm | 161.7 ± 8.6 | 162.6 ± 7.9 |
| Body mass index, kg/m2 | 25.5 ± 4.5 | 25.8 ± 4.5 |
| Blood pressure, mm Hg | ||
| Systolic | 139.4 ± 19.0 | 138.8 ± 16.0 |
| Diastolic | 74.5 ± 10.9 | 75.6 ± 8.8 |
| Renal function | ||
| Serum creatinine, mg/dl | 1.3 ± 0.3 | 1.4 ± 0.4 |
| Serum cystatin C, mg/l | 1.3 ± 0.3 | 1.4 ± 0.3 |
| Serum blood urea nitrogen, mg/dl | 19.6 ± 6.1 | 19.8 ± 6.6 |
| Estimated GFR, ml/min per 1.73 m2 | 49.7 ± 11.1 | 47.2 ± 11.9 |
| Estimated GFR, ml/min per 1.73 m2 category, n (%) | ||
| 45–60 | 20 (66.7) | 17 (54.8) |
| 30–44 | 7 (23.3) | 11 (35.5) |
| 15–29 | 3 (10.0) | 3 (9.7) |
| Extracellular water/total body water | 0.39 ± 0.01 | 0.40 ± 0.01 |
| Extracellular water/total body water category, n (%) | ||
| <0.36 | 0 | 0 |
| 0.36–0.40 | 22 (73.3) | 16 (51.6) |
| >0.40 | 8 (26.7) | 15 (48.4) |
| Left ventricular ejection fraction (%) | 61.9 ± 11.9 | 55.7 ± 8.5 |
| Underlying disease, n (%) | ||
| Diabetes mellitus | 12 (40.0) | 16 (51.6) |
| Hypertension | 29 (96.7) | 30 (96.8) |
| Dyslipidemia | 26 (86.7) | 26 (83.9) |
| Congestive heart failure | 3 (10.0) | 5 (16.1) |
| Previous percutaneous coronary | 11 (36.7) | 11 (35.5) |
| Intervention | ||
| Previous coronary artery bypass graft | 0 | 2 (6.5) |
| Medications, n (%) | ||
| RAAS-blocking agent | 19 (63.3) | 16 (51.6) |
| Diuretic | 4 (13.3) | 10 (32.3) |
| Statin | 24 (80.0) | 29 (93.6) |
| β-blocker | 19 (63.3) | 21 (67.7) |
| Calcium channel blocker | 16 (53.3) | 14 (45.2) |
| Insulin | 3 (10.0) | 5 (16.1) |
| Laboratory data | ||
| Hemoglobin concentration, g/dl | 12.4 ± 1.8 | 12.7 ± 1.5 |
| Hemoglobin A1C, % | 6.4 ± 0.7 | 7.3 ± 1.6 |
| Urine spot protein––creatinine ratio | 0.6 ± 1.4 | 0.5 ± 0.7 |
| Serum sodium, mEq/l | 135.6 ± 23.2 | 139.8 ± 2.4 |
| Serum uric acid, mg/dl | 6.8 ± 2.1 | 7.3 ± 1.7 |
| Serum albumin, g/dl | 36.7 ± 3.5 | 37.1 ± 4.0 |
| Procedural details | ||
| Contrast volume | 40 (30,65) | 45 (25,70) |
| Procedure duration | 42.5 (33,53) | 43 (32,65) |
| Percutaneous coronary intervention, n (%) | 8 (26.7) | 11 (35.5) |
| Number of vessel disease >1, n (%) | 11 (36.7) | 17 (54.8) |
| Left ventricular end-diastolic pressure, mm Hg | 23.7 ± 6.6 | 20.2 ± 6.5 |
| Total risk score category, n (%) | ||
| Low risk [0–5] | 13 (43.3) | 12 (38.7) |
| Moderate risk [6–10] | 14 (46.7) | 17 (54.8) |
| High risk [11–16] | 3 (10.0) | 2 (6.5) |
BIA, bioimpedance analysis; GFR, glomerular filtration rate; RAAS, renin–angiotensin–aldosterone system.
Figure 2.
Hydration volume of isotonic bicarbonate administered in each group. The box for each group represents the 25th percentile to the 75th percentile of the data (i.e., the interquartile range [IQR]). The line in the middle of the box indicates the median (50th percentile) of the data. The whiskers start from the edge of the box and extend to the farthest datapoint that is within 1.5 times the IQR. BIA, bioimpedance analysis.
Primary outcome
The overall incidence of CI-AKI was 4.9% (3 of 61) and was not significantly different between groups (Table 2).
Table 2.
Occurrence of contrast-induced acute kidney injury
| Endpoints | BIA-guided hydration group (n = 30) | Control group (n = 31) | Relative risk (95% CI) | P value |
|---|---|---|---|---|
| Primary endpoint | ||||
| ≥0.3 mg/dl or 1.5 times increase in serum creatinine | 1 (3.3) | 2 (6.5) | 0.52 (0.05–5.40) | 1.00 |
| Secondary endpoint | ||||
| ≥10% increase in serum cystatin C | 2 (6.7) | 5 (16.1) | 0.41 (0.09–1.97) | 0.43 |
| ≥0.3 mg/dl or 1.5 times increase in serum creatinine or ≥10% increase in serum cystatin C | 3 (10.0) | 6 (19.4) | 0.52 (0.14–1.88) | 0.47 |
BIA, bioimpedance analysis; CI, confidence interval.
Data are n (%).
Secondary Outcomes
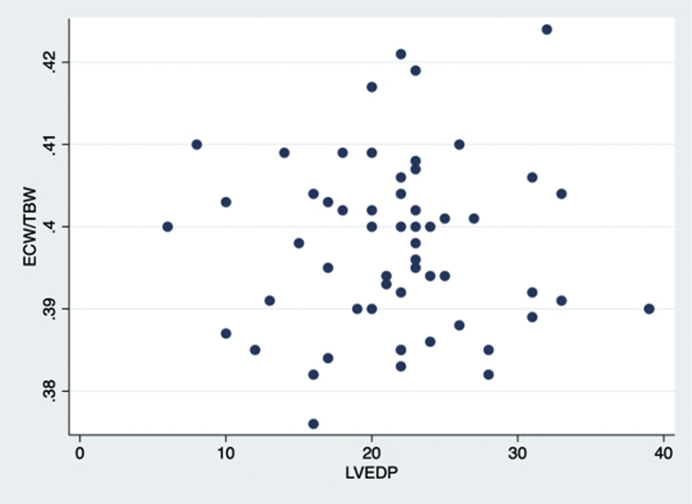
Similarly, there was no difference in patients with a 10% or greater increase in serum cystatin C concentration, or the composite end point of either an increase in serum creatinine or cystatin C (Table 2). No patients in either group required renal replacement therapy within 14 days after contrast agent exposure. There was no correlation between LVEDP and ECW/TBW (r = −0.02) (Figure 3).
Figure 3.
Correlations compared between left ventricular end-diastolic pressure (LVEDP) and ratio of extracellular water to total body water (ECW/TBW).
Adverse Events
Of the patients, 6.6% experienced a major adverse event, with no differences between groups (Table 3). One patient in each group discontinued i.v. fluids early because of pulmonary edema and received i.v. diuretics (P = 1.00). In both cases, the ECW/TBW ratio was >0.4.
Table 3.
Major adverse events in the study population
| Major adverse events | BIA-guided hydration group (n = 30) | Control group (n = 31) | P value |
|---|---|---|---|
| Any | 2 (6.7) | 2 (6.5) | 1.00 |
| Pulmonary edema | 1 (3.3) | 1 (3.2) | 1.00 |
| Arrhythmia | 1 (3.3) | 0 | 0.49 |
| Bleeding | 1 (3.3) | 1 (3.2) | 1.00 |
Data are n (%).
BIA, bioimpedance analysis.
Discussion
Previous studies have highlighted that CI-AKI is more likely to occur in patients who are volume depleted. However, patients with cardiac dysfunction are at increased risk for pulmonary edema, so rapid administration of fluids may be harmful. As such, our BELIEVE (Bioimpedance Analysis Guided Volume Expansion for the Prevention of Contrast- Induced Acute Kidney Injury) study was designed as a pilot randomized controlled trial to compare standard protocol fluid administration with isotonic bicarbonate guided by BIA, to determine whether individualized fluid administration would reduce the risk of CI-AKI. However we found no difference in the incidence of CI-AKI between the groups.
The overall incidence of CI-AKI in our study was lower (4.9%) than we had anticipated when comparing to earlier reports.4,5,10,13 This may have been due to a number of factors, including studying stable patients attending for outpatient routine cardiac catherization, who had a left ventricular ejection fraction of >40%, with stable chronic kidney disease stage 3 or 4, coupled with use of a small volume of radiocontrast in patients receiving hydration with isotonic bicarbonate. As such, our patients were classified as being at mild-to-moderate risk for developing CI-AKI using the Mehran score. In addition, they were compared to those in an earlier observational study that reported an association between dehydration and risk of CI-AKI; however, none of our patients were significantly dehydrated, with a ECW/TBW ratio of 0.36 or less, and as such the incidence of CI-AKI was lower22 and is keeping with some recent studies that also used hydration protocols.23
Bioimpedance has been reported to be a rapid, noninvasive, inexpensive, accurate tool for evaluating volume status. We were able to measure ECW/TBW in all patients, and, by using ECW/TBW to guide fluid balance, we administered more fluid to the bioimpedance group; although the incidence of CI-AKI was not statistically lower, there was trend for a reduction. As such, we cannot exclude a type II sttaistical error, in that for stable patients with chronic kidney disease stages 3 and 4 with a low risk of CI-AKI, much larger studies would be required to demonstrate that bioimpedance-guided fluid administration was not benficial over standard protocol fluid prescription. On the other hand, although more fluid was given, there was no difference in adverse effects such as pulmonary edema due to fluid administration. Thus, stratifying patients according to bioimpedance ECW/TBW measurements allows individualization of fluid management and thus improving hydration status without increasing the risk of volume overload.
There is currently no clear evidence to support the choice of optimal rate and duration of fluid administration to patients to prevent CI-AKI in those attending for cardiac angiography. We sought to develop a simple, pragmatic hydration protocol that can be used in the outpatient care setting. There have been a number of clinical trials, typically in high-risk patients, that evaluated various methods to determine optimal hydration for the prevention of CI-AKI.13,15,16,24 The Prevention of Contrast Renal Injury With Different Hydration Strategies (POSEIDON) trial also reported that LVEDP-guided fluid administration reduced the incidence of CI-AKI and major clinical adverse events in patients undergoing cardiac catheterization,13 and another study that used central venous pressure to guide fluid administration also reported an advantage in patients with congestive heart failure.16 We found no association between LVEDP and ECW/TBW, although patients would have received additional fluid between measuring ECW/TBW and then LVEDP during the cardiac catheterization. The LVEDP may better reflect intravascular volume, as LVEDP is dependent upon intracardiac volume but is also affected by left ventricular dysfunction and valvular heart disease, On the other hand, bioimpedance measures total ECW, rather than just plasma volume; but even so, a reduced ECW is strongly associated with a reduced plasma volume, although an increased ECW does not necessarily imply an increased plasma volume.25
The previous study revealed that an LVEDP below 22 mm Hg was associated with a higher incidence of CI-AKI; however, a recent study showed that an LVEDP above 20 mm Hg was associated with higher incidence of CI-AKI in the setting of poor left ventricular ejection fraction (LVEF <40%). These data uncover the effect of cardiac contractility on LVEDP. In this study, the participants had good LVEF and high LVEDP, although the BIA parameter showed low ECW/TBW. There is a possibility that LVEDP was measured after participants received the initial pre-procedural i.v. protocol of 3 ml/kg per hour of isotonic sodium bicarbonate.
Our study is the first randomized controlled trial to compare the administration of different rates of fluid administration guided by bioimpedance to patients with nonemergency cardiac catheterization. The primary results showed that the occurrence of CI-AKI was lower than expected and that the incidence of CI-AKI was lower in the BIA-guided group, although it not reach statistically significance. Moreover, the safety of the protocol was comparable to that of the standard protocol.
Our study has some limitations. First, our pilot trial was a single-center study with a relatively small sample size that may have been underpowered to detect a benefit. Although we administered more fluid in the bioimpedance group, the absolute volumes that we used were much less than in other studies, which reported a positive effect with a reduction in CI-AKI.22 Second, although the patients were blinded, the investigating physicians were not blinded. However, the cardiologists who performed the intervention were blinded to the treatment assignment, and the procedural time and contrast volume, which may affect the primary outcome, were comparable between groups. Third, we not only included low-risk patients, but most patients had normal or mildly increased ECW/TBW before fluid administration. This may be due to our policy of advising patients to discontinue diuretics before admission, in addition to potentially nephrotoxic medications, resulting in very low incidence of CI-AKI.
In conclusion, we found that the overall incidence of CI-AKI after cardiac catheterization in our study was much lower than that reported in other studies in outpatients with stable cardiac disease with mild-to-moderate chronic kidney disease attending for cardiac catherization. In our study, athough the incidence of CI-AKI was lower in the group given isotonic bicarbonate guided by bioimpedance analysis, this was not statistically different from results in our standard isotonic bicarbonate hydration protocol. Adverse effects of the protocol were comparable to those of the standard protocol.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding was provided by the Faculty of Medicine, Ramathibodi Hospital, Mahidol University. We would like to acknowledge valuable assistant from the cardiac catheterization team, Division of nutrition, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
Ethics Approval and Consent to Participate
This study was approved by the Committee on Human Rights Related to Research Involving Human Subjects’ Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. The written informed consent was obtained from all participants.
Availability of Data
The datasets generated during and/or analyzed during the current study are not publicly available due security reason but are available from the first author on reasonable request.
Author Contributions
SK, MA, and AN researched the idea and designed the study. SK, MA, SB, CK, KT, TL, and AN recruited patients. SK, KT, DW, and AN collected and measured samples. SK, AD, and AN analyzed statistics and interpreted data. SK, SB, AD, and AN prepared the manuscript. All authors have read and approved the final version of the manuscript.
Footnotes
STROBE Statement.
Supplementary Material
References
- 1.Parfrey P.S., Griffiths S.M., Barrett B.J. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 2.Mehran R., Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006;100:S11–S15. doi: 10.1038/sj.ki.5000368. [DOI] [PubMed] [Google Scholar]
- 3.Mehran R., Aymong E.D., Nikolsky E. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Rihal C.S., Textor S.C., Grill D.E. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 5.Maioli M., Toso A., Leoncini M. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation. 2012;125:3099–3107. doi: 10.1161/CIRCULATIONAHA.111.085290. [DOI] [PubMed] [Google Scholar]
- 6.Sadat U. Radiographic contrast-media-induced acute kidney injury: pathophysiology and prophylactic strategies. ISRN Radiol. 2013;2013:496438. doi: 10.5402/2013/496438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullough P.A. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Barrett B.J., Parfrey P.S. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379–386. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 9.Weisbord S.D., Palevsky P.M. Prevention of contrast-induced nephropathy with volume expansion. Clin J Am Soc Nephrol. 2008;3:273–280. doi: 10.2215/CJN.02580607. [DOI] [PubMed] [Google Scholar]
- 10.van der Molen A.J., Reimer P., Dekkers I.A. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients: recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2018;28:2856–2869. doi: 10.1007/s00330-017-5247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 12.Navaneethan S.D., Singh S., Appasamy S. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:617–627. doi: 10.1053/j.ajkd.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Brar S.S., Aharonian V., Mansukhani P. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. doi: 10.1016/S0140-6736(14)60689-9. [DOI] [PubMed] [Google Scholar]
- 14.Marenzi G., Ferrari C., Marana I. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovasc Interv. 2012;5:90–97. doi: 10.1016/j.jcin.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Briguori C., Visconti G., Focaccio A. Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II): RenalGuard System in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124:1260–1269. doi: 10.1161/CIRCULATIONAHA.111.030759. [DOI] [PubMed] [Google Scholar]
- 16.Qian G., Fu Z., Guo J. Prevention of contrast-induced nephropathy by central venous pressure-guided fluid administration in chronic kidney disease and congestive heart failure patients. JACC Cardiovasc Interv. 2016;9:89–96. doi: 10.1016/j.jcin.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Kyle U.G., Bosaeus I., De Lorenzo A.D. Bioelectrical impedance analysis─part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Nongnuch A., Campbell N., Stern E. Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients. Kidney Int. 2015;87:452–457. doi: 10.1038/ki.2014.276. [DOI] [PubMed] [Google Scholar]
- 19.Sethakarun S., Bijaphala S., Kitiyakara C. Effect of bioelectrical impedance analysis-guided dry weight adjustment, in comparison to standard clinical-guided, on the sleep quality of chronic haemodialysis patients (BEDTIME study): a randomised controlled trial. BMC Nephrol. 2019;20:211. doi: 10.1186/s12882-019-1405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronco C., Verger C., Crepaldi C. Baseline hydration status in incident peritoneal dialysis patients: the Initiative of Patient Outcomes in Dialysis (IPOD-PD study) Nephrol Dial Transplant. 2015;30:849–858. doi: 10.1093/ndt/gfv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maioli M., Toso A., Leoncini M. Pre-procedural bioimpedance vectorial analysis of fluid status and prediction of contrast-induced acute kidney injury. J Am Coll Cardiol. 2014;63:1387–1394. doi: 10.1016/j.jacc.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Maioli M., Toso A., Leoncini M. Bioimpedance-guided hydration for the prevention of contrast-induced kidney injury: the HYDRA study. J Am Coll Cardiol. 2018;71:2880–2889. doi: 10.1016/j.jacc.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Balemans C.E., Reichert L.J., van Schelven B.I. Epidemiology of contrast material-induced nephropathy in the era of hydration. Radiology. 2012;263:706–713. doi: 10.1148/radiol.12111667. [DOI] [PubMed] [Google Scholar]
- 24.Dorval J.F., Dixon S.R., Zelman R.B. Feasibility study of the RenalGuard balanced hydration system: a novel strategy for the prevention of contrast-induced nephropathy in high risk patients. Int J Cardiol. 2013;166:482–486. doi: 10.1016/j.ijcard.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Yoowannakul S., Kotecha T., Fontana M., Davenport A. Do pre-hemodialysis estimates of extracellular volume excess using bioimpedance and N-terminal brain natriuretic peptide correlate with cardiac chamber size measured by magnetic resonance imaging? Ther Apher Dial. 2019;23:362–368. doi: 10.1111/1744-9987.12779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due security reason but are available from the first author on reasonable request.