Abstract
Introduction
Calciprotein particles (CPPs) are potentially modifiable mediators of phosphate toxicity in patients with kidney disease. We compared the effects of calcium carbonate (CC) and the non–calcium-based phosphate binder sevelamer on CPP levels in patients undergoing hemodialysis (HD). We hypothesized that treatment with sevelamer would achieve greater reductions in amorphous calcium phosphate–containing CPP (CPP-1) and hydroxyapatite-containing CPP (CPP-2) owing to reduced calcium loading and anti-inflammatory pleiotropic effects.
Methods
We conducted an open-label, randomized controlled trial (RCT) in which 31 stable prevalent HD patients were allocated to receive either sevelamer hydrochloride (SH), sevelamer carbonate (SC), or CC for 24 weeks. Dual primary endpoints were the between groups differences in serum CPP-1 and CPP-2 levels at 24 weeks in SH + SC–treated versus CC-treated patients. Effects on aortic pulse wave velocity (aPWV), inflammatory cytokines (interleukin-6 and -8), and effects across individual treatment arms were also assessed.
Results
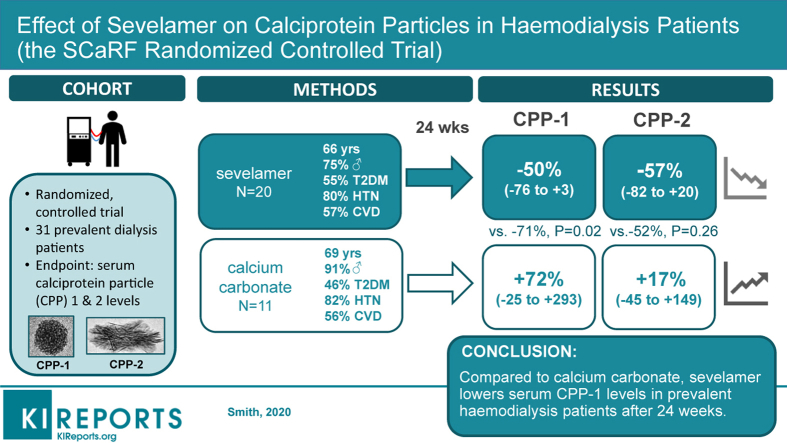
Serum CPP-1, but not CPP-2, levels were lower in those randomly assigned to the sevelamer (SH + SC) group compared with the CC group at 24 weeks (–70%, 95% confidence interval [CI] –90% to –15%, P = 0.02). In subgroup analysis, this effect was confined to those receiving SC (–83.4%, 95% CI –95.7% to –36.8%, P = 0.01). aPWV and interleukin-8 levels were also lower in those who received sevelamer compared with CC at 24 weeks (–2.0 m/s, 95% CI –2.9 to –1.1; –57%, 95% CI –73% to –30%, respectively, both P = 0.01). Conventional markers of mineral metabolism remained stable across all treatment groups.
Discussion
Compared with treatment with CC, use of sevelamer for 24 weeks was associated with lower serum CPP-1 levels and a reduction in aPWV and systemic inflammation.
Keywords: calciprotein particles, calcium carbonate, hemodialysis, inflammation, phosphate, phosphate binder, sevelamer
Graphical abstract
Hyperphosphatemia is common in patients with end-stage kidney disease and is associated with an increased risk of mortality and cardiovascular disease.1,2 Although any causal link between serum phosphate excess and mortality remains speculative and unproven, the widespread use of phosphate-lowering therapies in this population is common, particularly the prescription of intestinal phosphate binders.3 Although observational cohorts suggest lower mortality rates in patients treated with binders, no link with serum phosphate could be found in these studies,4, 5, 6 and prospective placebo-controlled trials are lacking. Calcium-based binders, such as calcium carbonate, are the most frequently prescribed class of binder worldwide, but the advantages of improved phosphate control may be offset by concurrent calcium loading. In some studies,7, 8, 9 but not all,10 their use is associated with progression, rather than retardation of cardiovascular calcification. Non–calcium-based binders, such as sevelamer, do not appear to carry this risk and may offer additional benefits unrelated to phosphate-lowering through anti-inflammatory and lipid-lowering effects.11, 12, 13, 14 Some analyses suggest that use of non–calcium-based binders may be associated with reduced risks of adverse events compared with therapy with calcium-based binders15,16; however, it is uncertain whether this represents true benefit of the former or relative harm from the latter.
Accumulating evidence suggests that mineral nanoparticles formed spontaneously postprandially, or as the result of efflux from bone, might better explain the harmful effects of phosphate excess than direct effects of phosphate ions themselves.17, 18, 19 Based on their concentrations in extracellular fluid, one might expect only relatively minor perturbations in levels of calcium and phosphate to result in precipitation. However, this does not occur in vivo due to the spontaneous formation of CPPs, colloidal complexes of calcium phosphate and the mineral-binding protein fetuin-A. These complexes chaperone nascent mineral nuclei in a manner analogous to that by which apolipoproteins carry insoluble lipid.20 Initially present as subnanometer clusters of calcium phosphate ions bound by fetuin-A, under permissive conditions these can coalesce to form 30- to 70-nm diameter particles with mineral held in a disordered amorphous phase (primary CPP or CPP-1), before transforming into larger denser particles with mineral in crystalline phases such as hydroxyapatite (secondary CPP or CPP-2).21 Ordinarily, CPPs are thought to buffer extracellular calcium phosphate, facilitating safe transport to sites of clearance and lessening the risk of ectopic deposition. However, recent studies suggest that these particles might also be toxic, inducing inflammation,22,23 endothelial damage,24,25 and ectopic mineralization,26,27 depending on the type and quantity of the particles present. Thus, measurement of CPP, and discrimination of the particle type, may provide additional insight into the mechanisms of phosphate-induced injury and a better therapeutic target than serum phosphate per se, which is a poor indicator of phosphate balance in patients with chronic kidney disease (CKD).28
Until recently, measurement of circulating CPPs has been hampered by the lack of specific and sensitive methodology. We developed a flow cytometric–based assay using small fluorescent mineral– and membrane-binding probes that permits direct quantitation of CPPs at endogenous levels, without further manipulation or prolonged incubation.29 Unlike other aggregate assays recently described,30 this method can distinguish CPPs from other mineral-containing debris as well as resolve populations of CPP-1 from CPP-2. Using this assay, we found that chronic dietary phosphate loading in rats increased CPP-1 levels, despite maintenance of normophosphatemia, whereas in animals with superimposed CKD, it resulted in marked elevations in CPP levels and frank hyperphosphatemia. Consistent with these observations, serum CPP levels appear elevated in patients undergoing dialysis compared with adults without CKD29,30 and increase after cessation of cinacalcet in patients with severe secondary hyperparathyroidism.31 Although work by other groups suggests that treatment of CKD rats with sucroferric oxyhydroxide (a non–calcium-based binder) lowers serum CPP levels,32 the effects of such pharmacologic interventions on CPPs in human CKD, and the comparative effects of calcium-containing binders, have yet to be examined in an RCT.
To address this issue, we designed a prospective RCT (the Sevelamer Versus Calcium to Reduce Fetuin-A-containing Calciprotein Particles in Dialysis [SCaRF] study) to compare the effect of the non–calcium-containing binder sevelamer with CC on serum CPP levels in prevalent HD patients over 24 weeks. Our primary hypothesis was that reductions in serum CPP-1 and CPP-2 levels would be greater among sevelamer-treated patients than those treated with CC for 24 weeks. Our secondary hypotheses were that reductions in CPP levels would be associated with a lowering in systemic inflammatory markers and an improvement in vascular function reflected by a reduction of aPWV. We also hypothesized that treatment with SH and SC, the 2 formulations of the binder currently on the market, may differ with respect to their effects on CPP levels and inflammation.
Methods
Study Design
The SCaRF trial was designed as a multicenter, 3-arm, parallel group, open-label, RCT trial of stable prevalent HD patients comparing the effects of SH, SC, and CC on serum CPP-1 and CPP-2 levels. The study was designed to enrol 90 patients across 3 centers, but owing to slow recruitment at the only active site (the Royal Melbourne Hospital), funding was withdrawn, and the recruitment terminated in August 2018 before reaching the randomization target. Hence, we report our single-center experience of patients who were recruited from dialysis units at Royal Melbourne Hospital from March 2017 to March 2018, with the last follow-up visit occurring in January 2019. SH and SC were supplied by Sanofi-Aventis (Singapore) Pte. Lld., and CC was obtained from the clinical trials pharmacy at Royal Melbourne Hospital. The study protocol was approved by the Melbourne Health Human Research Ethics Committee (HREC/15/MH/261) and registered with the Australian and New Zealand Clinical Trials Register under the identifier ACTRN12617000881336. All study activities were conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from all patients at enrollment. Complete methods and CONSORT statement checklist are provided in the Supplementary Material.
Study Participants
Key inclusion criteria were patients >18 years requiring HD 2 or 3 times per week for <6 hours per session; established vascular access; and a serum intact parathyroid hormone level ≤200 pmol/l, and if >100 pmol/l, alkaline phosphatase activity ≤200 IU/l. Key exclusion criteria included known allergy or intolerance to study medications, serum phosphate level >2.6 mmol/l despite optimized binder therapy, planned parathyroidectomy, or renal transplant within 36 weeks, or life expectancy <12 months. Other eligibility criteria are listed in the Supplementary Methods.
Study Procedure
Eligible participants began an active 13-week run-in period, which consisted of a 1-week washout followed by 12 weeks of treatment with CC. After the run-in period, participants were block randomized into 1 of 3 arms—CC, SH, or SC—in a 1:1:1 fashion with blocks of varying size. Randomization was performed using Sealed Envelope (Sealed Envelope Ltd., London, UK). To ensure allocation concealment, different investigators were independently responsible for randomization and participant enrolment.
Initial CC dosing during the run-in period was based on binder prescription at screening according to the following relative phosphate-binding coefficients: CC 1.0; SH or SC 0.75; and lanthanum carbonate 2.0. Participants who were not taking phosphate binders at screening commenced taking one 600 mg CC tablet per meal. At the completion of the CC run-in period, participants assigned to the SH or SC arms were converted from their CC dose to an equivalent dose of SH or SC. For those who continued with CC, the dose remained the same unless titration was required. Predialysis serum biochemistry was reviewed monthly with the dose of phosphate binder up-titrated by 1 tablet per day if the serum phosphate less was >2.0 mmol/l or decreased by 1 tablet per day if the level was <0.8 mmol/l. All participants received conventional bicarbonate HD using high-flux polysulfone membranes (Polyflux 210H, Gambro AB, Lund, Sweden) and were achieving their individual clearance targets. Standard dialysate calcium concentration was 1.25 mmol/l and the dialysate magnesium concentration was 0.5 mmol/l. Hypocalcaemia (<2.1 mmol/l) was treated by increasing the dialysate calcium concentration to 1.5 mmol/l. All other dialysis conditions were kept constant throughout the study. Initiation or dose changes to vitamin D, vitamin D analogs, magnesium-containing supplements, or warfarin were not permitted during the study.
Study visits were conducted at a midweek dialysis session (i.e., after a short 2-day interdialytic period) during week –13 (screening), week –12 (after washout), week 0 (baseline), week 12, and week 24. Adherence to the prescribed treatment was assessed with pill counts at each study visit. Patients were considered adherent if >80% of the prescribed study medications were taken.
Study Assessments
Participant demographics and baseline clinical data were collected at screening. The Charlson Comorbidity Index was used to assess overall comorbidity burden according to published scoring systems (Supplementary Table S1) validated for use in HD cohorts.33 Nonfasting venous blood samples were obtained <1 hour before starting hemodialysis at each study visit (i.e., predialysis). Analyses for serum phosphate, albumin, calcium, magnesium, bicarbonate, intact parathyroid hormone (1-84), alkaline phosphatase, and C-reactive protein were performed at the Department of Biochemistry and the Royal Melbourne Hospital using standard chemistries. The adequacy of dialysis was assessed using the urea reduction ratio. Blood samples for biomarker analysis (CPP, inflammatory cytokines, and serum calcification propensity, T50) were stored at –80° C for batched-blinded analysis at the completion of the study.
Calciprotein Particles
Serum levels of CPP-1 and CPP-2 were measured using a fluorescent probe–based assay running on a BD FACSVerse flow cytometer (Becton Dickinson, San Jose, CA) equipped with high-sensitivity fluidics and detectors, as previously described,29 but with minor modifications (see Supplementary Methods), where phosphatidyl serine–binding lactadherin-FITC (Haematologic Technologies Inc., Essex Junction, VT) was used to discriminate membrane-bound mineral-containing particles from CPP. A schematic for the method and representative examples of CPP analysis by flow cytometry are provided in Supplementary Figure S1. Analyses demonstrating the stability of CPP to freeze-thaw and dilution in TBS assay diluent are provided in Supplementary Figures S2 and S3, respectively.
Inflammatory Cytokines
Serum inflammatory cytokine concentrations (interleukin [IL]-1β, IL-6, IL-8, I-10, IL-17p20, and tumor necrosis factor [TNF]–α) were assayed by flow cytometry using a commercially available multiplex assay (BD Cytometric Bead Array Human Inflammatory Cytokines Kit, Becton Dickinson) according to manufacturer’s instructions. Samples were analyzed in triplicate and the data analyzed using FCAP Array software version 3 (Becton Dickinson).
Pulse Wave Velocity
The aPWV (measured in m/s) was determined by a single operator familiar with the technique using the Niccomo system (medis GmbH, Illmenau, Germany) at each study visit before starting HD. In this method, pulse transit time (in seconds) is provided by the time interval between the opening of the aortic valve detected by transthoracic impedance cardiography and oscillometric detection of the femoral pulse with a thigh cuff, which is then related to the distance (in meters) between the suprasternal notch and the middle of the thigh cuff. Signal quality assessment, peak detection, and pulse wave velocity (PWV) calculation are automated. Pulse waves with artifacts were automatically excluded with 10 evaluable waves used for calculations when the impedance cardiography waveform and signal stability quality criteria were met. This impedance thigh cuff-based method shows good agreement with PWV measurements made using carotid-femoral applanation tonometry and electrocardiography gating.34 For this study, measurements were made after a >10-minute rest with participants in the supine position and according to best practice guidelines.35 Mean arterial pressure and heart rate were recorded simultaneously using the same system. PWV readings were made in duplicate, and the mean value was used unless measurements differed by >0.5 m/s, in which case a third reading was taken and the median value was used in subsequent analyses.
Serum Calcification Propensity (T50 Test)
Serum T50 was measured by turbidimetry at 400 nm and 37 °C ± 0.1 °C using a Synergy HTX Multi-Mode plate reader (BioTek Instruments, Winooski, VT) running Gen5 version 3 data analysis software (BioTek), according to the method of Pasch and colleagues.36 The reference range for this assay is 270 to 460 minutes, as determined in 253 healthy Swiss adults.37
Study Endpoints
The dual primary endpoints of the study were the serum CPP-1 and CPP-2 levels at 24 weeks in all sevelamer-treated patients (SH + SC) versus CC-treated patients. Secondary endpoints compared (i) serum CPP-1 and CPP-2 levels in individual treatment arms, (ii) serum inflammatory cytokine concentrations (IL-1β, IL-6, IL-8, IL-10, IL17p20, and TNF–α), and (iii) aPWV at 24 weeks. All other analyses were considered exploratory.
Sample Size
Based on prior data we considered a 35% difference in CPP-1 or CPP-2 levels to be of interest,38,39 and assuming a within-group difference of 25% over time31 and an adjusted 2-sided significance level of 0.025, we estimated that 25 patients per group would yield 90% power. Allowing for a 17% attrition rate related to withdrawal or nonadherence, we aimed to recruit a total of 90 patients, with 30 patients to be randomized across each of the 3 arms.
Statistical Analyses
We analyzed all data in accordance with a modified intention-to-treat manner, wherein we included any randomized participant with at least 1 postrandomization sample for the primary endpoint. Variables with right-skewed distributions were log-transformed before analysis. To study changes in biochemical parameters over time, we used a direct restricted maximum likelihood–based repeated measures approach. Mixed-effect models were constructed that included fixed effects of treatment group, visit, treatment group-by-visit interaction, as well as fixed continuous covariates of baseline measurement and baseline measurement-by-visit interaction. Each model incorporated random intercepts, and an unstructured covariance structure was used to model within-subject errors. The primary treatment comparison was the difference in CPP-1 and CPP-2 levels between all sevelamer-treated patients (SH + SC) and CC-treated patients at week 24. Given the dual primary endpoint of CPP-1 and CPP-2 levels, we prespecified a conservative 2-sided significance level of 0.025 (α = 0.05/2) to control for multiple comparisons. For all other comparisons, 2-tailed P values <0.05 were considered significant. To assess the potential effects of nominal differences in baseline characteristics, we performed post hoc adjustment for serum magnesium concentrations, dialysis vintage, diabetic status, and sex. All models included baseline CPP levels as a covariate. We also performed a series of post hoc analyses in sevelamer-treated patients to test whether changes in CPP-1 levels were associated with changes in other continuous time-varying biochemical parameters from baseline to week 24. We used multiple imputation to assess the potential bias arising from missing data and performed additional sensitivity analyses based on completely observed data (i.e., complete case analysis) using analysis of covariance. On-treatment analyses of the primary and secondary endpoints were also performed in adherent participants. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Enrollment, Baseline Characteristics, and Study Conduct
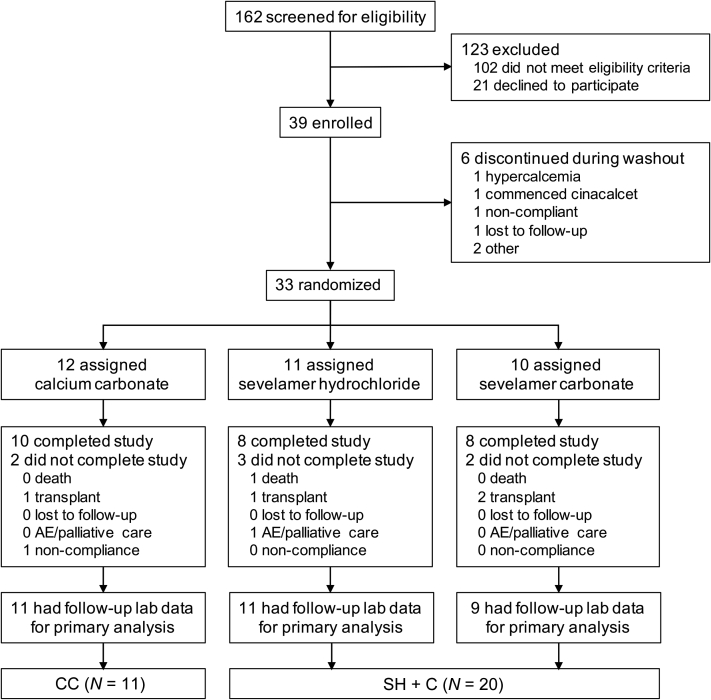
Patient disposition is shown in Figure 1. Thirty-nine of the 162 patients screened were eligible to participate and consented with 33 undergoing randomization after run-in. Study exclusions are summarized in Supplementary Table S2; the majority of exclusions (85%) were due to severe hyperparathyroidism (n = 30), frailty/life expectancy <12 months (n = 21), psychiatric or cognitive issues (n = 18), or physician refusal because of the washout requirement or use of a calcium-containing binder (n = 17). One patient each in the CC and SH treatment arms did not have postrandomization samples available for analysis, yielding 31 patients in the modified intention-to-treat dataset. As prespecified in the protocol, SH- and SC-treated participants were grouped together (SH + SC) for the primary analysis. Secondary analyses considered effects across individual sevelamer treatment arms versus the CC group. Baseline characteristics (Table 1) and biochemical parameters (Table 2) were reasonably well balanced across treatment groups apart from nominal differences in serum phosphate, magnesium, CPP levels, dialysis vintage, and sex balance that did not reach significance in formal statistical testing. Overall, participants were predominantly male (81%) and white (68%) with a mean age of 67 ± 14 years. Diabetes mellitus and underlying cardiovascular disease were prevalent in both treatment arms, although the prevalence of diabetes appeared modestly numerically greater in the CC arm. The overall comorbidity burden as judged by Charlson Comorbidity Index scores was similar across treatment groups. All patients were receiving thrice-weekly HD with equivalent adequacy based on the urea reduction ratio. Baseline data for individual sevelamer treatment arms are summarized in Supplementary Tables S3 and S4. Mean doses of study medication were 2.2 g/d for the CC group, 2.8 g/d for the SH group, and 3.0 g/d for the SC group. Discontinuation rates were similar across arms with kidney transplantation accounting for 4 of the 7 study withdrawals. No patients were lost to follow-up and none experienced a serious adverse event. One patient in the SH group remained hypocalcemic despite supplementing oral calcium; this patient’s dialysate calcium concentration was increased from the standard 1.25 mmol/l to 1.5 mmol/l. All other patients were maintained on 1.25 mmol/l dialysate calcium concentration throughout the study. No other laboratory-based safety endpoints were recorded. One patient in the SH group was intolerant to study medication. One death also occurred in the SH group, which was attributed to bacterial endocarditis but considered unrelated to the study intervention. Average adherence was high (>85%) across all treatment arms.
Figure 1.
CONSORT diagram showing patient disposition through consent, randomization, and dropout in the Sevelamer Versus Calcium to Reduce Fetuin-A-containing Calciprotein Particles in Dialysis (SCaRF) trial. Primary analyses were conducted on a modified intention-to-treat basis in which randomized patients with at least 1 postrandomization follow-up visit were included. AE, adverse events; CC, calcium carbonate; SC, sevelamer carbonate; SH, sevelamer hydrochloride.
Table 1.
Baseline characteristics of the modified ITT cohort by treatment arm
| Characteristics | Total (N = 31) | CC (n = 11) | SH + SC (N = 20) |
|---|---|---|---|
| Age, yr | 67 (14) | 69 (13) | 66 (15) |
| Male sex | 25 (81%) | 10 (91%) | 15 (75%) |
| Ethnicity | |||
| Caucasian | 21 (68%) | 7 (64%) | 14 (70%) |
| Middle Eastern | 7 (23%) | 3 (27%) | 4 (20%) |
| Asian | 3 (9%) | 1 (9%) | 2 (10%) |
| BMI, kg/m2 | 26.6 (23.9, 30.1) | 26.4 (24.7, 28.4) | 27.4 (23.7, 30.8) |
| Dialysis vintage, mo | 23 (8, 50) | 19 (7, 95) | 26 (10, 46) |
| HD frequency, per wk | Thrice | Thrice | Thrice |
| Treatment length, hr | 4.2 (0.4) | 4.3 (0.5) | 4.2 (0.3) |
| Urea reduction ratio, % | 76.3 (5.5) | 76.8 (6.9) | 76.1 (4.7) |
| Diabetes mellitus | 16 (52%) | 5 (46%) | 11 (55%) |
| Hypertension | 25 (81%) | 9 (82%) | 16 (80%) |
| MAP, mm Hg | 93 (13) | 87 (6) | 97 (15) |
| Heart rate, beats/min | 74 (13) | 73 (13) | 75 (10) |
| Smoker (past or present) | 15 (48%) | 5 (46%) | 10 (50%) |
| Coronary artery disease | 16 (52%) | 5 (46%) | 11 (55%) |
| Peripheral vascular disease | 1 (3%) | 1 (9%) | 0 |
| Cerebral vascular disease | 5 (16%) | 3 (27%) | 2 (10%) |
| Charlson Comorbidity Index | 4.16 (1.36) (range, 2–7) |
4.18 (1.60) (range, 2–7) |
4.15 (1.27) (range, 2–7) |
| Previous parathyroidectomy | 5 (16%) | 2 (18%) | 3 (15%) |
| Previous renal transplants | 3 (10%) | 2 (18%) | 1 (5%) |
| Primary renal disease | |||
| Diabetic nephropathy | 12 (39%) | 4 (36%) | 8 (40%) |
| Glomerulonephritis | 8 (26%) | 3 (27%) | 5 (20%) |
| Hypertension/renovascular disease | 6 (19%) | 3 (27%) | 3 (15%) |
| Polycystic kidney disease | 1 (3%) | 1 (9%) | 0 |
| Congenital/reflux nephropathy | 1 (3%) | 0 | 1 (5%) |
| Light chain nephropathy | 1 (3%) | 0 | 1 (5%) |
| Unknown | 2 (6%) | 0 | 2 (10%) |
| Medical Therapy | |||
| Phosphate binder at screening | |||
| Calcium carbonate | 15 (48%) | 6 (55%) | 9 (45%) |
| Sevelamer hydrochloride | 12 (39 %) | 4 (36%) | 8 (40%) |
| Lanthanum carbonate | 3 (10%) | 1 (9%) | 2 (10%) |
| No phosphate binder | 9 (29%) | 5 (46%) | 4 (20%) |
| Vitamin D therapy | |||
| Cholecalciferol | 10 (32%) | 3 (27%) | 7 (35%) |
| Calcitriol or other active sterols | 14 (45%) | 6 (55%) | 8 (40%) |
| Magnesium supplements | 2 (6%) | 1 (9%) | 1 (5%) |
| Statin | 18 (58%) | 7 (64%) | 11 (55%) |
| Proton pump inhibitors | 13 (42%) | 6 (55%) | 7 (35%) |
| Antihypertensive medications | |||
| ACE inhibitor or ARB | 7 (23%) | 3 (27%) | 4 (20%) |
| β-blocker | 16 (52%) | 5 (45%) | 11 (55%) |
| Calcium channel blocker | 7 (23%) | 3 (27%) | 4 (20%) |
| Loop diuretic | 12 (39%) | 7 (64%) | 5 (25%) |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; CC, calcium carbonate; HD, hemodialysis; ITT, intention to treat; MAP, mean arterial pressure; SC, sevelamer carbonate; SH, sevelamer hydrochloride.
Data are expressed as mean (SD), median (25th, 75th percentile), or number (%).
Table 2.
Baseline serum biochemistry in the SCaRF trial by treatment arm
| Variable | Total (N = 31) | CC (n = 11) | SH + SC (n = 20) | Reference interval |
|---|---|---|---|---|
| Phosphate, mmol/l | 1.41 (0.40) | 1.49 (0.46) | 1.37 (0.37) | 0.75–1.50 |
| Calcium, mmol/l | 2.18 (0.22) | 2.10 (0.18) | 2.22 (0.23) | 2.10–2.60 |
| Albumin, g/l | 32 (3) | 32 (2) | 33 (4) | 35–50 |
| Bicarbonate, mmol/l | 24 (2) | 24 (2) | 25 (2) | 22–32 |
| Magnesium, mmol/l | 0.92 (0.15) | 0.86 (0.15) | 0.93 (0.14) | 0.7–1.10 |
| Intact PTH, pmol/l | 51 (33, 67) | 48 (33, 67) | 54 (31, 63) | 1.7–10.0 |
| ALP, IU/ml | 118 (90, 164) | 115 (90, 164) | 118 (89, 159) | 30–110 |
| T50, min | 274 (67) | 271 (60) | 275 (73) | 270–460 |
| CRP, mg/l | 5 (2, 8) | 6 (2, 8) | 5 (2, 9) | <5 |
ALP, alkaline phosphatase; CC, calcium carbonate; PTH, parathyroid hormone; SC, sevelamer carbonate; SCaRF, Sevelamer Versus Calcium to Reduce Fetuin-A-containing Calciprotein Particles in Dialysis; SH, sevelamer hydrochloride; T50, serum calcification propensity.
Data are expressed as mean (SD) or median (25th, 75th percentile).
Serum CPP-1
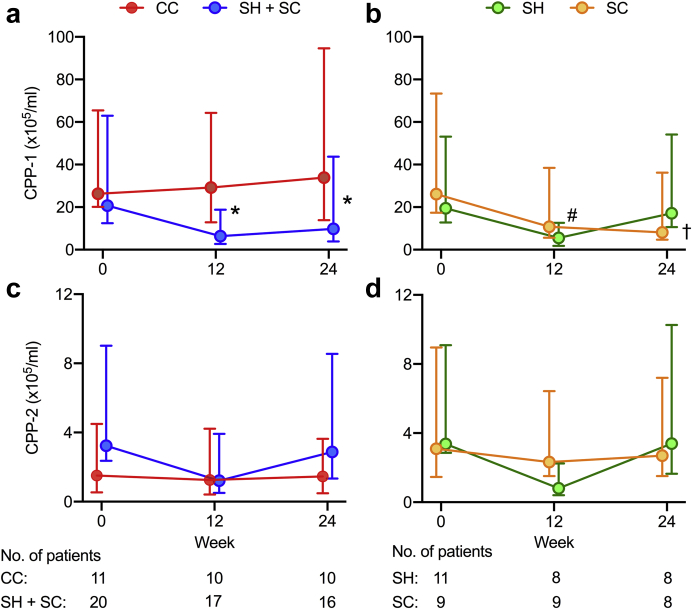
At 24 weeks, serum CPP-1 levels adjusted for baseline values were significantly lower in the sevelamer-treated group than in the CC-treated group (Table 3), with a mean difference of –71% (95% CI –90 to –15, P = 0.02; Table 3). Results were similar when models were adjusted for baseline serum phosphate concentration (–69%, 95% CI –88% to –17%, P = 0.02) and when multiple imputation was used to evaluate the impact of missing follow-up data (not shown). Effect estimates did not change (<1%) after post hoc adjustment for baseline magnesium concentration, diabetic status, dialysis vintage, and sex (all –71%, 95% CI –90% to –15%, P = 0.02) and were similar after exclusion of the single patient in the SH group in whom the dialysate calcium concentration was increased owing to persistent hypocalcemia (–73%, 95% CI –90% to –19%). Prespecified on-treatment analyses were expectedly similar given only 1 participant was found to be noncompliant with the intervention. Complete case analysis using data from the 26 participants who completed the 24-week study revealed comparable between-group treatment effects (–65%, 95% CI –86% to –12%, P = 0.03). The between-group difference in the serum CPP-1 level at 12 weeks was similar in magnitude to that at week 24 (–73%, 95% CI –88% to –38%, P = 0.002; Figure 2a). Although they did not reach significance (Table 3), trends in within-group treatment effects suggest that divergent trajectories of serum CPP-1 levels in the CC- and sevelamer-treated arms accounted for the differences at study end (Supplementary Figure S4).
Table 3.
Changes in serum CPP-1 and CPP-2 over 24 weeks in the SCaRF trial
| Treatment group | Baseline |
Week 12 |
Week 24 |
Within-group treatment effect (95% CI) | P Value | Treatment effect vs. CC (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |||||
| CPP-1, ×105/ml | ||||||||||
| CC | 11 | 26.3 (6.2, 39.1) | 10 | 29.3 (16.3, 35.1) | 10 | 33.9 (20.0, 60.7) | 71.9% (–24.8% to 293%) | 0.20 | — | — |
| SH + SC | 20 | 18.7 (7.2, 41.3) | 17 | 6.4 (3.8, 12.4) | 16 | 9.8 (5.9, 33.9) | –49.7% (–75.8% to 4.3%) | 0.07 | –70.6% (–89.8 to –15.2) | 0.02 |
| CPP-2, ×105/ml | ||||||||||
| CC | 11 | 1.5 (1.0, 3.0) | 10 | 1.3 (0.8, 3.0) | 10 | 1.5 (1.0, 2.2) | 17.4% (–44.8% to 149%) | 0.68 | — | — |
| SH + SC | 20 | 3.2 (0.9, 5.8) | 17 | 1.2 (0.7, 2.7) | 16 | 2.9 (1.5, 5.7) | –56.5% (–84.2% to 20.1%) | 0.11 | –52.2% (–86.7 to 71.35) | 0.26 |
CC, calcium carbonate; CPP, calciprotein particles; IQR, interquartile range; SC, sevelamer carbonate; SCaRF, Sevelamer Versus Calcium to Reduce Fetuin-A-containing Calciprotein Particles in Dialysis; SH, sevelamer hydrochloride; 95% CI, 95% confidence interval.
Serum CPP levels are expressed as median (25th, 75th percentiles) using all available data (N). CPP-1 and CPP-2 values were log-transformed before analysis. Estimated treatment effects are expressed as the percentage change from baseline or relative to CC treatment group (reference category). Mixed-effects analyses are expressed after controlling for the baseline level of the parameter tested.
P values < 0.025 are considered statistically significant owing to multiple comparisons.
Figure 2.
Changes in serum calciprotein particle (CPP)-1 and CPP-2 levels over 24 weeks in response to treatment with calcium carbonate or sevelamer. (a) Changes in serum CPP-1 levels in calcium carbonate (CC) and sevelamer hydrochloride (SH) + sevelamer carbonate (SC) treatment arms. (b) Changes in serum CPP-1 levels by individual SH and SC treatment arms. (c) Changes in serum CPP-2 in CC and SH + SC treatment arms. (d) Changes in serum CPP-2 levels by individual SH CPP and SC treatment arms. Data are presented as median values with error bars depicting interquartile ranges. Data series were offset to aid visualization. Mixed-effects models with repeated measures were used to test for between-group differences: ∗P < 0.02 for CC versus SH + SC; †P < 0.01 for CC versus SC, and #P < 0.02 for SH versus CC.
Secondary analyses across the individual sevelamer treatment arms revealed a significant reduction in CPP-1 at 24 weeks in the SC-treated arm compared with the CC arm, but not in the SH arm (Figure 2b; Supplementary Table S5). However, serum CPP-1 levels were reduced in those receiving SH versus CC at week 12 (–74%, 95% CI –89% to –40%, P = 0.002; Figure 2b). Again, results were similar after additional adjustment for baseline phosphate or imputation of missing data (not shown).
Serum CPP-2
At 24 weeks, serum CPP-2 levels adjusted for baseline values did not differ between groups (Figure 2c) and remained nonsignificant after adjustment for baseline serum phosphate (P = 0.28) or with imputation of missing data (P = 0.58). Null effects remained after further adjustment for baseline magnesium, dialysis vintage, presence of diabetes, and sex (P > 0.28). Likewise, complete case and on-treatment analyses did not attain significance (P > 0.28). Changes observed in individual sevelamer-treated arms were also nonsignificant at 24 weeks (Supplementary Table S5) compared with the CC group (Figure 2c).
Serum Inflammatory Cytokines
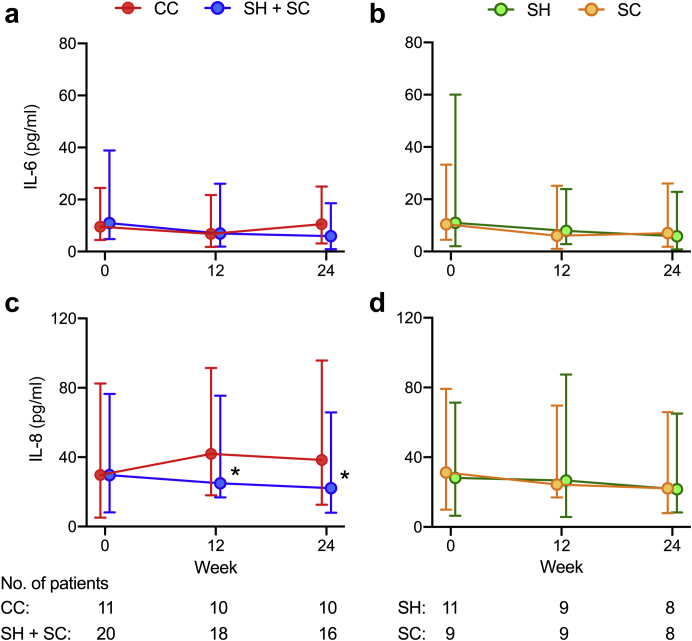
Four of the 6 cytokines tested by multiplex bead array (IL-1β, IL-10, IL17p20, and TNF–α) were undetectable at baseline and follow-up study visits, consistent with the low levels of C-reactive protein observed at baseline (Table 2) and over the 24-week study period (Supplementary Table S6). Serum IL-6 and IL-8 were detectable in all participants, but with missing data (9%) owing to study dropout. At 24 weeks, both IL-6 and IL-8 levels were significantly lower in sevelamer-treated patients compared with the CC-treated arm (Table 4, Figure 3a and 3c). Results were similar after adjustment for baseline phosphate and after imputation of missing data (P < 0.02) but differed with respect to effects across individual sevelamer-treatment arms (Supplementary Table S6). Although only treatment with SC favored reductions in serum IL-6 levels (Figure 3b), serum IL-8 levels were reduced in both SH- and SC-treated arms compared with CC-treated patients (Figure 3d). Serum C-reactive protein levels did not differ between CC- and sevelamer-treated arms (P = 0.32) or across individual groups (Supplementary Table S6). Within-group analyses revealed that between-group effects were driven by a decrease in IL-8 levels in the sevelamer-treated arm and a parallel increase in cytokine concentrations in the CC-treated arm.
Table 4.
Changes in serum inflammatory markers and calcification propensity over 24 weeks in the SCaRF trial
| Treatment group | Baseline |
Week 12 |
Week 24 |
Within-group treatment effect estimate (95% CI) | P value | Treatment effect estimate vs. CC (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |||||
| IL-6, pg/ml | ||||||||||
| CC | 11 | 9.5 (5.0, 15.0) | 10 | 6.8 (5.0, 14.9) | 10 | 10.6 (7.5, 14.5) | 6.5% (–14.2 to 32.0) | 0.57 | — | — |
| SH + SC | 20 | 11.0 (6.2, 27.9) | 18 | 6.9 (5.0, 19.2) | 16 | 5.9 (5.0, 12.7) | –48.5% (–68.0 to –17.2) | 0.01 | –51.8% (–74.7 to –8.2) | 0.03 |
| IL-8, pg/ml | ||||||||||
| CC | 11 | 29.8 (24.6, 52.7) | 10 | 41.9 (23.9, 49.5) | 10 | 38.4 (25.8, 57.3) | 35.1% (5.2 to 73.6) | 0.02 | — | — |
| SH + SC | 20 | 29.7 (21.5, 46.8) | 18 | 25.0 (8.2, 50.4) | 16 | 22.1 (14.1, 43.7) | –45.5% (–63.8 to –17.9) | 0.01 | –6.6% (–73.3 to –29.6%) | 0.01 |
CC, calcium carbonate; IL, interleukin; IQR, interquartile range; SC, sevelamer carbonate; SCaRF, Sevelamer Versus Calcium to Reduce Fetuin-A-containing Calciprotein Particles in Dialysis; SH, sevelamer hydrochloride;95% CI, 95% confidence interval.
Serum IL-6 and IL-8 concentrations are expressed as median (25th, 75th percentiles) using all available data (N). IL-6, and IL-6 values were log-transformed before analysis. Within-group estimated treatment effects for IL-6 and IL-6 are as reported as percentage change from baseline in the geometric mean. Mixed-effects analyses are expressed after controlling for the baseline level of the parameter tested.
Figure 3.
Changes in serum interleukin (IL)-6 and IL-8 over 24 weeks in response to treatment with calcium carbonate or sevelamer. (a) Changes in serum IL-6 in calcium carbonate (CC) and sevelamer hydrochloride (SH) + sevelamer carbonate (SC) treatment arms. (b) Changes in serum IL-6 by individual SH and SC treatment arms. (c) Changes in serum IL-8 in CC and SH + SC treatment arms. (d) Changes in serum IL-8 by individual SH and SC treatment arms. Data are presented as median values with error bars depicting interquartile ranges. Data series were offset to aid visualization. Mixed-effects models with repeated measures were used to test for between-group differences: ∗P < 0.02 for CC versus SH + SC.
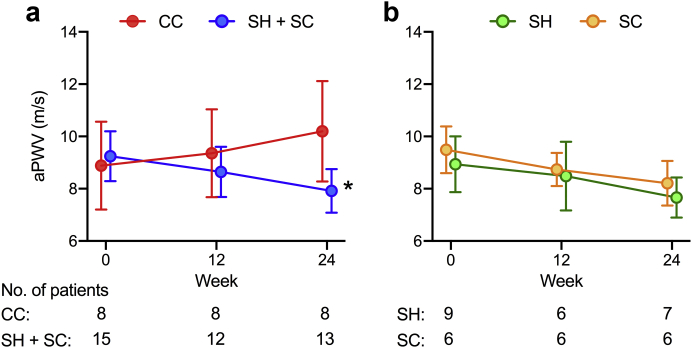
aPWV
PWV measurements were not technically evaluable in a minority of patients (CC, n = 3; SH + SC, n = 5) because of arrhythmias, severe leg cramps, or both. Of those with evaluable measurements, 31% had missing data at follow-up due to dropout. Apart from a slightly higher mean age and prevalence of diabetes, baseline characteristics of the entire study cohort and those with available aPWV measurements were similar (Supplementary Table S7). At 24 weeks, aPWV adjusted for mean arterial pressure, heart rate, and baseline aPWV was lower in sevelamer-treated patients compared with those who received CC (Table 5 and Figure 4a). Sensitivity analysis with imputation for missing data revealed a significant but slightly attenuated treatment effect (–1.7, 95% CI –2.8 to –0.8, P = 0.02). Treatment effects in individual SH and SC arms versus CC were equivalent (Figure 4b) and persisted after post hoc adjustment for serum phosphate (–1.9 m/s, 95% CI –2.9 to –1.0, P = 0.01). Within-group analyses showed an increase in aPWV in CC-treated patients (mean +1.1 m/s) and a similar magnitude decrease in those using sevelamer (mean –0.9 m/s) over 24 weeks (Table 5).
Table 5.
Changes in aortic pulse wave velocity over 24 weeks in the SCaRF trial
| Treatment group | Baseline |
Week 12 |
Week 24 |
Within-group treatment effect estimate (95% CI) | P Value | Treatment effect estimate vs. CC (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |||||
| PWV, m/s | ||||||||||
| CC | 8 | 8.9 (1.7) | 8 | 9.4 (1.7) | 8 | 10.2 (1.9) | 1.1 (0.2 to 2.0) | 0.01 | — | — |
| SH+SC | 15 | 9.2 (1.0) | 12 | 8.6 (1.0) | 13 | 7.9 (0.8) | –0.9 (–1.4 to –0.5) | 0.01 | –2.0 (–2.9 to –1.1) | 0.01 |
| SH | 9 | 9.5 (0.9) | 6 | 8.7 (0.6) | 7 | 8.2 (0.9) | –1.0 (–1.7 to –0.3) | 0.01 | –2.0 (–3.0 to –0.9) | 0.01 |
| SC | 6 | 8.9 (1.1) | 6 | 8.5 (1.3) | 6 | 7.7 (0.8) | –1.0 (–1.7 to –0.3) | 0.01 | –2.1 (–3.2 to –1.0) | 0.01 |
CC, calcium carbonate; SC, sevelamer carbonate; SCaRF, Sevelamer Versus Calcium to Reduce Fetuin-A-containing Calciprotein Particles in Dialysis; SH, sevelamer hydrochloride; PWV, pulse wave velocity; 95% CI, 95% confidence interval;
PWV values are expressed as mean (SD) using all available data (N). Mixed-effects analyses are adjusted for mean arterial pressure, heart rate, and baseline PWV.
Figure 4.
Changes in aortic pulse wave velocity (aPWV) over 24 weeks in response to treatment with calcium carbonate or sevelamer. (a) Changes in aPWV in calcium carbonate (CC) and sevelamer hydrochloride (SH) + sevelamer carbonate (SC) treatment arms. (b) Changes in aPWV by individual SH and SC treatment arms. Data are presented as mean values with error bars depicting standard deviation. Data series were offset to aid visualization. Mixed-effects models with repeated measures were used to test for between-group differences: ∗P < 0.01 for CC versus SH + SC.
Exploratory Endpoints
CKD–Mineral and Bone Disorder Parameters
Concentrations of serum phosphate, calcium, albumin, intact parathyroid hormone, magnesium, and alkaline phosphatase were stable over the study period in both the CC- and sevelamer-treated groups (Supplementary Figure S5) with no significant differences between groups or across individual treatment arms at 24 weeks (data not shown). Serum bicarbonate levels were stable in the SC-treated arm (P = 0.78), but decreased from baseline in CC- and SH-treated patients by week 24 (CC –1.64 mmol/l [95% CI –3.27 to –0.01], P = 0.049; SH –0.50 mmol/l [95% CI –0.97 to –0.03], P = 0.039). Despite these within-group differences, serum bicarbonate levels adjusted for baseline concentrations did not differ between CC- and sevelamer-treated groups (–1.7 mmol/l, 95% CI, –3.9 to +0.55, P = 0.14) or across individual sevelamer-treatment arms at week 24.
Serum calcification propensity (T50-test), a functional readout of the ability of a patient’s serum to resist de novo CPP-2 formation when enriched with exogenous calcium and phosphate ex vivo, increased (i.e., reduced propensity) from baseline over 24 weeks in both the CC- and sevelamer-treated groups (CC +30 minutes [95% CI +4 to +46 minutes], P = 0.022; SH + SC +34 minutes [95% CI, +10 to +57 minutes, P = 0.005]; Supplementary Figure S6 and Supplementary Table S8), and did not significantly differ between groups at 24 weeks (P = 0.66). Serum T50 was not associated with native CPP-1 or CPP-2 levels at baseline or at 12 or 24 weeks (data not shown).
Factors Associated With Changes in CPP-1 in Sevelamer-Treated and CC-Treated Patients
To gain insight into factors that might be related to the observed reduction in serum CPP-1 levels in sevelamer-treated patients, we used mixed-effects models to estimate the association of different biochemical variables with CPP-1 levels over the 24-week study period (Supplementary Table S9). We used the observed intraindividual variation of each parameter to define unit change and to enable meaningful comparison of effect sizes. Because no interactions by binder formulation were detected (P > 0.20), we analyzed pooled results in the combined SH + SC group. Interestingly, although the majority of these variables did not show significant between-group or within-group changes in aggregate analyses (Supplementary Figure S5), nominal increases in serum magnesium, bicarbonate, alkaline phosphatase, as well as decreases in phosphate, C-reactive protein, and IL-8 concentrations, were all associated with greater reductions in serum CPP-1 levels. After additional adjustment for serum phosphate, associations with magnesium, bicarbonate, and IL-8 persisted (Supplementary Table S9). In contrast, corresponding analyses in those treated with CC revealed that changes in CPP-1 levels were associated only with variability in serum phosphate (4.3% increase in CPP-1 per 0.2-mmol/l increase [0.6% to 16.5%], P = 0.007) and serum calcium (8.1% increase in CPP–1 per 0.15-mmol/l increase [4.0% to 13.3%], P = 0.001). Mixed-effect models revealed no significant association of serum T50 with either CPP-1 or CPP-2 levels in CC- or sevelamer-treated patients (all comparisons P > 0.5).
Discussion
To our knowledge, this is the first RCT to compare the effects of sevelamer and calcium carbonate on serum CPP levels in patients with end-stage kidney disease requiring maintenance HD therapy. After 24 weeks, serum CPP-1 levels were on average 70% lower in those treated with sevelamer (SH + SC) than those randomly assigned to receive CC. This finding was robust to adjustment for baseline CPP-1 levels and serum phosphate, replicated in sensitivity analyses evaluating the effect of missing data or adherence to the study medication, and maintained after adjustment for potential confounders that were nominally different across groups at baseline. In secondary analyses, treatment with sevelamer (SH or SC) was associated with lower serum IL-8 concentrations and aPWV compared with treatment with CC, but only those treated with SC had lower serum CPP-1 levels at 24 weeks. Serum CPP-1 levels were, however, significantly lower in SH-treated patients at 12 weeks compared with the CC group, suggesting a difference in the kinetics of action on CPP between sevelamer formulations.
Prior cross-sectional studies have revealed strong associations between serum CPP-1 and CPP-2,29, 31 and here we also observed a significant association between the 2 arms over the study period (r = 0.45, 95% CI 0.26 to 0.60, P < 0.001). We expected that changes in 1 arm would be reflected in the other. Surprisingly, however, a reduction in CPP-1 was not accompanied by a similar magnitude reduction in CPP-2. In fact, CPP-2 levels appeared stable over the 24-week study. Accounting for the differential effects of sevelamer on CPP-1 and CPP-2 is challenging. Although CPP-2 appears pro-inflammatory and pro-calcific in vitro, the significance in vivo is uncertain because artifacts of sample storage and processing30 may potentially mask true biological variation. However, as in other cohorts of stable prevalent HD patients,40 CPP-2 levels were only a minor fraction of the total circulating CPP pool at baseline (∼9%). Likewise, despite the substantial reduction in CPP-1 in response to sevelamer, CPP-2 still accounted for only ∼20% of total CPP levels at 24 weeks. Nonetheless, routes of synthetic CPP-1 and CPP-2 clearance differ in vivo—the former principally via liver sinusoidal endothelial cells and the latter through the reticuloendothelial system.23 A partial dissociation of CPP-1 and CPP-2 levels is therefore biologically plausible if sevelamer impacts these clearance mechanisms differently. However, it is important to stress that underrecruitment to the study means that our analysis is underpowered to detect the substantially smaller prespecified changes in CPP-2 levels (35%) on which the sample size calculations were predicated. Thus, we cannot exclude smaller, but potentially important, effects on CPP-2 due to binder treatment that could not be detected here.
Because intestinal phosphate binding should be equivalent across treatment arms owing to the design of the study, we considered 2, not mutually exclusive, explanations for the divergent effects of sevelamer and calcium carbonate on CPP-1 levels: a reduction in calcium loading as a result of withdrawal of CC and direct pleiotropic (e.g., anti-inflammatory) effects of sevelamer.
Given that calcium is one of the main biochemical drivers for CPP formation, it might be expected that dietary calcium loading would augment circulating levels if adaptations in mineral handling cannot maintain homeostasis. Indeed, there is compelling evidence that treatment with oral calcium carbonate results in positive calcium balance in patients with CKD and is temporally linked to increased extraosseous calcium deposition over a relatively short time frame.41 Although within-group analyses in this study showed a nonsignificant change in CPP-1 levels over 24 weeks from baseline in those treated with CC (95% CI –25% to +300%, P = 0.2), clearly a significant subset of individuals in this group did experience a substantial increase in CPP-1 levels given the confidence intervals. In exploratory analyses, variations in serum calcium and phosphate levels also remained the only factors related to changes in the CPP-1 levels in the CC treatment arm. Post hoc analysis of serum CPP-1 levels during the 13-week study run-in showed a comparable increase in levels across both arms during CC treatment, but strikingly different variation in levels during the preceding washout phase depending on pre-existing binder therapy. In this study, CPP-1 levels were observed to increase markedly in those originally prescribed non–calcium-containing phosphate binders, whereas those originally treated with CC experienced much more modest increments more in keeping with the magnitude to changes observed in those not receiving binder therapy. These findings are consistent with another recent prospective observational study of 24 HD patients, in which serum CPP levels were reported to significantly decrease after a switch from CC to lanthanum carbonate, another non–calcium-based binder.42 Together these observations imply that the relative lowering of CPP-1 levels observed in those receiving sevelamer in this study may in part reflect withdrawal of CC rather than direct suppressive effects of the sevelamer.
Although the proinflammatory properties of CPP-2 have garnered considerable interest,22,26 recent data suggest that CPP-1 may be even more inflammatory in vitro for the same calcium load.23 Consistent with such effects, we observed a concomitant reduction in CPP-1 and IL-8 levels in sevelamer-treated patients, whereas serum IL-8 levels were observed to increase in those randomly assigned to receive CC in whom CPP-1 also showed a trend toward higher levels. However, the directionality of such relationships, if causal, remains uncertain as the interplay of inflammation and mineral metabolism may be bidirectional, with inflammation also driving uncoupling of bone turnover, favoring net resorption and resulting in increased release of CPP from bone into the circulation. The latter supposition is supported by recent data from our group showing that increasing dialysate magnesium was associated with a reduction in inflammatory and osteoclastic markers and an attendant lowering of serum CPP levels.40 Another post hoc analysis of an RCT evaluating the effect of sevelamer and sucroferric oxyhydroxide on CKD–mineral and bone disorder parameters in dialysis patients are also consistent with effects on bone turnover, showing a reduction in osteoclastic markers and an increase in osteoblastic markers after 24 weeks of therapy.43 Therefore, the well-documented anti-inflammatory effects of sevelamer could provide a mechanism of CPP lowering that is independent of effects on phosphate or avoidance of calcium but rather through effects on bone. Unfortunately, measurements of bone markers were not available to explore this hypothesis empirically in this cohort. Regardless, in the absence of a control (placebo) arm it is not possible to delineate effects related to withdrawal of calcium from direct anti-inflammatory actions of sevelamer.
As far as we are aware, this is the first report of effects of phosphate binders on IL-8. Data on IL-8 in the context of end-stage kidney disease are sparse, but earlier studies suggested that levels were predictive or poor outcomes.44 IL-8 (or chemokine [C-X-C motif] ligand 8) is a prototypical chemokine of the C-X-C motif family that is chiefly secreted by macrophage as part of the early inflammatory response, where it functions to recruit neutrophil and monocyte to sites of injury.45 IL-8 is considered to have established roles not only in early atherogenesis but also in the persistence of inflammation in vascular lesions.46 High phosphate conditions that engender CPP formation in vitro have also been reported to induce IL-8 secretion from endothelial cells, which reportedly has a pro-calcifying effect on vascular smooth muscle cells through suppression of osteopontin,47 a potent mineralization inhibitor. Given other recent data elucidating the liver sinusoidal endothelial cells as a major site of CPP-1 clearance in vivo23 and the temporal relationship of IL-8 concentrations observed by us, further work is warranted to explore the potential effects of CPP on the endothelium and downstream vascular pathology. Interestingly, there are also reports that IL-8 may have direct effects on bone by stimulating osteoclastogensis,48,49 providing another possible link between the effects of different phosphate binders on inflammation, bone, and CPP.
Our finding that aortic PWV was attenuated in sevelamer-treated patients compared with those receiving CC is consistent with previous reports of other small, prospective HD cohorts where sevelamer was found to reduce PWV over 6 to 11 months.50,51 Given the relatively short-term nature of this and these other studies, such reductions in PWV are unlikely to reflect structural changes (i.e., calcification), which are thought to occur over longer times scales, but may instead relate to functional changes (i.e., endothelial function). Conceivably, reduced calcium loading as well as anti-inflammatory effects may contribute to improvement in this integrative parameter and explain the divergent trajectories in the sevelamer- and CC-treatment arms.
With respect to other biochemical parameters, all but serum bicarbonate remained stable over the study period and at comparable levels across treatment arms. Whereas serum bicarbonate levels remained stable in SC-treated patients, levels declined over 24 weeks in both the SH and CC arms. Differential effects of SH and SC on bicarbonate are well described and explained by the exchange of chloride for phosphate with SH, resulting in a dose-dependent metabolic acidosis, compared with neutral effects with SC that exchange carbonate for each phosphate bound by the resin.52 Interestingly, despite the small numbers, secondary analyses across individual sevelamer treatments suggest that reductions in serum CPP-1 levels at week 24 were observed only in those randomly assigned to receive SC. Reductions in both IL-6 and IL-8 levels also appeared more robust in the SC arm compared with the SH arm. Indeed, in exploratory analyses, changes in bicarbonate levels, in addition to those of magnesium and IL-8, were the only variables associated with the changes in the CPP-1 level that remained significant after adjustment for phosphate. Given the potential role of metabolic acidosis in the development of bone disease in CKD,53 the effect of changes in acid-base status on CPP metabolism warrants further investigation. Differences in the effects of SH and SC also argue against withdrawal of calcium as the only mechanism driving the divergent trajectories of serum CPP levels in our patients, but again, a control group is needed to provide conclusive evidence of such effects.
A novel aspect of this report is the concurrent measurement of native CPP levels in serum by flow cytometry and serum calcification propensity (T50 test) by turbidimetry: 2 contemporary measures of “mineral stress.” Although both notionally involve measurement of CPP, it is important to emphasize the fundamental distinctions between the 2 methods. Whereas nano flow cytometry enumerates and discriminates major subsets of native mineral-containing nanoparticles (e.g., CPP-1, CPP-2, membrane-bound mineral-containing particles) in biological fluids,29 the T50 test monitors the crystallization of CPP synthesized in serum after addition of supersaturating amounts of calcium and phosphate.36 The kinetic parameter T50 is the time taken for half-maximal conversion of CPP-1 to CPP-2 and depends on the balance of various promoting and inhibitory substances present in serum.54 The faster this transformation occurs, the lower the T50 value, denoting enhanced calcification propensity. Within the T50 test environment CPPs are generated de novo to levels >1014/ml depending on the exact conditions used. In contrast, native CPP levels are ∼105 to 107/ml and sedimentation of native CPP from serum has minimal impact on the T50 test readout.36 Although they are measured in serum or plasma, it is also important to appreciate that native CPPs are unlikely to be formed there in appreciable amounts because of various kinetic constraints and, instead, originate from extravascular sites of high mineral ion flux (bone, kidney, and intestine).29,55 Thus, CPP made in serum ex vivo in the T50 test and those present endogenously differ in their composition.56 We found that neither serum CPP-1 nor CPP-2 levels were associated T50 measurements at baseline or over time. Although the small sample size might account for these null findings, the evidence presented in our study is consistent with the view that these 2 measures capture different aspects of mineral homeostasis and hence risk. Interestingly, unlike native CPP levels, which appear convincingly associated with ambient calcium levels,31,57 calcium has relatively weak effects on T50 and thus is relatively insensitive to the chronic effects of calcium loading,36,58 which may explain the lack of observed differences in response to CC and sevelamer treatment we found.
Strengths of our study include accurate measurement of CPP, and discrimination of CPP-1 from CPP-2 and other mineral-containing debris in serum using a novel flow cytometry–based assay. High rates of adherence to study medications, stringent control of other medications that may impact mineral metabolism (e.g., calcitriol, magnesium supplementation), and a long active run-in to render the patients as similar as possible at baseline are also strengths.
The major limitation of this study is the small sample size owing to low recruitment (34% of target), rendering it underpowered to detect the prespecified primary endpoint: a 35% difference in CPP-1 and CPP-2 levels at 24 weeks. Fortuitously, the observed treatment effect with respect to CPP-1 levels was approximately double this predetermined level (70%), but we are unable to exclude more modest effects on CPP-2. Although the overall comorbidity burden appears similar across treatment arms, the small sample size limited the effectiveness of the randomization procedure, which resulted in imbalances between treatment groups in certain characteristics that could potentially confound some of the comparisons. Although our modeling strategy and post hoc adjustments account for some of these factors, we cannot exclude the possibility that a degree of uncontrolled confounding remains. We also acknowledge that the open-label (unblinded) design is suboptimal and the relatively short duration of the trial may have biased the results. Because of the nature of the study design, we can provide limited mechanistic insight into the factors driving the clearly divergent trajectories of CPP-1 in those receiving sevelamer compared with CC. In particular, we cannot conclusively differentiate effects related to withdrawal of CC therapy in those subsequently randomly assigned to receive sevelamer from direct anti-inflammatory actions of this binder. Regrettably, the perceived lack of equipoise in this setting prevents us from addressing these questions explicitly with a placebo-controlled trial. Indeed, the need for washout and run-in with CC was a reason for physician disinclination and exclusion from the trial. Finally, it is also uncertain to what extent our reported findings are representative of the wider HD population, given the selection of mostly stable well-functioning HD patients without biochemical evidence of severe secondary hyperparathyroidism or bone disease. The predominance of elderly white men enrolled in the trial also potentially limits generalizability to other patient groups given possible racial and sex-related differences in mineral and bone metabolism.59,60
In conclusion, 24-week treatment with sevelamer resulted in a reduction in levels of serum CPP-1 compared to treatment with CC, with effects most robustly observed in those randomly assigned to receive SC. Treatment with sevelamer was associated with a concomitant decrease in IL-8 and aPWV, suggesting that it may help reduce systemic inflammation and improve vascular function. We speculate that the potentially beneficial effects of sevelamer may, in part, work through direct suppressive effects on CPP-1 as well as avoidance of calcium-loading associated with calcium-based binders. Given the encouraging results of this study, we hope that it may prompt larger trials in this setting to validate these findings and to ascertain whether lowering CPP levels in HD patients may improve patient outcomes.
Disclosure
ERS reports research funding from Amgen, Baxter, and Sanofi and owns stock in Calciscon, the company that specializes in performing the T50 test. FFMP reports research funding from Sanofi. TDH reports research funding from Sanofi. NDT reports honoraria, travel support, and research funding from Amgen, Shire, and Sanofi. SGH reports honoraria, travel support, and research funding from Amgen, Astra Zenica, Baxter, and Sanofi.
Acknowledgments
Study funding and investigational drug was kindly provided by Sanofi-Aventis (Singapore) Pte Ltd. The investigators are solely responsible for the design, conduct, collection, analysis, and interpretation of the data, writing of the report, and the decision to submit for publication, with no input from Sanofi-Aventis (Singapore) Pte Ltd.
Preliminary data from this study were previously presented at the International Society of Nephrology World Congress of Nephrology, April 12-15, 2019, in Melbourne, Australia, and the World Congress of Nephrology, March 26-29, 2020, in Abu Dhabi, United Arab Emirates.
The investigators thank the study participants and the staff at the Coburg and Essendon dialysis units who assisted with data and sample collection. This trial was registered with the Australian and New Zealand Clinical Trials Register under the identifier ACTRN12617000881336.
Author Contributions
ERS, TDH, and SGH designed the study; FFMP managed the study; FFMP performed the majority of measurements with help from ERS; all authors analyzed the data; ERS drafted and revised the manuscript; all authors critically revised and approved the final version.
Footnotes
Complete Methods.
CONSORT statement checklist.
Table S1. Charlson Comorbidity Index scoring system and ICD-10 codes.
Table S2. SCaRF trial exclusions.
Table S3. Baseline characteristics of the modified ITT cohort in individual sevelamer treatment arms in the SCaRF trial.
Table S4. Baseline serum biochemistry in individual sevelamer treatment arms in the SCaRF trial.
Table S5. Changes in serum CPP-1 and CPP-2 levels over 24 weeks in individual treatment arms in the SCaRF trial.
Table S6. Changes in serum inflammatory markers over 24 weeks in individual treatment arms in the SCaRF trial.
Table S7. Baseline characteristics in all randomized patients and those with PWV measurements in the SCaRF trial.
Table S8. Changes in serum calcification propensity over 24 weeks in the SCaRF trial.
Table S9. Biochemical factors associated with reduction in serum CPP-1 from baseline to 24 weeks in sevelamer-treated patients.
Figure S1. Flow cytometric analysis of serum CPP.
Figure S2. The effect of freeze-thaw cycling on synthetic and native serum CPP.
Figure S3. Stability of synthetic and native serum CPP in assay diluent.
Figure S4. (A) Trajectory of serum CPP-1 levels during study run-in and randomized phase of the SCaRF trial. (B) Changes in serum CPP-1 during binder washout categorized by prescribed binder therapy at screening.
Figure S5. Changes in serum CKD-MBD parameters over 24 weeks in response to treatment with calcium carbonate or sevelamer in the SCaRF trial.
Figure S6. Changes in serum calcification propensity (T50) over 24 weeks in response to treatment with calcium carbonate or sevelamer in the SCaRF trial.
Supplementary Material
References
- 1.Fukagawa M., Kido R., Komaba H. Abnormal mineral metabolism and mortality in hemodialysis patients with secondary hyperparathyroidism: evidence from marginal structural models used to adjust for time-dependent confounding. Am J Kidney Dis. 2014;63:979–987. doi: 10.1053/j.ajkd.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Tentori F., Blayney M.J., Albert J.M. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Floege J. Phosphate binders in chronic kidney disease: a systematic review of recent data. J Nephrol. 2016;29:329–340. doi: 10.1007/s40620-016-0266-9. [DOI] [PubMed] [Google Scholar]
- 4.Cannata-Andia J.B., Fernandez-Martin J.L., Locatelli F. Use of phosphate-binding agents is associated with a lower risk of mortality. Kidney Int. 2013;84:998–1008. doi: 10.1038/ki.2013.185. [DOI] [PubMed] [Google Scholar]
- 5.Isakova T., Gutiérrez O.M., Chang Y. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20:388–396. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopes A.A., Tong L., Thumma J. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis. 2012;60:90–101. doi: 10.1053/j.ajkd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chertow G.M., Burke S.K., Raggi P., Treat to Goal Working Group Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 8.Block G.A., Spiegel D.M., Ehrlich J. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 9.Raggi P., Bommer J., Chertow G.M. Valvular calcification in hemodialysis patients randomized to calcium-based phosphorus binders or sevelamer. J Heart Valve Dis. 2004;13:134–141. [PubMed] [Google Scholar]
- 10.Qunibi W., Moustafa M., Muenz L.R. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 11.Ferramosca E., Burke S., Chasan-Taber S. Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J. 2005;149:820–825. doi: 10.1016/j.ahj.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Brandenburg V.M., Schlieper G., Heussen N. Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol Dial Transplant. 2010;25:2672–2679. doi: 10.1093/ndt/gfq053. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-González J.F., Mora-Fernández C., Muros de Fuentes M. Effect of phosphate binders on serum inflammatory profile, soluble CD14, and endotoxin levels in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2272–2279. doi: 10.2215/CJN.01650211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peres A.T., Dalboni M.A., Canziani M.E. Effect of phosphate binders on oxidative stress and inflammation markers in hemodialysis patients. Hemodial Int. 2009;13:271–277. doi: 10.1111/j.1542-4758.2009.00369.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel L., Bernard L.M., Elder G.J. Sevelamer Versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11:232–244. doi: 10.2215/CJN.06800615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habbous S., Przech S., Acedillo R. The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32:111–125. doi: 10.1093/ndt/gfw312. [DOI] [PubMed] [Google Scholar]
- 17.Villa-Bellosta R., Sorribas V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler Thromb Vasc Biol. 2009;29:761–766. doi: 10.1161/ATVBAHA.108.183384. [DOI] [PubMed] [Google Scholar]
- 18.Ewence A.E., Bootman M., Roderick H.L. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–e34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 19.Sage A.P., Lu J., Tintut Y. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79:414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahnen-Dechent W., Schafer C., Ketteler M. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med (Berl) 2008;86:379–389. doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 21.Heiss A., DuChesne A., Denecke B. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 22.Smith E.R., Hanssen E., McMahon L.P. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppert S., Büscher A., Babler A. Cellular clearance and biological activity of calciprotein particles depend on their maturation state and crystallinity. Front Immunol. 2018;9:1991. doi: 10.3389/fimmu.2018.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutikhin A.G., Velikanova E.A., Mukhamadiyarov R.A. Apoptosis-mediated endothelial toxicity but not direct calcification or functional changes in anti-calcification proteins defines pathogenic effects of calcium phosphate bions. Sci Rep. 2016;6:27255. doi: 10.1038/srep27255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shishkova D., Velikanova E., Sinitsky M. Calcium phosphate bions cause intimal hyperplasia in intact aortas of normolipidemic rats through endothelial injury. Int J Mol Sci. 2019;20:5728. doi: 10.3390/ijms20225728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghagolzadeh P., Bachtler M., Bijarnia R. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis. 2016;251:404–414. doi: 10.1016/j.atherosclerosis.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 27.Cai M.M.X., Smith E.R., Tan S.J. The role of secondary calciprotein particles in the mineralisation paradox of chronic kidney disease. Calcif Tissue Int. 2017;101:570–580. doi: 10.1007/s00223-017-0313-0. [DOI] [PubMed] [Google Scholar]
- 28.Stremke E.R., McCabe L.D., McCabe G.P. Twenty-four-hour urine phosphorus as a biomarker of dietary phosphorus intake and absorption in CKD: a secondary analysis from a controlled diet balance study. Clin J Am Soc Nephrol. 2018;13:1002–1012. doi: 10.2215/CJN.00390118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith E.R., Hewitson T.D., Cai M.M.X. A novel fluorescent probe-based flow cytometric assay for mineral-containing nanoparticles in serum. Sci Rep. 2017;7:5686. doi: 10.1038/s41598-017-05474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura Y., Iwazu Y., Shiizaki K. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci Rep. 2018;8:1256. doi: 10.1038/s41598-018-19677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruderman I., Smith E.R., Toussaint N.D. Longitudinal changes in bone and mineral metabolism after cessation of cinacalcet in dialysis patients with secondary hyperparathyroidism. BMC Nephrol. 2018;19:113. doi: 10.1186/s12882-018-0910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemoto Y., Kumagai T., Ishizawa K. Phosphate binding by sucroferric oxyhydroxide ameliorates renal injury in the remnant kidney model. Sci Rep. 2019;9:1732. doi: 10.1038/s41598-018-38389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chae J.W., Song C.S., Kim H. Prediction of mortality in patients undergoing maintenance hemodialysis by Charlson Comorbidity Index using ICD-10 database. Nephron Clin Pract. 2011;117:c379–c384. doi: 10.1159/000321525. [DOI] [PubMed] [Google Scholar]
- 34.Brinkmann J., Jordan J., Tank J. A pilot study comparison of a new method for aortic pulse wave velocity measurements using transthoracic bioimpedance and thigh cuff oscillometry with the standard tonometric method. J Am Soc Hypertens. 2015;9:293–298. doi: 10.1016/j.jash.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Townsend R.R., Wilkinson I.B., Schiffrin E.L. Recommendations for improving and standardizing vascular research on arterial stiffness: a Scientific Statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasch A., Farese S., Gräber S. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol. 2012;23:1744–1752. doi: 10.1681/ASN.2012030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bundy J.D., Cai X., Scialla J.J. Serum calcification propensity and coronary artery calcification among patients with CKD: the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2019;73:806–814. doi: 10.1053/j.ajkd.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith E.R., Ford M.L., Tomlinson L.A. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant. 2012;27:1957–1966. doi: 10.1093/ndt/gfr609. [DOI] [PubMed] [Google Scholar]
- 39.Smith E.R., Ford M.L., Tomlinson L.A. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol. 2014;25:339–348. doi: 10.1681/ASN.2013060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bressendorff I., Hansen D., Pasch A. The effect of increasing dialysate magnesium on calciprotein particles, inflammation and bone markers: post hoc analysis from a randomized controlled clinical trial. Nephrol Dial Transplant. 2019:gfz234. doi: 10.1093/ndt/gfz234. [DOI] [PubMed] [Google Scholar]
- 41.Hill K.M., Martin B.R., Wastney M.E. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2013;83:959–966. doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura K., Nagata Y., Hiroyoshi T. The effect of lanthanum carbonate on calciprotein particles in hemodialysis patients. Clin Exp. Nephrol. 2019;24:323–329. doi: 10.1007/s10157-019-01832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ketteler M., Sprague S.M., Covic A.C. Effects of sucroferric oxyhydroxide and sevelamer carbonate on chronic kidney disease-mineral bone disorder parameters in dialysis patients. Nephrol Dial Transplant. 2019;34:1163–1170. doi: 10.1093/ndt/gfy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panichi V., Taccola D., Rizza G.M. Interleukin-8 is a powerful prognostic predictor of all-cause and cardiovascular mortality in dialytic patients. Nephron Clin Pract. 2006;102:c51–c58. doi: 10.1159/000088923. [DOI] [PubMed] [Google Scholar]
- 45.Gimbrone M.A., Jr., Obin M.S., Brock A.F. Endothelial interleukin-8: a novel inhibitor of leukocyte-endothelial interactions. Science. 1989;246:1601–1603. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- 46.Apostolakis S., Vogiatzi K., Amanatidou V. Interleukin 8 and cardiovascular disease. Cardiovasc Res. 2009;84:353–360. doi: 10.1093/cvr/cvp241. [DOI] [PubMed] [Google Scholar]
- 47.Bouabdallah J., Zibara K., Issa H. Endothelial cells exposed to phosphate and indoxyl sulphate promote vascular calcification through interleukin-8 secretion. Nephrol Dial Transplant. 2019;34:1125–1134. doi: 10.1093/ndt/gfy325. [DOI] [PubMed] [Google Scholar]
- 48.Bendre M.S., Montague D.C., Peery T. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 49.Kopesky P., Tiedemann K., Alkekhia D. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol Open. 2014;3:767–776. doi: 10.1242/bio.20148128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takenaka T., Suzuki H. New strategy to attenuate pulse wave velocity in haemodialysis patients. Nephrol Dial Transplant. 2005;20:811–816. doi: 10.1093/ndt/gfh656. [DOI] [PubMed] [Google Scholar]
- 51.Othmane Tel H., Bakonyi G., Egresits J. Effect of sevelamer on aortic pulse wave velocity in patients on hemodialysis: a prospective observational study. Hemodial Int. 2007;11(suppl 3):S13–S21. doi: 10.1111/j.1542-4758.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 52.Pai A.B., Shepler B.M. Comparison of sevelamer hydrochloride and sevelamer carbonate: risk of metabolic acidosis and clinical implications. Pharmacotherapy. 2009;29:554–561. doi: 10.1592/phco.29.5.554. [DOI] [PubMed] [Google Scholar]
- 53.Kraut J.A., Madias N.E. Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr Nephrol. 2011;26:19–28. doi: 10.1007/s00467-010-1564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasch A., Jahnen-Dechent W., Smith E.R. Phosphate, calcification in blood, and mineral stress: the physiologic blood mineral buffering system and its association with cardiovascular risk. Int J Nephrol. 2018;2018:9182078. doi: 10.1155/2018/9182078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith E.R., Hewitson T.D., Jahnen-Dechent W. Calciprotein particles: mineral behaving badly? Curr Opin Nephrol Hypertens. 2020;29:378–386. doi: 10.1097/MNH.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 56.Smith E.R., Hewitson T.D., Hanssen E. Biochemical transformation of calciprotein particles in uraemia. Bone. 2018;110:355–367. doi: 10.1016/j.bone.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 57.Cai M.M.X., Smith E.R., Kent A. Calciprotein particle formation in peritoneal dialysis effluent is dependent on dialysate calcium concentration. Perit Dial Int. 2018;38:286–292. doi: 10.3747/pdi.2017.00163. [DOI] [PubMed] [Google Scholar]
- 58.Smith E.R., Hewitson T.D., Holt S.G. Diagnostic tests for vascular calcification. Adv Chronic Kidney Dis. 2019;26:445–463. doi: 10.1053/j.ackd.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Yan L., Prentice A., Zhou B. Age- and gender-related differences in bone mineral status and biochemical markers of bone metabolism in Northern Chinese men and women. Bone. 2002;30:412–415. doi: 10.1016/s8756-3282(01)00676-7. [DOI] [PubMed] [Google Scholar]
- 60.Scialla J.J., Parekh R.S., Eustace J.A. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol. 2015;42:25–34. doi: 10.1159/000438999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.