Introduction
Severe microangiopathic hemolytic anemia and thrombocytopenia during pregnancy or during the postpartum period requires a differential diagnosis between hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, thrombotic thrombocytopenic purpura, and complement-mediated thrombotic microangiopathy. The 3 diseases may merge into one another, overlap syndromes are increasingly described, and the differential diagnosis may sometimes be allowed only by genetic analysis.1,2
Atypical hemolytic uremic syndrome (aHUS) is a rare variant accounting for 5%–10% of all cases of HUS; it is characterized by an increased activity of the alternative pathway of the complement caused by an acquired or genetic dysregulation.3 Genetic causes include different mutations in regulatory complement genes. The most common loss of function mutations involve complement factor H (CFH) and CFH-related hybrid genes (CFHR1–CFHR5), complement factor I (CFI), and monocyte chemoattractant protein (MCP); gain of function mutations of complement-activating genes, such as complement factor B (CFB) or C3, are less common. Overall, pathogenic mutations are described in 50%–60% of patients with aHUS and mainly regard CFH.3
aHUS is a highly heterogeneous disease: different haplotypes in complement genes and various pathogenic genetic variants determine different levels of alternative pathway activity, as measured by different tests, and modulate the risk of developing aHUS and its severity.
Pregnancy has been identified as an important trigger for aHUS, with a reported incidence of 1 in 25,000 gestations. The majority of cases present in the postpartum period, although pregnancy-associated aHUS (p-aHUS) may develop during pregnancy.4,5 p-aHUS is a severe disease, with high maternal mortality, a risk of fetal loss, and a risk of rapid progression to end-stage renal disease (ESRD).4,5
Conventional management of aHUS, including plasma exchange and hemodialysis in patients with severe acute kidney injury, may not be effective in patients with severe or recurrent disease.6 Eculizumab, a humanized monoclonal antibody directed against C5, is presently the gold standard treatment to control the overamplification of complement alternative pathway, and a growing body of literature supports its use in p-aHUS.7, 8, 9
The largest available series of patients with p-aHUS reports on 87 patients diagnosed from 1983 to 2013. In this multicenter cohort, only 4 patients observed since 2011 received eculizumab as second-line therapy after plasma exchanges, with complete kidney function recovery in 3 patients. Overall, genetic mutations were detected in 49 (56%) patients, mainly concerning CFH (30%) and CFI (9%).4 Before eculizumab became available, treatment was based on plasma therapy and corticosteroids; the prognosis was grim, leading to ESRD in 53% of cases and to predialytic chronic kidney disease in 19% of cases. Twenty-eight percent of patients relapsed over a mean follow-up of 7.2 years, including half of those who had received a kidney transplant.4 Fetal or neonatal death occurred in 14% of cases, and dialysis was required, at least temporarily, in 71% of patients.4
The case here described may stress the importance of early diagnosis and prompt treatment but suggests also that the challenge of pregnancy may represent a unique occasion for the diagnosis of rare diseases.
Case Presentation
We describe the case of a 39-year-old woman who developed p-aHUS during a twin pregnancy.
The patient’s family history was negative for renal or other relevant diseases. The patient underwent in vitro fertilization resulting in twin pregnancy, whose development was unremarkable until gestational week 32, when she was referred to the obstetrics and gynecology unit for hypertension (blood pressure, 150/95 mm Hg). At referral, her serum creatinine level was 1.05 mg/dl, no proteinuria was detected at urinalysis, hemoglobin was 13.8 g/dl, haptoglobin was 30 mg/dl, and the platelet count was slightly lower than normal (139 × 103/μl); liver enzymes were normal as were uterine and umbilical Doppler flows; gestational hypertension was therefore diagnosed, on the account of the lack of proteinuria; however, in retrospect, a mild increase in serum creatinine was overlooked.S1–S3
The patient was started on α-methyldopa (250 mg twice a day), but 4 days later she presented to the hospital complaining of strong epigastrium pain. Elevated liver enzymes (aspartate aminotransferase, 1575 U/l; alanine aminotransferase, 1175 U/l), acute hemolytic anemia (hemoglobin 9.5 g/dl), increased lactate dehydrogenase (5574 U/l), and thrombocytopenia (platelets, 61 × 103/μl) were found, in addition to persistent hypertension. Serum creatinine and urea were still in the normal range, but urinalysis demonstrated high-grade proteinuria with a high albumin-to-creatinine ratio (300 mg/g). The diagnosis of HELLP syndrome was made and Cesarean section was performed, giving birth to healthy dizygotic twins, weighing 2.190 Kg (97 percentile) and 2.050 Kg (90 percentile).
After Cesarean section, severe bleeding occurred, and subtotal hysterectomy was needed. The patient was admitted to the intensive care unit. Her blood pressure remained high (180/110 mm Hg) and anemia persisted despite blood transfusions. She was treated by plasma infusion on postpartum days 1 and 2 (4 units of frozen fresh plasma). Nephrology was consulted 5 days postpartum because of a rapid increase in serum creatinine (up to 5.5 mg/dl), worsening of anemia (hemoglobin 6.6 g/dl) with low haptoglobin level (30 mg/dl) and low platelets (174 × 103/μl) with high lactate dehydrogenase levels (1150 U/l). Schistocytes were not found in the peripheral smear. ADAMTS-13 activity assay was not feasible because of the interference with the previous plasma infusions. However, the lack of response to plasma infusion and the low C3 level (53 mg/dl) suggested p-aHUS; on account of the severity of the clinical picture, eculizumab was started according to the protocol described by Fakhouri et al.,S4 which consisted of intravenous infusion of 900 mg of eculizumab every week for 4 weeks, followed by 1200 mg every 2 weeks.
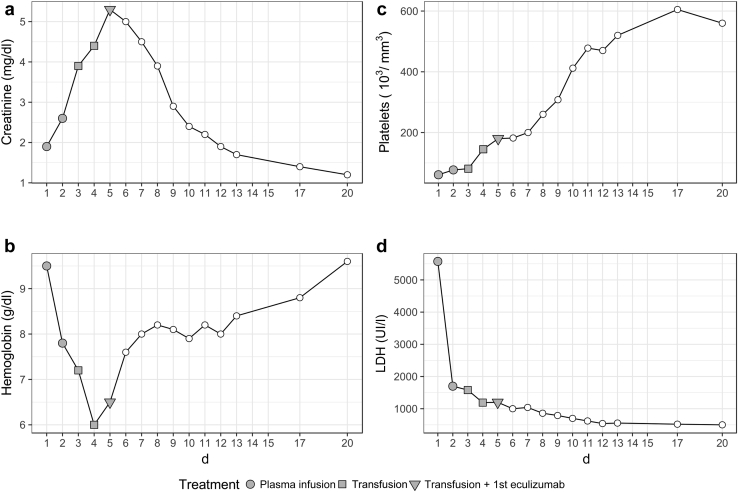
After the first eculizumab infusion the clinical conditions and laboratory tests rapidly and dramatically improved. Treatment with eculizumab was continued for 1 year and discontinued thereafter (Figure 1). One year after discontinuation, kidney function remained normal (Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate, 98 ml/min per 1.73 m2) without proteinuria or hematuria; the children are on regular pediatric follow-up and have attained the expected developmental milestones.
Figure 1.
Trend of biochemical parameters (creatinine [a], hemoglobin [b], platelets [c], and lactate dehydrogenase [LDH; d]) after delivery.
Genetic Analysis
After acquiring written informed consent, genetic analysis was performed by direct sequencing to investigate the genetic variants coding for proteins regulating alternative complement pathway, as CFH, MCP, C3, CFI, CFB, and THBD, using primers yet described in the published literature.S5,S6 Resulting sequences were evaluated with Sequencher software (Gene Codes Corporation, Ann Arbor, MI).
We identified a novel heterozygous G-to-A mutation [NM_000186.3(CFH): c.1873+1G>A] in CFH that has never been described in the Human Gene Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) nor in the 1000 Genomes database (ftp://ftp.1000genomes.ebi.ac.uk/vol1/ftp/).
In addition, we identified other variants in the genes that encode the alternative pathway of complement that have already been described in the database (http://www.fh-hus.org/). In particular, we identified a H3 risk haplotype for aHUS on the CFH gene3 and 2 genetic variants on the MG3 region of C3 [NM_000064.3(C3): c.1001T>C p. Prol314Leu], and the CFI gene [NM_000204.4 (CFI): c.898G>A p.Ala300Thr]. Conversely, MPC, CFB, and THBD genes resulted wild type.
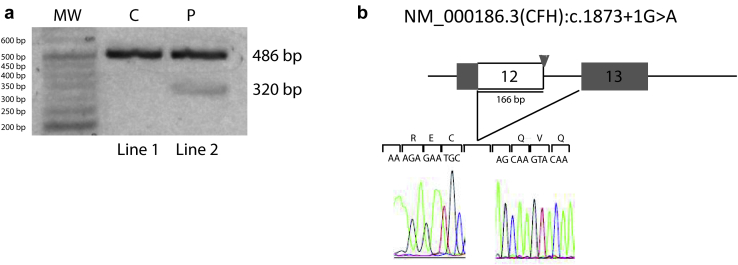
To confirm the abnormal splicing according to the standard protocol,S7 we extracted the RNA from peripheral blood mononuclear cells of a control subject (C) and from our patient (P) and performed reverse transcriptase–polymerase chain reaction using primer sequences to amplify the region between exons 12 and 13 of CFH. Reverse transcriptase–polymerase chain reaction generated a single fragment of 486 bp in the control subject, while in the patient reverse transcriptase–polymerase chain reaction displayed also a 320-bp band (Figure 2a), that was sequenced and revealed a 166-bp deletion of the 3ʹ end of exon 12 (Figure 2b).
Figure 2.
Analysis of the splice mutation IVS12+1G>A [NM_000186.3(CFH): c.1873+1G>A]. (a) Reverse transcriptase–polymerase chain reaction from the control (C) (line 1) generated a single band of 486 bp. Reverse transcriptase–polymerase chain reaction from the patient (P) carrying a heterozygous IVS12+1G>A mutation (line 2) generated a 320-bp band that was sequenced (b) and revealed a 166-bp deletion of the 3ʹ end of exon 12. IVS, intervening sequence; MW, molecular weight.
The splicing defect would lead to loss of 55 amino acids, a change in reading frame, and a premature termination codon in exon 13. This transcript may undergo nonsense-mediated decay and could therefore lead to haploinsufficiency of CFH products or lead to an abnormal protein missing residues 570–1231. The most likely outcome from this canonical splice variant is skipping of exon 12. Skipping of exon 12 would be in-frame and lead to only the deletion of residues encoded by exon 12. This transcript would not undergo nonsense-mediated mRNA decay, and could possibly retain some function depending on what is lost. Repeating the reverse transcriptase–polymerase chain reaction experiment with 1 primer upstream and 1 downstream of exon 12 is planned, to further characterize the anomaly in our patient.
Discussion
Pregnancy is an important trigger for several immunologic diseases and represents an important occasion for diagnosis by giving a chance of advancing the understanding of their pathogenesis. Many kidney and immunologic diseases present with a clinical picture that is initially interpreted as belonging to the spectrum of the hypertensive disorders of pregnancy. This case may be an example of the importance of the differential diagnosis between pre-eclampsia–HELLP, primary thrombotic microangiopathy, such as p-aHUS or thrombotic thrombocytopenic purpura, and overlap cases, which may be more frequent than previously thought; notably, this case gave the occasion for discovering a potentially pathogenic genetic variant of the complement pathway that has not been previously described.S8
Data reported in the literature show that about 70% of CFH mutations identified in patients with dysregulation of complement alternative pathway are heterozygous and located on short consensus repeats (SCR) 19-20, resulting in a CFH unable to exert its regulatory activity, especially on endothelial cells and platelets. To date, few mutations have been identified in the remaining domains.3
We report here a new CFH splicing mutation located in a domain never previously involved in the development of p-aHUS. Of note, the splicing mutation causes a partial deletion of exon 12 that should affect SCR 10 central modules, leading to a misfolding similar to the bend of a hinge, which appear to be more compact than wild-type central SCR conformation and whose functional role has not yet been elucidated.S9 We speculate that the mutation found in our patient affects the CFI-mediated regulatory activity, contributing to massive fluid-phase activation of the alternative complement pathway. The clinical phenotype of our patient is probably the result of a strong environmental trigger (twin pregnancy) and of her genetic background; of note, she is also carrier of a C3 variant already described in the literature but whose pathogenic role is not well established, which is located in the conserved and only partially known MG3 domain.S9
Finally, according to what has been reported in the published literature, aHUS risk haplotype H3 [NM_000186.3(CFH)er SNPs −331C>T (rs3753394), c.184G>A Val62Ile (rs800292), c.1204T>C p.Tyr402His (rs1061170), c.2016A>G p.Gln672Gln (rs3753396), IVS15 −543G>A intron 15 (rs1410996) and c.2808G>T p.Glu936Asp (rs1065489)],S10 together with the 2 mutations, may increase the penetrance of disease.3
The rapid and almost complete kidney function recovery, with rapid rise in both hemoglobin and platelets levels after the first eculizumab administration led to continue this therapy for a year. During this follow-up period, the estimated glomerular filtration rate was normal and the patient did not develop hypertension or urinary abnormalities. Moreover, we considered that her pregnancy had triggered aHUS; the patient could not have additional pregnancies because of the hysterectomy, and therefore eculizumab was discontinued after 1 year of treatment. Normalization of the kidney function persists 1 year later.
In conclusion, our case underlines the importance of considering thrombotic microangiopathy in the differential diagnosis of hemorrhagic and microangiopathy syndromes in pregnancy and puerperium (Table 1). The relationship with hypertensive disorders of pregnancy, and in particular with HELLP syndrome, is not fully clear, and a clear-cut differentiation is not always feasible; the encouraging results obtained with eculizumab in some severe cases of HELLP syndrome further underline the pathogenesis overlap. S11–S13
Table 1.
Teaching points
| Microangiopathic hemolytic anemia and thrombocytopenia during pregnancy or the postpartum period requires a differential diagnosis between HELLP syndrome, TTP, and complement-mediated TMA or HUS. |
| This differential diagnosis is not easy, because HELLP, TTP, and TMA or HUS may merge into one another, and overlap syndromes are described. |
| Atypical hemolytic uremic syndrome is a rare variant of HUS characterized by an increased activity of the alternative pathway of the complement. Genetic causes include mutations in different regulatory complement genes. |
| Differential diagnosis analysis may be possible only by genetic analysis, considering that new mutations are continuously reported. |
| Timely treatment with eculizumab may be critical to prevent irreversible kidney damage. |
HELLP, hemolysis, elevated liver enzymes, low platelets; HUS, hemolytic uremic syndrome; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.
From a diagnostic point of view, our case suggests that in patients affected by aHUS sequencing should not be limited to hot spot regions because new mutations may be identified in uncommon domains, and these domains may be of high relevance for both patients and their offspring. From the clinical point of view, our case suggests that timely treatment, based on clinical data, even before the genetic confirmation, has probably prevented irreversible kidney damage.S14,S15
Disclosure
All the authors declared no competing interests.
Authorship
DS, ALR, GT, AP, RG, RB, and VP conceived of the study. ALR, GT, AP, RB, SL, and DL designed the work. DS and AV acquired and analyzed the data. DS, ALR, GT, AP, RB, and GBP interpreted the data. DS, ALR, RS, and GBP created the first draft and revised the text.
Footnotes
Supplemental References.
Supplementary Material
References
- 1.Sibasi S.M. Imitators of severe preeclampsia. Obstet Gynecol. 2007;10:956–966. doi: 10.1097/01.AOG.0000258281.22296.de. [DOI] [PubMed] [Google Scholar]
- 2.Scully M., Thomas M., Underwood M. Thrombotic thrombocytopenic purpura and pregnancy: presentation, management, and subsequent pregnancy outcomes. Blood. 2014;124:211–219. doi: 10.1182/blood-2014-02-553131. [DOI] [PubMed] [Google Scholar]
- 3.Fakhouri F., Zuber J., Frémeaux-Bacchi V. Haemolytic uraemic syndrome. Lancet. 2017;390:681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 4.Bruel A., Kavanagh D., Noris M. Uremic syndrome in pregnancy and postpartum. J Am Soc Nephrol. 2017;12:1237–1247. doi: 10.2215/CJN.00280117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaggl M., Aigner C., Csuka D. Maternal and fetal outcomes of pregnancies in women with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2018;29:1020–1029. doi: 10.1681/ASN.2016090995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noris M., Remuzzi G. Managing and preventing atypical hemolytic uremic syndrome recurrence after kidney transplantation. Curr Opin Nephrol Hypertens. 2013;22:704–712. doi: 10.1097/MNH.0b013e328365b3fe. [DOI] [PubMed] [Google Scholar]
- 7.Rathbone J., Kaltenthaler E., Richards A. A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS) BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mussoni M.P., Veneziano F.A., Boetti L. Innovative therapeutic approach: sequential treatment with plasma exchange and eculizumab in a pregnant woman affected by atypical hemolytic-uremic syndrome. Transfus Apher Sci. 2014;51:134–136. doi: 10.1016/j.transci.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Sarno L., Tufano A., Maruotti G.M. Eculizumab in pregnancy: a narrative overview. J Nephrol. 2019;32:17–25. doi: 10.1007/s40620-018-0517-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.