Introduction
Anti–brush border antibody disease (ABBAD) is a rare autoimmune disease associated with acute kidney injury and tubulointerstitial damage. The autoantibody target was recently identified as low-density lipoprotein receptor–related protein 2 (LRP2), also known as megalin.1 LRP2 is a large 517-kDa transmembrane glycoprotein present along the apical brush border of the renal proximal tubular epithelium where it functions as an endocytic receptor for more than 30 substances including vitamin-binding proteins, apolipoproteins, low-molecular-weight peptides, hormones, enzymes, drugs, and ions.2 ABBAD, also known as anti-LRP2 nephropathy, is usually diagnosed in individuals older than 65 years who present with unexplained renal failure. The disease is highly aggressive, with the majority progressing to end-stage renal disease within a year, and it responds poorly to various attempted treatment strategies.1
Histologic evaluation of a kidney biopsy reveals a pattern of severe but nonspecific tubulointerstitial injury, without significant glomerular alterations. However, the pattern of immunofluorescence staining for IgG serves as an initial clue to the presence of ABBAD. In approximately half of patients, abnormal IgG positivity along the proximal tubular epithelial brush border will be present, the native location of the LRP2 protein in the kidney.1 Although the majority also have granular IgG staining along the subepithelial aspect of the glomerular capillary loop (segmental membranous pattern), all patients show granular IgG staining along the tubular basement membranes (TBMs) and Bowman capsules.1 Therefore, extraglomerular IgG staining, in concert with light microscopic evidence of acute tubular injury, are critical to identification of ABBAD.
The pathophysiologic mechanism whereby basal tubular IgG deposits develop because of an autoantibody directed against an apical brush border protein in ABBAD is unclear and possibly related to transcytotic mechanisms of antigen-antibody complexes.1 However, detection of ectopic LRP2 serves as a highly sensitive and specific diagnostic tool for ABBAD. Pathologists utilize a commercial antibody against LRP2 conjugated to a fluorescent marker to colocalize IgG and LRP2 deposits along the TBMs. Confirmation of the presence of a circulating anti–brush border antibody via serologic assay is also currently available.
To our knowledge, this is the first report of ABBAD associated with proliferative lupus nephritis in a nonelderly patient, and only a second case of ABBAD that responded well to treatment. We believe this case highlights (i) the difficulty in diagnosing ABBAD when associated with other autoimmune nephritides, (ii) the possibility that ABBAD is underdiagnosed in younger individuals, and (iii) that ABBAD responds to combination immunosuppressive therapy.
Case Report
The patient is a 55-year-old Caucasian male with a medical history of hypertension, diabetes mellitus type 2, and hyperlipidemia who presented with a 2-day history of left-sided chest pain radiating to his shoulders. He endorsed several weeks of dry cough, fatigue, lower extremity edema, arthralgias, poor appetite, and mild weight loss. The patient’s primary care provider noted a decrease in hemoglobin (10 g/dl from 15.8 g/dl) and referred the patient for a colonoscopy, which was unremarkable. Imaging workup on hospital admission was negative for pulmonary embolism, but revealed mild pulmonary vascular congestion, ground-glass opacification of lungs, cardiomegaly, mild concentric left ventricular hypertrophy, a moderate pericardial effusion (without tamponade), and a small amount of intra-abdominal and pelvic ascites. Electrocardiogram was consistent with pericarditis. Real ultrasound showed normal cortical echogenicity and was otherwise unremarkable.
The patient was admitted for treatment of pericarditis and pericardial effusion and was started on aspirin and colchicine with significant early symptomatic improvement. Laboratory values from the date of admission showed acute renal failure, nephrotic-range proteinuria, hypocomplementemia and positive autoimmune serologies (summarized in Table 1). The patient subsequently met the Systemic Lupus International Collaborating Clinics (SLICC) criteria for systemic lupus erythematosus with clinical (renal, serositis) and immunologic (+ANA, +dsDNA, +anti-Smith, hypocomplementemia) criteria.3 He was discharged on prednisone (60 mg once daily) and mycophenolate mofetil (1.5 g every 12 hours) pending renal biopsy in the outpatient setting.
Table 1.
Laboratory parameters at the time of biopsy
| Laboratory parameter | Value | Reference range |
|---|---|---|
| Serum creatinine, mg/dl | 1.24 | 0.6–1.2 |
| BUN, mg/dl | 12 | 11–23 |
| Proteinuria, mg/24 h | 5717 | 10–150 |
| Hematuria, urinalysis | Moderate, 2+ | Negative |
| C3, mg/dl | 65 | 90–170 |
| C4, mg/dl | <8 | 19–52 |
| Serum albumin, g/dl | 2.9 | 3.2–4.8 |
| ESR, mm/h | 63 | 0–26 |
| CRP, mg/l | 4 | 0–0.5 |
| Ferritin, ng/ml | 450 | 22–322 |
| ANA, | Positive, >1:640 | <1:40 |
| Anti-dsDNA, IU/ml | Positive, 727 | <30 |
| RF, IU/ml | Positive, 18 | 0–14 |
| Anti-Smith, U | Positive, 1.1 | <1.1 |
| Anti-histone, U | Positive, 9.4 | 0–0.9 |
| Anti-SSA/Ro, AI | Negative, <0.2 | 0–0.9 |
| Anti-SSB/La, AI | Negative, <0.2 | 0–0.9 |
| Anti-cardiolipin, U/ml | Negative, <9 | 0–12 |
| ANCA, U | Negative, <0.2 | <0.4 |
| White blood cells, 1000/μl | 5 | 4.5–11 |
| Hemoglobin, g/dl | 10.7 | 14–18 |
| Platelets, 1000/μl | 270 | 150–400 |
| HIV | Negative | Negative |
| Hepatitis panel | Negative | Negative |
ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; BUN, blood urea nitrogen; CRP, C-reactive protein; dsDNA, double-stranded DNA; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor; SSA, Sjögren syndrome–related antigen A; SSB, Sjögren syndrome–related antigen B.
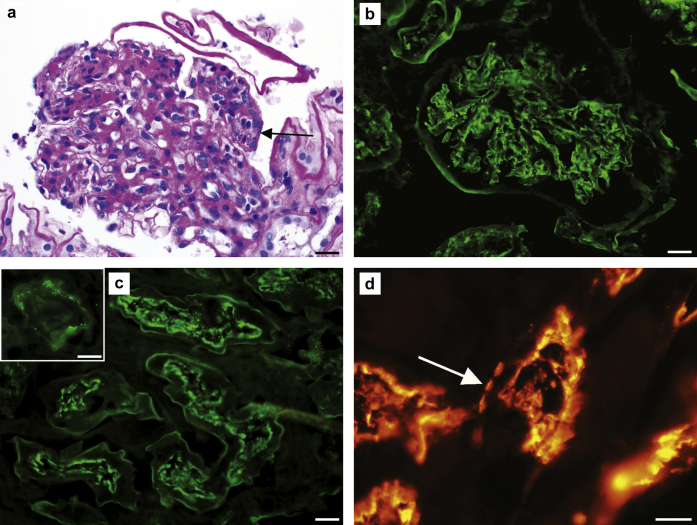
Major renal biopsy findings are summarized in Figure 1. The biopsy contained 26 glomeruli, 1 of which was obsolescent. Light microscopic examination revealed glomeruli with diffuse mesangial hypercellularity associated with mesangial matrix expansion. Two of 12 glomeruli showed segmental endocapillary hypercellularity with predominantly mononuclear cells and lesser neutrophils with karyorrhexis. One glomerulus demonstrated segmental fibrinoid necrosis with an associated cellular crescent. Focal red blood cell casts and acute tubular injury were also present, but the interstitium was devoid of a significant inflammatory infiltrate. No significant chronic injury was present, with only mild interstitial fibrosis (10%) and minimal tubular atrophy (<10%). Immunofluorescence showed diffuse, granular, predominantly mesangial and lesser capillary loop “full-house” staining for IgG (3+), IgA (3+), IgM (1–2+), C3 (3+), C1q (2+), kappa (1–2+), and lambda (2–3+). Neural epidermal growth factor-like 1 protein (NELL1) and exostosin (EXT1 and EXT2) stains were negative in glomeruli (Supplementary Figure S1). Extraglomerular granular IgG deposits were focally present along the TBMs and Bowman capsules. IgG also highlighted the apical brush border of numerous proximal tubules. Immunofluorescence with the anti-LRP2 antibody showed positivity along the apical brush border of the proximal tubules (internal positive control) and focal granular positivity along the TBMs. Glomerular ultrastructural evaluation revealed numerous mesangial electron-dense deposits, but no discrete electron-dense deposits were seen along the peripheral glomerular basement membranes. A diagnosis of focal lupus nephritis (ISN/RPS Class III) and ABBAD (anti-LRP2 nephropathy) was rendered, with a total modified NIH lupus nephritis activity score of 6 of 24 and chronicity score of 2 of 12.4 The diagnosis of ABBAD was confirmed via serologic testing, revealing positive anti-kidney tubular brush border antibodies by indirect immunofluorescence with a titer of 1:10 (negative < 1:10).
Figure 1.
Anti–brush border antibody disease and lupus nephritis. (a) Glomerular mesangial and endocapillary (arrow) hypercellularity (periodic acid–Schiff stain; original magnification ×400; bar = 20 μm). (b) Glomerular staining for IgG (original magnification ×400; bar = 20 μm). (c) IgG staining along the tubular epithelial apical brush border (original magnification ×400; bar = 20 μm). Inset: granular IgG staining along the tubular basement membrane (original magnification ×600; bar = 20 μm). (d) LRP2/megalin immunofluorescence with normal staining along the apical brush border and ectopic staining along the tubular basement membrane (arrow; original magnification ×600; bar = 20 μm).
Despite treatment with mycophenolate mofetil and prednisone, the patient was readmitted approximately 18 days following renal biopsy with elevated serum creatinine (1.93 mg/dl) and hyperkalemia. Mycophenolate mofetil was discontinued and therapy was switched to methylprednisolone pulse (1 g, 3-day pulse) and intravenous cyclophosphamide. Three days later, serum creatinine improved to 1.10 mg/dl and the patient was discharged on prednisone (60 mg, qd) with slow taper and monthly intravenous cyclophosphamide infusions. Following 4 (out of 6) infusions of cyclophosphamide, serum creatinine decreased to within normal limits (1.0–1.1 mg/dl), together with a marked reduction in proteinuria (urine protein-creatinine ratio 0.8 g/g). Ultrasonography showed only a minimal residual pericardial effusion. Repeat serologic testing was negative for anti–kidney tubular brush border antibodies performed 5 months following the initial positive result.
Discussion
Our patient represents a rare case of anti-LRP2 nephropathy in a nonelderly individual (55 years old), and the first reported case of ABBAD in association with lupus nephritis. Of all the known cases of ABBAD in the English literature, only 1 was in a patient younger than ours.5 In the largest-to-date case series of ABBAD, the youngest patient was 66 years old, with the average cohort age of 72.9 years (range: 66–80 years).1 In the same series, 3 patients showed serologic positivity for antinuclear antibody, but none met diagnostic criteria for systemic lupus erythematosus.1 All other known case reports of ABBAD are in patients 59 years of age or older,6, 7, 8, 9 and none carried an association with systemic lupus erythematosus or lupus nephritis. Therefore, our patient is a highly unusual case of ABBAD in a relatively young individual associated with another concurrent autoimmune nephritis.
ABBAD is likely significantly underdiagnosed in all patient populations for a number of reasons. The clinical features of ABBAD are entirely nonspecific. The typical patient presentation is that of an older individual with progressively worsening renal function (mean serum creatinine 6.2 mg/dl) and variable subnephrotic proteinuria.1 The clinical differential diagnosis of such patients is broad and most are not biopsied. Furthermore, the pathologic features of ABBAD are non-specific and could easily lead to misdiagnosis on biopsy. For instance, the presence of segmental subepithelial glomerular deposits could lead to a misdiagnosis of ABBAD as membranous glomerulonephritis. In such cases, the absence of phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain containing 7A (THSD7a) positivity portends the possibility of a secondary etiology of membranous glomerulonephritis such as malignancy or drug-induced etiologies, both of which are relatively common in elderly individuals. The presence of severe acute tubular injury on light microscopy is common in elderly patients who are frequently on multipharmacy because of comorbidities. The presence of tubular deposits is also not specific and is often seen in association with a wide variety of both common and rare conditions including nonsteroidal anti-inflammatory drug use, cardiac valve replacement, IgG-4–related renal disease, membranous nephropathy, idiopathic hypocomplementemic tubulointerstitial nephritis, polyomavirus nephritis, Kimura disease, Sjögren syndrome, and lupus nephritis.7 Lastly, the awareness and familiarity with ABBAD is low among both clinicians and pathologists. The disease went largely unrecognized for 35 years since first descriptions of a tubulointerstitial disease with TBM and glomerular deposits with patient sera reactive to the renal proximal tubular brush border, reported independently in 1981 by Morrison9 and Douglas.5 Renewed interest in this disease arose only recently with a case report of an immune-complex tubulointerstitial nephritis with antibodies to the proximal tubular brush border in 20168 and discovery of LRP2 as the target antigen in 2017, confirmed in a small series of 10 patients.1 Since then, only 2 case reports of ABBAD have emerged,6,7 and no large case series have been published.
The association of ABBAD with other autoimmune diseases should not be surprising. More than 34% of patients with an autoimmune disease show polyautoimmunity, defined as the presence of more than 1 autoimmune disease in a single patient.S1 Development of autoantibodies to LRP2 is also not surprising if one considers that LRP2 is expressed by a wide variety of nonrenal absorptive epithelia. Expression of LRP2 in the lung, epididymis, parathyroid, thyroid, placenta, choroid plexus, labyrinthic cells of the inner ear, ciliary epithelium of the eye, small intestine, endometrium, fallopian tube, and breast increases the likelihood of autoantibody formation.2,S2 Indeed, LRP2 is a common autoantigen in a wide range of autoimmune conditions. Ooka and colleagues identified LRP2 autoantibodies in up to 40% of lupus, 87% of rheumatoid arthritis, 35% of systemic sclerosis, and 3% of Behçet disease patients.S3 LRP2 autoantibodies have also been identified in patients with Crohn disease,S4 ulcerative colitis,S5 and autoimmune thyroiditis.S6 In the small case series of 10 ABBAD patients reported by Larsen and colleagues, 4 showed at least 1 positive autoimmune serology, and 1 even had positive ANA and dsDNA titers (none met criteria for systemic lupus erythematosus).1
Furthermore, Dinesh and colleagues reported a case of anti-LRP2 nephropathy in a 90-year-old female with an associated plasma cell–rich interstitial inflammatory infiltrate, with up to 40 IgG4-positive plasma cells per high-power field.7 This feature raised the possibility of concomitant IgG4-related tubulointerstitial nephritis, a disease that shares the common feature of tubular basement membrane deposits, Bowman capsule deposits, and segmental peripheral glomerular capillary loop deposits. Our case did not have a notable tubulointerstitial inflammatory infiltrate, and the modified NIH (National Institutes of Health) activity index score for interstitial inflammation was zero (on a scale of 0–3).
As autoimmunity tends to commonly affect nonelderly patients, it is likely that ABBAD is therefore underrecognized in this patient population. Although most patients with autoimmunity do not suffer renal sequelae and are thus not biopsied, more than half of patients with systemic lupus erythematosus develop lupus nephritis at some point in their disease courseS7 and are thus likely to undergo kidney biopsy. The difficulty of identifying superimposed ABBAD in those patients is confounded by the fact that many lupus nephritis cases will have granular TBM deposits (33%–67% of lupus nephritis casesS8,S9) and segmental subepithelial glomerular capillary loop deposits (22%–31% of lupus nephritis casesS10,S11). In fact, the presence of extraglomerular immune deposits carries a high sensitivity (93%) and specificity (85%) in distinguishing lupus from nonlupus nephritis.S12 As extraglomerular staining is often the only clue to the presence of ABBAD, it is likely that extraglomerular staining in lupus nephritis masks the presence of anti-LRP2 nephropathy in many cases. One possible clue for the pathologist to the presence of ABBAD in these instances, as in the present case, is IgG staining along the apical brush border of the proximal tubule, a finding not seen in normal biopsies. However, it should be noted that IgG staining of the apical brush border is only present in 50% of ABBAD cases1; thus, a high index of suspicion should be maintained with patients showing significant tubular injury and TBM deposits on both lupus and nonlupus biopsies. Once the diagnosis of ABBAD is rendered on biopsy, serologic testing may then be employed to identify the presence of circulating anti–brush border autoantibodies and to determine titer for monitoring of disease activity.
Because of a low number of identified cases, the optimal treatment for ABBAD and disease outcome is currently unclear. In a retrospective series of 10 cases with an average follow-up of 11 months, 5 patients reached end-stage renal disease and required renal replacement.1 Treatment was variable and consisted of observation only, prednisone only, combination of prednisone and cyclophosphamide, and rituximab only. Immunologic remission occurred at 12 months in a single patient treated with a combination of prednisone and cyclophosphamide, with a stabilization of serum creatinine and resolution of proteinuria. One patient treated with prednisone received a kidney transplant in which the disease recurred. These data, albeit limited, suggest that ABBAD leads to a rapid decline in renal function and that prednisone and rituximab are not efficacious as single agents. Our patient responded favorably to a combination of corticosteroids and cyclophosphamide (as he did not respond favorably to corticosteroids and mycophenolate mofetil), with massive improvement in systemic symptomology, proteinuria, and renal function that returned to normal. Pre-biopsy treatment with corticosteroid and mycophenolate mofetil is the most likely explanation for the initial low positive serologic titer (1:10) for anti–kidney brush border antibodies in this case. Repeat serologic testing 5 months later and following change of treatment to corticosteroid and cyclophosphamide yielded a negative result. In combination, these observations contribute to preliminary evidence that treating ABBAD similarly to proliferative lupus nephritis (classes III and IV) with a combination of corticosteroid and cyclophosphamide may lead to best outcomes.
Conclusion
To our knowledge, this is the first report of ABBAD in combination with lupus nephritis, and a rare case of ABBAD in a nonelderly patient that responded to treatment (Table 2). This case report highlights the need for pathologists to maintain a high index of suspicion for ABBAD as it may occur independently or in combination with other autoimmune nephritides. In particular, this case underlines the need for heightened awareness of extraglomerular staining on biopsies in patients of all ages, and in particular in those with unexplained acute tubular injury with serologic or clinical evidence of autoimmunity. Furthermore, our case represents a second known case of ABBAD that responded favorably to a treatment combination of corticosteroids and cyclophosphamide. Larger studies are needed to clarify the natural progression, optimal treatment, and outcomes for patients with this rare but aggressive autoimmune tubulointerstitial kidney disease.
Table 2.
Key teaching points
| 1. ABBAD is a rare autoimmune tubulointerstitial renal disease with autoantibodies against the tubular apical brush border protein LRP2/megalin. |
| 2. ABBAD typically affects older individuals (>65 yr of age) and leads to ESRD in the majority within 12 mo and recurs in transplants. |
| 3. ABBAD is diagnosed on kidney biopsy by the presence of LRP2-positive TBM deposits. |
| 4. A serologic test of the presence of circulating anti–brush border antibodies is available. |
| 5. ABBAD may occur in nonelderly individuals and it may coexist with autoimmune diseases such as lupus nephritis. |
| 6. Optimal treatment is unclear, but favorable outcomes are seen with a combination of corticosteroids and cyclophosphamide. |
ABBAD, anti–brush border antibody disease; ESRD, end-stage renal disease; LRP2, low-density lipoprotein receptor–related protein 2; TBM, tubular basement membrane.
Disclosure
All the authors declared no competing interests.
Footnotes
Figure S1. Neural epidermal growth factor-like 1 protein (NELL1) and exostosin (EXT1/2) stains are negative in glomeruli on formalin-fixed tissue (original magnification ×400; bar = 20 μm).
Supplementary References.
Supplementary Material
References
- 1.Larsen C.P., Trivin-Avillach C., Coles P. LDL receptor-related protein 2 (Megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol. 2018;29:644–653. doi: 10.1681/ASN.2017060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen E.I., Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280:F562–F573. doi: 10.1152/ajprenal.2001.280.4.F562. [DOI] [PubMed] [Google Scholar]
- 3.Petri M., Orbai A.M., Alarcon G.S. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajema I.M., Wilhelmus S., Alpers C.E. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Douglas M.F., Rabideau D.P., Schwartz M.M. Evidence of autologous immune-complex nephritis. N Engl J Med. 1981;305:1326–1329. doi: 10.1056/NEJM198111263052206. [DOI] [PubMed] [Google Scholar]
- 6.Campbell R.E., Uhlenhopp D., Shaw M. Anti-brush border antibody (ABBA) QJM. 2020;113:561–562. doi: 10.1093/qjmed/hcaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinesh K.P., Raniele D., Michels K. Anti-LRP2 nephropathy with abundant IgG4-positive plasma cells: a case report. Am J Kidney Dis. 2019;74:132–137. doi: 10.1053/j.ajkd.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Rosales I.A., Collins A.B., do Carmo P.A. Immune complex tubulointerstitial nephritis due to autoantibodies to the proximal tubule brush border. J Am Soc Nephrol. 2016;27:380–384. doi: 10.1681/ASN.2015030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison E.B., Kozlowski E.J., McPhaul J.J. Primary tubulointerstitial nephritis caused by antibodies to proximal tubular antigens. Am J Clin Pathol. 1981;75:602–609. doi: 10.1093/ajcp/75.4.602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.