Abstract
Introduction
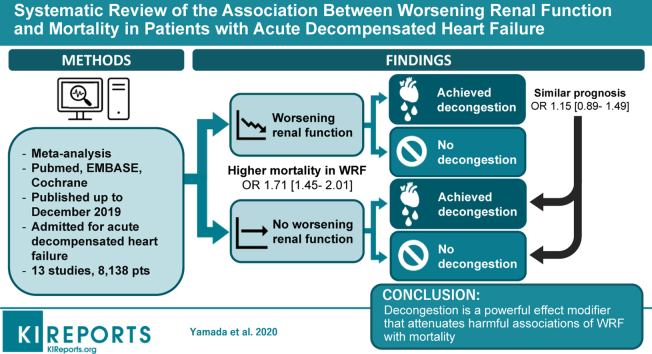
Outcomes in acute decompensated heart failure (ADHF) have remained poor. Worsening renal function (WRF) is common among patients with ADHF. However, the impact of WRF on the prognosis is controversial. We hypothesized that in patients with ADHF, the achievement of concomitant decongestion would diminish the signal for harm associated with WRF.
Methods
We performed a systematic search of PubMed, EMBASE, and the Cochrane Library up to December 2019 for studies that assessed signs of decongestion in patients with WRF during ADHF admission. The primary outcome was all-cause mortality and heart transplantation.
Results
Thirteen studies were selected with a pooled population of 8138 patients. During the follow-up period of 60–450 days, 19.2% of patients died. Unstratified, patients with WRF versus no WRF had a higher risk for mortality (odds ratio [OR], 1.71 [95% confidence interval {CI}, 1.45–2.01]; P < 0.0001). However, patients who achieved decongestion had a similar prognosis (OR, 1.15 [95% CI, 0.89–1.49]; P = 0.30). Moreover, patients with WRF who achieved decongestion had a better prognosis compared with those without WRF or decongestion (OR, 0.63 [95% CI, 0.46–0.86]; P = 0.004). This tendency persisted for the sensitivity analyses.
Conclusions
Decongestion is a powerful effect modifier that attenuates harmful associations of WRF with mortality. Future studies should not assess WRF as an endpoint without concomitant assessment of achieved volume status.
Keywords: cardiorenal syndrome, heart failure, meta-analysis, mortality/survival
Graphical abstract
Heart failure (HF) is a major public health problem with a high prevalence1 and a substantial economic burden.2 Management of ADHF presents a major challenge. Although survival from chronic HF has improved,3 outcomes in ADHF have changed little because there continues to be a high risk of mortality and rehospitalization.4
Chronic kidney disease (CKD) is present in 17%–30% of patients with ADHF and portends a poorer prognosis.5 However, the impact of WRF during ADHF admission on prognosis is controversial. While it is well known that WRF is associated with poorer outcomes,6,7 several studies claim that WRF is unrelated to increased mortality in some patients.8,9 These findings suggest that this population is heterogeneous and that WRF may be caused by derangements in hemodynamics that are reversible in some patients with ADHF.10
We hypothesized that in patients with ADHF, the achievement of concomitant decongestion including hemoconcentration, a decrease of B-type natriuretic peptide (BNP), and the absence of signs of congestion on physical examination would diminish the signal for harm associated with WRF.
Methods
Literature Search
The search strategy was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses.11 We performed a systematic search of PubMed, EMBASE, and the Cochrane Library from inception to December 2019. The following keywords were applied: (“heart failure” [MeSH] OR HF OR “acute heart failure” OR AHF OR “acute decompensated heart failure” OR ADHF) AND (“acute kidney injury” [MeSH] OR AKI OR “worsening renal function” OR WRF OR creatinine) AND (edema [MeSH] OR “edema, cardiac” [MeSH] OR “pulmonary edema” OR congestion OR decongestion OR “natriuretic peptide, brain” OR BNP OR “N-terminal pro b-type natriuretic peptide” [NT-proBNP] OR “NT-proBNP” OR hemoconcentration OR hemoglobin OR hematocrit) AND (mortality [MeSH] OR “all-cause mortality” OR “hospital mortality” OR “heart transplantation” OR prognosis). We restricted the search to human studies. There were no language restrictions. Further manual searches of bibliographies for all relevant studies and review articles were conducted by 2 investigators (TYamad, HU).
Study Selection and Data Extraction
We included all studies that involved adult patients (>18 years of age) who were admitted for ADHF where the outcomes were comparing patients with or without decongestion between patients with WRF and those without WRF. The primary outcome was a composite of all-cause mortality and heart transplantation. Studies were excluded if (i) they included nonhuman subjects and (ii) no crude mortality data or ORs for the study groups were available even after contact with the authors. All data from eligible studies were independently extracted by 2 investigators (TYamad, HU). Discrepancies were resolved by discussion among the 2 reviewers and by referencing the original report. The Newcastle-Ottawa Scale12 and the Newcastle-Ottawa Scale adapted for cross-sectional studies13 were used to assess the quality of nonrandomized studies. We considered studies to be of high quality if they had a score ≥6.
Statistical Analysis
All analyses were conducted using Review Manager version 5.314 and Comprehensive Meta-Analysis version 3 (Biostat, Englewood, NJ). ORs and 95% CIs were obtained directly from individual articles or by calculating from crude mortality using Mantel–Haenszel methods. A random effects model was used to determine the risk associated with the presence of WRF/decongestion and all-cause mortality or heart transplantation. All reported probability values were 2-sided, with significance set at P < 0.05. Heterogeneity was assessed by the probability value of the χ2 statistic and I2.15,16 We regarded an I2 of <40% as “heterogeneity might not be important” and >50% as “may represent substantial heterogeneity” based on the suggestion of the Cochrane Handbook for Systemic Review of Interventions.17 Sensitivity analyses were performed for (i) the definition of WRF, (ii) short (≤180 days) versus long (>180 days) follow-up periods, (iii) the definition of decongestion, and (iv) prospective versus retrospective studies. Univariable meta-regression analysis was conducted to examine the effect of study-level variables: study size, age, sex, left ventricular ejection fraction, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, beta-blockers (BBs), diuretics, creatinine, blood urea nitrogen, estimated glomerular filtration rate, hemoglobin level, proportion of WRF, achievement of decongestion, and presence of diabetes, hypertension, CKD, HF, coronary artery disease (CAD), and atrial fibrillation. The general linear method was used for meta-regression, weighting by study sample size.
Publication bias of studies with different sample sizes was assessed by the Begg and Mazumdar rank correlation test18 and the Egger regression test.19
Results
Literature Search and Included Studies
A diagram of the study selection is shown in Figure 1. Initially, a total of 4303 studies were obtained in the primary database search and 16 studies were identified through references. We removed 358 duplicate studies; 3961 studies were screened. By screening titles and abstracts, 3947 articles were excluded. By assessing full-text articles, 13 studies published up to December 2019 were selected for our meta-analysis according to the inclusion criteria.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The pooled population consisted of 8138 patients. The prevalence of WRF was 27.8%. More than half (55.4%) of those with WRF and 58.1% of those without WRF experienced decongestion.
Figure 1.
Flow diagram for study selection.
Study Characteristics and Quality Assessment
The definitions of terms and characteristics of the included studies are listed in Tables 1 and 2, respectively.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The median ages of patients in the included studies ranged from 56–78 years. The proportion of the history of HF varied from 46%–78% and left ventricular ejection fraction ranged from 20%–45%. Four prospective studies were identified in the meta-analysis, while the other 9 studies were retrospective or post hoc studies. Most studies regarded WRF as an increase in creatinine of >0.3 mg/dl from baseline, except studies by Stolfo et al.24 and Testani et al.,21 in which WRF was defined as a decrease in estimated glomerular filtration rate of ≥20%. The definition of decongestion varied by study. Six studies defined decongestion based on physical examination findings (such as jugular venous distention, hepatomegaly, edema, pulmonary rales, third heart sound, and a decrease in blood pressure); 5 studies regarded decongestion as a decrease in BNP or NT-proBNP; and 2 studies determined decongestion as hemoconcentration, such as an increase in hemoglobin or hematocrit. According to the Newcastle-Ottawa Scale, all studies were of high quality and had scores ≥6 (Supplementary Table S1).
Table 1.
Definitions of terms in included studies
| Author, yr | Study design | Definition of decongestion | Definition of WRF | Follow-up, d | Outcome |
|---|---|---|---|---|---|
| Breidhardt et al.,26 2017 | Prospective cohort | Increase >3 of the parameters (Hgb, Hct, Alb, and TP) after day 4 | Increase Cr ≥0.3 mg/dl | 90 | All-cause mortality |
| Brisco et al.,32 2016 | Post hoc analysis | NT-proBNP reduction >30% | Increase Cr ≥0.3 mg/dl | 60 | All-cause mortality |
| Fudim et al.,22 2018 | Post hoc analysis | No physical signs of congestion | Increase Cr ≥0.3 mg/dl | 180 | All-cause mortality |
| Martins et al.,27 2018 | Retrospective analysis | Increase in Hgb during hospitalization | Increase Cr ≥0.3 mg/dl | 180 | All-cause mortality |
| Metra et al.,20 2012 | Prospective cohort | No physical signs of congestion (third heart sound, pulmonary rales, jugular venous distention, hepatomegaly, or edema) | Increase Cr ≥0.3 mg/dl | 365 | Death, heart transplantation |
| Metra et al.,29 2018 | Post hoc analysis | No physical signs of congestion (orthopnea, edema, or jugular venous distention) | Increase Cr ≥0.3 mg/dl | 90 | All-cause mortality |
| Rao et al.,31 2019 | Post hoc analysis | NT-pro BNP reduction >30% | Increase Cr ≥0.3 mg/dl | 60 | All-cause mortality |
| Salah et al.,25 2015 | Retrospective cohort | NT-pro BNP reduction >30% | Increase Cr >0.3 mg/dl and >25% | 180 | All-cause mortality |
| Skolski et al.,28 2019 | Prospective cohort | Not needed increase of i.v. diuretics or ultrafiltration | Increase Cr ≥0.3 mg/dl or eGFR decrease >25% | 365 | All-cause mortality |
| Stolfo et al.,24 2017 | Prospective cohort | BNP reduction >40% | ≥20% decrease eGFR | 390 | All-cause mortality |
| Testani et al.,21 2011 | Retrospective analysis | SBP reduction over the median | ≥20% decrease eGFR | 180 | All-cause mortality |
| Wattad et al.,23 2015 | Post hoc analysis | No physical signs of congestion (jugular venous distention, hepatomegaly, edema, pulmonary rales, and third heart sound) | Increase Cr ≥0.3 mg/dl | 450 | All-cause mortality |
| Wettersten et al.,30 2019 | Retrospective analysis | BNP reduction >30% | Increase Cr ≥0.3 mg/dl or ≥50% | 365 | All-cause mortality |
Alb, albumin; BNP, brain natriuretic peptide; Cr, creatinine; eGFR, estimated glomerular filtration rate; Hct, hematocrit; Hgb, hemoglobin; NT-proBNP, N-terminal pro-brain natriuretic peptide; SBP, systolic blood pressure; TP, total protein; WRF, worsening renal function.
Table 2.
Baseline characteristics of included studies
| Author, yr | Patients, n | Age, yr | Male, % | DM, % | HTN, % | CKD, % | HF, % | CAD, % | Afib, % | LVEF, % | ACEi/ARB, % | BB, % | Diuretic, % | Cr, mg/dl | eGFR, ml/min per 1.73 m2 | Hgb, g/dl | WRF, % | Decongestion (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breidhardt et al.,26 2017 | 1019 | 77.9 | 54.9 | 30.6 | 76.2 | 44 | 49.4 | 24.9 | N/A | 45 | 56.1 | 51.7 | 54 | N/A | N/A | N/A | 33.2 | 21.3 |
| Brisco et al.,32 2016 | 301 | 66.0 | 73.4 | 52.5 | 80.4 | N/A | 74.4 | N/A | N/A | 34.5 | 63.8 | 83.4 | 100 | 1.6 | 51.8 | 11.7 | 15.5 | 46.1 |
| Fudim et al.,22 2018 | 206 | N/A | 73.8 | 31.1 | N/A | N/A | 54.9 | 44.8 | N/A | 20 | 78.2 | 64.6 | 98.5 | N/A | N/A | N/A | 44.8 | 47.8 |
| Martins et al.,27 2018 | 618 | 79 | 41.9 | 36.2 | 60.7 | 22.5 | N/A | 20.5 | N/A | 43 | 65 | 32.4 | 66.7 | 1.4 | N/A | 12.2 | 49 | 43.2 |
| Metra et al.,20 2012 | 594 | 69.1 | 74 | 35 | 53 | 35 | 66 | 56 | 36 | 33.3 | 76 | 60 | 99 | 1.6 | 42.8 | 12.8 | 50.2 | 87.2 |
| Metra et al.,29 2018 | 1537 | 70 | 66.4 | 45.3 | 79.4 | N/A | 66.4 | 38.6 | 53.5 | 30.3 | N/A | N/A | N/A | 1.3 | 49 | 12.7 | 20.5 | 77.4 |
| Rao et al.,31 2019 | 188 | 67.5 | 70.5 | 62.0 | N/A | N/A | 73.3 | 60.6 | 51.5 | 32.5 | 51.0 | 73.5 | 88.0 | 1.99 | N/A | N/A | 26.4 | 41.1 |
| Salah et al.,25 2015 | 1232 | 74 | 60 | 32 | 50 | N/A | 74 | 49 | 43 | N/A | 66 | 57 | 95 | 1.52 | 56.3 | 12.57 | 10.9 | 56.8 |
| Skolski et al.,28 2019 | 266 | 67 | 70.7 | 36.8 | 56 | 38.7 | 72.9 | 51.5 | 48.5 | 35 | 65.8 | 69.2 | 7.9 | 1.2 | 54.3 | 13 | 14.3 | 71.8 |
| Stolfo et al.,24 2017 | 122 | 69.3 | 74 | 28 | N/A | 61 | N/A | 53 | 31 | 34.7 | N/A | N/A | N/A | 1.44 | 55.1 | 12.4 | 23 | 59.8 |
| Testani et al.,21 2011 | 386 | 56.4 | 74.1 | 31.9 | 46.6 | N/A | 50.3 | 50.3 | N/A | 19.5 | N/A | N/A | N/A | N/A | 56.9 | 12.5 | 20.5 | 50 |
| Wattad et al.,23 2015 | 762 | 77.1 | 49.7 | 45 | 87.7 | N/A | 46 | 66 | 47.1 | N/A | 71 | 66.1 | N/A | 1.3 | 51 | 11.7 | 27 | 66.8 |
| Wettersten et al.,30 2019 | 814 | 69 | 63 | 44 | 80.1 | 26 | N/A | 47 | N/A | N/A | 31.4 | 72 | 70.8 | 1.2 | 60 | 11.7 | 32.2 | 51.7 |
ACEi, angiotensin-converting enzyme inhibitor; Afib, atrial fibrillation; ARB, angiotensin II receptor blocker; BB, beta blocker; BUN, blood urea nitrogen; CAD, coronary artery disease; CKD, chronic kidney disease; Cr, creatinine; DM, diabetes; eGFR, estimated glomerular filtration rate; HF, heart failure; Hgb, hemoglobin; HTN, hypertension; LVEF, left ventricular ejection fraction; N/A, not available; NOS, Newcastle-Ottawa scale; WRF, worsening renal function.
WRF and All-Cause Mortality
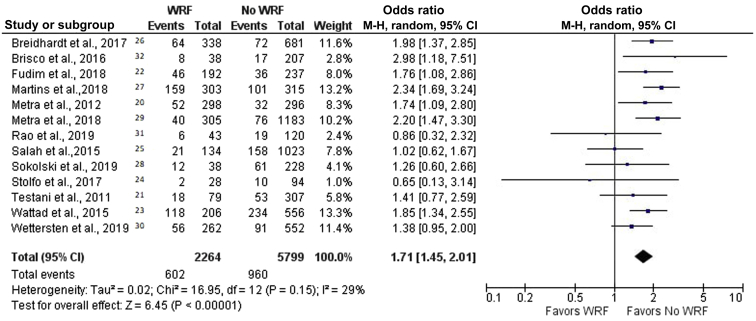
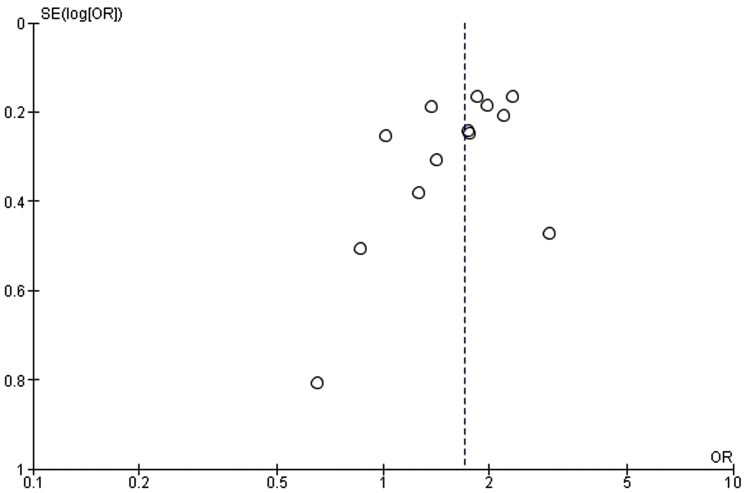
After a follow-up period of 60–450 days, 19.2% of patients died; the crude mortality rates for patients with and without WRF were 26.6% and 16.6%, respectively. This resulted in a combined unadjusted OR for mortality of 1.71 (95% CI, 1.45–2.01, P < 0.00001; I2 = 29%) (Figure 2).20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The funnel plot is symmetric for the overall effect (Figure 3). The Begg and Mazumdar rank correlation test and the Egger regression test indicated no statistically significant publication bias (2-tailed P values of 0.54 and 0.82, respectively).
Figure 2.
Forest plot of the association between worsening renal function (WRF) and mortality in patients with acute decompensated heart failure. Odds ratios are presented as means and 95% confidence intervals (CIs). M-H, Mantel–Haenszel.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
Figure 3.
Funnel plot of worsening renal function and all-cause mortality. OR, odds ratio; SE, standard error.
Effect Modification of Decongestion on the Association Between WRF and All-Cause Mortality in Patients with ADHF
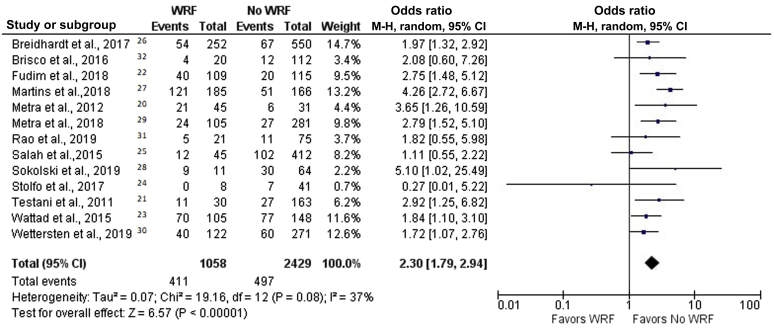
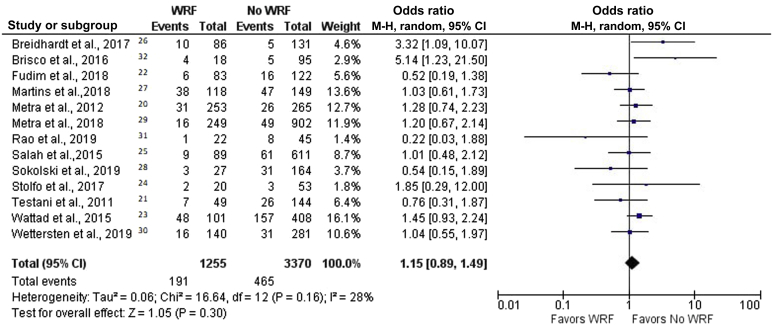
We divided patients with WRF into 2 groups: patients with or patients without decongestion, as defined as above. The crude mortality rates for patients with WRF with and without decongestion were 15.2% and 38.8%, respectively. In patients without decongestion, WRF was associated with a higher risk of mortality (OR, 2.30 [95% CI, 1.79–2.94]; P < 0.00001; I2 = 37%) (Figure 4).20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 On the other hand, the harmful effect of WRF was nullified by decongestion. In patients with WRF who achieved decongestion, mortality was not inferior to that in patients who did not have WRF (OR, 1.15 [95% CI, 0.89–1.49]; P = 0.30; I2 = 28%) (Figure 5).20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 Moreover, patients with WRF who achieved decongestion were found to have lower mortality than those without WRF who did not reach decongestion (OR, 0.63 [95% CI, 0.46–0.86]; P = 0.04; I2 = 46%).
Figure 4.
Forest plot of the association between worsening renal function (WRF) and mortality in patients without decongestion. Odds ratios are presented as means and 95% confidence intervals (CIs). M-H, Mantel–Haenszel.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
Figure 5.
Forest plot of the association between worsening renal function (WRF) and mortality in patients with decongestion. Odds ratios are presented as means and 95% confidence intervals (CIs). M-H, Mantel–Haenszel.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
Sensitivity Analyses
In the 11 studies that defined WRF as an increase in creatinine of >0.3 mg/dl from baseline, WRF was associated with higher mortality overall (OR, 1.64 [95% CI, 1.36–1.98]; P < 0.0001; I2 = 49.2%), but the effect was not seen in patients with decongestion (OR, 1.17 [95% CI, 0.89–1.53]; P = 0.27; I2 = 38.1%). However, in 2 studies that defined WRF as a decrease in estimated glomerular filtration rate of ≥20%, WRF did not show a statistically significant difference (OR, 1.28 [95% CI, 0.73–2.25]; P = 0.39; I2 = 0%).
Second, we performed a sensitivity analysis stratified by follow-up period. The results are consistent in studies with a short period (≤180 days: overall OR, 1.80 [95% CI, 1.44–2.25]; P < 0.0001; I2 = 39.3%; in decongested patients: OR, 1.15 [95 % CI, 0.76–1.74]; P = 0.50; I2 = 51.3%) and a long period (>180 days: overall OR, 1.33 [95% CI, 1.12–1.59]; P = 0.001; I2 = 0%; in decongested patients: OR, 1.17 [95% CI, 0.88–1.56]; P = 0.28; I2 = 0%).
Third, we divided studies according to the definitions of decongestion. In 6 studies that defined decongestion based on physical examination findings, WRF was related to poor outcomes (OR, 1.55 [95% CI, 1.27–1.90]; P < 0.0001; I2 = 22.8%) but not in the decongested group (OR, 1.10 [95% CI, 0.82–1.47]; P = 0.51; I2 = 26.2%). This tendency was similar in the 2 studies that defined decongestion as hemoconcentration (overall: OR, 2.17 [95% CI, 1.70–2.77]; P < 0.0001; I2 = 0%; in decongested patients: OR, 1.66 [95% CI, 0.54–5.12]; P = 0.38; I2 = 71.3%). However, in 5 studies that regarded decongestion as a decrease in BNP or NT-proBNP, WRF did not show a statistically significant difference in either overall patients (OR, 1.29 [95% CI, 0.94–1.77]; P = 0.11; I2 = 19.6%) or decongested patients (OR, 1.23 [95% CI, 0.63–2.40]; P = 0.54; I2 = 42.4%).
Lastly, we separated prospective and retrospective studies. In 4 prospective studies, WRF was associated with poor prognosis, but not in decongested group (OR, 1.74 [95% CI, 1.33–2.27]; P < 0.0001; I2 = 0%; and OR, 1.28 [95% CI, 0.63–2.58]; P = 0.49; I2 = 43.1%, respectively). The results were similar in 9 retrospective studies as well but with high heterogeneity (overall: OR, 1.59 [95% CI, 1.27–1.99]; P < 0.0001; I2 = 55.3%; decongested group: OR, 1.13 [95% CI, 0.85–1.49]; P = 0.40; I2 = 33.6%).
Meta-regression
Meta-regression analysis for all 11 studies suggested that the proportion of CAD (P = 0.0004) and BB (P = 0.0023) contributed to overall heterogeneity (Table 3). In 11 studies that defined WRF as an increase in creatinine >0.3 mg/dl, meta-regression suggested that WRF accounted for heterogeneity but CAD and BB did not (P values for CAD, BB, and WRF were 0.20, 0.057, and 0.0013, respectively).
Table 3.
Meta-regression analyses of mortality on predictors
| Covariate | Coefficient | Standard error | 95% lower limit | 95% upper limit | Z value | Two-sided P value |
|---|---|---|---|---|---|---|
| Study size | 0.0001 | 0.0002 | −0.0003 | 0.0006 | 0.59 | 0.56 |
| Age | 0.0046 | 0.013 | −0.021 | 0.031 | 0.35 | 0.73 |
| Male, % | −0.005 | 0.0085 | −0.022 | 0.012 | −0.58 | 0.56 |
| Diabetes, % | −0.0048 | 0.012 | −0.028 | 0.018 | −0.42 | 0.68 |
| Hypertension, % | 0.002 | 0.0073 | −0.012 | 0.016 | 0.27 | 0.78 |
| CKD, % | −0.011 | 0.014 | −0.039 | 0.018 | −0.74 | 0.46 |
| HF, % | −0.0007 | 0.0093 | −0.019 | 0.018 | −0.07 | 0.94 |
| CAD, % | −0.014 | 0.0039 | −0.021 | −0.006 | −3.6 | 0.0004 |
| Afib, % | 0.011 | 0.021 | −0.030 | 0.052 | 0.51 | 0.61 |
| LVEF, % | 0.011 | 0.0092 | −0.0070 | 0.029 | 1.20 | 0.23 |
| ACEi/ARB, % | 0.0020 | 0.0076 | −0.013 | 0.017 | 0.26 | 0.79 |
| BB, % | −0.016 | 0.0052 | −0.026 | −0.0056 | −3.05 | 0.0023 |
| Diuretics, % | −0.008 | 0.0046 | −0.0098 | 0.0082 | −0.18 | 0.86 |
| Cr, mg/dl | −0.18 | 0.64 | −1.44 | 1.08 | −0.28 | 0.78 |
| BUN, mg/dl | −0.079 | 0.0057 | −0.0033 | 0.019 | 1.39 | 0.17 |
| eGFR, ml/min per 1.73 m2 | −0.024 | 0.018 | −0.058 | 0.011 | −1.33 | 0.18 |
| Hgb, g/dl | 0.048 | 0.24 | −0.42 | 0.51 | 0.2 | 0.84 |
| Proportion of WRF, % | 0.011 | 0.0064 | −0.0020 | 0.023 | 1.65 | 0.099 |
| Achievement of decongestion, % | −0.030 | 0.0051 | −0.013 | 0.0069 | −0.59 | 0.55 |
ACEi, angiotensin-converting enzyme inhibitor; Afib, atrial fibrillation; ARB, angiotensin II receptor blocker; BB, beta blocker; BUN, blood urea nitrogen; CAD, coronary artery disease; CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; HF, heart failure; Hgb, hemoglobin; LVEF, left ventricular ejection fraction; WRF, worsening renal function.
Discussion
In this systematic review and meta-analysis we examined the effect modification of decongestion on the association between WRF and mortality in patients with ADHF. Unstratified analysis showed that WRF was associated with higher mortality, a finding that is well known.6 This study divided WRF patients into 2 groups: patients with and without signs of decongestion at the time of hospital discharge. The results of the pooled analyses demonstrated that decongestion was associated with the mitigation of the harmful effects of WRF in patients with ADHF. This fact suggests that WRF in ADHF can be heterogeneous in terms of prognosis. Moreover, our study revealed that patients with WRF who achieved decongestion had a better prognosis compared with patients without WRF who did not accomplish decongestion.
Multiple mechanisms are involved in the pathophysiology of WRF; among them, venous congestion plays a central role. Studies have shown that elevated central venous pressure is associated with a higher risk of WRF.10,33 Venous congestion can lead to WRF via several mechanisms, including activation of the renin-angiotensin-aldosterone system, an increase in renal interstitial pressure, and sympathetic nervous system stimulation.34 Renal dysfunction resulting from neurohormonal or hemodynamic abnormalities (also known as vasomotor nephropathy) can be reversible.
This meta-analysis suggests that WRF caused by vasomotor nephropathy should be distinguished from WRF related to intrinsic kidney disease. It also indicates that aggressive diuresis can be warranted even though it may cause WRF because it is related to a better prognosis. Despite aggressive therapy, congestion at discharge is frequent and is associated with a higher risk of death or rehospitalization.35
Meta-regression analysis suggested that the proportion of CAD, BB, and WRF could affect the result. It has been reported that the presence of CAD is independently associated with increased mortality in patients with ADHF,36 which is a possible explanation. A cohort study suggested that the use of BB was protective for in-hospital mortality in patients with ADHF who were complicated by WRF.37 Further studies can be warranted to assess the association between BB and mortality in patients with ADHF and WRF.
As far as we know, this is the first systematic review and meta-analysis that stratified patients with WRF into decongestion and nondecongestion groups.
The large number of patients analyzed is a strength of our study. We included 7730 patients, enough to show the statistically significant differences. The results are consistent with sensitivity analyses, which strengthens our findings.
Our study has several limitations. First, this analysis contained retrospective data that are subject to bias. Second, we used the reported crude data to calculate effect estimates for most of the studies, since multivariate-adjusted data were not available in most articles. Therefore, the results should be interpreted with caution because the data may be biased by confounding factors. Third, the definitions of decongestion varied among studies, based on physical examinations, hemoconcentration, or change in BNP. Moreover, the definition of WRF used in the main analysis and meta-regression varied as well. These factors may lead to heterogeneity. Finally, patients who achieve decongestion are likely less diuretic resistant and represent a less severe phenotype of cardiorenal syndrome.
In conclusion, decongestion is a powerful effect modifier that attenuates the harmful associations of WRF with mortality in ADHF. Future studies should not assess WRF in isolation as an endpoint without concomitant assessment of the volume status that accompanied the WRF.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by grants from the Japan Society for the Promotion of Science, Salt Science Research Foundation (20C4), Japan Agency for Medical Research and Development (AMED), and Yokohama City University “KAMOME Project.”
Author Contributions
TYamad designed the study, collected the data, contributed to the statistical analysis, and served as the primary author of the manuscript. HU collected the data, contributed to the statistical analysis, and served as an author of the manuscript (equivalent contributor). NC, KA, RK, SK, HW, and KT contributed to the statistical analysis and assisted with the writing of the manuscript. TYamaj, SU, TS, EA, PP, AB, and MAR contributed to the data collection and assisted with the writing of the manuscript. YS and JI-M contributed to the data collection and data analysis. CAB contributed to the data collection and assisted with the data analysis and writing of the manuscript. JT and SC contributed to study design and assisted with the writing of the manuscript.
Footnotes
Table S1. Newcastle-Ottawa scale for assessment of quality of included studies (each asterisk represents if individual criterion within the subsection was fulfilled).
Contributor Information
Kouichi Tamura, Email: tamukou@yokohama-cu.ac.jp.
Steven Coca, Email: steven.coca@mssm.edu.
Supplementary Material
References
- 1.Benjamin E.J., Muntner P., Alonso A. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Cook C., Cole G., Asaria P. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–376. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Barker W.H., Mullooly J.P., Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970-1974 and 1990-1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 4.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 5.Cleland J.G., Carubelli V., Castiello T. Renal dysfunction in acute and chronic heart failure: prevalence, incidence and prognosis. Heart Fail Rev. 2012;17:133–149. doi: 10.1007/s10741-012-9306-2. [DOI] [PubMed] [Google Scholar]
- 6.Damman K., Valente M.A., Voors A.A. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 7.Raichlin E., Haglund N.A., Dumitru I. Worsening renal function in patients with acute decompensated heart failure treated with ultrafiltration: predictors and outcomes. J Card Fail. 2014;20:376.e325–376.e332. [PubMed] [Google Scholar]
- 8.Ather S., Bavishi C., McCauley M.D. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol. 2013;167:1912–1917. doi: 10.1016/j.ijcard.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanfear D.E., Peterson E.L., Campbell J. Relation of worsened renal function during hospitalization for heart failure to long-term outcomes and rehospitalization. Am J Cardiol. 2011;107:74–78. doi: 10.1016/j.amjcard.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullens W., Abrahams Z., Francis G.S. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G.A., Shea B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at:
- 13.Modesti P.A., Reboldi G., Cappuccio F.P. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One. 2016;11:e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Review Manager (RevMan) [computer program]. Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2014. [Google Scholar]
- 15.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; London, UK: 2011. [Google Scholar]
- 18.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metra M., Davison B., Bettari L. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 21.Testani J.M., Coca S.G., McCauley B.D. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. 2011;13:877–884. doi: 10.1093/eurjhf/hfr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fudim M., Loungani R., Doerfler S.M. Worsening renal function during decongestion among patients hospitalized for heart failure: findings from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. Am Heart J. 2018;204:163–173. doi: 10.1016/j.ahj.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Wattad M., Darawsha W., Solomonica A. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Am J Cardiol. 2015;115:932–937. doi: 10.1016/j.amjcard.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Stolfo D., Stenner E., Merlo M. Prognostic impact of BNP variations in patients admitted for acute decompensated heart failure with in-hospital worsening renal function. Heart Lung Circ. 2017;26:226–234. doi: 10.1016/j.hlc.2016.06.1205. [DOI] [PubMed] [Google Scholar]
- 25.Salah K., Kok W.E., Eurlings L.W. Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail. 2015;3:751–761. doi: 10.1016/j.jchf.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Breidthardt T., Weidmann Z.M., Twerenbold R. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail. 2017;19:226–236. doi: 10.1002/ejhf.667. [DOI] [PubMed] [Google Scholar]
- 27.Martins J.L., Santos L., Faustino A. Worsening or ‘pseudo-worsening’ renal function? The prognostic value of hemoconcentration in patients admitted with acute heart failure. Rev Port Cardiol. 2018;37:595–602. doi: 10.1016/j.repc.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Sokolski M., Zymlinski R., Sokolska J.M. True worsening renal function identifies patients with acute heart failure with an ominous outcome. Pol Arch Intern Med. 2019;129:357–360. doi: 10.20452/pamw.4453. [DOI] [PubMed] [Google Scholar]
- 29.Metra M., Cotter G., Senger S. Prognostic significance of creatinine increases during an acute heart failure admission in patients with and without residual congestion: a post hoc analysis of the PROTECT data. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004644. [DOI] [PubMed] [Google Scholar]
- 30.Wettersten N., Horiuchi Y., van Veldhuisen D.J. B-type natriuretic peptide trend predicts clinical significance of worsening renal function in acute heart failure. Eur J Heart Fail. 2019;21:1553–1560. doi: 10.1002/ejhf.1627. [DOI] [PubMed] [Google Scholar]
- 31.Rao V.S., Ahmad T., Brisco-Bacik M.A. Renal effects of intensive volume removal in heart failure patients with preexisting worsening renal function. Circ Heart Fail. 2019;12:e005552. doi: 10.1161/CIRCHEARTFAILURE.118.005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brisco M.A., Zile M.R., Hanberg J.S. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. 2016;22:753–760. doi: 10.1016/j.cardfail.2016.06.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damman K., van Deursen V.M., Navis G. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 34.Damman K., Navis G., Smilde T.D. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Lala A., McNulty S.E., Mentz R.J. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF) Circ Heart Fail. 2015;8:741–748. doi: 10.1161/CIRCHEARTFAILURE.114.001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purek L., Laule-Kilian K., Christ A. Coronary artery disease and outcome in acute congestive heart failure. Heart. 2006;92:598–602. doi: 10.1136/hrt.2005.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu W., He W., Liu W. Risk factors and prognosis of cardiorenal syndrome type 1 in elderly Chinese patients: a retrospective observational cohort study. Kidney Blood Press Res. 2016;41:672–679. doi: 10.1159/000447936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.