Abstract
The diagnosis of coronavirus disease−19 (COVID-19) relies on the detection of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) RNA by real-time reverse-transcription polymerase chain reaction in respiratory samples. Rapid increase in the COVID-19 cases across the world requires fast and efficient testing as testing capacity is a bottleneck in diagnosis. In this context, pooling strategy can be opted for rapid testing in a cost-effective manner. In this study, the authors have optimized and compared the effect of pooling (5 and 10 samples) before and after nucleic acid extraction. It was concluded that there was no significant difference in the SARS CoV-2 RNA detection in the pools prepared at sample or RNA level. Even after pooling, 10-fold dilution was detectable with 3–cycle threshold value change in both type of pools when compared with individual samples. Hence, sample pool size of 10 can be used in low-prevalent areas, and testing capacity can be substantially increased.
Keywords: COVID-19, Pool testing, Nucleic acid testing, Deconvolution, Cycle threshold
Highlights
-
•
Utility of pooling samples in COVID-19 testing to save time, cost, and manpower.
-
•
A change of 3 cycle threshold values is observed at a dilution of a sample up to 10-fold.
-
•
No significant change is observed before and after nucleic acid extraction in pools.
1. Introduction
Coronavirus disease−19 (COVID-19) is a pandemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) which originated in the Wuhan city of China, spreading across 215 countries and affecting more than 7.2 million people worldwide, and cases are still increasing further (https://www.worldometers.info/coronavirus/). Human-to-human transmission occurs mainly through respiratory droplets (while coughing and sneezing); however, possible transmission through aerosol route and feco-oral route has also been postulated (Chen et al., 2020). There is also evidence of presence of virus in the saliva and breast milk, which may also be a possible source of transmission (Azzi et al., 2020; Groß et al., 2020). It has been seen that persons who are presymptomatic or asymptomatic can also transmit the virus.
The gold standard for the laboratory diagnosis of SARS CoV-2 is real-time reverse-transcription polymerase chain reaction panel (rRT-PCR) recommended by the Centers for Disease Control and Prevention (CDC), Atlanta, due to its high sensitivity and specificity which is carried out in throat/nasopharyngeal swabs. There is emphasis to increase the testing capacity so as to identify the infectious cases and quarantine them, thereby preventing the transmission to their contacts. This testing is a constraint in terms of limitations of reagents and cost of testing specially in resource-limited developing countries. In such scenarios, pool testing is beneficial for screening purposes, contact tracing, and population-based studies where the positivity of SARS CoV-2 is likely to be less than <2% (https://www.mohfw.gov.in/pdf/letterregguidanceonpoolingsamplesfortesting001.pdf).
The concept of pool testing is well described and utilized in surveillance programs for many viral diseases, screening in blood banks, outbreaks, and population-based studies (Laurin et al., 2019; Yogurtcu et al., 2019). Nucleic acid testing is used to detect various viral pathogens (hepatitis C, hepatitis B, human immunodeficiency virus) (Hourfar et al., 2008) in pooled samples (6–8 numbers). A pool of 10 samples was used to increase the testing capacity for influenza virus (Van et al., 2012). Pool testing preserves the cost and time compared to individual testing (Chandrashekar, 2014). The size of the pool depends upon the type of virus and prevalence in the country, but each positive pool needs to be deconvoluted individually.
The current study focused on the standardization and validation of pool testing before and after nucleic acid extraction for the diagnosis of SARS CoV-2. The initial standardization was carried out by performing the rRT-PCR in 2 mini pools (size of 5 and 10) prepared before and after nucleic acid extraction, which was further validated in a large number of pools. The study would be beneficial in policy making to increase the testing capacity with low budget in low–COVID-19 prevalent areas and in asymptomatic individuals and population-based studies.
2. Materials and methods
2.1. Site of the study
The study was carried out in the Department of Virology in a tertiary care hospital in North India. The department has a dedicated Biosafety level class II (BSL class II) facility, and the testing for SARS CoV-2 was carried out as per Indian Council of Medical Research (ICMR) testing guidelines.
2.2. Sample collection and processing
The throat swabs (TS)/nasopharyngeal swabs (NS) collected in viral transport medium (VTM) and received in cold chain from suspected COVID 19 patients were used for the study. These samples had been stored in aliquots in −80 °C subsequent to initial diagnostic testing, and no additional sampling was performed for the study purpose. The study was approved by Institute Ethical Committee (IEC).
2.3. Nucleic acid extraction
The nucleic acid extraction of samples and pools was performed using NucliSens™- easyMAG (Biomeurix, France) or Qiagen viral mini kit (Qiagen, Germany) according to manufacturer's protocol. The elution was done in 60 μL of elution buffer, and RNA was stored at 4 °C and used the same day for real-time PCR.
2.4. Standardization of pool testing
2.4.1. Positive samples (PS)
Thirteen samples already tested by TaqPath COVID-19 Combo Kit (“Taq Path kit”: Applied Biosystems, Foster City, CA) were selected based on cycle threshold (Ct) values. For the preliminary standardization of pooling strategy, 3 samples having Ct values near the highest, medium, and lowest viral copy number based on the standard curve of positive control were selected. High-positive sample (HPS) with a Ct value of 17 (low Ct value), medium-positive sample (MPS) with a Ct value of 27 (medium Ct value), and low-positive sample (LPS) with a Ct value of 31 (high Ct value) were selected.
For further standardization of pooling results, subsequently, 10 samples with Ct values (≥27) were selected to detect false negativity rate due to logarithmic rise of Ct value after pooling of borderline samples. Two hundred microliters of each aliquot was subjected to RNA extraction individually before pooling.
2.4.2. Negative samples (NS)
A total of 120 TS/NS tested by Taq Path kit and confirmed to be negative were also included in the study. These NS were used for creating the various pools used in the study per se. Approximately 200 μL of each of the above sample was mixed in the pool, and 200 μL of each aliquot was subjected to RNA extraction individually before pooling.
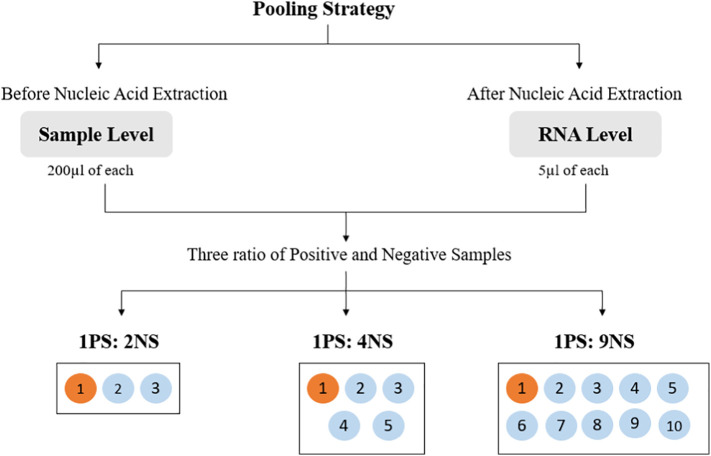
2.4.3. Pooling strategy
For the study, the pooling strategy was set at pool size of 3, 5, and 10 at both sample and RNA levels as shown in Fig. 1 .
-
a)
Pooling before nucleic acid extraction (sample level): For each pool, 200 μL of PS of each Ct value category was pooled with 200 μL of NS in the ratios of 1PS:2NS, 1PS:4NS, and 1PS:9NS. Each pool was subjected to RNA extraction.
-
b)
Pooling after nucleic acid extraction (RNA level): After nucleic acid extraction of individual sample, 5 μL of extracted RNA from NS was spiked with 5 μL of extracted RNA from PS in the ratio of 1PS:2NS, 1PS:4NS, and 1PS:9NS.
Fig. 1.
Graphical representation of pol strategy at pool size of 3, 5, and 10 at both sample and RNA levels.
2.5. Real-time PCR
Detection of SARS CoV-2 by real-time PCR was performed by Taq Man probe–based commercial kit (Taq Path kit) in Applied Biosystem 7500 real-time machine (ABI, USA). For calculation of the lower limit of detection, positive control of known concentration (1 × 104 copies/μL) for all the 3 genes already available with the kit was 10-fold serially diluted, and the standard curve was prepared. The lower limit of detection was found to be 5 copies/reaction with a Ct value of 34.15, 33.76, and 33.02 for N gene, ORF-1ab, and S gene, respectively.
2.6. Data analysis
Criteria of positivity were set as per manufacturer's instructions of positivity in 2 of 3 target genes, i.e., Nucleocapsid (N), Open Reading Frame (ORF-1ab), and Spike (S) genes. Each individual positive sample and spiked pools were performed in triplicate for rRT-PCR.
2.7. Validation of the pool study
2.7.1. Samples
A total of 500 samples were consecutively pooled in groups of 5 (total 100 pools; 11 positive pools and 89 negative pools) as per ICMR guidelines of pooling 5 samples (https://www.mohfw.gov.in/pdf/letterregguidanceonpoolingsamplesfortesting001.pdf). All the positive pools and 5% of the negative pools were deconvoluted and tested individually. The 5 μL RNA of positive pools (pool of 5 sample) was again diluted with 20 μL of nuclease-free water to get a dilution of 10, which was further used for the detection of SARS CoV-2 genes.
2.7.2. Statistical analysis
All statistical analyses were performed using Graph Pad PRISM version 5.03 where quantitative variables were represented in the form of mean and standard deviation. Analysis of variance test was used to estimate the difference in Ct values of undiluted and diluted samples (pool samples) before and after nucleic acid extraction. Only P value <0.05 was considered significant in all the tests.
3. Results
3.1. Standardization of pool testing
3.1.1. Pooling of sample/RNA in ratio of 1:2
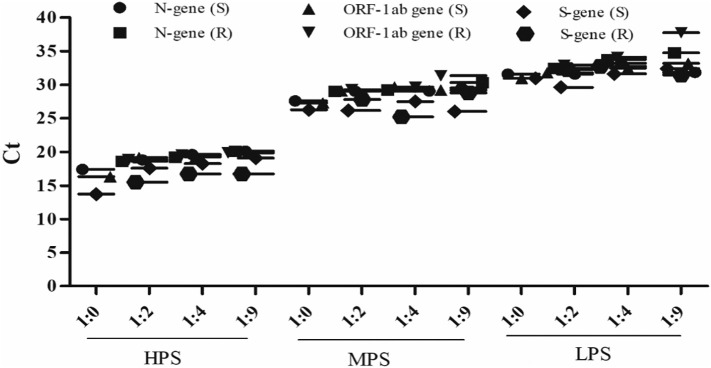
All the 3 genes (N, ORF-1ab, S) were detected in a high-, medium-, and low-positive sample pool with a 1- or 2-cycle change in Ct value in both sample and RNA levels when compared to individual values as shown in Fig. 2 and Supplementary Table 1.
Fig. 2.
Graphical representation of the 3 genes (N, ORF-1ab, S) with high-, medium-, and low-positive samples (HPS, MPS, and LPS) in ratio of 1:2, 1:4, and 1:9 done at both sample (S) and RNA (R) levels. Results depict mean ± SD of the triplicates.
3.1.2. Pooling of sample/RNA in ratio of 1:4
All the 3 genes (N, ORF-1ab, S) were detected in a high-, medium-, and low-positive sample pool with a change of 2 or 3 cycles in Ct value in both sample and RNA levels when compared to individual values as shown in Fig. 2 and Supplementary Table 1.
3.1.3. Pooling of sample/RNA in ratio of 1:9
All the 3 genes (N, ORF-1ab, S) were detected in a high-, medium-, and low-positive sample pool with a change of 3 or 4 cycles in Ct value in both sample and RNA levels when compared to individual values as shown in Fig. 2 and Supplementary Table 1.
3.2. Standardization of pool testing in low-positive samples
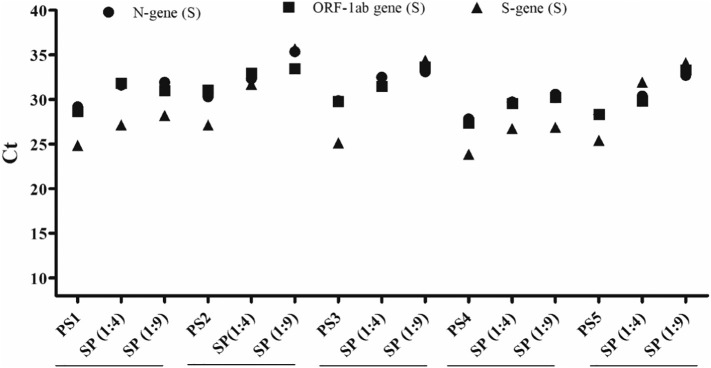
The preliminary standardization results confirmed that, at 1:9 dilution, Ct value could be easily detection in high– and medium–viral load samples. Considering that the main challenge lies in the detection of samples in low-positive samples with a high Ct value (≥27) as they may not be detected with higher dilution, the subsequent study was carried out in these samples. However, similar results were obtained for N and ORF-1ab genes in 10 samples with Ct values (≥27) at sample and RNA levels when pooled in 1:4 and 1:9 as compared to their individual positive Ct values (Supplementary Table 2). A gradual increase in Ct values at dilution 1:4 and 1:9 can be seen in Fig. 3 , showing only 5 representative spiked sample pools (SP) (1:4 and 1:9) with their individual samples (PS).
Fig. 3.
Graphical representation of SARS CoV-2 genes in representative 5 positive samples (PS) and their spiked pools (SP) with Ct value ≥27 in ratio of 1:4 and 1:9 at sample level (S). Results represent mean ± SD of the triplicates.
Moreover, a 3-cycle change in Ct value was observed at a dilution of 10 compared to the individual Ct of PS at both RNA and sample levels in all 10 low PS as shown in Fig. 4 .
Fig. 4.
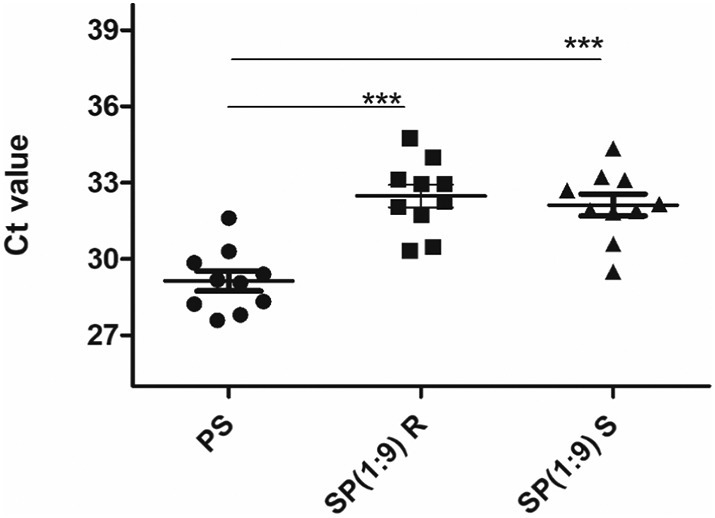
Graphical representation of SARS CoV-2 N genes in spiked pool (SP) (1:9) at RNA (R) and sample (S) level compared to individual positive samples (PS). Results represent mean ± SD of the triplicates.
3.3. Comparison of Ct values before and after nucleic acid extraction (sample level versus RNA level)
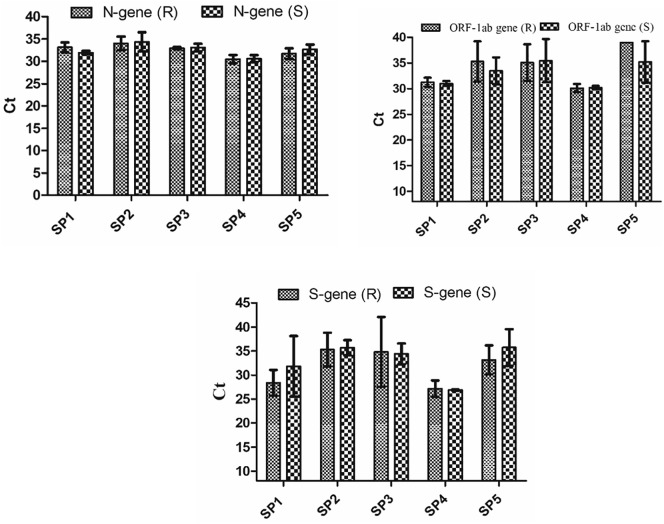
The Ct values of high-, medium-, and low-positive samples in all the 3 genes in 3 ratios were approximately the same at sample level and RNA level (Table 1 ). Also, in the 10 samples with Ct values (≥27), the Ct values at sample and RNA level were approximately the same in all the genes and in ratio of 1:4 and 1:9 (Supplementary Table 2). Only 5 representative spiked pools at 1:9 dilution at sample and RNA levels were shown in Fig. 5 , and there was no significant difference (P value > 0.05) observed in Ct values of pools at sample level and RNA level.
Table 1.
Representative Ct values of a pool having 4 positive samples and 1 negative sample for SARS CoV-2 virus.
| Sample | N gene | ORF1ab gene | S gene |
|---|---|---|---|
| 1 | 24.86 | 23.73 | 21.92 |
| 2 | 23 | 22.43 | 20.74 |
| 3 | 20.69 | 20.08 | 18.52 |
| 4 | 24.68 | 23.86 | 22.61 |
| 5 | Neg | Neg | Neg |
| Poola | 21.41 ± 1.78 | 20.54 ± 0.28 | 20.91 ± 0.41 |
Mean ± SD of triplicate.
Fig. 5.
Graphical representation of SARS CoV-2 genes in representative 5 spiked pools (SP) (1:9) at sample level (S) and at RNA level (R). Each Ct value (threshold cycle) corresponds to mean ± SD of the triplicates.
3.4. Validation of pool testing in random testing
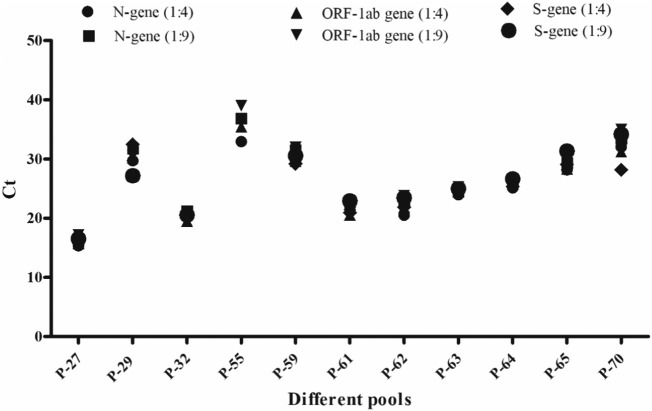
A total of 500 samples were pooled in a group of 5 (n = 100 pools). All were subjected for the detection of SARS CoV-2 genes. Out of 100 consecutive pools, 11 (11%) were found to be positive for at least 1 of 3 genes, and they were deconvoluted for the individual testing. Out of the total 11 positive pools, 4 samples were positive in pool no. 61; 3 samples were positive in pool nos. 27, 62, 63, and 64; 2 samples were positive in pool nos. 32 and 55; and 1 sample was positive in pool nos. 29, 59, 65, and 70. All the genes were detected in all positive pools after obtaining 10-fold dilution in RNA pools as shown in Fig. 6 .
Fig. 6.
Graphical representation of SARS CoV-2 A) N-gene, B) ORF-1ab gene, and C) S-gene in 11 positive pools in 5- and 10-fold dilutions at RNA level.
The 5% negative pools with Ct value above kit cutoff (>37) of N, ORF-1ab, and S gene were subjected to deconvolution, and none of the genes was found to be positive for SARS CoV-2.
3.5. To check the inhibition of rRT-PCR when more than 1 positive sample is present in pools
When the pool samples were deconvoluted and tested, it was found that even in positive pools having 4 positive samples, no inhibition of any gene target was observed.
4. Discussion
Pooling strategy is being increasingly adopted for diagnostic purposes and has been found to be beneficial in resource-limited countries to reduce the test cost. To date, more than 13.3 million people have been infected with COVID-19 worldwide, and testing capacity has to be increased with constraint of resources. Pool testing has been recommended in the pandemic of SARS CoV-2 by ICMR in areas with less than 2% prevalence. According to the 2018 census, the Indian population is 1.3526 billion, and the positivity rate of COVID-19 in our area was less than 2% at the time the study was conducted, i.e., March–May 2020 (unpublished data); hence, in this low-prevalence scenario, sample pooling can be beneficial for screening large population in limited time and with less resources. However, once the positivity increases, then additional resources will be required as all positive pools need to be deconvoluted, and thus, individual testing will add to the cost of testing.
A pool of 5 samples has been considered to be optimal by ICMR in terms of dilution effects particularly in high–Ct value samples. To increase the testing capacity up to 0.2 million tests per day, pooling of 10 samples if found to be optimum will accelerate the testing capacity. Based on this, the authors had tried to see the utility of pool testing using 10 samples in routine diagnosis of COVID-19 disease.
Yelin et al. have shown that positive samples can be detected in a pool of 32 negative RNA samples just before rRT-PCR with a false negativity of 10%. They have also suggested that positive samples diluted in even up to 64 samples may also be possible with additional amplification cycles (Täufer, 2020; Yelin et al., 2020).
However, the labor-intensive procedure of RNA extraction of each sample would lead to delay in detection and decision-making apart from using additional resources, thereby adding to the cost. To reduce this, we have compared the pool testing before and after nucleic acid extraction. It was observed that the pooling of samples in a group of 3, 5, and 10 did not affect the detection rate by PCR. The sensitivity of detection was not affected in pooling (up to 10 samples) even when the pool had a single sample with Ct value >27. The results are similar to the study by Van et al. where the authors have shown the detection of influenza virus in a pool of 10 samples (Van et al., 2012).
The pooling before nucleic acid extraction (sample pool) will contribute more in terms of increasing testing capacity and decreasing cost and labor. To the best of our literature search, this study was the first attempt to compare the testing efficiency of pooling samples before and after nucleic acid extraction for SARS CoV-2. Combined results reveal that the detection of SARS CoV-2 in clinical sample pools could be beneficial and reproducible similar to RNA pool and individual samples.
Our data had demonstrated that the individual sample with high Ct value could be efficiently detected in both pools (pool of 5 and pool of 10) prepared before (at sample level) and after nucleic acid extractions (at RNA level).
In contrast to Van et al., presence of even 4 positive samples in a single pool did not have an inhibitory effect on the detection by rRT-PCR (Van et al., 2012). The RNA obtained from these positive pools was diluted to achieve 10-fold dilution. Similar to previous study, it has been observed that the SARS CoV-2 RNA can be detected in a pool of 10 samples (Hogan et al., 2020). The average Ct values were shown in the table for both types of pools (5 and 10) at the RNA level, showing comparable detection in the pool of 10.
As expected, it has been observed that the 500 asymptomatic blinded clinical samples were efficiently tested by adopting a pooling strategy (5 blinded sequential samples) for the presence of SARS CoV-2. These positive pools (11%, n = 11/100) were tested individually, and it was found that the Ct values are not affected due to presence of more than 1 positive sample in a single pool.
Pool testing should be done in a small pool size as it is easy to screen out negative samples in small pools compared to large pools. It will be easy to deconvolute the positive pool in less turnaround time and repeat testing will be fast if the pool size is small.
To our knowledge, our study is unique in that aspect that we had looked into the effect of pooling samples on multiple genes, namely, N, ORF-1ab, and S gene, of SARS CoV-2. Pooling even in the ratio 1:9 did not affect the detection of samples in terms of N and ORF-1ab genes. Thus, kits involving these 2 genes may be useful in diagnosing SARS CoV-2 in pooled samples without any effect of dilution. However, validating such kits will require studies with larger sample size.
CDC, Atlanta, have recommended various kits for the detection of SARS CoV-2 RNA. In the present study, a single tube assay was used for the detection of SARS CoV-2 genes. However, in different public health laboratories, different COVID testing kits (each with different panel combinations) are available, and the sensitivity might suffer in pool testing, but kits with single tube assay targeting multiple genes will be useful for reducing resources and increasing sensitivity in pool samples.
In conclusion, we emphasize that TS/NS can be pooled in a pool of 10 without sacrificing the sensitivity of SARS CoV-2 testing. Moreover, pooling of 10 samples before nucleic acid extraction will increase the testing capacity with minimum resources in the pandemic era of infectious diseases in areas with low prevalence.
Uniqueness: In this study, we have targeted 3 genes using multiplex single tube testing and found more consistent results with N gene as the screening gene and ORF-1ab as the confirmatory gene in contrast to S gene. Another important aspect of the study is investigating pooled and deconvoluted clinical samples before and after nucleic acid extraction.
Acknowledgments
Acknowledgments
This work was carried out under the activity of Regional VRDL at PGIMER, Chandigarh. The support from ICMR is duly acknowledged.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Ethical consideration
The study was approved by Post Graduate Institute of Medical Education and Research Ethical Committee vide IEC no. NK/6377/Study/591 dated 14.07.2020.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diagmicrobio.2020.115206.
Appendix A. Supplementary data
Supplementary tables
References
- Azzi L., Carcano G., Gianfagna F., Grossi P., Dalla Gasperina D., Genoni A. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;14 doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar S. Half a decade of mini-pool nucleic acid testing: cost-effective way for improving blood safety in India. Asian journal of transfusion science. 2014;8(1):35. doi: 10.4103/0973-6247.126688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;3 doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Groß R., Conzelmann C., Müller J.A., Stenger S., Steinhart K., Kirchhoff F. Detection of SARS-CoV-2 in human breast milk. The Lancet. 2020;395(10239):1757–1758. doi: 10.1016/S0140-6736(20)31181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan C.A., Sahoo M.K., Pinsky B.A. Sample pooling as a strategy to detect community transmission of SARS-CoV-2. JAMA. 2020;6 doi: 10.1001/jama.2020.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourfar M.K., Jork C., Schottstedt V. Experience of German Red Cross blood donor services with nucleic acid testing: results of screening more than 30 million blood donations for human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus. Transfusion. 2008;48(8):1558–1566. doi: 10.1111/j.1537-2995.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- Laurin E., Thakur K., Mohr P.G., Hick P., Crane M.S., Gardner I.A. To pool or not to pool? Guidelines for pooling samples for use in surveillance testing of infectious diseases in aquatic animals. J Fish Dis. 2019;42(11):1471–1491. doi: 10.1111/jfd.13083. [DOI] [PubMed] [Google Scholar]
- Täufer M. Rapid, large-scale, and effective detection of COVID-19 via non-adaptive testing. J Theor Biol. 2020;506:110450. doi: 10.1016/j.jtbi.2020.110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T.T., Miller J., Warshauer D.M., Reisdorf E., Jernigan D., Humes R. Pooling nasopharyngeal/throat swab specimens to increase testing capacity for influenza viruses by PCR. J Clin Microbiol. 2012;50(3):891–896. doi: 10.1128/JCM.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin I., Aharony N., Shaer-Tamar E., Argoetti A., Messer E., Berenbaum D. Evaluation of COVID-19 RT-qPCR test in multi-sample pools. Clin Infect Dis. 2020:ciaa531. doi: 10.1093/cid/ciaa531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogurtcu O.N., Yang H., Chancey C., Forshee R.A., Eder A.F. Predictive model for Zika virus RNA minipool nucleic acid testing in outbreak scenarios. Transfusion. 2019;59(7):2211–2217. doi: 10.1111/trf.15296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables