Abstract
The family Cichlidae contains approximately 2000 species that live in diverse freshwater habitats including murky lakes, turbid rivers, and clear lakes from both the Old and New Worlds. Their visual systems are similarly diverse and have evolved specific sensitivities that differ along several axes of variation. Variation in cornea and lens transmission affect which wavelengths reach the retina. Variation in photoreceptor number and distribution affect sensitivity, spectral sensitivity and resolution. Probably their most dynamic characteristic is the variation in visual pigment peak sensitivities. Visual pigments can be altered through changes in chromophore, opsin sequence and opsin expression. Opsin expression varies by altering which of the seven available cone opsins in their genomes are turned on. These opsins can even be coexpressed to produce seemingly infinitely tunable cone sensitivities. Both chromophore and opsin expression can vary on either rapid (hours or days), slower (seasonal or ontogenetic) or evolutionary timescales. Such visual system shifts have enabled cichlids to adapt to different habitats and foraging styles. Through both short term plasticity and longer evolutionary adaptations, cichlids have proven to be ecologically successful and an excellent model for studying organismal adaptation.
Keywords: Visual pigments, photoreceptors, opsins, gene expression, cichlid fishes
1. Introduction
The path to understanding the construction of visual systems has been paved by elegant work done in model systems. Studies in the fly, the mouse, and the fish have revealed similar reliance on photoreceptors that detect different wavelengths of light and the downstream neural comparisons that allow organisms to infer brightness and color [1, 2]. Model teleosts (cavefish, Astyanax mexicanus, goldfish, Carassius auratus, zebrafish, Danio rerio, and medaka, Oryzias latipes) were some of the first species whose visual sensitivities were linked to opsin gene sequences [3–6]. In fish, genes from all of the known vertebrate families are present including rhodopsin (RH1) in rods and short wavelength sensitive (SWS1, SWS2), rhodopsin like (RH2) and long wavelength sensitive (LWS) opsins in cones [7]. Fishes have duplicated and lost both rod and cone opsins many times, leading to significant visual system diversity [8, 9].
Ecology is likely an important driver of visual system diversity. Variation may result from the variable light quality found across diverse aquatic habitats in which fish live, from clear to turbid streams, lakes, and rivers, as well as estuarine to clear ocean wate s [10, 11]. Foraging and mating strategies may also drive the evolution of visual ca abilities [12]. These disparate ecologies may select for a diversity of visual sensitivities and aquatic organisms may have some of the most variable visual sensitivities across animals [12–14].
Historically, we have harnessed the power and tools that teleost model visual systems provide to inform us about questions ranging from genetic regulation to neural wiring and behavior [15–18]. These studies demonstrate the power of models to inform us how a few species operate and can provide a framework of how those functions may exist in other teleosts. However, compared to our knowledge of lab organisms, the ecological context that informs us how these mechanisms evolved is less well-understood [19]. In addition, with a taxonomic group as ancient as teleosts, it can be difficult to draw conclusions about the evolution of traits when comparing distant model systems across the phylogeny. Zebrafish and medaka alone are approximately 230 MY divergent (timetree.org; [20]). Studying a single species of a given taxonomic group with little ecological context provides only an abbreviated picture of species diversity and the mechanisms by which phenotypes evolve.
One way to understand mechanisms for phenotypic evolution is to study many closely related species across a diverse taxonomic group. One such taxa whose visual diversity has been examined more than any other are cichlid fishes. Studies have documented the variety of cichlid visual systems set against a backdrop of the varied light environments and ecologies which they inhabit [21–26]. This has led researchers to examine the proximate molecular mechanisms that contribute to this variation and the ultimate evolutionary pressures that led to the diversification of cichlid visual sensitivities.
2. Cichlid fishes
The family Cichlidae is comprised of ~2000 species distributed across the world in a Gondwanan distribution, occupying various freshwater habitats in Central and South America, Africa, Madagascar, and even India [27, 28]. Estimates of the divergence between New and Old World cichlids are ~95mya [20] (Fig. 1). In comparison to the teleost models, cichlids are approximately 230 MY divergent from zebrafish but are closer to medaka, being only 120 MY divergent [20]. Cichlids and medaka display a lot of chromosomal synteny [29]. This includes having similar homologs for all opsin genes except for a duplicate LWS opsin in medaka that is missing in cichlids [30, 31].
Figure 1.
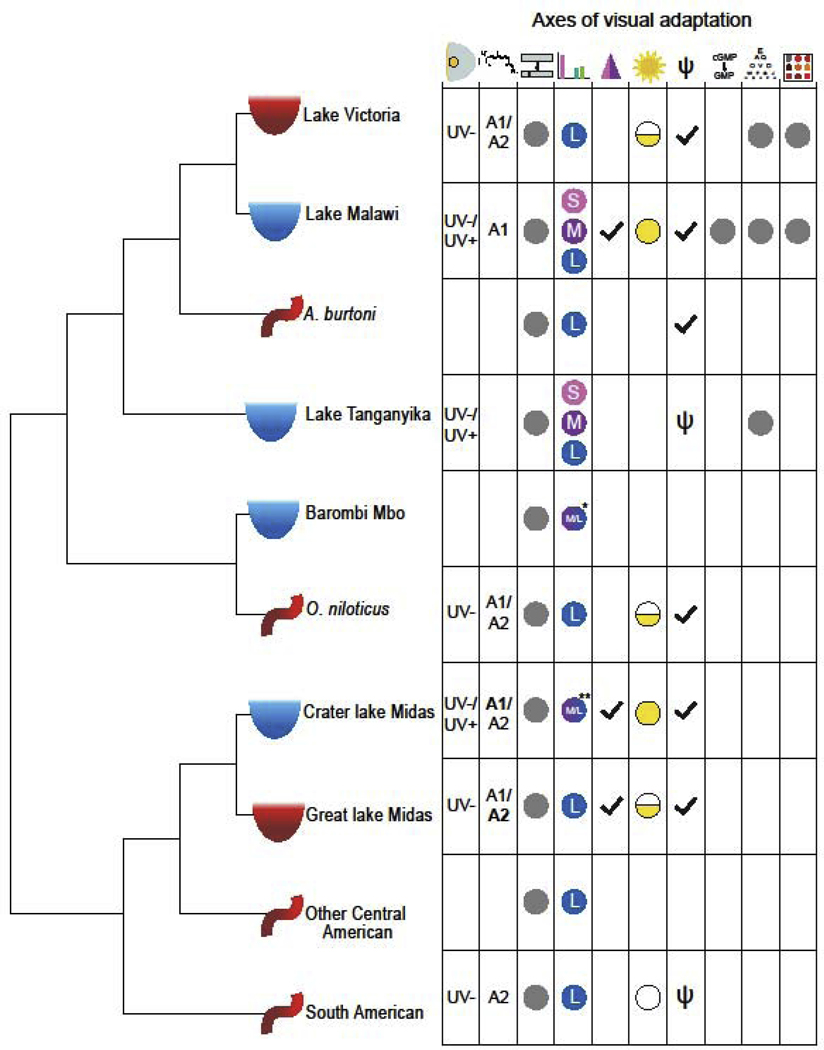
Cichlid phylogeny showing the ecological and geographic diversity and the corresponding diversity of visual tuning mechanisms. Icons noted on the phylogeny show the habitat of each species or group: turbid lakes (red semi-circle), clear lakes (blue semi-circle), and rivers (red curved line). Icons across the top show axes of visual adaptation from left to right: lens and cornea transmission, chromophore, opsin coding sequence, opsin expression, coexpression, environmental plasticity, gene loss, phototransduction, visual acuity, and color sensitivity and discrimination. Grey dots or symbols denote that this axis has been investigated in this group on some level, blank spaces denote areas needing further study. Results for some axes are shown. Symbols: Lens and cornea type are UV transmissive (UV+) or blocking (UV-); Chromophore type (A1 or A2 with predominant in bold); Adult opsin expression palettes are short (S), medium (M), and long (L); Coexpression presence (checkmark); Plasticity demonstrated for development (half-circle), development and adults (filled circle), or absent (empty circle); Opsin gene complement is full (checkmark) or has gene loss (psi). *Some Barombi Mbo species express a dichromatic palette that differs from the typical trichromatic palettes. **Some crater lake Midas cichlids have expression midway between M and L.
As riverine cichlids colonized newly forming rivers and lakes, they repeatedly speciated on different continents. Most of cichlid diversity occurs within the African great lakes (1500 species; [32]). However, New World cichlids also show significant variation with 400–500 species [33]. With this many species, cichlids represent nearly 10% of teleost fishes.
Cichlids are found in turbid rivers, clear deep lakes, murky lakes, and small crater lakes. Many cichlid species thrive in riverine environments, which are typically more turbid and red-shifted than clear environments of deeper lakes. This includes Amazonian cichlids that must adapt to diverse environments which include white and black waters that show seasonal variation [34]. Therefore, cichlids are found across a most the full range of aquatic habitats, allowing us to investigate the link between ecology and visual adaptation.
3. Axes of visual adaptation
Correlated with their diverse habitats, cichlids have some of the most diverse visual sensitivities found in vertebrates. Incredibly, some of these differences in visual sensitivities have arisen rapidly after invasion of new niches such as the African Great Lakes (eg. <5 MY in Lake Malawi; [35, 36]). This amazing diversity results from the tweaking of several visual tuning mechanisms (Fig. 2). First, cichlids can have variable cornea and lens transmission because of deposited pigments that reduce the amount of short wavelength light reaching the retina. Second, they can vary the type and number of their photoreceptors. Third, cichlids can alter the peak absorbance of the visual pigments in these photoreceptors depending on the combination of chromophore and opsin protein, thereby significantly shifting visual sensitivity.
Figure 2.

Comparison of visual pigment sets for cichlids using the short (left) or long (right) palette. (The medium palette shows similar effects). A) The original visual pigment sets for either the short (SWS1:468nm, RH2B: 488nm, RH2A: 528nm) or long (SWS2A: 455, RH2A: 528nm, LWS: 565nm) palettes. B) The effects of having a UV transparent (solid) or UV blocking (dashed) lens. The lens cutoff only effects wavelengths shorter than 400 nm. C) The effects of chromophores shifts from pure A1 (solid) to pure A2 (dashed) chromophore. Longer visual pigments have larger shifts than shorter wavelength pigments.
3.1. Lens and cornea transmission
The lens and cornea collect and focus light onto the retinal photoreceptors. As in most aquatic species, cichlid focal power depends on a large spherical lens [37]. Studies of the riverine Astatotilapia burtoni, show that cichlids’ spherical lens is layered like an onion, with each layer varying in index of refraction. This produces a graded index that varies as light passes from the edge to the center of the lens to correct for spherical aberration [38, 39]. Another aspect of both the lens and the cornea is that either can contain pigments that absorb light [40, 41]. These pigments prevent shorter wavelengths from reaching the retina, producing effective long pass filters. In green or turbid South American waters, cichlids have corneas and lenses containing carotenoid pigments. These pigments absorb UV to blue wavelengths and may help reduce the deleterious effects of short wavelength scattered light [21, 42, 43].
More subtle differences in lens transmission have been observed in species in the African (Fig. 2B) and New World lakes. Their seemingly transparent lenses can differ in whether they transmit or block UV wavelengths [23, 24, 44, 45]. In these cases, co0072neas are UV transparent, so that the lens determines which wavelengths reach the retina. Lens transmission can vary with age, with UV transparency decreasing in older fish [46, 47]. UV transparency may have ecological benefits. In cichlids from both Lakes Malawi and Tanganyika, UV transparent lenses occur in species with UV sensitive cones [23, 45]. UV sensitivity is thought to enhance zooplanktivory in species and developmental stages more likely to be zooplanktivorous feeders. Species which feed in other ways do not benefit from UV sensitivity and so may have UV blocking lenses to prevent potentially harmful UV light from damaging the retina.
3.2. Photoreceptor type and arrangement
Cichlids are strongly diurnal and have duplex retina with both rod and cone photoreceptors. This includes two types of cones: short wavelength sensitive single cones and longer wavelength sensitive double cones. The double cones often have two different pigments, which in combination with the single cones produce a trichromatic visual system. This trichromacy has been behaviorally documented [48]. Cones are typically arranged in a square mosaic with one single cone surrounded by 4 pairs of double cones [49, 50]. This produces cone ratios of 1:2:2 for cones containing short : medium : long wavelength visual pigments. Rods are interspersed among the cones with 8 to 30 rods per cone, depending on the species [49, 50].
3.3. Chromophore
Embedded in the outer segment membrane of photoreceptors are the visual pigments, which consist of a light absorbing chromophore bound to an opsin protein. Like many fish, cichlids utilize chromophores derived from either vitamin A1 (11-cis retinal) or vitamin A2 (3,4 didehydroretinal). A2 based visual pigments have slightly longer wavelength peak absorbance than those with A1 chromophores (Fig. 2C). Peak shifts in absorbance are wavelength dependent, being small (10 nm) for SWS based pigments, but increasing up to 60 nm for LWS based pigments [51, 52]. For cichlids living in clearer habitats, A1 pigments are most common [53, 54]. A2 chromophore usage may help cichlids adapt to red-shifted murky waters and depth. Such chromophore shifts occur in turbid environments including Lake Victoria [55], Lake Managua [24, 56] and the Panama canal [43].
The enzyme that converts A1 to A2 chromophore is a cytochrome P450 family gene, cyp27c1 [57]. Increased cyp27c1 expression has been found in cichlids from murkier Lake Nicaragua as compared to clearer crater lake species [24, 56]. However, cyp27c1 expression differences were not confirmed in Cichla monoculus located in clear versus murky parts of the Panama canal [43]. Chromophore shifts could be an important art of light adaptation because the A1 to A2 switch can occur relatively rapidly, over the course of a few days to weeks [58]. However, the speed of chromophore switching has not y t b n quantified for cichlids.
3.4. Opsins
The second critical component of visual pigments are the opsin proteins. Shifts in visual pigment absorbance depend on properties of the opsins, including the polarity of the amino acids (AA) closest to the chromophore [59]. Opsin dependent cichlid visual diversity result from several mechanisms including changes to opsin AA sequence, changes in which opsin gene is expressed, coexpression of opsins in a single photoreceptor, and opsin gene losses. While changes in opsin sequence or gene number require long evolutionary times, changes in opsin expression can be q ite rapid and are a main mechanism behind the diversity in cichlid visual sensitivities. Such rapid changes result in environmental plasticity, with opsin expression responding to the environment in a similar manner to changes in chromophore. Below we describe how these different factors contribute to visual sensitivity shifts, facilitating cichlid adaptation.
3.4.1. Opsin coding sequence
Changes in opsin AA sequence can shift visual pigment absorbance. Changes in one or more AA can cause 1–15 nm shifts (e.g. [60]). These shifts may result in visual pigments that are better matched to the local light environment. Opsin sequence changes have been found for cichlids differing in depth or water turbidity. Species moving from rivers into clearer lakes have acquired changes that blue shift their RH1 opsins [25, 61, 62]. Deep living species in clear Malawi and Tanganyika have evolved RH1 and RH2 genes that match the blue shifted waters at depth [63–65]. In murky Lake Victoria, opsins sequence changes shift visual pigments to longer wavelength with depth [60, 66]. Sensitivity shifts may have significant impacts on cichlid behaviors such as mate choice, and may contribute to speciation [66, 67].
3.4.2. Opsin gene expression
The majority of cichlids maintain seven cone opsin genes but differentially express a subset as adults. The three most common expression combinations include the short (SWS1, RH2B, RH2A), medium (SWS2B, RH2B, RH2A) and long (SWS2A, RH2A, LWS) visual palettes (short and long palettes shown in Fig. 2A). In clear Lakes Malawi and Tanganyika, all three palettes occur and are likely convergent in the these lakes, with sister taxa sometimes differing in expressed palette [22, 23]. However, species from murkier Lake Victoria or from rivers in Africa and the New World utilize the long palette [21, 22, 43, 68]. Deep dwelling species in clear lakes may express medium palette opsins as found in Lak s Malawi and Barombi Mbo, since water absorbs the shortest and longest wavelengths [26, 69]. Species that have colonized clear Central American crater lakes express shorter wavelength opsins than murkier lakes nearby [24, 56].
Some species utilize all three palettes as part of a developmental progression, shifting from the short to medium to long palette with age [68, 70, 71]. During the developmental transitions, multiple opsins are coexpressed in the same cell (see 3.4.3). Halting the progression can alter the final adult palette, and may be one way that some species adapt to new lakes or foraging styles.
Opsin expression also varies diurnally. Cone opsin expression is highest in the afternoons, with all cone opsins following the same cycle [72, 73]. This may be part of a daily photoreceptor renewal found in many animals [74–76] and suggests that all cone opsins share a common diurnal regulatory mechanism.
3.4.3. Opsin coexpression and plasticity
In addition to shifts that completely replace one cone opsin with another, partial expression shifts can occur, resulting in coexpression of two cone opsins in a single photoreceptor at the end point [24, 77–79]. This coexpression has been found in both African and New World cichlids and produces cone sensitivities that are intermediate between the coexpressed pair [78]. Interestingly, the coexpressed pigments are always spectral nearest neighbors rather than the opsin genes that are adjacent in the genome. These sizable sensitivity shifts in cichlids are comparable to some of the most plastic teleost species [80, 81].
Short term environmental shifts can lead to increased or decreased coexpression [82, 83]. In this way, altered lighting environments upregulate certain opsins that increase sensitivity in the new conditions. Coexpression changes quickly in just a few days to a few weeks and is reversible [70, 83, 84]. This plastic response varies between species with some species showing considerable plasticity and others none at all [43, 85]. Plasticity is more common for shorter wavelength palette species and younger animals. Adults with the long palette seem to have lost plasticity [70, 86], although slight changes have been found for Lake Victorian cichlids [87]. Plasticity may be the result of hormone levels that provide environmental sensitivity.
Plasticity-induced coexpression also varies spatially across the retina (Fig. 3). In the Lake Malawi cichlid Metriaclima zebra, the higher acuity, area centralis has minimal c expression, but the periphery shows higher coexpression [77, 78]. Increased coexpression might improve contrast detection useful for motion perception, but may hinder object discrimination needed in the central retina. Variation in spectral sensitivities across the retina has been documented in other fishes and may play an important role in performing different visual tasks using different parts of the retina [16, 88].
Figure 3.
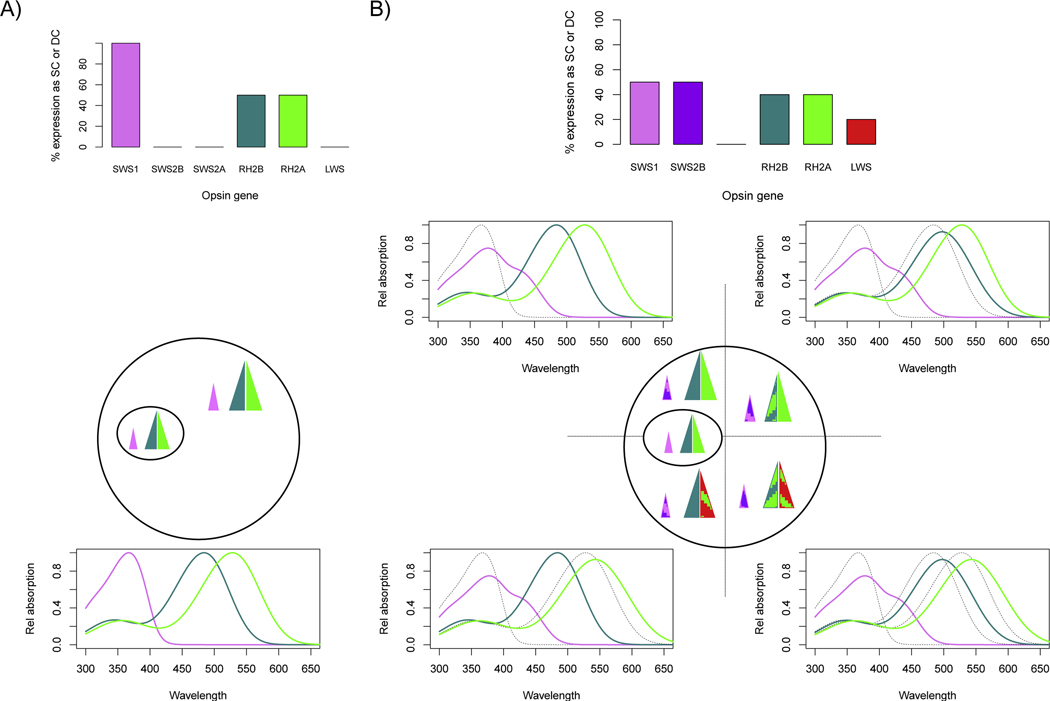
Opsin coexpression resulting from environmental plasticity modifies cone sensitivities in short palette species [77, 78]. Shown are the relative expression levels for the different opsin classes expressed as % of single cones (all SWS genes) or % of double cones (RH2 and LWS genes) as well as the resulting visual pigments. A) Wild caught or UV laboratory lighting. All cones express a single opsin gene to produce the pure short palette (SWS1, RH2B and RH2A opsins) across the retina. B) Fluorescent lab raised individuals (and some wild caughts) show increased coexpression that varies across the retina. In the central area centralis, the pure short palette opsins as shown in (A). Single cones outside of this region coexpress SWS1 and SWS2B. For double cones, RH2B/RH2Ab coexpression increases in the temporal region while RH2Aa/LWS coexpression increases in the ventral region. This produces 4 different coexpression regions. For the coexpressed pigments, the visual pigment sum is shown in color, as compared to the pure pigment it replaces shown in dotted gray.
3.4.4. Opsin gene losses
While the majority of cichlids retain all seven cone opsin genes, there is some evidence for opsin gene losses. A few pseudogenes have been observed in cichlids from Lake Tanganyika [89] Tanganyika is the oldest of the African Great Lakes and so may have had more evolutionary time to acquire gene losses. Evidence for gene loss is more prevalent in New World cichlids [21, 90]. Different lineages in these murky habitats show loss of SWS1, SWS2B, or RH2B genes. Species’ reliance on the long palette in these more turbid habitats may diminish the need for expressing shorter wavelength genes.
3.5. Phototransduction
Light absorption by visual pigments activates the phototransduction pathway that converts photons into a neural response. As a G protein coupled receptor, opsin interacts with G proteins that turn on the effector protein, phosphodiesterase. This effector alters cGMP concentrations closing ion channels and causing cell hyperpolarization [91, 92]. Additional proteins help shut down the activated states of the opsins (G protein receptor kinase and arrestin) and G proteins, returning the cell to its resting state ready to detect more light. The efficiency with which the proteins activate or deactivate each other can affect the sensitivity and speed of photoreception. Fish living in different habitats might trade off speed and sensitivity, depending on the needs of their ecology and local environment. Studies have compared cichlids that live in shallow versus deep habitats. They found evidence for selection on coding sequence of several phototransduction genes including the cone pathway G protein, G protein receptor kinase and arrestin [63]. These adaptations may enhance sensitivity under the low light conditions at depth. This compliments the many changes in opsin sequence and expression levels observed in adapting to both depth and turbidity (see 3.4.1). Future studies of phototransduction protein in New World cichlids or deep living crater lake species might prove interesting.
3.6. Visual acuity
Visual acuity quantifies the retina’s ability to spatially distinguish objects. Smaller, more closely spaced cones (and their associated ganglion cells) enable higher visual acuity, but lower overall light sensitivity. Larger cones have the reverse tradeoff, increasing sensitivity while sacrificing acuity. Acuity is quantified from the finest alternating bright and dark bars that can be distinguished from a uniform gray, and is termed spatial r solving power (SRP, in cycles per degree). Cichlids differ in visual acuity, with SRP varying from 3–7 cycles per degree, with values based on ganglion cells lower than for photoreceptors (Fig. 4). In Lake Victoria cichlids, visual acuity does not correlate with species depth, but has some relationship with size of food items [93] Overall, cichlid visual acuities are intermediate among fishes [94], but are sufficient for the kinds of tasks cichlids perform, from discriminating cichlid stripe patterns to detecting small food items such as zooplankton [77].
Fig 4.
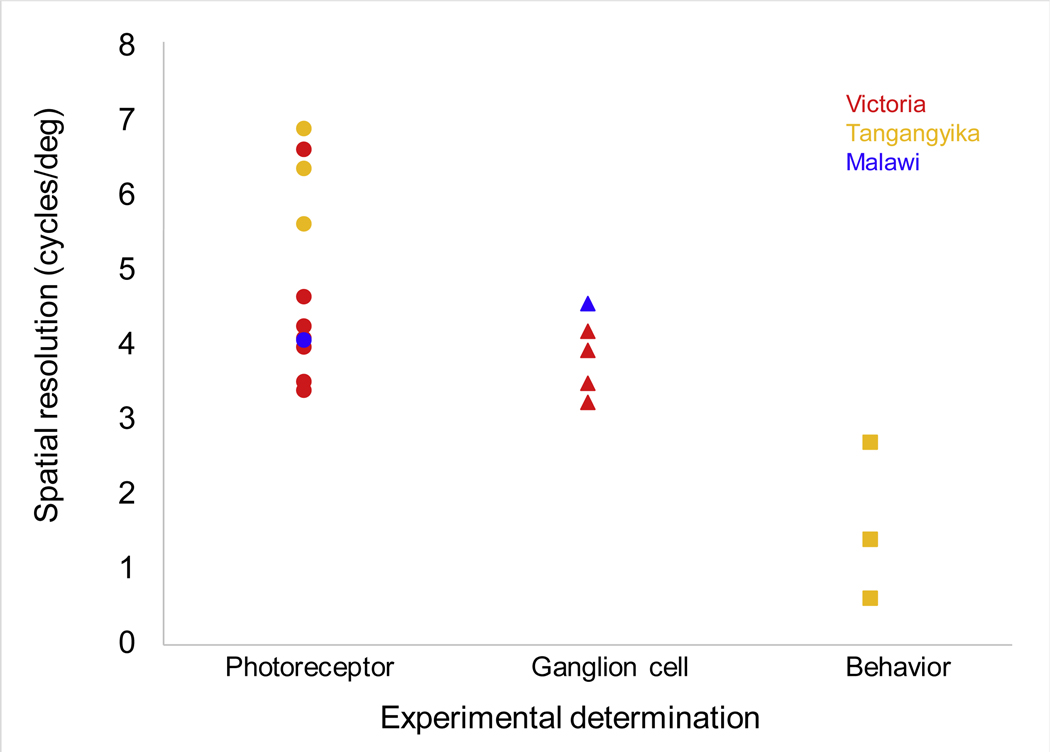
Visual acuity measured for African cichlids. Methods include quantifying photoreceptor or ganglion cell densities [77, 97, 118] as well as behavioral measures [98]. Points are color coded based on the lake where species live. Data in Supp Table S1.
In murkier or deeper habitats, cichlids alter the number and types of photoreceptors, thereby sacrificing visual acuity to enhance sensitivity. Double cones switch from containing different visual pigments to containing the same pigment, essentially doubling light sensitivity to the prevailing light spectrum. For example, Lake Victoria species in the clearest waters have all RH2/LWS double cones while fish from more turbid habitats have over 50% LWS/LWS cones [95] Variation also occurs across the retina with more LWS/LWS cones in the downward looking dorsal retina and more RH2/RH2 cones in the upward looking ventral retina [96]. This provides a better match to the long wavelength light coming up from below versus the broader light downwelling from above. In very murky environments, cichlids can even lose single cones all together, making room for more long wavelength sensitive double cones [93, 97].
Morphological measures of visual acuity are likely an overestimate of the actual behavioral resolution that fish have. Studies using two choice discrimination trials found Haplochromis argens discrimination ability for a checkerboard versus a gray target improved with age and eye size [97]. Although behavioral acuity was always worse than photoreceptor spacing would predict, it did improve rapidly with age, approaching the morphological limit set by photoreceptors.
Behavioral acuity has also been measured using the optomotor response (OMR), where fish swim to follow a moving stripe pattern, and the optokinetic response (OKR), where fish move their eyes in response to pattern movement. Using a combination of these responses, behavioral visual acuity was compared between four species of Lake Tanganyikan cichlids [98]. By varying the stripe spacing, the authors showed that a species that lives in rocky habitat had five times greater visual acuity than species that live in sand or intermediate habitat. They suggest that the complex rocky habitat benefits from improved spatial resolution for navigation while species over the sand may require better temporal resolution for predator avoidance. Therefore, habitat can impact visual system optimization.
3.7. Color sensitivity and discrimination
Several methods have been used to quantify color sensitivity and discrimination. OMR studies have shown that species can differ in color sensitivity as a result of differences in opsin sequence [99] and opsin expression [100]. These sensitivity differences could predispose cichlid preferences for particular mating colors. This might be one mechanism whereby sensory drive could promote cichlid speciation [66].
OMR studies test for color sensitivity, but they do not necessarily require the subjects to have neurally opponent color vision. Color vision can be confirmed using behavioral operant conditioning as shown for other fishes [101–103]. Such tests in the Lake Malawi species Metriaclima benetos found that they can distinguish blues and yellows apart from grays, independent of target brightness [48]. This confirms they are using color hues and not brightness to perform these tasks. In addition, discrimination threshold for M. benetos has been quantified in one part of color space where fish had to discriminate blues from purples [104]. Cichlids’ abilities to discriminate color pairs is quantified by neural circuit noise as this limits the best they can do. Noise estimates showed that these species have a noise value of v=0.16, significantly higher than typically assumed value of 0.05 [105], but comparable to other fishes [106]. Future work should examine other parts of the color spectrum as fish species have been shown to vary in their color discrimination abilities depending on the color of the targets [107]. In addition, studies of other cichlids with different visual palettes should be compared with those for the short palette M. benetos. It would be useful to know if visual pigment sensitivity impacts which part of the spectrum cichlids can best discriminate and how that relates to the colors that they prefer.
4. Power of cichlids as a genetic model
In addition to understanding the genetic mechanisms that tune cichlid visual sensitivities, cichlids can also serve as a model to define the network controlling opsin expression. Using the power of genetic crosses, quantitative trait loci (QTL) studies followed by fine mapping and transcriptomics have been used to identify several QTL and the underlying transcription factors that regulate the opsins [108–111]. This complements work in zebrafish and medaka that have identified key regulatory elements controlling opsin expression, including locus control regions [15, 112–114], and microRNAs [17]. The ultimate goal is to define the key genes and how they form a switchable opsin gene regulatory network. This would provide insights into how opsin expression changes through development within a species. Further, it could determine the types of mutations that evolutionarily modify the network to produce the diversity of cichlid visual sensitivities.
5. Unanswered questions
Many questions remain about the extensive diversity of visual systems. These questions are both specific to cichlids but also represent more general questions that extend across the majority of visual animals. The first question is whether visual system evolution is adaptive. Broader comparisons are needed across many taxa with ecological and visual system diversity to test for and identify mechanisms of adaptation. Cichlids are a great system to address this question on a smaller scale s they exhibit both immense visual and ecological variation. We can therefore determine whether visual variation evolved to maximize adaptive visual capabilities in cichlids and use that knowledge to inform findings across a greater number of taxa. In addition to documenting vis al systems variation, experiments are required to demonstrate functionality of the variation [13, 14]. There is evidence for selection on some key genes including opsins and other phototransduction proteins [63, 65, 89]. However, proof of functionality will require studies demonstrating that visual shifts provide true behavioral and reproductive fitness advantages [115].
In addition to considering long term evolutionary change, studies need to examine the role of short term visual plasticity in response to changes in environmental lighting This visual plasticity is found in some groups, but has not been investigated in others, including the common model systems (i.e. zebrafish, mammals). By studying mechanisms of plasticity we could investigate if and how it enables organisms to invade new habitats and speciate by improving visual tasks such as foraging or predator detection. Cichlids are again a useful model as they have invaded new habitats both over longer evolutionary times scales and with relatively recent introductions and exhibit a range of plasticity [24, 43]. Rapid changes in visual sensitivities in new habitats could lead to altered mate choice, and ultimately help drive speciation [116].
One area that requires much further study is neural wiring. With evidence for partitioning of the retina for different visual tasks [16, 88, 117], more work is needed to know how retinal wiring facilities such tasks. Very few systems have been examined in enough detail to understand the diversity of cell types or their wiring. In cichlids, studies of cell distributions and coexpression have identified an area centralis for object discrimination and a periphery for contrast detection. Although cichlids have the typical retinal layers of most vertebrates (Fig. 5), little is known about the cell types in these different layers or how their wiring might facilitate such a division of labor for different visual tasks. More rapid means of identifying the diversity of retinal cell types and circuit construction would help determine how a small set of photoreceptors contributes to processing the different aspects of discerning the visual world. Altered retinal wiring could enhance visual sensitivity and color discrimination, and could be an important part of mate choice. Since mate choice is thought to help drive r productive isolation and speciation, it would be interesting to know where female preferences lies. Does female preference depend on retinal wiring or is it inherent to the brain where multiple signals converge for analysis?
Fig 5.
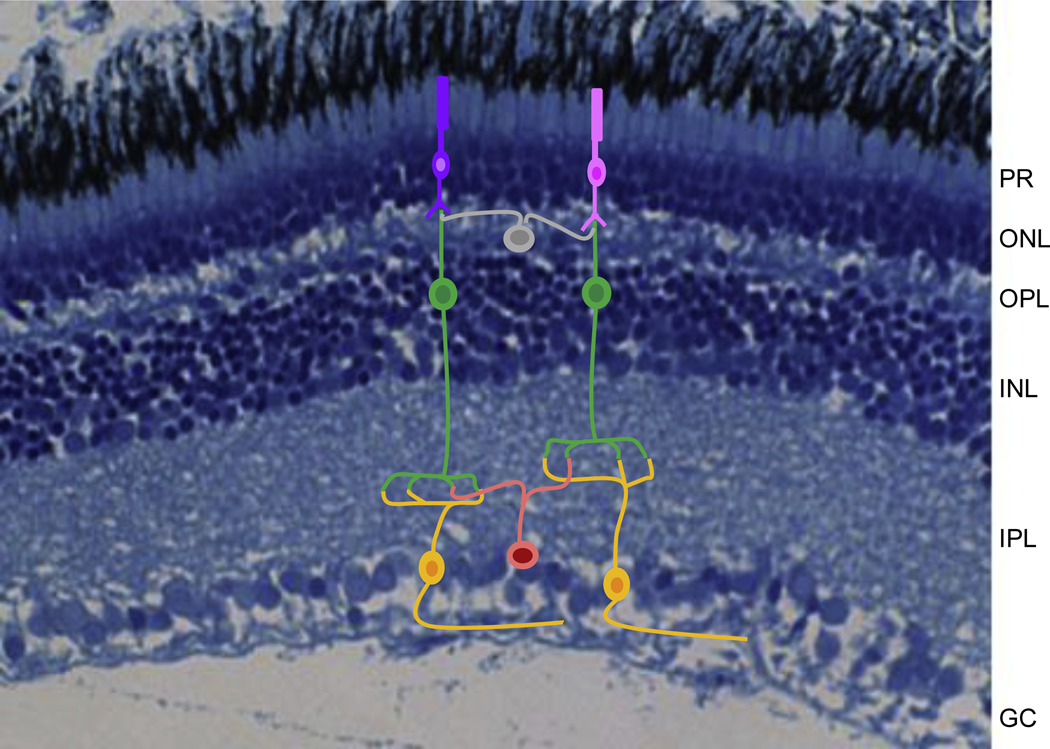
Retinal cross section of the tilapia Oreochromis aureus stained with toluidine blue (histology courtesy of Jacqueline Webb, University of Rhode Island). Superimposed are some possible retinal cells including photoreceptors (pink and purple), bipolar cells (green), ganglion cells (gold), horizontal cells (gray) and amacrine cells (orange). These interconnect to form the key retinal layers including the photoreceptor layer (PR), the outer nuclear layer (ONL), the outer plexiform layer (OPL), the inner nuclear layer (INL), the inner plexiform layer (IPL) and the ganglion cell layer (GC). Although we believe cichlids have all of these cell types, little is known about their diversity or connectivity.
A broad, comparative approach would provide benefits in understanding visual diversity, adaptation, and evolution, and could be carried out across a variety of phylogenetic levels. The breadth of the cichlid phylogeny offers possibilities in comparing species within lakes, within continents and across the globe. Identifying the similarities and differences within cichlids, but also within teleosts, and within vertebrates would help identify the constancies across visual systems and the parts that are more malleable, adapting to particular ecologies. Such comparisons need to consider multiple visual system attributes from visual sensitivities to visual acuity to color discrimination to behavioral choices such as foraging or mating preferences (Fig. 1).
6. Conclusions
Cichlid studies have made significant contributions to understanding the mechanisms that generate visual system diversity. Essentially every tuning mechanism that could play a role seems to contribute. This includes changes to cornea and lens transmission, photoreceptor number and distribution, and visual pigment sensitivities. Visual pigment sensitivities are altered through chromophore shifts as well as changes to opsin gene sequence, gene expression, and gene loss. Some of these changes are the result of developmental reprogramming to alter the expressed genes in adults. Considerable work is ongoing to unlock the genetic network that controls opsin expression, its developmental progression, and its evolution across the cichlid radiation. This work relies on the power of cichlid genetic crosses and genomic tools.
The work in cichlids compliments and informs studies in other fishes. Not much is known as to whether model fishes significantly change opsin expression through development or if they show environmental plasticity or coexpression. It may be that the models are more hardwired, but it is unclear if this is truly the case because of their more limited ecological niches, or simply a lack of available comparisons with closely related species. Therefore, more comparative work is needed for existing models and for new models. Cichlids have demonstrated their important role in documenting the proximate and ultimate mechanisms shaping visual sensitivity. They are therefore ready to take their place among such models based on their amazing phenotypic and ecological diversity
Supplementary Material
Highlights.
Cichlid visual sensitivities vary by changing cornea and lens transmission, photoreceptor densities, and photoreceptor sensitivities
Cichlid photoreceptor sensitivities shift through changes to chromophore, opsin gene sequence, opsin gene expression and opsin gene loss
Sensitivity differences are linked to differences in ecology including f raging and light environment
Cichlids are a powerful example of how the comparative a roach can unravel the proximate and ultimate causes of visual system variation
Acknowledgements
The authors would like to thank Prof. Tom Baden for the invitation to contribute to this issue. Thanks to all our collaborators and former lab members for their contributions in unravelling cichlid diversity. This work was supported by funding from the National Eye Institute of the National Institutes of Health (R01EY024639).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests.
References
- [1].Viets K, Eldred K, Johnston RJ Jr., Mechanisms of Photoreceptor Patterning in Vertebrates and Invertebrates, Trends Genet 32(10) (2016) 638–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baden T, Euler T, Berens P, Understanding the retinal basis of vision across species, Nat Rev Neurosci 21(1) (2020) 5–20. [DOI] [PubMed] [Google Scholar]
- [3].Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK, Expression of rod and cone visual pigments in goldfish and zebrafish: a rhodopsin-like gene is expressed in cones, Neuron 10(6) (1993) 1161–74. [DOI] [PubMed] [Google Scholar]
- [4].Register EA, Yokoyama R, Yokoyama S, Multiple origins of the green-sensitive opsin genes in fish, J Mol Evol 39(3) (1994) 268–73. [DOI] [PubMed] [Google Scholar]
- [5].Hisatomi O, Satoh T, Tokunaga F, The primary structure and distribution of killifish visual pigments, Vision Res 37(22) (1997) 3089–96. [DOI] [PubMed] [Google Scholar]
- [6].Vihtelic TS, Doro CJ, Hyde DR, Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins, Vis Neurosci 16(3) (1999) 571–85. [DOI] [PubMed] [Google Scholar]
- [7].Yokoyama S, Evolution of dim-light and color vision pigments, Annual review of genomics and human genetics 9 (2008) 259–282. [DOI] [PubMed] [Google Scholar]
- [8].Cortesi F, Musilova Z, Stieb SM, Hart NS, Siebeck UE, Malmstrom M, Torresen OK, Jentoft S, Cheney KL, Marshall NJ, Carleton KL, Salzburger W, Ancestral duplications and highly dynamic opsin gene evolution in percomorph fishes, Proc Natl Acad Sci U S A 112(5) (2015) 1493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Musilova Z, Cortesi F, Matschiner M, Davies WIL, Patel JS, Stieb SM, de Busserolles F, Malmstrom M, Torresen OK, Brown CJ, Mountford JK, Hanel R, Stenkamp DL, Jakobsen KS, Carleton KL, Jentoft S, Marshall J, Salzburger W, Vision using multiple distinct rod opsins in deep-sea fishes, Science 364(6440) (2019) 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jerlov NG, Marine Optics, Elsevier, Amsterdam, 1976. [Google Scholar]
- [11].Loew ER, McFarland WN, The underwater visual environment, in: Douglas RH, Djamgoz MBA (Eds.), The visual system of fish, Chapman and Hall, London, 1990, pp. 1–43. [Google Scholar]
- [12].Cronin TW, Johnsen S, Marshall NJ, Warrant EJ, Visual Ecology, Princeton University Press, Princeton, N.J., 2014. [Google Scholar]
- [13].Marshall J, Carleton KL, Cronin T, Colour vision in marine organisms, Curr Opin Neurobiol 34 (2015) 86–94. [DOI] [PubMed] [Google Scholar]
- [14].Carleton KL, Escobar-Camacho D, Stieb SM, Cortesi F, Marshall J, Seeing the rainbow: Mechanisms underlying spectral sensitivity in teleost fishes Journal of Experimental Biology in press (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsujimura T, Chinen A, Kawamura S, Identification of a locus control region for quadruplicated green-sensitive opsin genes in zebrafish, Proc Natl Acad Sci U S A 104(31) (2007) 12813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zimmermann MJY, Nevala NE, Yoshimatsu T, Osorio D, Nilsson DE, Berens P, Baden T, Zebrafish Differentially Process Color across Visual Space to Match Natural Scenes, Curr Biol 28(13) (2018) 2018–2032 e5. [DOI] [PubMed] [Google Scholar]
- [17].Daido Y, Hamanishi S, Kusakabe TG, Transcriptional co-regulation of evolutionarily conserved microRNA/cone opsin gene pairs: implications for photoreceptor subtype specification, Dev Biol 392(1) (2014) 117–29. [DOI] [PubMed] [Google Scholar]
- [18].Kamijo M, Kawamura M, Fukamachi S, Loss of red opsin genes relaxes sexual isolation between skin-colour variants of medaka, Behav Processes 150 (2018) 25–28. [DOI] [PubMed] [Google Scholar]
- [19].Parichy DM, Advancing biology through a deeper understanding of zebrafish ecology and evolution, Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kumar S, Stecher G, Suleski M, Hedges SB, TimeTree: A Resource for Timelines, Timetrees, and Divergence Times, Mol Biol Evol 34(7) (2017) 1812–1819. [DOI] [PubMed] [Google Scholar]
- [21].Escobar-Camacho D, Ramos E, Martins C, Carleton KL, The opsin genes of amazonian cichlids, Mol Ecol 26(5) (2017) 1343–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hofmann CM, O’Quin KE, Marshall NJ, Cronin TC, Seehausen O, Carleton KL, The eyes have it: Regulatory and structural changes both underlie cichlid visual pigment diversity, PLoS biology 7(12) (2009) e1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].O’Quin KE, Hofmann CM, Hofmann HA, Carleton KL, Parallel evolution of opsin gene expression in African cichlid fishes, Mol Biol Evol 27(12) (2010) 2839–54. [DOI] [PubMed] [Google Scholar]
- [24].Torres-Dowdall J, Pierotti MER, Harer A, Karagic N, Woltering JM, Henning F, Elmer KR, Meyer A, Rapid and Parallel Adaptive Evolution of the Visual System of Neotropical Midas Cichlid Fishes, Mol Biol Evol 34(10) (2017) 2469–2485. [DOI] [PubMed] [Google Scholar]
- [25].Hauser FE, Ilves KL, Schott RK, Castiglione GM, Lopez-Fernandez H, Chang BSW, Accelerated Evolution and Functional Divergence of the Dim Light Visual Pigment Accompanies Cichlid Colonization of Central America, Mol Biol Evol 34(10) (2017) 2650–2664. [DOI] [PubMed] [Google Scholar]
- [26].Musilova Z, Indermaur A, Bitja-Nyom AR, Omelchenko D, Klodawska M, Albergati L, Remisova K, Salzburger W, Evolution of the visual sensory system in cichlid fishes from crater lake Barombi Mbo in Cameroon, Mol Ecol 28(23) (2019) 5010–5031. [DOI] [PubMed] [Google Scholar]
- [27].Irisarri I, Singh P, Koblmuller S, Torres-Dowdall J, Henning F, Franchini P, Fischer C, Lemmon AR, Lemmon EM, Thallinger GG, Sturmbauer C, Meyer A, Phylogenomics uncovers early hybridization and adaptive loci shaping the radiation of Lake Tanganyika cichlid fishes, Nat Commun 9(1) (2018) 3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sparks JS, Molecular phylogeny and biogeography of the Malagasy and South Asian cichlids (Teleostei: Perciformes: Cichlidae), Mol Phylogenet Evol 30(3) (2004) 599–614. [DOI] [PubMed] [Google Scholar]
- [29].Conte MA, Joshi R, Moore EC, Nandamuri SP, Gammerdinger WJ, Roberts RB, Carleton KL, Lien S, Kocher TD, Chromosome-scale assemblies reveal the structural evolution of African cichlid genomes, Gigascience 8(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matsumoto Y, Fukamachi S, Mitani H, Kawamura S, Functional characterization of visual opsin repertoire in Medaka (Oryzias latipes), Gene 371(2) (2006) 268–78. [DOI] [PubMed] [Google Scholar]
- [31].O’Quin KE, Smith D, Naseer Z, Schulte J, Engel SD, Loh YH, Streelman JT, Boore JL, Carleton KL, Divergence in cis-regulatory sequences surrounding the opsin gene arrays of African cichlid fishes, BMC evolutionary biology 11 (2011) 120.21554730 [Google Scholar]
- [32].Genner MJ, Seehausen O, Lunt DH, Joyce DA, Shaw PW, Carvalho GR, Turner GF, Age of cichlids: new dates for ancient lake fish radiations, Mol Biol Ev l 24(5) (2007) 1269–82. [DOI] [PubMed] [Google Scholar]
- [33].Froese R, Pauly D, FishBase, 2019. [Google Scholar]
- [34].Costa MPF, Novo EMLM, Telmer KH, Spatial and temporal variability of light attenuation in large rivers of the Amazon, Hydrobiologia 702 (2013) 171–190. [Google Scholar]
- [35].Sturmbauer C, Baric S, Salzburger W, Ruber L, Verheyen E, Lake level fluctuations synchronize genetic divergences of cichlid fishes in African lakes, Mol Biol Evol 18(2) (2001) 144–54. [DOI] [PubMed] [Google Scholar]
- [36].Ivory SJ, Blome MW, King JW, McGlue MM, Cole JE, Cohen AS, Environmental change explains cichlid adaptive radiation at Lake Malawi over the past 1.2 million years, Proc Natl Acad Sci U S A 113(42) (2016) 11895–11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Matthiessen L, Ueber die beziehungen, welche zwischen dem brechungsindex des kerncentrums der krystalllinse und den dimensionen des auges bestehen, Pflugers Arch. 27 (1882) 510–523. [Google Scholar]
- [38].Kroger RH, Campbell MC, Fernald RD, The development of the crystalline lens is sensitive to visual input in the Afric n cichlid fish, Haplochromis burtoni, Vision Res 41(5) (2001) 549–59. [DOI] [PubMed] [Google Scholar]
- [39].Kroger RH, Campbell MC, Munger R, Fernald RD, Refractive index distribution and spherical aberration in the c ystalli e lens of the African cichlid fish Haplochromis burtoni, Vision Res 34(14) (1994) 1815–22. [DOI] [PubMed] [Google Scholar]
- [40].Lythgoe JN, The ecology of vision, Clarendon Press, Oxford, 1979. [Google Scholar]
- [41].Moreland JD, Lythgoe JN, Yellow corneas in fishes, Vision Res 8(10) (1968) 1377–80. [DOI] [PubMed] [Google Scholar]
- [42].Muntz WR, Yellow filters and the absorption of light by the visual pigments of some Amazonian fishes, Vision Res 13(12) (1973) 2235–54. [DOI] [PubMed] [Google Scholar]
- [43].Esc bar-Camacho D, Pierotti MER, Ferenc V, Sharpe DMT, Ramos E, Martins C, Carleton KL, Variable vision in variable environments: the visual system of an invasive cichlid (Cichla monoculus) in Lake Gatun, Panama, J Exp Biol 222(Pt 6) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Thorpe A, Douglas RH, Truscott RJW, Spectral transmission and short-wave absorbing pigments in the fish lens-- I. Phylogenetic distribution and identity, Vision Res 33(3) (1993) 289–300. [DOI] [PubMed] [Google Scholar]
- [45].Hofmann CM, O’Quin KE, Justin Marshall N, Carleton KL, The relationship between lens transmission and opsin gene expression in cichlids from Lake Malawi, Vision Res 50(3) (2010) 357–63. [DOI] [PubMed] [Google Scholar]
- [46].Thorpe A, Douglas RH, Spectral transmission and short-wave absorbing pigments in the fish lens--II. Effects of age, Vision Res 33(3) (1993) 301–7. [DOI] [PubMed] [Google Scholar]
- [47].Sabbah S, Hui J, Hauser FE, Nelson WA, Hawryshyn CW, Ontogeny in the visual system of Nile tilapia, J Exp Biol 215(Pt 15) (2012) 2684–95. [DOI] [PubMed] [Google Scholar]
- [48].Escobar-Camacho D, Marshall J, Carleton KL, Behavioral color vision in a cichlid fish: Metriaclima benetos, J Exp Biol 220(Pt 16) (2017) 2887–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Braekevelt CR, Smith SA, Smith BJ, Photoreceptor fine structure in Oreochromis niloticus L. (Cichlidae; Teleostei) in light- and dark-adaptation, Anat Rec 252(3) (1998) 453–61. [DOI] [PubMed] [Google Scholar]
- [50].Fernald RD, Chromatic organization of a cichlid fish retina, Vision Res 21 (1981) 1749–1753. [DOI] [PubMed] [Google Scholar]
- [51].Harosi FI, An analysis of two spectral properties of vertebrate visual pigments, Vision Res 34(11) (1994) 1359–67. [DOI] [PubMed] [Google Scholar]
- [52].Parry JW, Bowmaker JK, Visual pigment reconstitution in intact goldfish retina using synthetic retinaldehyde isomers, Vision Res 40(17) (2000) 2241–7. [DOI] [PubMed] [Google Scholar]
- [53].Jordan R, Kellogg K, Howe D, Juanes F, Stauffer JR, Loew ER, Photopigment spectral absorbance of Lake Malawi cichlids, J Fish Biology 68(4) (2006) 1291–9. [Google Scholar]
- [54].Terai Y, Mayer WE, Klein J, Tichy H, Okada N, The effect of selection on a long wavelength-sensitive (LWS) opsin gene of Lake Victoria cichlid fishes, Proc Natl Acad Sci U S A 99(24) (2002) 15501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Terai Y, Miyagi R, Aibara M, Mizoiri S, Imai H, Okitsu T, Wada A, Takahashi-Kariyazono S, Sato A, Tichy H, Mrosso HDJ, Mzighani SI, Okada N, Visual adaptation in Lake Victoria cichlid fishes: depth-related variation of color and scotopic opsins in species from sand/mud bottoms, BMC evolutionary biology 17(1) (2017) 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Harer A, Meyer A, Torres-Dowdall J, Convergent phenotypic evolution of the visual system via different molecular routes: How Neotropical cichlid fish s adapt to novel light environments, Evol Lett 2(4) (2018) 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Enright JM, Toomey MB, Sato SY, Templ SE, Allen JR, Fujiwara R, Kramlinger VM, Nagy LD, Johnson KM, Xiao Y, How MJ, Johnson SL, Roberts NW, Kefalov VJ, Guengerich FP, Corbo JC, Cyp27c1 Red -Shifts the Spectral Sensitivity of Photoreceptors by Converting Vitamin A1 into A2, Curr Biol 25(23) (2015) 3048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Munz FW, McFarland WN, Evolutionary adaptations of fishes to the photic environment, in: Crescitelli F (Ed.), The visual system in vertebrates, Springer-Verlag, New York, 1977, pp. 194–274. [Google Scholar]
- [59].Chang BS, Crandall KA, Carulli JP, Hartl DL, Opsin phylogeny and evolution: a model for blue shifts in wavelength regulation, Mol Phylogenet Evol 4(1) (1995) 31–43. [DOI] [PubMed] [Google Scholar]
- [60].Terai Y, Seehause O, Sasaki T, Takahashi K, Mizoiri S, Sugawara T, Sato T, Watanabe M, Konijnendijk N, Mrosso HD, Tachida H, Imai H, Shichida Y, Okada N, Divergent selection on opsins drives incipient speciation in Lake Victoria cichlids, PLoS biology 4(12) (2006) e433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schott RK, Refvik SP, Hauser FE, Lopez-Fernandez H, Chang BS, Divergent positive selection in rhodopsin from lake and riverine cichlid fishes, Mol Biol Evol 31(5) (2014) 1149–65. [DOI] [PubMed] [Google Scholar]
- [62].Torres-Dowdall J, Henning F, Elmer KR, Meyer A, Ecological and Lineage-Specific Factors Drive the Molecular Evolution of Rhodopsin in Cichlid Fishes, Mol Biol Evol 32(11) (2015) 2876–82. [DOI] [PubMed] [Google Scholar]
- [63].Malinsky M, Svardal H, Tyers AM, Miska EA, Genner MJ, Turner GF, Durbin R, Whole-genome sequences of Malawi cichlids reveal multiple radiations interconnected by gene flow, Nat Ecol Evol 2(12) (2018) 1940–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nagai H, Terai Y, Sugawara T, Imai H, Nishihara H, Hori M, Okada N, Reverse evolution in RH1 for adaptation of cichlids to water depth in Lake Tanganyika, Mol Biol Evol 28(6) (2011) 1769–76. [DOI] [PubMed] [Google Scholar]
- [65].Sugawara T, Terai Y, Imai H, Turner GF, Koblmuller S, Sturmbauer C, Shichida Y, Okada N, Parallelism of amino acid changes at the RH1 affecting spectral sensitivity among deep-water cichlids from Lakes Tanganyika and Malawi, Proc Natl Acad Sci U S A 102(15) (2005) 5448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HD, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, Imai H, Okada N, Speciation through sensory drive in cichlid fish, Nature 455 (2008) 620–6. [DOI] [PubMed] [Google Scholar]
- [67].Miyagi R, Terai Y, Aibara M, Sugawara T, Imai H, Tachida H, Mzighani SI, Okitsu T, Wada A, Okada N, Correlation between nuptial colors and visual sensitivities tuned by opsins leads to species richness in sympatric Lake Victoria cichlid fishes, Mol Biol Evol 29(11) (2012) 3281–96. [DOI] [PubMed] [Google Scholar]
- [68].Carleton KL, Spady TC, Streelman JT, Kidd MR, McFarland WN, Loew ER, Visual sensitivities tuned by heterochronic shifts in opsin gene expression, BMC biology 6(1) (2008) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Smith AR, D’Annunzio L, Smith AE, Sharma A, Hofmann CM, Marshall NJ, Carleton KL, Intraspecific cone opsin expression variation in the cichlids of Lake Malawi, Mol Ecol 20(2) (2011) 299–310. [DOI] [PubMed] [Google Scholar]
- [70].Harer A, Torres-Dowdall J, Meyer A, Rapid adaptation to a novel light environment: The importance of ontogeny and phenotypic plasticity in shaping the visual system f Nicaraguan Midas cichlid fish (Amphilophus citrinellus spp.), Mol Ecol 26(20) (2017) 5582–5593. [DOI] [PubMed] [Google Scholar]
- [71].O’Quin KE, Smith AR, Sharma A, Carleton KL, New evidence f r the r le f heterochrony in the repeated evolution of cichlid opsin expression, Evol Dev 13(2) (2011) 193–203. [DOI] [PubMed] [Google Scholar]
- [72].Halstenberg S, Lindgren KM, Samagh SP, Nadal-Vicens M, Balt S, Fernald RD, Diurnal rhythm of cone opsin expression in the teleost fish Haplochromis burtoni, Vis Neurosci 22(2) (2005) 135–41. [DOI] [PubMed] [Google Scholar]
- [73].Yourick MR, Sandkam BA, Gammerdinger WJ, Escobar-Camacho D, Nandamuri SP, Clark FE, Joyce B, Conte MA, Kocher TD, Carl ton KL, Diurnal variation in opsin expression and common housekeeping genes necessitates comprehensive normalization methods for quantitative real-time PCR analyses, Mol Ecol Resour 19(6) (2019) 1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dalal JS, Jinks RN, Cacciatore C, G eenbe g RM, Battelle BA, Limulus opsins: diurnal regulation of expression, Vis Neurosci 20(5) (2003) 523–34. [DOI] [PubMed] [Google Scholar]
- [75].Young RW, The renewal of photoreceptor cell outer segments, J Cell Biol 33(1) (1967) 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Young RW, The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina, Invest Ophthalmol Vis Sci 17(2) (1978) 105–16. [PubMed] [Google Scholar]
- [77].Dalton BE, de Busserolles F, Marshall NJ, Carleton KL, Retinal specialization through spatially varying cell densities and opsin coexpression in cichlid fish, J Exp Biol 220(Pt 2) (2017) 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dalton BE, Loew ER, Cronin TW, Carleton KL, Spectral tuning by opsin coexpression in retinal regions that view different parts of the visual field, Proc Biol Sci 281(1797) (2014) 20141980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Karagic N, Harer A, Meyer A, Torres-Dowdall J, Heterochronic opsin expression due to early light deprivation results in drastically shifted visual sensitivity in a cichlid fish: Possible role of thyroid h rm ne signaling, J Exp Zool B Mol Dev Evol 330(4) (2018) 202–214. [DOI] [PubMed] [Google Scholar]
- [80].Fuller RC, Noa LA, Strellner RS, Teasing apart the many effects of lighting environment on opsin expression and foraging preference in bluefin killifish, Am Nat 176(1) (2010) 1–13. [DOI] [PubMed] [Google Scholar]
- [81].Johnson AM, Stanis S, Fuller RC, Diurnal lighting patterns and habitat alter opsin expression and colour preferences in a killifish, Proc Biol Sci 280(1763) (2013) 20130796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dalton BE, Lu J, Leips J, Cronin TW, Carleton KL, Variable light environments induce plastic spectral tuning by regional opsin coexpression in the African cichlid fish, Metriaclima zebra, Mol Ecol 24(16) (2015) 4193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Harer A, Karagic N, Meyer A, Torres-Dowdall J, Reverting ontogeny: rapid phenotypic plasticity of colour vision in cichlid fish, R Soc Open Sci 6(7) (2019) 190841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nandamuri SP, Yourick MR, Carleton KL, Adult plasticity in African cichlids: Rapid changes in opsin expression in response to environmental light differences, Mol Ecol 26(21) (2017) 6036–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hofmann CM, O’Quin KE, Smith AR, Carleton KL, Plasticity of opsin gene expression in cichlids from Lake Malawi, Mol Ecol 19(10) (2010) 2064–74. [DOI] [PubMed] [Google Scholar]
- [86].Hornsby MA, Sabbah S, Robertson RM, Hawryshyn CW, Modulation of environmental light alters reception and production of visual signals in Nile tilapia, J Exp Biol 216(Pt 16) (2013) 3110–22. [DOI] [PubMed] [Google Scholar]
- [87].Wright DS, van Eijk R, Schuart L, Seehausen O, Groothuis TGG, Maan ME, Testing sensory drive speciation in cichlid fish: Linking light conditions to opsin expression, opsin genotype and female mate preference, J Evol Biol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Temple SE, Why different regions of the retina have different spectral sensitivities: a review of mechanisms and functional significance of intraretinal variability in spectral sensitivity in vertebrates, Vis Neurosci 28(4) (2011) 281–93. [DOI] [PubMed] [Google Scholar]
- [89].Spady TC, Seehausen O, Loew ER, Jordan RC, Kocher TD, Carleton KL, Adaptive molecular evolution in the opsin genes of rapidly speciating cichlid species, M l Bi l Evol 22(6) (2005) 1412–22. [DOI] [PubMed] [Google Scholar]
- [90].Weadick CJ, Loew ER, Rodd FH, Chang BS, Visual pigment molecular evolution in the Trinidadian pike cichlid (Crenicichla frenata): a less colorful world for neotropical cichlids?, Mol Biol Evol 29(10) (2012) 3045–60. [DOI] [PubMed] [Google Scholar]
- [91].Ebrey T, Koutalos Y, Vertebrate photoreceptors, Prog Retin Eye Res 20(1) (2001) 49–94. [DOI] [PubMed] [Google Scholar]
- [92].Lamb TD, Evolution of the genes mediating phototransduction in rod and cone photoreceptors, Prog Retin Eye Res (2019) 100823. [DOI] [PubMed] [Google Scholar]
- [93].van der Meer HJ, Bowmaker JK, Interspecific variation of photoreceptors in four co-existing haplochromine cichlid fishes, Brain Behav Evol 45(4) (1995) 232–40. [DOI] [PubMed] [Google Scholar]
- [94].Collin SP, Pettigrew JD, Retinal topography in reef teleosts. I. Some species with well-developed areae but poorly-developed streaks, B ain Behav Evol 31(5) (1988) 269–82. [DOI] [PubMed] [Google Scholar]
- [95].Carleton KL, Parry JW, Bowmaker JK, Hunt DM, Seehausen O, Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia, Mol Ecol 14(14) (2005) 4341–53. [DOI] [PubMed] [Google Scholar]
- [96].Levine JS, MacNichol EF Jr., Kraft T, Collins BA, Intraretinal distribution of cone pigments in certain teleost fishes, Science 204(4392) (1979) 523–6. [DOI] [PubMed] [Google Scholar]
- [97].van der Meer HJ, Visual resolution during growth in a cichlid fish: a morphological and behavioural case study, Brain Behav Evol 45(1) (1995) 25–33. [DOI] [PubMed] [Google Scholar]
- [98].Dobberfuhl AP, Ullm nn JF, Shumway CA, Visual acuity, environmental complexity, and social organization in African cichlid fishes, Behav Neurosci 119(6) (2005) 1648–55. [DOI] [PubMed] [Google Scholar]
- [99].Maan ME, Hofker KD, van Alphen JJ, Seehausen O, Sensory Drive in Cichlid Speciation, Am Nat 167(6) (2006). [DOI] [PubMed] [Google Scholar]
- [100].Smith AR, Ma K, Soares D, Carleton KL, Relative LWS cone opsin expression determines optomotor thresholds in Malawi cichlid fish, Genes, brain, and behavior 11(2) (2012) 185–92. [DOI] [PubMed] [Google Scholar]
- [101].Neumeyer C, Tetrachromatic color vision in goldfish: evidence from color mixture experiments, Journal of Comparative Physiology A 171 (1992) 639–649. [Google Scholar]
- [102].Pignatelli V, Champ C, Marshall J, Vorobyev M, Double cones are used for colour discrimination in the reef fish, Rhinecanthus aculeatus, Biology letters 6(4) (2010) 537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cheney KL, Newport C, McClure EC, Marshall NJ, Colour vision and response bias in a coral reef fish, J Exp Biol 216(Pt 15) (2013) 2967–73. [DOI] [PubMed] [Google Scholar]
- [104].Escobar-Camacho D, Taylor MA, Cheney KL, Green NF, Marshall NJ, Carleton KL, Color discrimination thresholds in a cichlid fish: Metriaclima benetos, J Exp Biol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Olsson P, Lind O, Kelber A, Chromatic and achromatic vision: parameter choice and limitations for reliable model predictions, Behavioral Ecology 29(2) (2018) 273–282. [Google Scholar]
- [106].Champ CM, Vorobyev M, Marshall NJ, Colour thresholds in a coral reef fish, Royal Society open science 3(9) (2016) 160399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cheney KL, Green NF, Vibert AP, Vorobyev M, Marshall NJ, Osorio DC, Endler JA, An Ishihara-style test of animal colour vision, J Exp Biol 222(Pt 1) (2019). [DOI] [PubMed] [Google Scholar]
- [108].Nandamuri SP, Dalton BE, Carleton KL, Determination of the Genetic Architecture Underlying Short Wavelength Sensitivity in Lake Malawi Cichlids, J Hered 108(4) (2017) 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].O’Quin KE, Schulte JE, Patel Z, Kahn N, Naseer Z, Wang H, Conte MA, Carleton KL, Evolution of cichlid vision via trans-regulatory divergence, BMC evolutionary biology 12 (2012) 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sandkam BA, Campello L, O’Brien C, Nandamuri SP, Gammerdinger WJ, Conte MA, Swaroop A, Carleton KL, Tbx2a modulates switching of RH2 and LWS opsin gene expression, Mol Biol Evol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Schulte JE, O’Brien CS, Conte MA, O’Quin KE, Carleton KL, Interspecific Variation in Rx1 Expression Controls Opsin Expression and Causes Visual System Diversity in African Cichlid Fishes, Mol Biol Evol 31(9) (2014) 2297–308. [DOI] [PubMed] [Google Scholar]
- [112].Tsujimura T, Hosoya T, Kawamura S, A single enhancer regulating the differential expression of duplicated red-sensitive opsin genes in zebrafish, PL S genetics 6(12) (2010) e1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tsujimura T, Masuda R, Ashino R, Kawamura S, Spatially differentiated expression of quadruplicated green-sensitive RH2 opsin genes in zebrafish is determined by proximal regulatory regions and gene order to the locus control region, BMC Genet 16 (2015) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mackin RD, Frey RA, Gutierrez C, Farre AA, Kawamura S, Mitchell DM, Stenkamp DL, Endocrine regulation of multichromatic color vision, Proc Natl Acad Sci U S A 116(34) (2019) 16882–16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Carleton KL, Escobar-Camacho D, Stieb SM, Cortesi F, Marshall NJ, Seeing the rainbow: Mechanisms underlying spectral sensitivity in t l ost fishes, J Exp Biol in press (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].West-Eberhard MJ, Developmental plasticity and the origin of species differences, Proceedings of the National Academy of Sciences of the United States of America 102 Suppl 1 (2005) 6543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Temple S, Hart NS, Marshall NJ, Collin SP, A spitting image: specializations in archerfish eyes for vision at the interface between air and water, Proc Biol Sci 277(1694) (2010) 2607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fernald RD, Wright SE, Growth of the visual system in the African cichlid fish, Haplochromis burtoni. Accommod tion, Vision Res 25(2) (1985) 163–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


