Abstract
The rate of translational effort of nanomedicine requires strategic planning of nanosafety research in order to enable clinical trials and safe use of nanomedicine in patients. In this communication, we will discuss the experiences that have emerged based on the safety data of classic liposomal formulations in the space of oncology, as well as describing the new challenges that need to be addressed according to the rapid expansion of nanomedicine platform beyond liposomes. It is valuable to consider the combined use of predictive toxicological assessment supported by deliberate investigation on aspects such as ADME (absorption, distribution, metabolism, and excretion) and toxicokinetic profiles, the risk that may be introduced during nano manufacture, unique nanomaterial properties, non-obvious nanosafety endpoints, etc. These efforts will allow the generation of IND (investigational new drug)-enabling safety data that can be incorporated into a rational infrastructure for regulatory decision-making. Since the safety assessment relates to nanomaterials, the investigation should cover the important physicochemical properties of the material that may lead to hazard when nanomedicine product is utilized in humans.
1. Introduction
The smart design and manufacture of engineered nanomaterials (NMs) is a major scientific achievement that impacts multiple areas, including biomedical and pharmaceutical fields in both research and industry [1–8]. Safety assessment is an integral component of any new technology or pharmaceutical product, and nano-enabled products are no exception [5, 9–13]. This notion is in agreement with a US Food and Drug Administration (FDA) draft guideline “Drug Products, Including Biological Products, that Contain Nanomaterials Guidance for Industry”, which includes statements such as, “FDA does not address, or presuppose, what ultimate regulatory outcome, if any, will result for a particular drug product that contains nanomaterials”; and, “Current [FDA] guidance documents and requirements for the evaluation and maintenance of quality, safety, and efficacy, apply to drug products containing nanomaterials” [14]. From a safety perspective, important considerations for biomedical NMs should include the complexity of NM structure, the mechanism by which NMs’ physicochemical properties impact biological effects in the clinical setting, physical/chemical stability, etc. Based on previous knowledge of nanotoxicology (nano-Tox) and nano environmental health and safety (EHS), it is necessary to contemplate the leveraged use of advanced strategies such as predictive toxicology, high throughput screening (HTS), nanomaterial library, multi-omics approach, adverse outcome pathways, in silico analysis and data mining, and “safe-by-design” in the context of safety assessment and safe use of nanomedicine in the clinic. It is also important to continue the enrichment of the safety knowledge toolbox, i.e. fate and transport, exposure assessment, toxicokinetics and biodistribution, active pharmaceutical ingredient (API)-specific toxicity, and process conditions and production scales, which are not fully covered, or even overlooked at times, in the existing nanotoxicology infrastructure. Therefore, the earlier we start gathering expertise and knowledge for the next-phase of nanosafety research, the more prepared we will be in dealing with the challenges of new nanomedicines that are being developed. In this short communication, we will focus on a major category of nanomedicine, i.e. anti-cancer drug nanocarriers, in order to discuss the critical role of nanosafety research in advancing nano drug delivery systems into clinic.
General perception of FDA-approved liposomal nanocarriers and their safety profile
Liposomes used for cancer drug delivery are generally 50~300 nm vesicles formed by one or more bilayers of natural or synthetic lipids [15]. While this type of nanocarrier has been studied in depth, it continues to constitute a field of robust research, leading to the most successful nanocarriers in terms of clinical translation [16–20]. Up to now, there have been ~2,400 clinical studies involving liposomes, ~1,800 of which are cancer-related (searched on clinicaltrials.gov in January 2020). Beginning from the prior knowledge on the FDA-approved liposomes, we will use Doxil® (for doxorubicin delivery), Onivyde® (for irinotecan delivery), and Vyxeos® (for cytarabine and daunorubicin co-delivery) to exemplify the preclinical and clinical observations on their safety profiles.
Liposomal doxorubicin (Doxil®)
Doxil® the first FDA-approved nano formulation for use in cancer in 1995, is a doxorubicin (DOX) hydrochloride liposome formulation with a diameter of ~85 nm and lipids composed of hydrogenated soy phosphatidylcholine (HSPC), cholesterol (Chol), and polyethylene glycol (PEG, Mw ~2,000) conjugated phospholipid (DSPE-PEG2k) (Fig. 1A) [21]. After its first approval for treating AIDS-related Kaposi’s sarcoma, it was further approved to treat ovarian cancer and multiple myeloma [21]. Three important principles are attributed to the unique Doxil® formulation, namely (i) use of surface PEGylation to prolong drug circulation time and avoid reticuloendothelial system (RES) clearance, (ii) use of a transmembrane ammonium sulfate gradient to achieve a high and stable remote loading of DOX by forming a crystal drug precipitate (Fig. 1A, cyroEM), and (iii) use of high transition temperature (Tm = 53 °C) phosphatidylcholine and cholesterol to make the liposome lipid bilayer in a stable “liquid ordered” phase [21].
Figure 1.
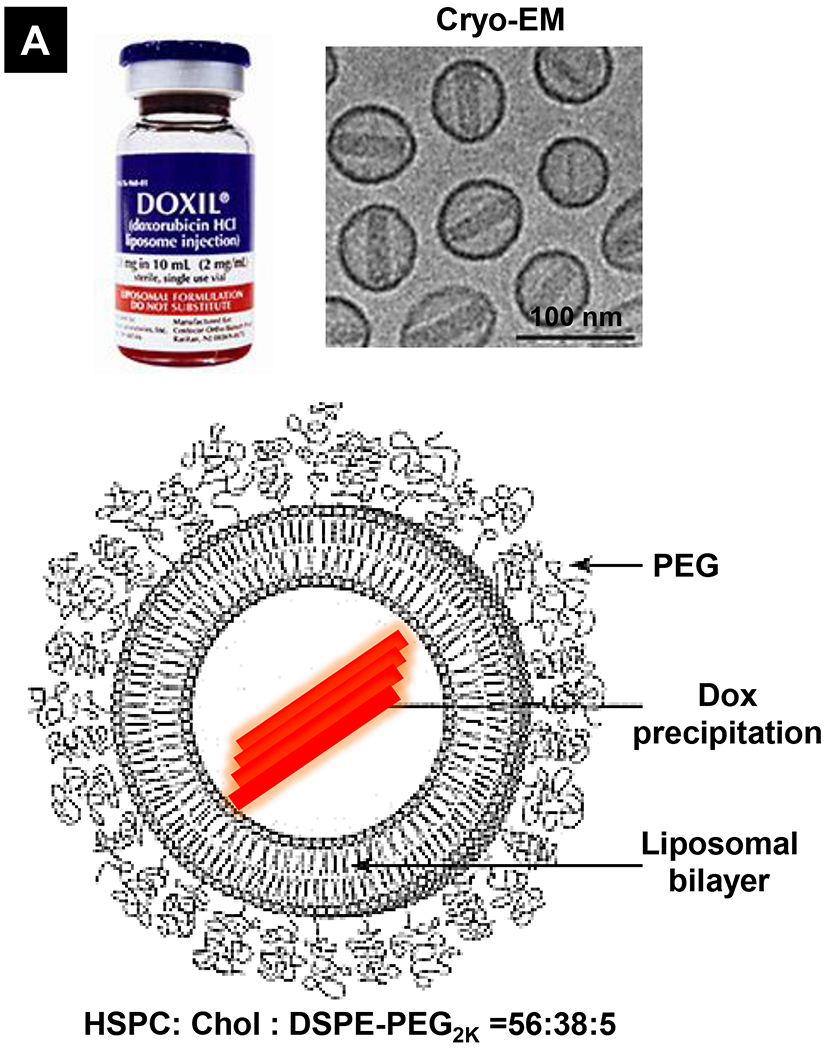
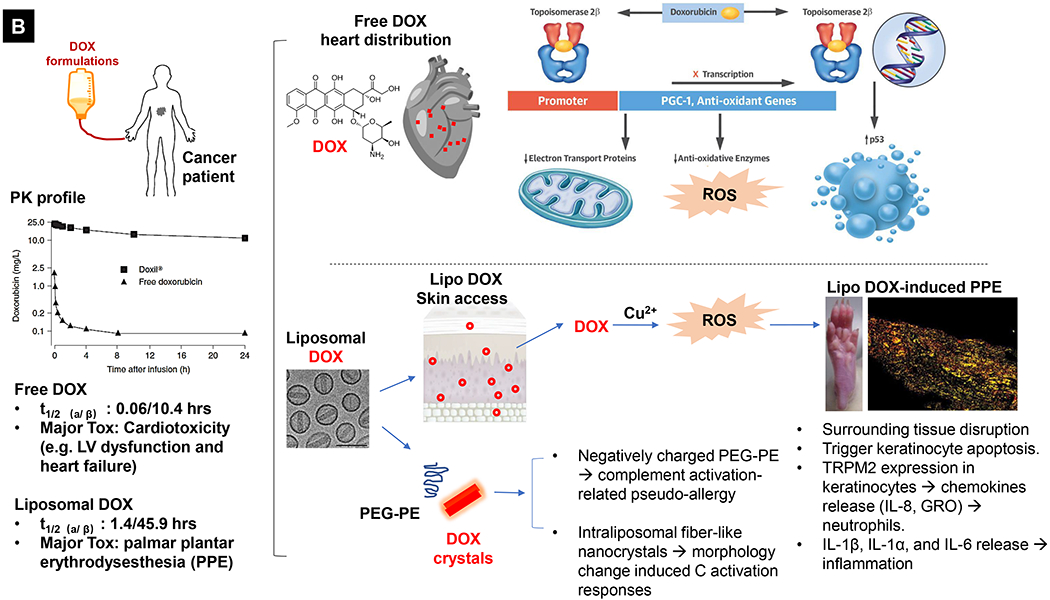
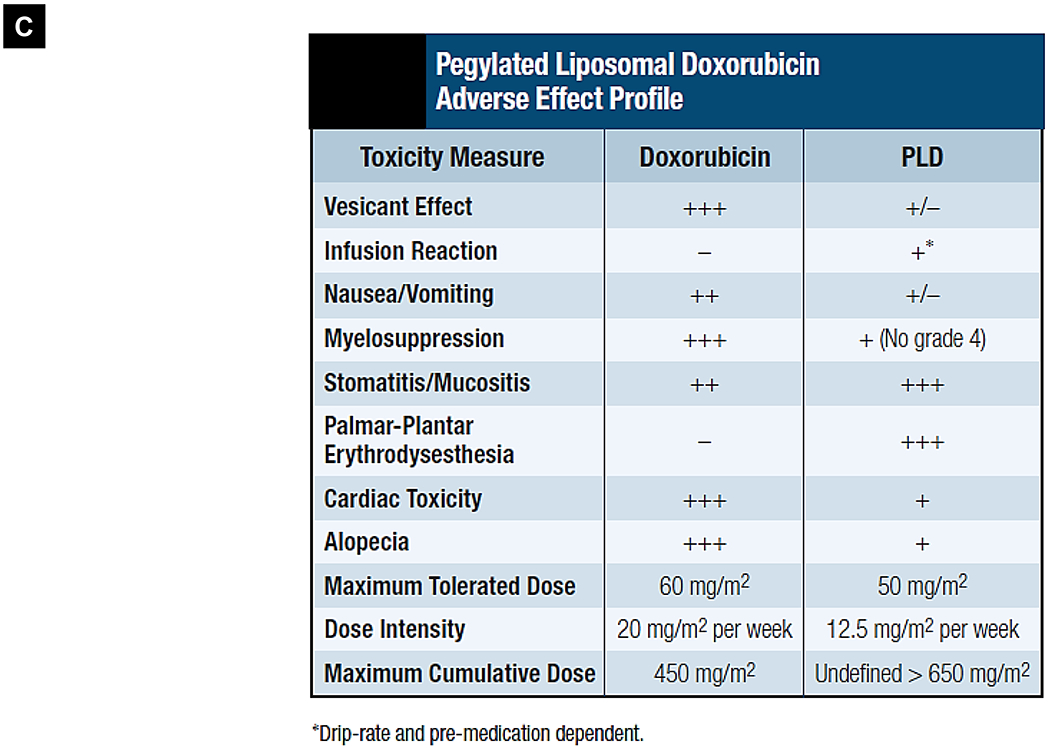
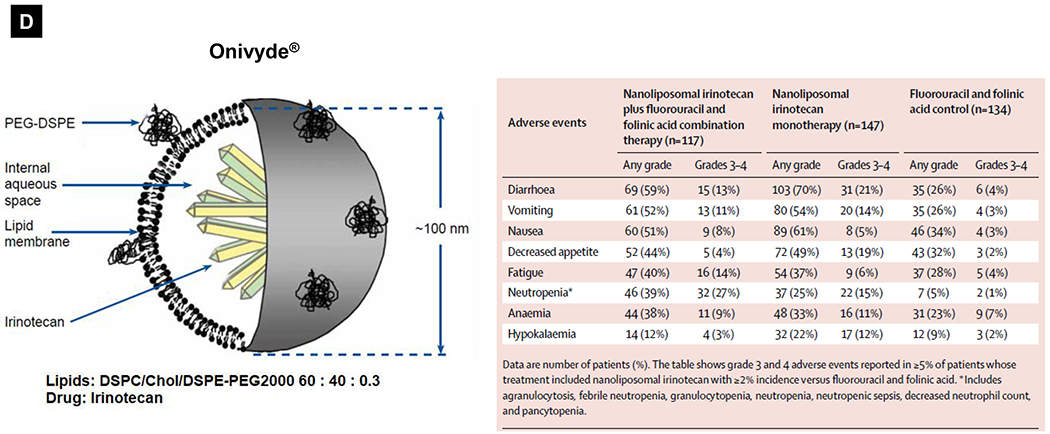
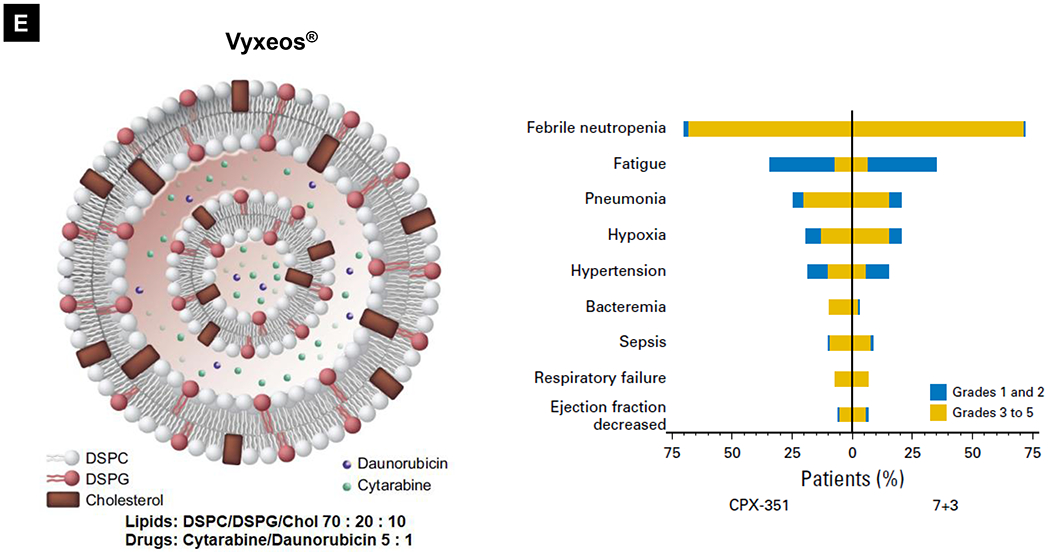
Comparative demonstration of free DOX and Doxil®’s PK/biodistribution, targeted organ and safety features. (A) Appearance, cryoEM image and scheme of Doxil® formulation. HSPC: hydrogenated soy phosphatidylcholine, Chol: cholesterol, DSPE-PEG2K:1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]. Reproduced with permission.[21] Copyright 2012, Elsevier. (B) Pharmacokinetics (PK) profiles and main cardiotoxicity and palmar plantar erythrodysesthesia (PPE) effects/mechanism of doxorubicin and Doxil® formulation. Reproduced with permission. [21, 23, 34, 37] Copyright 2012, Elsevier. Copyright 2003, Springer Nature. Copyright 2017, Elsevier. Copyright 2013, Springer Nature. (C) Clinical adverse effect profile of pegylated liposomal doxorubicin. Reproduced with permission.[24] Copyright 2008, Elsevier. (D) Scheme and clinical adverse effect profile of liposomal irinotecan Onivyde®. Reproduced with permission. [48, 60] Copyright 2014, Elsevier. Copyright 2016, Elsevier. (E) Scheme and clinical adverse effect profile of cytarabine and daunorubicin co-delivery liposome Vyxeos®. Reproduced with permission.[64, 71] Copyright 2018, Future Medicine Ltd. Copyright 2018, American Society of Clinical Oncology.
Generally, Doxil® demonstrated more favorable toxicity profiles than did non-encapsulated anthracyclines, such as improved cardiac safety feature, and less myelosuppression, alopecia, nausea, and vomiting [21–24]. This trend was quantitatively reflected in a meta-analysis in which 2,220 patients were included for comparison (1,112 liposome-treated versus 1,108 conventional anthracycline-treated patients), demonstrating that liposomal formulations have lower incidences of congestive heart failure (OR 0.34, 95% CI, 0.24~0.47) among other safety benefits [25]. As one of the most long-circulating liposomes [23, 26], the contrast safety feature of free DOX versus Doxil® is mainly due to the altered tissue distribution and pharmacokinetic (PK) profile (Fig. 1B, left). It was demonstrated that intravenously (IV) injected liposomes cannot easily escape from the tight capillary junctions in normal tissues, including heart muscle and gastrointestinal tract [27]. This is in contrast to free drug, which when infused, deploys deep tissue penetration of the drug and causes major cardiotoxicity through multiple mechanisms evidenced in a variety of experimental models, such as reactive oxygen species (ROS)-induced mitochondrial damage, elevated DNA damage, inhibition of cardiomyocyte specific genes, induction of cardiomyocyte apoptosis, and triggering of myofiber degeneration [28, 29]. In the setting of cancer patients, DOX treatment was more commonly associated with myocyte damage, leading to left ventricular dysfunction and heart failure, some of which were fatal [30–32]. The main mechanism is now believed to be through inhibition of topoisomerase 2β resulting in activation of cell death pathways and inhibition of mitochondrial biogenesis (Fig. 1B, upper right) [33, 34]. Therefore, for patients who are at increased risk of cardiotoxicity, Doxil® is an important alternative therapeutic option [21, 24, 35]. Use of Doxil®, however, does not necessarily mean that DOX’s toxicity is eliminated. In fact, new side effects were reported (Fig. 1C) [23, 24, 36]. The main dose limiting adverse effect is palmar plantar erythrodysesthesia (PPE), a.k.a. hand-foot syndrome (redness, inflammation, and sometimes blisters on the palms of the hands and/or the soles of the feet), which may be explained by long-circulating liposomes that accumulate in large amounts in various healthy and susceptible tissues such as skin [21, 23, 24, 36]. Fig. 1B (lower right) reveals the possible mechanisms of PPE induced by liposomal DOX. Molecular toxicology studies demonstrate the critical role of Cu2+ ions in the skin, which in turn, play a key role in ROS generation in the skin. This leads to skin tissue disruption including keratinocyte apoptosis, release of chemokines (IL-8, GRO, Fractalkine), and induction of inflammatory status (through the release of IL-1β, IL-1α, and IL-6) [37] (Fig. 1B, lower right). So far, the best way to overcome this effect is to increase the time interval between treatments, but this may lower the available maximal tolerated dose (MTD), resulting in decreased antitumor potency [21, 24, 36]. Another side effect related to Doxil® is infusion-related immediate hypersensitivity reactions (HSRs) that show up as flushing and shortness of breath [21, 24, 38]. These reactions are a type of complement activation-related pseudo-allergy which may be induced by the interaction with negatively charged PEG-PE in the presence of in Doxil® [24, 38, 39]. It was also shown that the morphology change of liposomes from spherical to non-spherical (oval-like) after forming the intraliposomal fiber-like drug crystals may contribute to the adverse events in patients, presumably owing to its low deformability [21, 40, 41]. Nevertheless, unlike life-threatening cardiotoxicity, PPE and HSRs due to Doxil® are manageable and frequently reversible with appropriate supportive care measures [35, 42–44]. Here, it is also necessary to mention Myocet®, a non-pegylated liposomal DOX that is approved for the treatment of metastatic breast cancer in combination with cyclophosphamide in Europe and Canada [45, 46]. Unlike Doxil®, the Myocet® liposome without PEG coating has been shown to have much lower risk of inducing Hand-Foot Syndrome [25, 45].
Liposomal Irinotecan (Onivyde®)
In addition to Doxil®, there are other liposomes that have been approved in the field of oncology [16–20]. Onivyde®, also known as MM-398 or PEP02, is a liposomal irinotecan injection approved by the FDA in 2015. These liposomes with a diameter of −100 nm are composed of distearoylphosphatidylcholine (DSPC), Chol, and DSPE-PEG2k (60: 40: 0.3 in molar ratio) bilayers with high loading of drug crystals or precipitates (Fig. 1D) [47, 48]. Currently, Onivyde® is indicated (in combination with fluorouracil and leucovorin but not as a single agent) for the treatment of patients with metastatic pancreatic ductal adenocarcinoma (PDAC) after disease progression following gemcitabine (GEM)-based therapy. In the setting of PDAC, irinotecan is a key component of FOLFIRINOX therapy (a 4-drug regimen that includes 5-FU, oxaliplatin, and irinotecan) [49]. While FOLFIRINOX is more clinically effective than GEM, this regimen is far more toxic, and is therefore restricted to select advanced stage PDAC patients with good functional status [49]. Irinotecan contributes in a major way to FOLFIRINOX toxicity due to effects on the bone marrow (e.g., ~70% incidence of neutropenia) and the gastrointestinal tract (GIT) (e.g., vomiting, diarrhea, nausea, anorexia in ~70% of patients) [49, 50]. In order to address this toxicity while maintaining therapeutic efficacy, liposomal encapsulation of irinotecan has been developed, e.g., (i) the MM-398 formulation, a liposome that stably incorporates irinotecan hydrochloride with the aid of a polyanionic trapping agent (triethylammonium sucrose octasulfate, TEA8SOS) [46], and (ii) liposomes that use active irinotecan loading through the generation of transmembrane proton gradients with an ionophore (A23187), ammonium sulfate, etc [51–56]. Similar to Doxil®, Onivyde® (although a lower percentage of PEG was used) still remains more advantageous in PK profiles. A much slower clearance, significant prolonged terminal t1/2 of circulating total irinotecan, and more favorable PK of its active metabolite SN-38 were observed in patients who received Onivyde® compared to free drug-treated patients [57, 58]. The dose-normalized AUC0-∞ value of active SN-38 was ~5x higher for Onivyde® than that of free irinotecan [57, 58]. Although great toxicity reduction with >4-fold increased maximum tolerated dose (MTD) was demonstrated by liposomal irinotecan compared to free drug in mice [47], there were no significant differences among the main adverse events observed in clinical trials [57, 59]. The grade 3 or 4 adverse events that occurred most frequently in the Onivyde® plus 5-FU/LV were neutropenia (27%), diarrhea (13%), vomiting (11%), and fatigue (14%) (Fig. 1D)[60]. While irinotecan liposomes could achieve high irinotecan loading capacity, instability of liposomal nanocarriers under shear and osmotic stress from bloodstream conditions, as well as bilayer disruption by serum proteins, have been shown to result in toxicity due to premature drug release[61–63]. We surmise that this could explain the frequently observed GIT and bone marrow toxicity, including the “black box warning” for life-threatening diarrhea and neutropenia of patients receiving Onivyde® treatment. Interestingly, there were no reports of Onivyde®-related hand-foot syndrome side effects in this study, which can be associated with irinotecan and Doxil® therapy [60].
Ratiometric cytarabine and daunorubicin co-delivery liposome (Vyxeos®)
The majority of FDA-approved liposome carriers deliver one active pharmaceutical ingredient (API). The recent success of Vyxeos® (also known as CPX-351 and carries cytarabine and daunorubicin at a 5:1 molar ratio, Fig. 1E) shows the impact of nano-enabled drug synergy for acute myeloid leukemia (AML) management [64] The classic drug combination was frequently tested by adding one drug at incremental doses to the recommended dose of the other until the aggregate toxicity became dose-limiting [65]. A key advantage of ratiometric co-encapsulation is the ensured in vivo drug synergy through synchronized PK/biodistribution profile, which turns out to be essential because the extent of synergy (or antagonism) frequently depends on the ratio and concentrations of APIs in the mixture. Another advantage is to avoid the maximal dose of each drug, serving as an important safeguard from the side effect reduction perspective. In a bone marrow (BM) P388 leukemia tumor model, it was demonstrated that the CPX-351 liposomes could not only maintain the synergistic drug ratio in plasma but also in BM, which exhibited superior therapeutic activity compared to free-drug cocktails [66]. In another BM-engrafting CCRF-CEM leukemia model, it was shown that CPX-351 liposomes had superior activity against BM leukemia cells than the free-drug cocktail [67]. The drug distribution results also demonstrated that drug concentrations were selectively and markedly elevated for liposome formulation over free-drug cocktail in leukemia-laden BM [67]. Interestingly, it was demonstrated that the CPX-351 formulation, as compared to free drug combination, showed enhanced selective in vitro cytotoxicity for AML cells rather than normal progenitors from patients [68]. These pharmacologic advantages, preferential uptake of liposomes by leukemia cells but reduced drug exposure in off-target tissues, may explain the robust efficacy and manageable safety profile of Vyxeos® observed in clinical trials [69, 70]. The Vyxeos® liposome significantly improved median overall survival versus the standard-of-care cytarabine plus daunorubicin chemotherapy (a.k.a. “7+3”), i.e. 9.56 versus 5.95 months (P = 0.003). Moreover, there is limited information on the toxicity mechanism for Vyxeos®, which was only approved in 2017. In fact, the side effect profile was comparable between co-delivery Vyxeos® and free drug mixture in patients (Fig. 1E)[71]. However, it is necessary to consider toxicity as a consequence. When ratiometric co-delivery is used because of the deliberately-optimized drug synergy that hypothetically ensures the in vivo high potency (killing effect), this may exert unexpected adverse effects in the normal organs.
What do we learn from these approved liposomes? Ample evidence shows that the safety profiles of liposomal nanocarriers is structure-dependent. Along the technology maturation process, we learn that the quality, efficacy, and safety of a liposomal carrier (and drug products containing nanomaterials; see below) can, however, be very sensitive to the manufacturing process [72]. Important considerations should include lipid composition, size, lamellarity, charge, drug loading approach, drug loading condition (feed ratio, temperature, and time), type/concentration of trapping agent, intraliposomal osmotic pressure and pH, drug localization (interior, surface attached, or lipid incorporation), drug release profile, w/wo targeting design, etc. In the case of Doxil®, the main component HSPC has a high Tm. Thus, making the liposome membrane in a gel state below this Tm prevents drug diffusion through the bilayer at the human body temperature [21]. Another popular design is the use of a trapping agent, such as ammonium sulfate for DOX or TEA8SOS for irinotecan. To do this, liposomes are usually prepared at the desired concentration of trapping agent. In the case of Doxil®, after removing the free ammonium sulfate from the external liposome medium, intraliposomal NH4+ dissociates into NH3, which then diffuses out from the liposome, leading to a lower pH aqueous phase inside of the liposome as the H+ ions are retained by the bilayer. The weak base non-ionized form of DOX can pass through the lipid bilayer at temperature above Tm, and become ionized upon entering the liposome. The cationic form further complexes with the SO42− anions to form long and fiber-like salt crystals. Doxil’s success (including safety feature) is partially due to the trapping agent’s triad effect: reduced premature DOX release, minimized intraliposomal osmotic pressure issue, and augmented loading capacity and efficiency. This highlights the necessity of a critically integrated nano manufacture and high standard quality control (QC) criteria. This serves as the basis of “nano Chemistry Manufacturing and Controls” (nano-CMC), which may generate profound impact on nanosafety.
Consideration beyond liposomes - New challenges for the next stage nano-Tox research in nanomedicine
There is a large number of research labs and startups working on a wide variety of nano-enabled solutions in medicine, creating job opportunities, fund-raising, Investigational New Drug (IND) filing, and clinical trials [73, 74]. A keyword search of “nano” in the clinical.gov database produced 282 results/records (searched in January 2020). Approximately one third of the clinical records are cancer-related. From the pipeline perspective, ample evidence showed that the expedited developmental work leads to the versatile utilization of biomedical NMs, beyond just liposomes. This includes polymer[75–81], metal and metal oxides[82–84], carbonaceous materials[85], silica-based materials[86], two-dimensional nanostructures[87, 88], up-conversion materials[89], metal organic framework[90], etc [16–18, 90–92]. An elevated degree of unpredictability in biocompatibility and prospective safety issues, however, created new challenges along their translational route. The level of sophistication demands a paradigm shift from understanding classic toxicology (“what happens” approach) to how mechanistic nano/bio interactions can facilitate hazard identification and risk assessment, ultimately leading to a safe-by-design nanomedicine (Fig. 2). Spurred on by the ~15 years of development in nanotoxicology and nano EHS, it is a practical approach to consider the existing experimental strategies, such as physicochemical characterization [93, 94], high-throughput and high-content screening [95, 96], nano quantitative structure-activity relationship (QSAR) [95, 97–100], and understanding of nano-associated molecular patterns (NAMP) and adverse outcome pathways (AOP) [101–103], with an agenda to address the safety issue in the clinic (also see Fig. 4). These components serve as the basis of “predictive toxicology,” which was advocated as a paradigm shift for the assessment of nanomaterial hazards [100, 104–107]. The existing assays, which were summarized in Fig. 2, cover a decent amount of mechanistic endpoints that are frequently involved in NMs’ safety, such as oxidative stress, particle dissolution, surface membrane injury, lysosomes, inflammasomes, autophagy activation/blockade, mitochondria damage, pro-inflammatory response, etc [104, 107, 108]. The most efficient approach that could reasonably address the complexity of nanomaterials is to use mechanistic cellular injury responses that can be linked to disease pathogenesis (Fig. 3). Quantitative study of these cellular toxicological endpoints would allow HTS to be used for in vivo safety prediction, thereby limiting animal use as the primary test platform (Fig. 2). At this point, the examples of mechanistic injury include heavy metal ion shedding (metal and metal oxide) [109–112], membrane damage (cationic nanoparticles) [113–119], lysosome damage (long aspect ratio NMs) [120–124], cell membrane disruption (NMs with reactive surface) [125–130], bio-transformation (rare earth NMs) [131–133], etc (Fig. 3).
Figure 2.
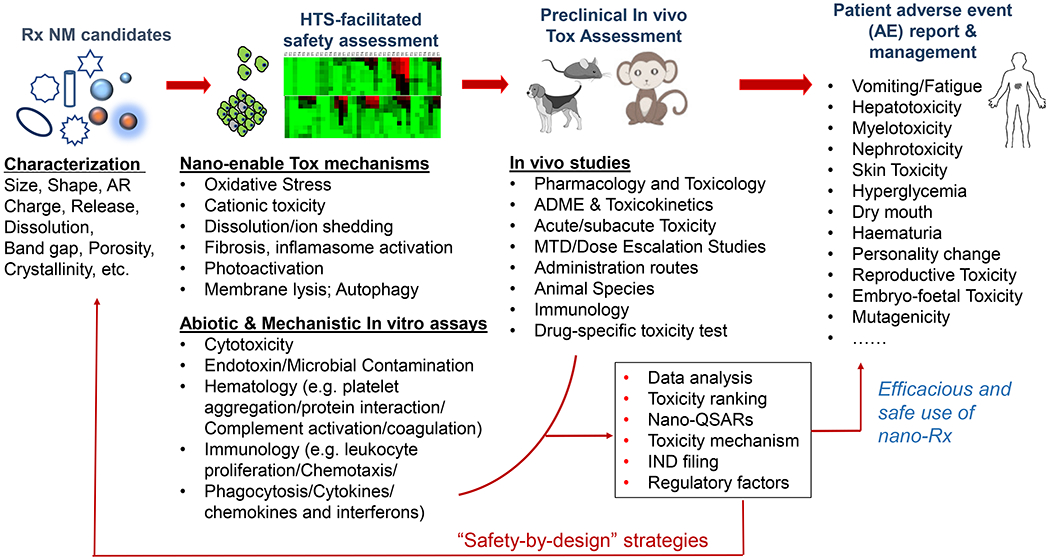
Overall blueprint of safety assessment of nanomedicine. Therapeutic nanomaterials of various chemical compositions demand comprehensive characterization, such as size, morphology, charge, surface area, porosity, crystal structure, API concentration, release profiles, etc. It is necessary to consider HTS assays and response readouts in cells to obtain mechanistic toxicity insight with a view to plan and prioritize the animal assessments for the IND filing. The in vivo toxicity assessment should be designed and implemented with necessary consideration on ADME, toxicokinetics, administration routes, and acute vs chronic toxicity, which may not be fully covered in the in vitro assays. The output of these assays needs to be analyzed to facilitate the decision-making with respect to the technology translation. If necessary, a safe-by-design approach could be helpful to improve nanomedicine’s safety features. The preclinical data can be used to as a reference for the clinical safety assessment, i.e. patient adverse event report and management, for the efficacious and safe use of nanomedicine in patients.
Figure 4.

Critical research aspects that are required to strengthen nanomedicine nanosafety investigation.
Figure 3.
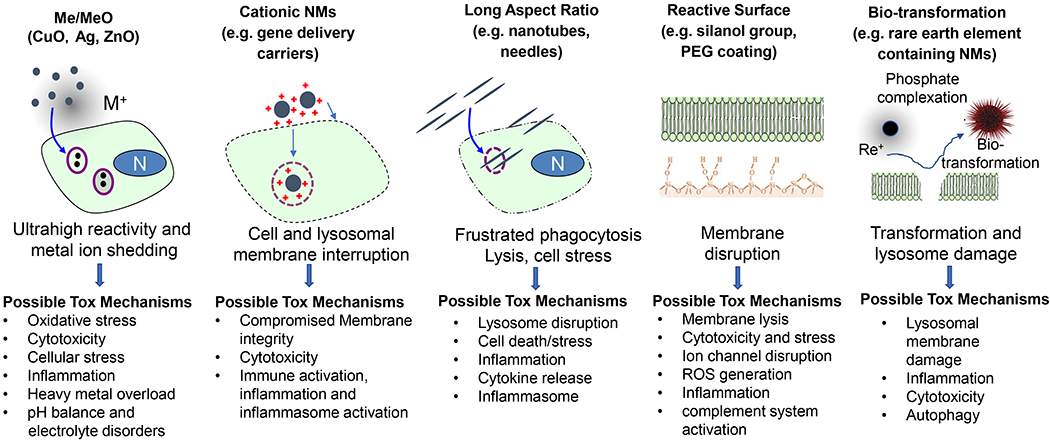
Mechanistic injury responses that are frequently involved in nanomaterials. This includes dissolution and release of toxic metal ions from metal or metal oxide nanoparticles, cationic injury to surface membrane and organelles, frustrated phagocytosis and inflammasome activation by long aspect ratio nanomaterials, reactive surface that may lead to membrane disruption, and bio-transformation response in rare earth nanoparticles.
The question then becomes whether or not the existing nano EHS and nanotoxicology knowledge is well-developed enough for nanomedicine’s safety assessment. While useful, the existing knowledge needs to be further incorporated into a rational infrastructure for further data generation and regulatory decision-making, premised on the use of quantitative assessment of nanotoxicological mechanisms that are linked to acute and chronic side effects and adverse effects in patients. Despite the promise of leveraging a mechanistic approach for nanomedicine safety, new studies with a view to facilitate IND filing and comprehensive safety assessment in patients are still needed. We believe the next phase of research should pay extra attention to the following aspects (Fig. 4):
The consideration of absorption, distribution, metabolism, excretion (ADME), and toxicokinetics profiles of NMs and APIs is required. New safety issues may emerge since nano-Rx may enhance the delivery of payload molecules to certain tissues in which the free drug has limited access.
Quality, safety, and efficacy of nano-Rx that are very sensitive to manufacturing conditions, i.e. correlative study of nano-CMC and nano-safety.
Consideration of unique (non-obvious) NM’s properties that determine critical nanosafety issue, such as reactive surface, irregular shape and long aspect ratio, bio-transformative composition, etc.
Use of standardized safety assessment approach (i.e. ISO guidelines, SOPs, and engage a CRO entity).
Plan and implement effective IND-enabling toxicology study for nano-Rx.
Use of multi-omics approaches to reveal non-obvious safety-related observation of nano-Rx.
Predictive nano-Rx safety and biomarker-guided safe use of nano-Rx.
Nano adverse event management in patients.
Nanomedicine that may interact with classic drugs in human.
Development of new experimental models with augmented predictive value for rapid capture of nanomedicine safety profile (i.e. lab-on-a-chip, organ-on-a-chip approaches).
Limitation of pre-clinical nanosafety assessment. While findings in animal safety data are generally applicable to patients, the responses of animals and humans may differ. This disparity has been attributed to many factors, i.e. different levels of cytochrome P450s and detoxification enzymatic system. In terms of dose, use of body weight for linear dosimetry extrapolation frequently leads to less accurate animal-human conversion, while allometric scaling on the basis of body surface area is generally believed more accurate. Moreover, animal studies cannot reveal subjective effects such as headache, dizziness, nausea, or mental effect, which frequently involve idiosyncratic reactions and inter-individual responses in a complex real-life patient population.
In our opinion, there are no simple solutions to nanomedicine’s safety assessment during the translation phase. It is likely to be case-by-case. It should fit into the existing infrastructure governed by the FDA. At this stage, the combined use of predictive toxicology supplemented with ADME and toxicokinetics analyses could be a reasonable approach. From the feasibility aspect, implementation of physicochemical characterization plus HTS predictive toxicity profiling will facilitate important decision-making and determine the scope (and the focus) of the safety assessment in vivo. For nanotherapeutics, it is important to address the administration route and exposure likelihood with the view to understand toxicokinetics profile, ADME, and targeting of specific organs. Depending on the mechanism and degree of potential toxicity, it is also important to use appropriate animal models [134, 135]. For example, the spontaneously hypertensive rat and the beagle dog are considered the most suitable small and large animal models, respectively, to use for anthracycline cardiotoxicity [136–138]. The rat model is regarded to be more sensitive than mice in predicting both chemotherapy and targeted therapy induced GIT toxicity in humans [139–141]. Beyond liposomes, we want to mention inorganic nanoparticles that have emerged as new therapy. A frequent safety concern was raised in the category of the inorganic nanoparticles because they are generally thought to be more difficult to degrade and to be cleared out than organic particles like liposomes and micelles. While this could be true, it may also just be a stereotype perception because nanomaterial properties are obviously more complicated than “inorganic” or “organic”.
There are multiple successful inorganic nanoparticles that have moved into clinical studies[91, 142], such as C-dots (a silica particle for imaging and treatment purpose)[86], metal-organic frameworks (MOFs) [143–146], nanoscale coordination polymers (NCPs) [147–149] and gold nanoparticles [150–153]. We want to discuss a gold-silica nanoshells (GSNs or AuroShell, 155 nm in diameter with a coating of polyethylene glycol 5000) (Fig. 5A) in more detail [154]. This platform recently passed a clinical pilot device study for local photothermal ablation of prostate tumors [152]. The dielectric silica/gold shell structure was designed to absorb light at wavelengths of high tissue transparency (principally at near infrared (NIR) wavelengths ~ 800 nm), which can convert absorbed NIR light to heat and induce highly localized hyperthermia, resulting in cell death and tumor remission for photothermal cancer therapy [153]. As the therapeutic mechanism of action is physical and not biological or chemical, the AuroShell particles are regulated as a medical device by the FDA [154]. Before clinical trials, the AuroShell particles were comprehensively evaluated for preclinical biocompatibility, toxicity, and biodistribution by both in vitro and in vivo assays. There was no indication of toxicity in a complete good laboratory practice (GLP) compliant International Organization for Standardization (ISO)-10993 biocompatibility program, including cytotoxicity, pyrogenicity, genotoxicity, in vitro hemolysis, intracutaneous reactivity, sensitization, and USP/ISO acute systemic toxicity assays (Fig. 5B) [154]. Impressively, after a single IV injection, the amount of gold in the body did not decrease significantly even at >1 year post infusion (Fig. 5C). The nanoshells are too large to be cleared through standard excretion pathways and are chemically inert. Despite the long-term persistence in the body, a series of studies in mice, Sprague-Dawley rats, and Beagle dogs for time durations of up to 404 days identified no toxicities, lack of tolerance, or immunological effects to these particles [154]. The following safety profile of AuroShell particles in clinical trial was excellent, matching previous preclinical findings where the blood/hematology/urinalysis assays indicated no particles-related toxicity [152]. More and more encouraging clinical safety profiles are being confirmed for emerging inorganic biomedical nanoparticles, such as the ~27 nm polyethylene glycol-coated gold nanoparticles binding recombinant human tumor necrosis factor alpha (rhTNF) (CYT-6091) [150, 151] and the ~6- to 7-nm ultrasmall inorganic hybrid silica nanoparticles (Cornell dots, C dot)[86].
Figure 5.
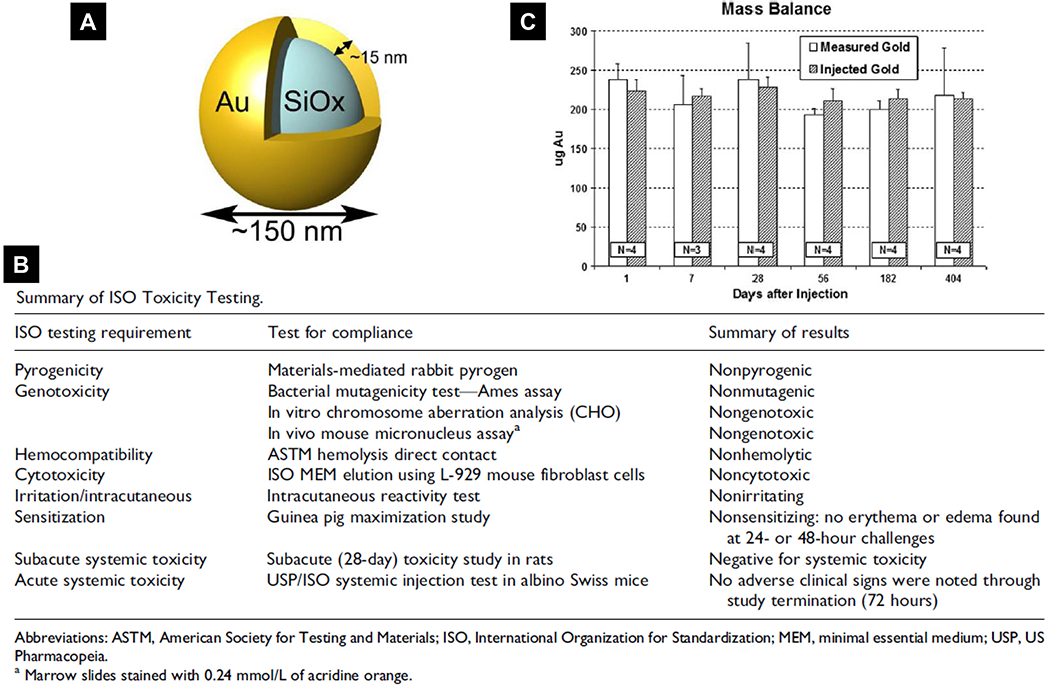
Safety assessment of Auroshell nanoparticles, an example for inorganic biomedical nanomaterials. (A) Scheme of the silica/gold core-shell structure of Auroshell nanoparticle. (B) Preclinical International Organization for Standardization (ISO) toxicological testing performed for Auroshell particles. (C) Mass balance data of mice receiving a single injection of Auroshell particles and sacrificed at the time points indicated. Gold content was detected by Neutron activation analysis (NAA). Reproduced with permission.[154] Copyright 2012, SAGE Publishing.
Here, we also want to illustrate our view by sharing the developmental experiences of a novel nanocarrier, i.e. “silicasome”, for pancreatic cancer (PDAC) treatment (Fig. 6A) [155, 156]. We have developed a mesoporous silica nanoparticle (MSNP) platform to efficiently deliver a protected high-dose PDAC chemotherapy using a supported lipid bilayer (LB) for payload encapsulation (Fig. 6B). The name is given as “silicasome” to distinguish it from liposomes, which contain a non-supported LB. This platform was used to load a highly potent but toxic PDAC cancer drug irinotecan (a key component in the “FOLFIRINOX” regimen; Fig. 6A), which is a weak base that can be imported by a proton-releasing trapping agent, triethylammonium sucrose octasulfate. After experimenting with ~70 reaction conditions in an iterative optimization approach, it is possible to accomplish ~120 g batch sizes of biocompatible MSNPs in ~20 L volume (Fig. 6C) [157]. Since the LB is supported, the improved stability of the silicasome was associated with less API leakage systemically, which was experimentally proven in the case of irinotecan delivery [156]. We have tested our irinotecan silicasome in an orthotopic Kras-derived PDAC model as well as a colorectal cancer (CRC) orthotopic model [157]. The latter model was selected because irinotecan was approved as a monotherapy for CRC management. The data demonstrated that the silicasome delivered significantly increased drug concentrations at the orthotopic tumor sites compared to liposomes (in-house synthesized or commercial irinotecan liposome Onivyde®), leading to superior anticancer effect (including treating metastases) in vivo (Fig. 6D) [156, 157]. Equally important, the reduced leakage and slower rate of drug release by the silicasome carrier dramatically reduced the rate of bone marrow, gastrointestinal, and liver toxicity compared to the liposomal carrier (Fig. 6D) [156, 157]. The superb safety feature also comes from MSNP itself, a platform that is generally devoid of biohazard including previous data with respect to its biocompatibility, biodegradability, and bio-elimination from living animals [158–161]. A mechanisms study shows the safety feature to the profound surface chemistry on the silica [125]. The intrinsic safety of MSNP (and Stöber silica), which are synthesized under low temperature conditions, is due to the lack of high energy and strained siloxane rings. This differs from the silica nanomaterials that are made at high temperature, such as quartz and fumed silica. The latter type of silica nanoparticles frequently leads to toxicity, such as pulmonary toxicity (silicosis) due to surface reconstruction and display of H-bonded silanols [125].
Figure 6.
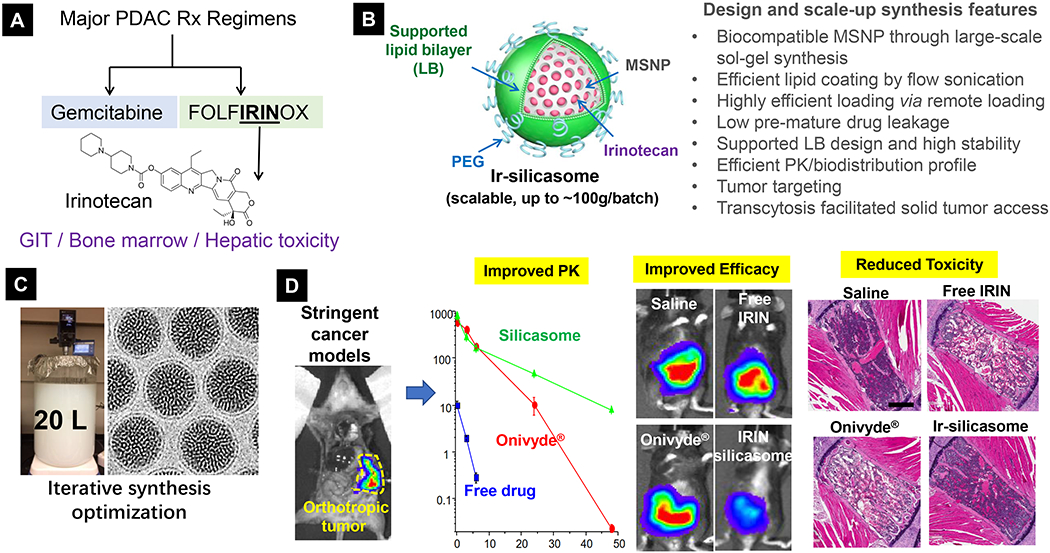
Development of silicasome drug nanocarrier to address irinotecan safety and efficacy for pancreatic and colon cancer treatment. (A) In the setting of PDAC, while FOLFIRINOX is more potent than GEM, this regimen is far more toxic, in which irinotecan contributes in a major way to its toxicity on the bone marrow and the GIT. (B) Scheme and unique characteristics of silicasome nanocarrier. (C) Successful scale-up development of silicasome for translational study. (D) Use of highly stringent orthotopic cancer models to study PK, efficacy and safety features of silicasome nanocarrier. Irinotecan delivery by silicasome led to a major improvement of efficacy and safety over free drug and commercial liposomes for PDAC and colon cancer. In order to study irinotecan’s toxicity in diffident formulations, liver, sternal bone marrow, and intestinal tissues were collected from the mice. Histological examination of these organs showed reduced hepatocyte necrosis and GIT apoptosis. Panel D highlights the H&E staining result of bone marrow. While animals treated with free drug or commercial liposomes generated strong effects on bone marrow damage (i.e. evidenced by ~30% of the space being filled by hematopoietic cells), there was no major reduction in silicasome treated mice. This correlates to peripheral blood neutropenia in patients receiving either free drug or Onivyde®. No neutropenia was observed in mice receiving irinotecan silicasome. Reproduced with permission.[155, 164] Copyright 2017, American Society for Clinical Investigation. All other figures. Copyright 2019, American Chemical Society.
Lastly, we want to briefly mention the safety assessment of non-cancer nanomedicine. The blueprint of safety assessment should be applicable to any nanomaterials irrespective of the indication. However, it is necessary to pay extra attention to API-specific safety endpoints. One example is nano-enabled gene editing in rare genetic diseases. Although the nanocarrier itself could be highly biocompatible[6], the safety feature of the gene editing platform itself (i.e. off-target effect) is still controversia[162, 163].
Conclusions
It is a timely effort to “rethink” the major thrust of nanotoxicology (nanosafety) research. Like traditional toxicology research, it is always relevant to address the safety and hazardous features of emerging new nano substances. In the context of clinical nanomedicine safety, it is necessary to consider the leveraged use of predictive toxicology expertise with a view to correlate the NM properties and manufacture process to its safety features, including the use of safe-by-design approach. Moreover, the existing knowledge needs to be further incorporated into a clinical infrastructure for further data generation and regulatory decision-making, premised on the use of quantitative assessments of nanotoxicological mechanisms that are linked to acute and chronic side effects and adverse effects in patients.
Acknowledgements
This work was supported by the U.S. Public Health Service Grant, 1U01CA198846.
References
- [1].Chen HB; Gu ZJ; An HW; Chen CY; Chen J; Cui R; Chen SQ; Chen WHH ; Chen XS; Chen XY; Chen Z; Ding BQ; Dong Q; Fan Q; Fu T; Hou DY; Jiang Q; Ke HT; Jiang XQ; Liu G; Li SP; Li TY; Liu Z; Nie GJ; Ovais M; Pang DW; Qiu NS; Shen YQ; Tian HY; Wang C; Wang H; Wang ZQ; Xu HP; Xu JF; Yang XL; Zhu S; Zheng XC; Zhang XZ; Zhao YB; Tan WH; Zhang X; Zhao YL, Sci China Chem 2018, 61, 1503–1552. [Google Scholar]
- [2].Bamrungsap S; Zhao ZL; Chen T; Wang L; Li CM; Fu T; Tan WH, Nanomedicine 2012, 7, 1253–1271. [DOI] [PubMed] [Google Scholar]
- [3].Mitragotri S; Anderson DG; Chen X; Chow EK; Ho D; Kabanov AV; Karp JM; Kataoka K; Mirkin CA; Petrosko SH; Shi JJ; Stevens MM; Sun SH; Teoh S; Venkatraman SS; Xia YN; Wang ST; Gu Z; Xu CJ, ACS Nano 2015, 9, 6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sun Q; Zhou Z; Qiu N; Shen Y, Adv Mater 2017, 29, 1606628. [DOI] [PubMed] [Google Scholar]
- [5].Wang Y; Cai R; Chen C, Accounts of chemical research 2019, 52, 1507–1518. [DOI] [PubMed] [Google Scholar]
- [6].Niidome T; Huang L, Gene Ther 2002, 9, 1647–52. [DOI] [PubMed] [Google Scholar]
- [7].Liu D; Yang F; Xiong F; Gu N, Theranostics 2016, 6, 1306–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen K; Liu B; Yu B; Zhong W; Lu Y; Zhang J; Liao J; Liu J; Pu Y; Qiu L; Zhang L; Liu H; Tan W, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology 2017, 9, e1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Accomasso L; Cristallini C; Giachino C, Front Pharmacol 2018, 9, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wolfram J; Zhu M; Yang Y; Shen J; Gentile E; Paolino D; Fresta M; Nie G; Chen C; Shen H; Ferrari M; Zhao Y, Curr Drug Targets 2015, 16, 1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yan L; Zhao F; Wang J; Zu Y; Gu Z; Zhao Y, Advanced Materials 2019, 31, 1805391. [DOI] [PubMed] [Google Scholar]
- [12].Pelaz B; Charron G; Pfeiffer C; Zhao Y; De La Fuente JM; Liang XJ; Parak WJ; Del Pino P, Small 2013, 9, 1573–1584. [DOI] [PubMed] [Google Scholar]
- [13].Kabanov AV, Advanced Drug Delivery Reviews 2006, 58, 1597–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. https://www.fda.gov/media/109910/downloadDrug.
- [15].Sharma A; Sharma US, International Journal of Pharmaceutics 1997, 154, 123–140. [Google Scholar]
- [16].Pillai G, SOJ Pharm Pharm Sci 2014, 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tinkle S; McNeil SE; Muhlebach S; Bawa R; Borchard G; Barenholz YC; Tamarkin L; Desai N, Ann N Y Acad Sci 2014, 1313, 35–56. [DOI] [PubMed] [Google Scholar]
- [18].Anselmo AC; Mitragotri S, Bioeng Transl Med 2016, 1, 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bulbake U; Doppalapudi S; Kommineni N; Khan W, Pharmaceutics 2017, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ventola CL, Pharmacy and Therapeutics 2017, 42, 742.29234213 [Google Scholar]
- [21].Barenholz YC, Journal of controlled release 2012, 160, 117–134. [DOI] [PubMed] [Google Scholar]
- [22].Working P; Dayan A, Pharmacological-toxicological expert report. CAELYX. (Stealth liposomal doxorubicin HCl). Hum Exp Toxicol.: 1996; Vol. 15, pp 752–785. [PubMed] [Google Scholar]
- [23].Gabizon A; Shmeeda H; Barenholz Y, Clin Pharmacokinet 2003, 42, 419–36. [DOI] [PubMed] [Google Scholar]
- [24].Solomon R; Gabizon AA, Clin Lymphoma Myelom 2008, 8, 21–32. [DOI] [PubMed] [Google Scholar]
- [25].Rafiyath SM; Rasul M; Lee B; Wei G; Lamba G; Liu D, Exp Hematol Oncol 2012, 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Drummond DC; Noble CO; Hayes ME; Park JW; Kirpotin DB, J Pharm Sci 2008, 97, 4696–740. [DOI] [PubMed] [Google Scholar]
- [27].Tardi PG; Boman NL; Cullis PR, J Drug Target 1996, 4, 129–40. [DOI] [PubMed] [Google Scholar]
- [28].Lim CC; Zuppinger C; Guo X; Kuster GM; Helmes M; Eppenberger HM; Suter TM; Liao R; Sawyer DB, The Journal of biological chemistry 2004, 279, 8290–9. [DOI] [PubMed] [Google Scholar]
- [29].Papoian T; Lewis W, Exp Mol Pathol 1991, 54, 112–21. [DOI] [PubMed] [Google Scholar]
- [30].Singal PK; Iliskovic N, N Engl J Med 1998, 339, 900–5. [DOI] [PubMed] [Google Scholar]
- [31].Chatterjee K; Zhang J; Honbo N; Karliner JS, Cardiology 2010, 115, 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bloom MW; Hamo CE; Cardinale D; Ky B; Nohria A; Baer L; Skopicki H; Lenihan DJ; Gheorghiade M; Lyon AR, Circulation: Heart Failure 2016, 9, e002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Henriksen PA, Heart 2018, 104, 971–977. [DOI] [PubMed] [Google Scholar]
- [34].Chang ΗM; Moudgil R; Scarabelli T; Okwuosa TM; Yeh ETH, Journal of the American College of Cardiology 2017, 70, 2536–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].O’Brien ΜE; Wigler N; Inbar M; Rosso R; Grischke E; Santoro A; Catane R; Kieback D; Tomczak P; Ackland S, Annals of oncology 2004,15, 440–449. [DOI] [PubMed] [Google Scholar]
- [36].Lyass O; Uziely B; Ben-Yosef R; Tzemach D; Heshing NI; Lotem M; Brufman G; Gabizon A, Cancer 2000, 89, 1037–47. [DOI] [PubMed] [Google Scholar]
- [37].Yokomichi N; Nagasawa T; Coler-Reilly A; Suzuki H; Kubota Y; Yoshioka R; Tozawa A; Suzuki N; Yamaguchi Y, Hum Cell 2013, 26, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chanan-Khan A; Szebeni J; Savay S; Liebes L; Rafique NM; Alving CR; Muggia FM, Annals of oncology : official journal of the European Society for Medical Oncology 2003, 14, 1430–7. [DOI] [PubMed] [Google Scholar]
- [39].Szebeni J; Baranyi L; Savay S; Milosevits J; Bunger R; Laverman P; Metselaar JM; Storm G; Chanan-Khan A; Liebes L; Muggia FM; Cohen R; Barenholz Y; Alving CR, J Liposome Res 2002,12, 165–72. [DOI] [PubMed] [Google Scholar]
- [40].Szebeni J; Muggia F; Gabizon A; Barenholz Y, Adv Drug Deliv Rev 2011, 63, 1020–30. [DOI] [PubMed] [Google Scholar]
- [41].Szebeni J; Bedocs P; Rozsnyay Z; Weiszhar Z; Urbanics R; Rosivall L; Cohen R; Garbuzenko O; Bathori G; Toth M; Bunger R; Barenholz Y, Nanomedicine 2012, 8, 176–84. [DOI] [PubMed] [Google Scholar]
- [42].Titgan M In Prevention of palmar-plantar erythrodysesthesia associated with liposome-encapsulated doxorubicin (Doxil) by oral dexamethasone, Proc Am Soc Clin Oncol, 1997; p 82a. [Google Scholar]
- [43].Vail DM; Chun R; Thamm DH; Garrett LD; Cooley AJ; Obradovich JE, Clinical Cancer Research 1998, 4, 1567–1571. [PubMed] [Google Scholar]
- [44].Honecker F; Mayer F; Hilger R, J Cancer Res Clin Oncol 2002,128, 848. [Google Scholar]
- [45].Harris L; Batist G; Belt R; Rovira D; Navari R; Azamia N; Welles L; Winer E; Group TDS, Cancer 2002, 94, 25–36. [DOI] [PubMed] [Google Scholar]
- [46].Batist G; Ramakrishnan G; Rao CS; Chandrasekharan A; Gutheil J; Guthrie T; Shah P; Khojasteh A; Nair ΜK; Hoelzer K; Tkaczuk K; Park YC; Lee LW, Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2001,19, 1444–54. [DOI] [PubMed] [Google Scholar]
- [47].Drummond DC; Noble CO; Guo ZX; Hong K; Park JW; Kirpotin DB, Cancer Research 2006, 66, 3271–3277. [DOI] [PubMed] [Google Scholar]
- [48].Von Hoff D; Li C; Wang-Gillam A; Bodoky G; Dean A; Jameson G; Macarulla T; Lee K; Cunningham D; Blanc J, Annals of Oncology 2014, 25, ii105–ii106. [Google Scholar]
- [49].Conroy T; Desseigne F; Ychou M; Bouche O; Guimbaud R; Becouarn Y; Adenis A; Raoul JL; Gourgou-Bourgade S; de la Fouchardiere C; Bennouna J; Bachet JB; Khemissa-Akouz F; Pere-Verge D; Delbaldo C; Assenat E; Chauffert B; Michel P; Montoto-Grillot C; Ducreux M; Groupe Tumeurs Digestives of, U.; Intergroup, P., N Engl J Med 2011, 364, 1817–25. [DOI] [PubMed] [Google Scholar]
- [50].Ueno H; Okusaka T; Funakoshi A; Ishii H; Yamao K; Ishikawa O; Ohkawa S; Saitoh S, Cancer Chemotherapy and Pharmacology 2007, 59, 447–454. [DOI] [PubMed] [Google Scholar]
- [51].Chou TH; Chen SC; Chu IM, J Biosci Bioeng 2003, 95, 405–8. [PubMed] [Google Scholar]
- [52].Messerer CL; Ramsay EC; Waterhouse D; Ng R; Simms EM; Harasym N; Tardi P; Mayer LD; Bally MB, Clin Cancer Res 2004,10, 6638–49. [DOI] [PubMed] [Google Scholar]
- [53].Ramsay E; Alnajim J; Anantha M; Taggar A; Thomas A; Edwards K; Karlsson G; Webb M; Bally M, Pharm Res 2006, 23, 2799–808. [DOI] [PubMed] [Google Scholar]
- [54].Dicko A; Tardi P ; Xie X; Mayer L, Int J Pharm 2007, 337, 219–28. [DOI] [PubMed] [Google Scholar]
- [55].Hattori Y; Shi L; Ding WX; Koga K; Kawano K; Hakoshima M; Maitani Y, Journal of Controlled Release 2009,136, 30–37. [DOI] [PubMed] [Google Scholar]
- [56].Yang W; Yang Z; Fu J; Guo M; Sun B; Wei W; Liu D; Liu H, Biomaterials science 2019, 7, 419–428. [DOI] [PubMed] [Google Scholar]
- [57].Roy AC; Park SR; Cunningham D; Kang YK; Chao Y; Chen LT; Rees C; Lim HY; Tabernero J; Ramos FJ; Kujundzic M; Cardie MB; Yeh CG; de Gramont A, Annals of Oncology 2013, 24, 1567–1573. [DOI] [PubMed] [Google Scholar]
- [58].Chiang NJ; Chang JY; Shan YS; Chen LT, Expert Opin Pharmacother 2016,17, 1413–20. [DOI] [PubMed] [Google Scholar]
- [59].Chibaudel B; Maindrault-Gcebel F; Bachet JB; Louvet C; Khalil A; Dupuis O; Hammel P ; Garcia ML; Bennamoun M; Brusquant D, Cancer medicine 2016, 5, 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang-Gillam A; Li CP; Bodoky G; Dean A; Shan YS; Jameson G; Macarulla T; Lee KFL; Cunningham D; Blanc JF; Hubner RA; Chiu CF; Schwartsmann G; Siveke JT; Braiteh F; Moyo V; Belanger B; Dhindsa N; Bayever E; Von Hoff DD; Chen LT; Group N-S, Lancet 2016, 387, 545–557. [DOI] [PubMed] [Google Scholar]
- [61].Liu DZ; Chen WY; Tasi LM; Yang SP, Colloid Surface A 2000,172, 57–67. [Google Scholar]
- [62].Heurtault B; Saulnier P; Pech B; Proust JE; Benoit JP, Biomaterials 2003, 24, 4283–300. [DOI] [PubMed] [Google Scholar]
- [63].Sabin J; Prieto G; Ruso JM; Hidalgo-Alvarez R; Sarmiento F, The European Physical Journal E 2006, 20, 401–408. [DOI] [PubMed] [Google Scholar]
- [64].Tolcher AW; Mayer LD, Future Oncol 2018,14, 1317–1332. [DOI] [PubMed] [Google Scholar]
- [65].Meng H; Wang ΜY; Liu ΗY; Liu XS; Situ A; Wu B; Ji ZX; Chang CH; Nel AE, Acs Nano 2015, 9, 3540–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tardi P; Johnstone S; Harasym N; Xie S; Harasym T; Zisman N; Harvie P; Bermudes D; Mayer L, Leak Res 2009, 33, 129–39. [DOI] [PubMed] [Google Scholar]
- [67].Lim WS; Tardi PG; Dos Santos N; Xie X; Fan M; Liboiron BD; Huang X; Harasym TO; Bermudes D; Mayer LD, Leak Res 2010, 34, 1214–23. [DOI] [PubMed] [Google Scholar]
- [68].Kim HP; Gerhard B; Harasym TO; Mayer LD; Hogge DE, Experimental Hematology 2011, 39, 741–750. [DOI] [PubMed] [Google Scholar]
- [69].Mayer LD; Tardi P; Louie AC, Int J Nanomedicine 2019,14, 3819–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chen EC; Fathi AT; Brunner AM, Oncotargets and Therapy 2018,11, 3425–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lancet JE; Uy GL; Cortes JE; Newell LF; Lin TL; Ritchie EK; Stuart RK; Strickland SA; Hogge D; Solomon SR; Stone RM; Bixby DL; Kolitz JE; Schiller GJ; Wieduwilt MJ; Ryan DH; Hoering A; Banerjee K; Chiarella M; Louie AC; Medeiros BC,J Clin Oncol 2018, 36, 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wibroe PP; Ahmadvand D; Oghabian MA; Yaghmur A; Moghimi SM, J Control Release 2016, 221, 1–8. [DOI] [PubMed] [Google Scholar]
- [73].Hua S; de Matos MBC; Metselaar JM; Storm G, Front Pharmacol 2018, 9, 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].http://www.cancer.gov/nano/research/alliance.
- [75].Autio KA; Dreicer R; Anderson J; Garcia JA; Alva A; Hart LL; Milowsky MH ; Posadas EM; Ryan CJ; Graf RP; Dittamore R; Schreiber NA; Summa JM; Youssoufian H; Morris MJ; Scher HI, JAMA Oncol 2018, 4, 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang J; Mao W; Lock LL; Tang J; Sui M; Sun W; Cui H; Xu D; Shen Y., ACS Nano 2015, 9, 7195–206. [DOI] [PubMed] [Google Scholar]
- [77].Zhou Q; Shao S; Wang J; Xu C; Xiang J; Piao Y; Zhou Z; Yu Q; Tang J; Liu X, Nature nanotechnology 2019, 14, 799–809. [DOI] [PubMed] [Google Scholar]
- [78].Wang HX; Zuo ZQ; Du JZ; Wang YC; Sun R; Cao ZT; Ye XD; Wang JL ; Leong KW; Wang J, Nano Today 2016, 11, 133–144. [Google Scholar]
- [79].Xu CF; Iqbal S; Shen S; Luo YL; Yang X; Wang J, Small 2019, 15, 1900055. [DOI] [PubMed] [Google Scholar]
- [80].Cabral H; Miyata K; Osada K; Kataoka K, Chem Rev 2018, 118, 6844–6892. [DOI] [PubMed] [Google Scholar]
- [81].Mochida Y; Cabral H; Kataoka K, Expert Opin Drug Deliv 2017, 14, 1423–1438. [DOI] [PubMed] [Google Scholar]
- [82].Giljohann DA; Seferos DS; Daniel WL; Massich MD; Patel PC; Mirkin CA, Angewandte Chemie 2010, 49, 3280–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Xiong F; Huang S; Gu N, Drug Dev Ind Pharm 2018, 44, 697–706. [DOI] [PubMed] [Google Scholar]
- [84].Liu G; Gao J; Ai H; Chen X, Small 2013, 9, 1533–45. [DOI] [PubMed] [Google Scholar]
- [85].Liu Y; Zhao Y; Sun B; Chen C, Acc Chem Res 2013, 46, 702–13. [DOI] [PubMed] [Google Scholar]
- [86].Phillips E; Penate-Medina O; Zanzonico PB; Carvajal RD; Mohan P; Ye Y; Humm J; Gonen M; Kalaigian H; Schoder H; Strauss HW; Larson SM; Wiesner U; Bradbury MS, Sci Transl Med 2014, 6, 260ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yang K; Feng L; Liu Z, Adv Drug Deliv Rev 2016, 105, 228–241. [DOI] [PubMed] [Google Scholar]
- [88].Chen Y; Wu Y; Sun B; Liu S; Liu H, Small 2017, 13, 1603446. [DOI] [PubMed] [Google Scholar]
- [89].Gu ZJ; Yan L; Tian G; Li SJ; Chai ZF; Zhao YL, Advanced Materials 2013, 25, 3758–3779. [DOI] [PubMed] [Google Scholar]
- [90].Liu Y; Zhao Y; Chen X, Theranostics 2019, 9, 3122–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Etheridge ML; Campbell SA; Erdman AG; Haynes CL; Wolf SM; McCullough H , Nanomedicine 2013, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhu S; Tian R; Antaris AL; Chen X; Dai H, Advanced Materials 2019, 31, 1900321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fadeel B; Fornara A; Toprak MS; Bhattacharya K, Biochem Biophys Res Commun 2015, 468, 498–503. [DOI] [PubMed] [Google Scholar]
- [94].Maynard AD; Warheit DB; Philbert MA, Toxicological sciences : an official journal of the Society of Toxicology 2011, 120 Suppl 1, S109–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Oh E; Liu R; Nel A; Gemill KB; Bilal M; Cohen Y; Medintz IL, Nat Nanotechnol 2016, 11, 479–86. [DOI] [PubMed] [Google Scholar]
- [96].George S; Xia TA; Rallo R; Zhao Y; Ji ZX; Lin SJ; Wang X; Zhang HY; France B; Schoenfeld D; Damoiseaux R; Liu R; Lin S; Bradley KA; Cohen Y; Nel AE, Acs Nano 2011, 5, 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhang HY; Ji ZX; Xia T; Meng H; Low-Kam C; Liu R; Pokhrel S; Lin SJ; Wang X; Liao YP; Wang MY; Li LJ; Rallo R; Damoiseaux R; Telesca D; Madler L; Cohen Y; Zink JI; Nel AE, Acs Nano 2012, 6, 4349–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Fourches D; Pu D; Tassa C; Weissleder R; Shaw SY; Mumper RJ; Tropsha A, ACS Nano 2010, 4, 5703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Puzyn T; Rasulev B; Gajewicz A; Hu XK; Dasari TP; Michalkova A; Hwang HM; Toropov A; Leszczynska D; Leszczynski J, Nature Nanotechnology 2011, 6, 175–178. [DOI] [PubMed] [Google Scholar]
- [100].Burello E; Worth AP, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology 2011, 3, 298–306. [DOI] [PubMed] [Google Scholar]
- [101].Gerloff K; Landesmann B; Worth A; Munn S; Palosaari T; Whelan M, Computational Toxicology 2017, 1, 3–11. [Google Scholar]
- [102].Villeneuve DL; Crump D; Garcia-Reyero N; Hecker M; Hutchinson TH; LaLone CA; Landesmann B; Lettieri T; Munn S; Nepelska M; Ottinger MA; Vergauwen L; Whelan M, Toxicological sciences : an official journal of the Society of Toxicology 2014, 142, 312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Muller EB; Lin S; Nisbet RM, Environ Sci Technol 2015, 49, 11817–24. [DOI] [PubMed] [Google Scholar]
- [104].Meng H; Xia T; George S; Nel AE, ACS Nano 2009, 3, 1620–7. [DOI] [PubMed] [Google Scholar]
- [105].Meng H; Leong W; Leong KW; Chen CY; Zhao YL, Biomaterials 2018, 174, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhao F; Meng H; Yan L; Wang B; Zhao YL, Science Bulletin 2015, 60, 3–20. [Google Scholar]
- [107].Nel A; Xia T; Meng H; Wang X; Lin SJ; Ji ZX; Zhang HY, Accounts of Chemical Research 2013, 46, 607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhu M; Nie G; Meng H; Xia T; Nel A; Zhao Y, Acc Chem Res 2013, 46, 622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Gojova A; Guo B; Kota RS; Rutledge JC; Kennedy IM; Barakat AI, Environ Health Perspect 2007, 115, 403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lin S; Zhao Y; Xia T; Meng H; Ji Z; Liu R; George S; Xiong S; Wang X; Zhang H; Pokhrel S; Madler L; Damoiseaux R; Lin S; Nel AE, ACS Nano 2011, 5, 7284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ivask A; Titma T; Visnapuu M; Vija H; Kakinen A; Sihtmae M; Pokhrel S; Madler L; Heinlaan M; Kisand V; Shimmo R; Kahru A, Curr Top Med Chem 2015, 15, 1914–29. [DOI] [PubMed] [Google Scholar]
- [112].Peng GT; He Y; Zhao M; Yu TY; Qin Y; Lin SJ, Environ Sci-Nano 2018, 5, 1200–1207. [Google Scholar]
- [113].Frohlich E, International Journal of Nanomedicine 2012, 7, 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu XS; Huang N; Li H; Jin Q; Ji J, Langmuir 2013, 29, 9138–9148. [DOI] [PubMed] [Google Scholar]
- [115].Ruenraroengsak P; Novak P; Berhanu D; Thorley AJ; Valsami-Jones E; Gorelik J; Korchev YE; Tetley TD, Nanotoxicology 2012, 6, 94–108. [DOI] [PubMed] [Google Scholar]
- [116].Bexiga MG; Varela JA; Wang F; Fenaroli F; Salvati A; Lynch I; Simpson JC; Dawson KA, Nanotoxicology 2011, 5, 557–67. [DOI] [PubMed] [Google Scholar]
- [117].Zhang H; Xia T; Meng H; Xue M; George S; Ji Z; Wang X; Liu R; Wang M; France B; Rallo R; Damoiseaux R; Cohen Y; Bradley KA; Zink JI; Nel AE, ACS Nano 2011, 5, 2756–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Li R; Wang X; Ji Z; Sun B; Zhang H; Chang CH; Lin S; Meng H; Liao YP; Wang M; Li Z; Hwang AA; Song TB; Xu R; Yang Y; Zink JI; Nel AE; Xia T, ACS Nano 2013, 7, 2352–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Xia T; Kovochich M; Liong M; Zink JI; Nel AE, ACS Nano 2008, 2, 85–96. [DOI] [PubMed] [Google Scholar]
- [120].Ji Z; Wang X; Zhang H; Lin S; Meng H; Sun B; George S; Xia T; Nel AE; Zink JI, ACS Nano 2012, 6, 5366–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sun B; Wang X; Ji Z; Li R; Xia T, Small 2013, 9, 1595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lin S; Wang X; Ji Z; Chang CH; Dong Y; Meng H; Liao YP; Wang M; Song TB; Kohan S; Xia T; Zink JI; Lin S; Nel AE, ACS Nano 2014, 8, 4450–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhu W; von dem Bussche A; Yi X; Qiu Y; Wang Z; Weston P; Hurt RH; Kane AB; Gao H, Proc Natl Acad Sci U S A 2016, 113, 12374–12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Meng H; Yang S; Li Z; Xia T; Chen J; Ji Z; Zhang H; Wang X; Lin S; Huang C; Zhou ZH; Zink JI; Nel AE, ACS Nano 2011, 5, 4434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhang H; Dunphy DR; Jiang X; Meng H; Sun B; Tarn D; Xue M; Wang X; Lin S; Ji Z; Li R; Garcia FL; Yang J; Kirk ML; Xia T; Zink JI; Nel A; Brinker CJ, J Am Chem Soc 2012, 134, 15790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Havrdova M; Hola K; Skopalik J; Tomankova K; Petr M; Cepe K; Polakova K; Tucek J; Bourlinos AB; Zboril R, Carbon 2016, 99, 238–248. [Google Scholar]
- [127].Park SJ; Park YC; Lee SW; Jeong MS; Yu KN; Jung H; Lee JK; Kim JS; Cho MH, Toxicol Lett 2011, 207, 197–203. [DOI] [PubMed] [Google Scholar]
- [128].Rogers NJ; Franklin NM; Apte SC; Batley GE; Angel BM; Lead JR; Baalousha M, Environmental Chemistry 2010, 7, 50–60. [Google Scholar]
- [129].Meng H; Chen Z; Xing G; Yuan H; Chen C; Zhao F; Zhang C; Zhao Y, Toxicol Lett 2007, 775, 102–10. [DOI] [PubMed] [Google Scholar]
- [130].Dowding JM; Das S; Kumar A; Dosani T; McCormack R; Gupta A; Sayle TX; Sayle DC; von Kalm L; Seal S; Self WT, ACS Nano 2013, 7, 4855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Han Y; Lee DK; Kim SH; Lee S; Jeon S; Cho WS, Nanotoxicology 2018, 12, 712–728. [DOI] [PubMed] [Google Scholar]
- [132].Li R; Ji Z; Qin H; Kang X; Sun B; Wang M; Chang CH; Wang X; Zhang H; Zou H, ACS nano 2014, 8, 10280–10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Li R; Ji Z; Chang CH; Dunphy DR; Cai X; Meng H; Zhang H; Sun B; Wang X; Dong J; Lin S; Wang M; Liao YP; Brinker CJ; Nel A; Xia T, ACS Nano 2014, 8, 1771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Parasuraman S, J Pharmacol Pharmacother 2011, 2, 74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Dixit R; Boelsterli UA, Drug Discov Today 2007, 12, 336–42. [DOI] [PubMed] [Google Scholar]
- [136].Lamberti M; Giovane G; Garzillo EM; Avino F; Feola A; Porto S; Tombolini V; Di Domenico M, BiomedRes Int 2014, 2014, 240642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Robert J, Cell Biol Toxicol 2007, 25, 27–37. [DOI] [PubMed] [Google Scholar]
- [138].Herman EH; Ferrans VJ, Progress in Pediatric Cardiology 1997, 8, 49–58. [Google Scholar]
- [139].Shankaran H; Cronin A; Barnes J; Sharma P; Tolsma J; Jasper P; Mettetal JT, CPT Pharmacometrics Syst Pharmacol 2018, 7, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Bowen JM, Scientifica 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gibson RJ; Bowen JM; Inglis MRB; Cummins AG; Keefe DMK, Journal of Gastroenterology and Hepatology 2003, 18, 1095–1100. [DOI] [PubMed] [Google Scholar]
- [142].Gil PR; Hühn D; Loretta L; Sasse D; Parak WJ, Pharmacological research 2010, 62, 115–125. [DOI] [PubMed] [Google Scholar]
- [143].Della Rocca J; Liu D; Lin W, Accounts of chemical research 2011, 44, 957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Lu K; He C; Guo N; Chan C; Ni K; Lan G; Tang H; Pelizzari C; Fu Y-X; Spiotto MT, Nature biomedical engineering 2018, 2, 600–610. [DOI] [PubMed] [Google Scholar]
- [145].Ni K; Lan G; Chan C; Duan X; Guo N; Veroneau SS; Weichselbaum RR; Lin W, Matter 2019, 1, 1331–1353. [PMC free article] [PubMed] [Google Scholar]
- [146].Ni K; Lan G; Veroneau SS; Duan X; Song Y; Lin W, Nat Commun 2018, 9, 4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].He C; Poon C; Chan C; Yamada SD; Lin W, J Am Chem Soc 2016, 158, 6010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].He C; Duan X; Guo N; Chan C; Poon C; Weichselbaum RR; Lin W, Nat Commun 2016, 7, 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Duan X; Chan C; Han W; Guo N; Weichselbaum RR; Lin W, Nat Commun 2019, 10, 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Paciotti GF; Kingston DG; Tamarkin L, Drug development research 2006, 67, 47–54. [Google Scholar]
- [151].Libutti SK; Paciotti GF; Byrnes AA; Alexander HR Jr.; Gannon WE; Walker M; Seidel GD; Yuldasheva N; Tamarkin L, Clin Cancer Res 2010, 16, 6139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Stern JM; Kibanov Solomonov VV; Sazykina E; Schwartz JA; Gad SC; Goodrich GP, Int J Toxicol 2016, 55, 38–46. [DOI] [PubMed] [Google Scholar]
- [153].Rastinehad AR; Anastos H; Wajswol E; Winoker JS; Sfakianos JP; Doppalapudi SK; Carrick MR; Knauer CJ; Taouli B; Lewis SC; Tewari AK; Schwartz JA; Canfield SE; George AK; West JL; Halas NJ, Proc Natl Acad Sci U S A 2019, 116, 18590–18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gad SC; Sharp KL; Montgomery C; Payne JD; Goodrich GP, International journal of toxicology 2012, 31, 584–594. [DOI] [PubMed] [Google Scholar]
- [155].Liu X; Lin P; Perrett I; Lin J; Liao YP; Chang CH; Jiang J; Wu N; Donahue T ; Wainberg Z; Nel AE; Meng H, J Clin Invest 2017, 127, 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Liu XS; Situ A; Kang YA; Villabroza KR; Liao YP; Chang CH; Donahue T; Nel AE; Meng H, Acs Nano 2016, 10, 2702–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Liu X; Jiang J; Chan R; Ji Y; Lu J; Liao YP; Okene M; Lin J; Lin P; Chang CH; Wang X; Tang I; Zheng E; Qiu W; Wainberg ZA; Nel AE; Meng H, ACS Nano 2019, 13, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhang Q; Wang X; Li PZ; Nguyen KT; Wang XJ; Luo Z; Zhang H; Tan NS; Zhao Y, Advanced Functional Materials 2014, 24, 2450–2461. [Google Scholar]
- [159].Argyo C; Weiss V; Brauchle C; Bein T, Chemistry of Materials 2014, 26, 435–451. [Google Scholar]
- [160].Lu J; Liong M; Li Z; Zink JI; Tamanoi F, Small 2010, 6, 1794–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].He QJ; Shi JL, Journal of Materials Chemistry 2011, 21, 5845–5855. [Google Scholar]
- [162].Liu C; Zhang L; Liu H; Cheng K, J Control Release 2017, 266, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Abou-El-Enein M; Cathomen T; Ivics Z; June CH; Renner M; Schneider CK; Bauer G, Cell Stem Cell 2017, 21, 427–430. [DOI] [PubMed] [Google Scholar]
- [164].Liu XS; Jiang JH; Chan R; Ji Y; Lu JQ; Liao YP; Okene M; Lin JS; Lin P; Chang CH; Wang X; Tang I; Zheng E; Qiu W; Wainberg ZA; Nel AE; Meng H, Acs Nano 2019, 13, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


