Figure 1.
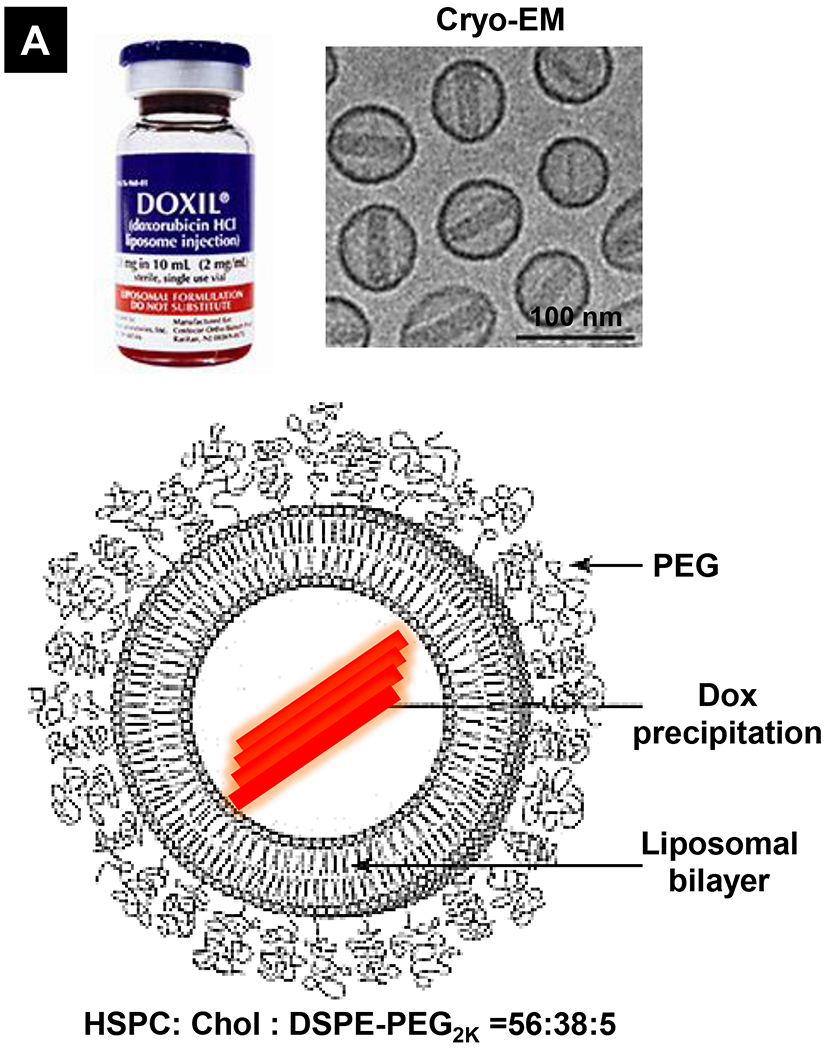
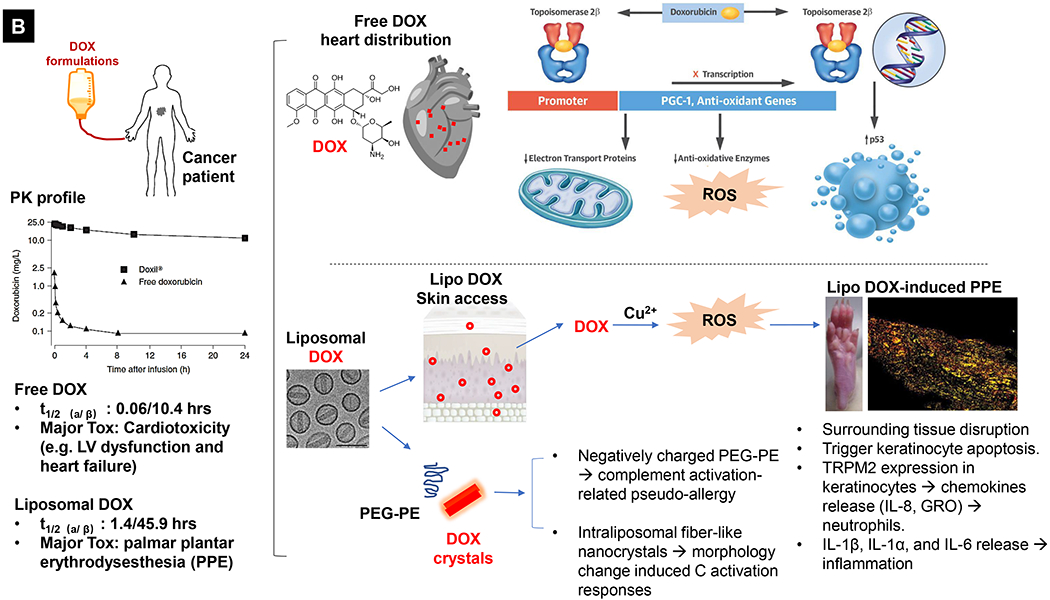
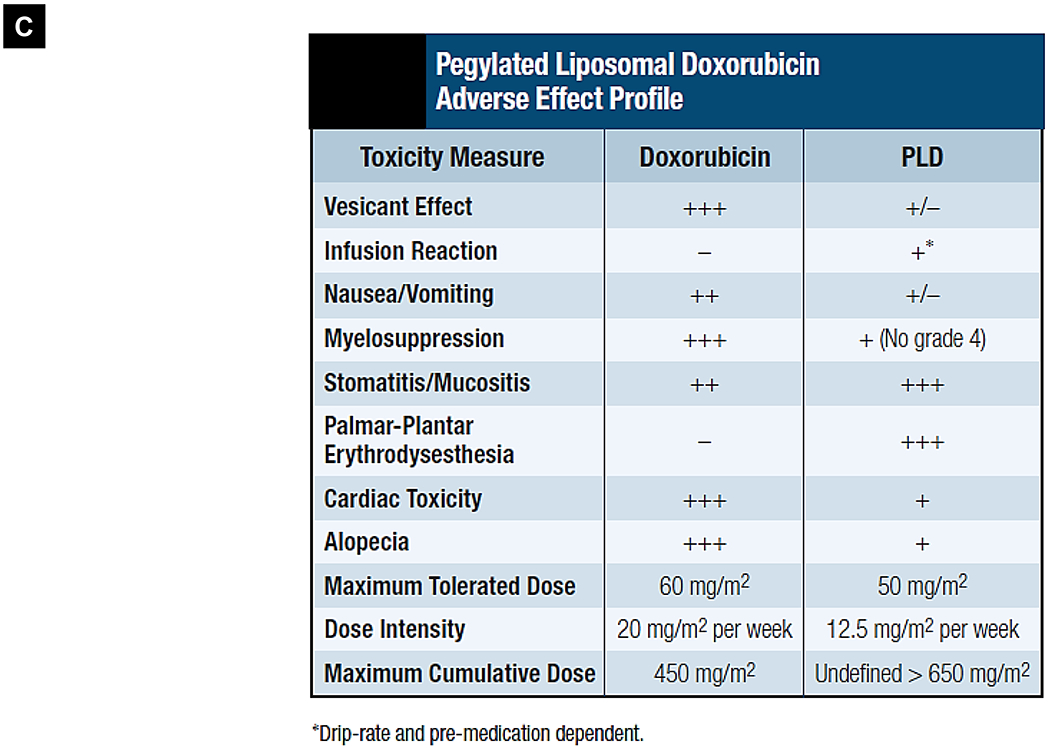
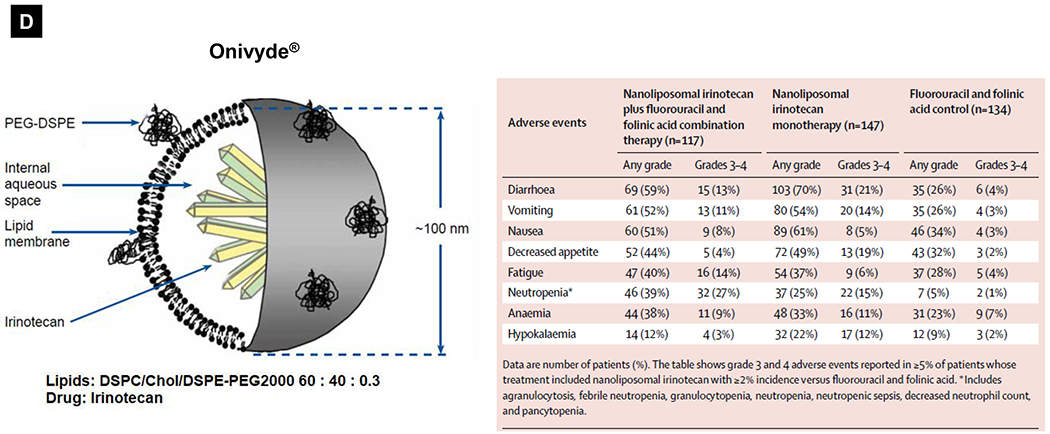
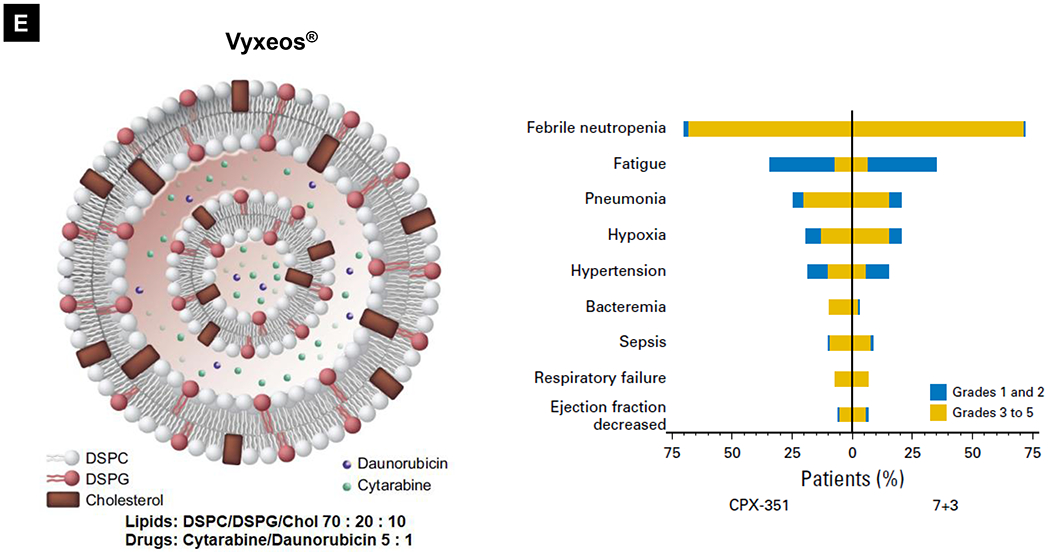
Comparative demonstration of free DOX and Doxil®’s PK/biodistribution, targeted organ and safety features. (A) Appearance, cryoEM image and scheme of Doxil® formulation. HSPC: hydrogenated soy phosphatidylcholine, Chol: cholesterol, DSPE-PEG2K:1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000]. Reproduced with permission.[21] Copyright 2012, Elsevier. (B) Pharmacokinetics (PK) profiles and main cardiotoxicity and palmar plantar erythrodysesthesia (PPE) effects/mechanism of doxorubicin and Doxil® formulation. Reproduced with permission. [21, 23, 34, 37] Copyright 2012, Elsevier. Copyright 2003, Springer Nature. Copyright 2017, Elsevier. Copyright 2013, Springer Nature. (C) Clinical adverse effect profile of pegylated liposomal doxorubicin. Reproduced with permission.[24] Copyright 2008, Elsevier. (D) Scheme and clinical adverse effect profile of liposomal irinotecan Onivyde®. Reproduced with permission. [48, 60] Copyright 2014, Elsevier. Copyright 2016, Elsevier. (E) Scheme and clinical adverse effect profile of cytarabine and daunorubicin co-delivery liposome Vyxeos®. Reproduced with permission.[64, 71] Copyright 2018, Future Medicine Ltd. Copyright 2018, American Society of Clinical Oncology.
