Abstract
Introduction:
Improvement of walking performance is a primary goal for individuals post-stroke or with Parkinson disease (PD) who receive physical therapy. More data about day-to-day variability of walking performance is critical for determining if changes in performance have occurred.
Methods:
Baseline assessments were utilized from an ongoing, observational, prospective cohort study including 84 individuals post-stroke (n=37) or with PD (n=47) receiving outpatient physical therapy services to improve mobility. Participants wore step activity monitors for up to 7 days to measure walking performance (steps per day, walking duration, max 30-min output, peak activity index) in daily life. Correlation analyses evaluated relationships between both capacity and performance measures as well as the relationships between mean performance variables and day-to-day variability. Regression analyses explored factors that contribute to variability in day-to-day performance variables.
Results:
Mean steps per day for participants post-stroke (5376 ± 2804) and with PD (8149 ± 4490) were consistent with previously reported cohorts. Greater amounts of walking were related to more day-to-day variability, with moderate correlations found between the mean and day-to-day variability of each performance measure, regardless of medical diagnosis or walking speed. Day-to-day variability is large (upwards of 50% of the mean), with the amount of walking performance serving as the primary predictor of day-to-day variability in walking performance.
Conclusion:
The results of this study elucidate the factors that are related to and predict day-to-day variability of performance. Walking performance metrics should be evaluated over multiple days and greater variability should be anticipated with greater amounts of performance.
INTRODUCTION
People post-stroke or with Parkinson disease (PD) are two of the largest populations seeking neurorehabilitation services1,2, and improvement in walking is the most common rehabilitation goal.3,4 The capacity for walking can be readily assessed in the clinic following best practice recommendations.5 Patients engage in rehabilitation care, however, to improve their walking performance (i.e. walking activity in daily life), not just their capacity to walk as measured in the clinic. There is strong support to capture walking performance as a measure of patient mobility outside the clinic.6,7
Multiple devices with embedded sensors are now on the market to assist patients, clinicians, and researcher consumers in capturing walking performance. Most devices have an accelerometer that quantifies steps per day and other performance variables. These variables can then be used to establish a starting point from which to set goals and track progress.7 Efforts have begun to standardize days of wear and classifications of levels of activity.8,9 Little is known, however, about day-to-day variability in walking performance or factors that may affect the variability in walking for people post stroke or with PD. Clinicians need this information to anticipate expected variation and to differentiate that from improvement or regression. Until more is understood about variability, it will be challenging to set realistic goals and determine if changes in walking performance have occurred.
The purposes of this paper are to: 1) describe day-to-day variability in walking performance, 2) examine relationships between variability and mean performance over the recording period, and 3) evaluate the influence of other factors (e.g. walking capacity, medical diagnosis, socioeconomic disadvantage) on variability. Data are examined at the individual level to characterize more precisely how amount of daily activity is related to variability of walking performance. As clinicians begin to capture walking performance in their daily practice, an understanding of amount vs. variability and the factors that influence variability will enable accurate and individualized goals and interventions for people post-stroke or PD.
METHODS
This paper reports on data from an ongoing longitudinal, prospective cohort of persons with stroke or PD. The larger study evaluates changes in capacity and performance in upper limb function and mobility over the course of physical and occupational therapy services. Following the World Health Organization ICF model, capacity and performance are both activity-level measures, with the former capturing activity in a structured setting, and the latter capturing activity in the unstructured, free-living environment.10 Data analyzed here are from the initial assessment, within 10 days of starting outpatient services, in a subset of 84 participants who were receiving physical therapy and had goals to improve mobility, i.e. walking.
Participants
Individuals with a diagnosis of stroke or idiopathic PD (Hoehn & Yahr Stage 2 or 3) were recruited into this cohort study. Recruitment occurred across five outpatient therapy clinics in the United States (3 clinics in St. Louis MO, 1 clinic in Boston MA, 1 clinic in Chicago IL) via treating therapist referral or by participant contact of the study team in response to approved recruitment materials. Inclusion criteria were: 1) within 10 days of initial evaluation for physical therapy; 2) documented therapy goals to improve mobility; 3) anticipated duration of therapy services of at least one month; and 4) ability to follow 2-step commands. Exclusion criteria were: 1) other neurological or psychiatric conditions, including deep brain stimulation implants; 2) other orthopaedic conditions that limited mobility or potential progress in mobility; 3) other co-morbid conditions such that the physician or therapy documentation indicates minimal chance for improvement in function; and 4) self-selected gait speed ≥ 1.2 m/s. All procedures were approved by each site’s local Institutional Review Board, with written informed consent provided by each participant, and data were stored in Research Electronic Data Capture (REDCap).11
Study Assessments
Descriptive characteristics of the sample including age, sex, days since stroke, and years since PD diagnosis were collected. In addition, socioeconomic disadvantage was captured by the Area of Deprivation Index (ADI).12,13 The ADI is derived from analysis of census data on education, income, employment, housing, and household characteristics and is scored on a scale of 0–100 percent. Individuals who reside in areas with a higher ADI tend to be more economically disadvantaged. Living in areas that are more economically disadvantaged has been associated with a variety of detrimental health effects including higher incidence and poorer management of chronic disease (often due to lack of access to services, healthy food sources, etc.)14, earlier mortality15, and higher hospital re-admission rates16. We chose ADI because of its comprehensive quantification of socioeconomic disadvantage and because it is possible that socioeconomic disadvantage could influence walking performance (e.g. lack of safe walking spaces, crumbled sidewalks). The ADI was accessed through the University of Wisconsin School of Medicine and Public Health’s Neighborhood Atlas17 by entering participants’ 9- digit zip codes.
Study assessments were typically collected around the time of the participants’ therapy appointments, and participants with PD were assessed while on their usual medication regimen. The primary measure of walking capacity was as-fast-as-possible walking speed during the 10 MWT.5 Self- selected walking speed was also assessed. Participants walked over a 14- meter course with their customary assistive device and any required bracing, where the middle 10 meters was timed. The three trials were averaged and are reported in meters/second. Participants requiring physical assistance were given a walking speed score of 0 m/s. Individuals who were unable to walk as defined by a gait speed of 0.0m/s were excluded from this analysis.
Walking performance, i.e. mobility in daily life, was recorded via a validated accelerometer (StepWatch Activity Monitor, Modus Inc, Washington DC) worn on the least affected limb.18–21 The Step Activity Monitor was calibrated to participant height and walking characteristics in accordance with manufacturer instructions. Calibration was verified by having the participant walk 40 strides at their self-selected pace. Recalibration was performed when the step activity count was off by ≥ 2 strides. Number of strides was captured over 60-second intervals and converted to steps. Data were captured over the course of seven consecutive days during all waking hours, except while participants were bathing.20–23 Each participant was provided instructions for wearing the device and a log for documenting time when they did not wear the device. Data from the first and last days of the recording period were excluded from the analysis, as the devices were only worn for part of those days (e.g. started wearing at 1 pm, removed at 1 pm).
Here, we report on four variables derived from the step activity monitor recordings that reflect walking performance in daily life. We report on four instead of only one (e.g. steps per day) because it is unlikely that a single variable can completely capture the whole construct of performance in daily life.24 Variables were chosen based on how commonly they are seen in the literature, their use in both stroke and PD populations, and objectivity in calculations (e.g. bouts of walking was not calculated because that is dependent on the definition of a bout). Each variable was calculated per day as well as an average over the recording period. The primary variable was total steps per day, as is most commonly seen in rehabilitation literature.7,20,25,26 The three other variables were walking duration, maximum 30- minute output, and peak activity index. Walking duration is the sum of the minutes of walking and includes every minute where the step count was > 1.7,22,25,27 Note that walking duration determined from one- minute periods potentially overestimates walking duration over the course of a day. This is because any minute where walking is recorded is counted as a minute of walking (e.g. five steps at the beginning of one minute = one minute of walking). The maximum 30- minute output is the average number of steps per minute during the 30 consecutive most active minutes over a day.25 The peak activity index is the average number of steps per minute over the 30 most active minutes of the day, regardless of when they occurred.25 Both maximum 30- minute output and peak activity index were included because participants may have spread their more intense activities throughout the day vs. within a single time period during the day.
Analyses
All statistical analyses were completed in R (version 3.3.2), an open source statistical computing program, and alpha level of significance was set at 0.05. Histograms were generated for each variable to examine the distributions, and tests confirmed normality of these distributions. Descriptive statistics were then calculated for each variable. To quantify day-to-day variability in walking performance, the standard deviation and coefficient of variation (i.e. standard deviation/mean) of each variable were computed for each person from their values for each day during the recording period. Relationships between the standard deviation and mean values were evaluated using Pearson correlation coefficients. Regression modeling was used to determine the influence of multiple variables on the variability in walking performance. For the regression modeling, linear models were generated with the ‘lm’ function in R separately with each of the walking performance variables serving as the dependent variable in their own model. The modeling was structured such that the first model tested predicted the standard deviation from the mean. For each walking performance variable, additional models were constructed adding potential modifiers (diagnosis [stroke or PD]), fast walk speed, and ADI.) Models containing each additional modifying variable were compared to the previous model using Chi squared analyses, and the added modifying variable was retained only when the more complex model was significantly different from the simpler model.
RESULTS
One hundred and three participants enrolled in the mobility portion of the study. Of these, five individuals were excluded on initial evaluation (self-selected walking speed ≥ 1.2 m/s), seven individuals had no stepping data available for analysis (e.g. lost the SAM, non-compliance), four individuals were excluded for having only one day of stepping data, and three individuals who could not walk without physical assistance and had inaccurate SAM data due to wheelchair use. This resulted in 84 individuals with usable data. Table 1 provides descriptive information about the sample. As expected in a sample of participants undergoing outpatient therapy services, there was a wide range of time since stroke or PD diagnosis, economic disadvantage, and capacity for walking. Table 2 provides the group statistics for the number of recording days and the walking performance variables. The majority of individuals had five days of recording. Because we found no relationship between number of days of recording and individual variability (r = −0.15, p = 0.18), data from all participants were pooled together. Group means for steps per day are consistent with previously reported values in other cohorts of individuals with chronic stroke28 and similar to other cohorts with PD.21,29 Walking duration, maximum 30- minute output, and peak activity index group means are less widely reported, but appear consistent or slightly lower in value compared with other published reports.22,30
Table 1.
Descriptive characteristics of the sample.
| Stroke (n=37) | Parkinson disease (n=47) | |
|---|---|---|
| Age, yrs | 63 ± 13 (29–85) | 71 ± 7 (55–87) |
| Sex | 13 female (35%) | 21 female (45%) |
| Time since stroke, months | 6.8 ± 0.4 (0.5–65) | -- |
| Side affected by stroke | 21 Left, 16 Right | -- |
| Time since PD diagnosis, yrs | -- | 5.4 ± 5 (0–22) |
| Area of Deprivation Index | 49 ± 33 (4–100) | 20 ± 20 (1–82) |
| Fast-as-possible velocity, m/s | 0.8 ± 0.3 (0.2–1.3) | 1.3 ± 0.3 (0.5–1.8) |
| Self-selected velocity, m/s | 0.6 ± 0.2 (0.1–1.1) | 0.9 ± 0.2 (0.4–1.2) |
| Utilized Assistive Device and/or Orthotic | 25 (68%) | 8 (17%) |
Values are mean±SD (range), or number (%).
Table 2.
Group statistics for walking performance variables collected by the Step Activity Monitor.
| Stroke (n=37) | Parkinson disease (n=47) | |
|---|---|---|
| # SAM days recorded 2 3 4 5 |
1 3 3 30 |
3 3 3 38 |
| Steps per day | 5464 ± 2714 | 8149 ± 4490 |
| Walking duration, min | 192 ± 83 | 247 ± 108 |
| Max 30-min output, steps/min | 27 ± 11 | 42 ± 24 |
| Peak activity index, steps/min | 55 ± 16 | 70 ± 22 |
Values are mean ± SD, unless otherwise indicated.
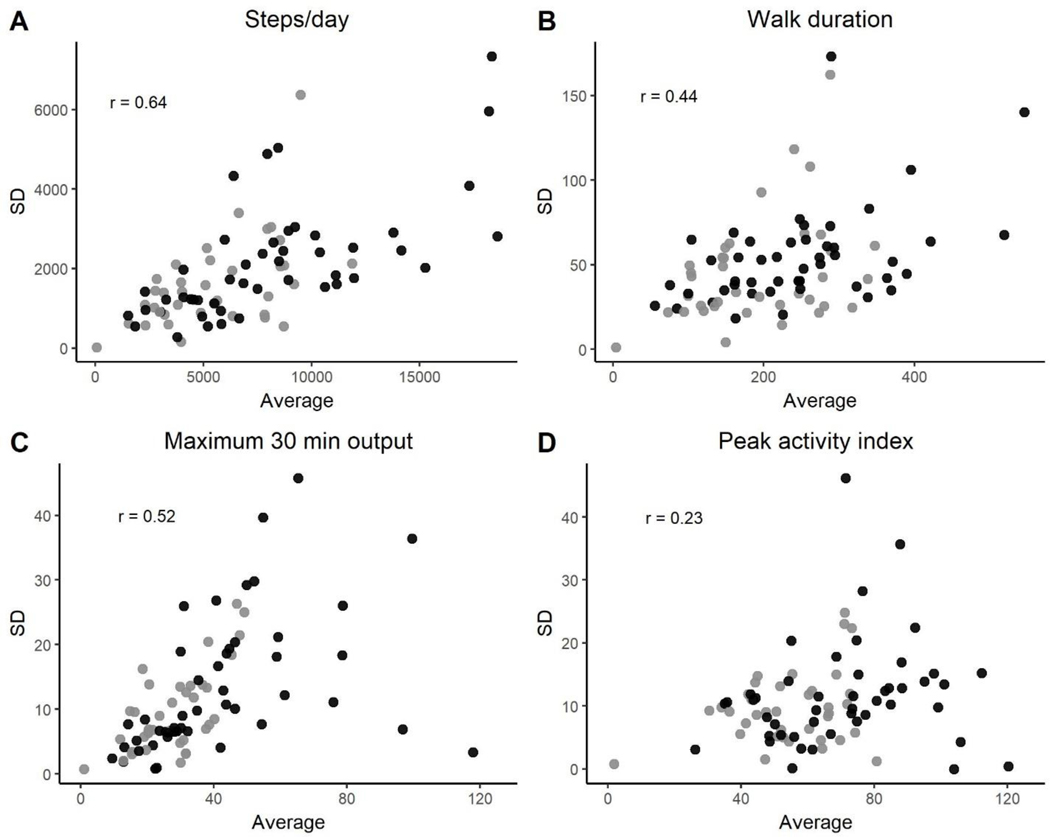
At the individual level, greater amounts of walking were related to more day-to-day variability in walking performance. Figure 1 shows scatterplots of individual means versus individual standard deviations for the four walking performance variables, with participants with stroke marked by gray circles and those with PD marked by black circles. Moderate associations were found between how much walking occurred and how variable the day-to-day amounts were for steps per day (1A; r = 0.64, p < 0.0001), walking duration (1B; r = 0.44, p < 0.0001), and maximum 30 minute output (1C; r = 0.52, p < 0.0001). A weaker relationship was evident for peak activity index (1D; r = 0.23, p = 0.019). An important point seen within the graphs is that an individual’s average amount informs day-to-day variability but medical diagnosis does not, as depicted by the intermingling of the gray (stroke) and black (PD) circles.
Figure 1.
Relationships between individual means and standard deviations (over days) of gait performance variables for all participants. Black circles = PD participants, gray circles = participants with stroke. Walking duration is in minutes, while maximum 30-minute output and peak activity index are in steps/min. For correlations coefficients, p < 0.0001 for A-C and p = 0.015 for D.
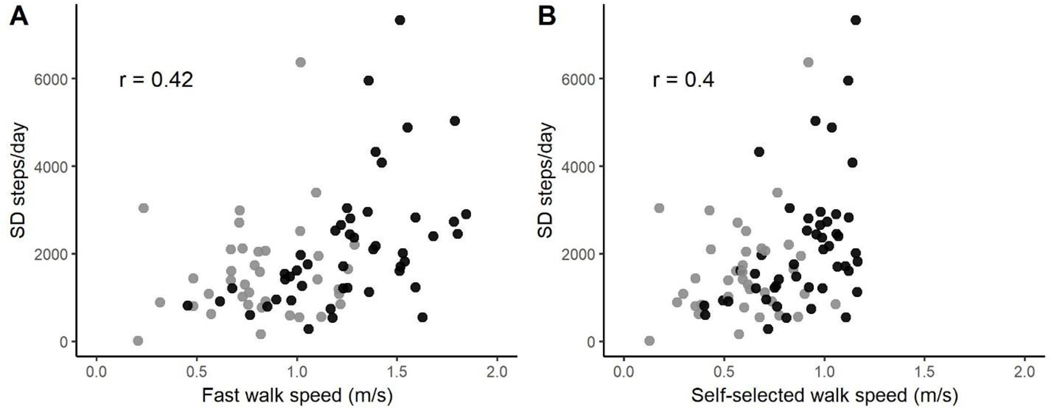
Given that activity measures of walking capacity are readily accessible in the clinic, we also looked to see if there was a relationship between walking capacity, as indexed by gait speed, and individual mean and day-to-day variability of walking performance. Individual gait speed was moderately correlated to individual steps per day (fast, r = 0.42, p < 0.0001; self-selected, r = 0.40, p < 0.0001), as has been shown by others.31 Of more interest, Figure 2 shows scatterplots of fastest (2A) and self-selected walking speed (2B) for each individual versus their individual standard deviations of steps per day. Both pictures reveal moderate relationships, with correlation coefficients of 0.42 and 0.40 (both p <0.0001) for fastest and self-selected walking speed, respectively. Thus, individuals that have a greater capacity to walk, measured via fast or self-selected walking speed, tend to have more day-to-day variability in their walking performance.
Figure 2.
Relationships between walking speed and standard deviation of steps per day. A: Fastest walking speed; B: self-selected walking speed. Black circles = PD participants, gray circles = participants with stroke. Note that an inclusion criterion of the study was self-selected walking speed ≤ 1.2 m/s, so no one has a value above that in panel B. For correlations coefficients, p < 0.0001.
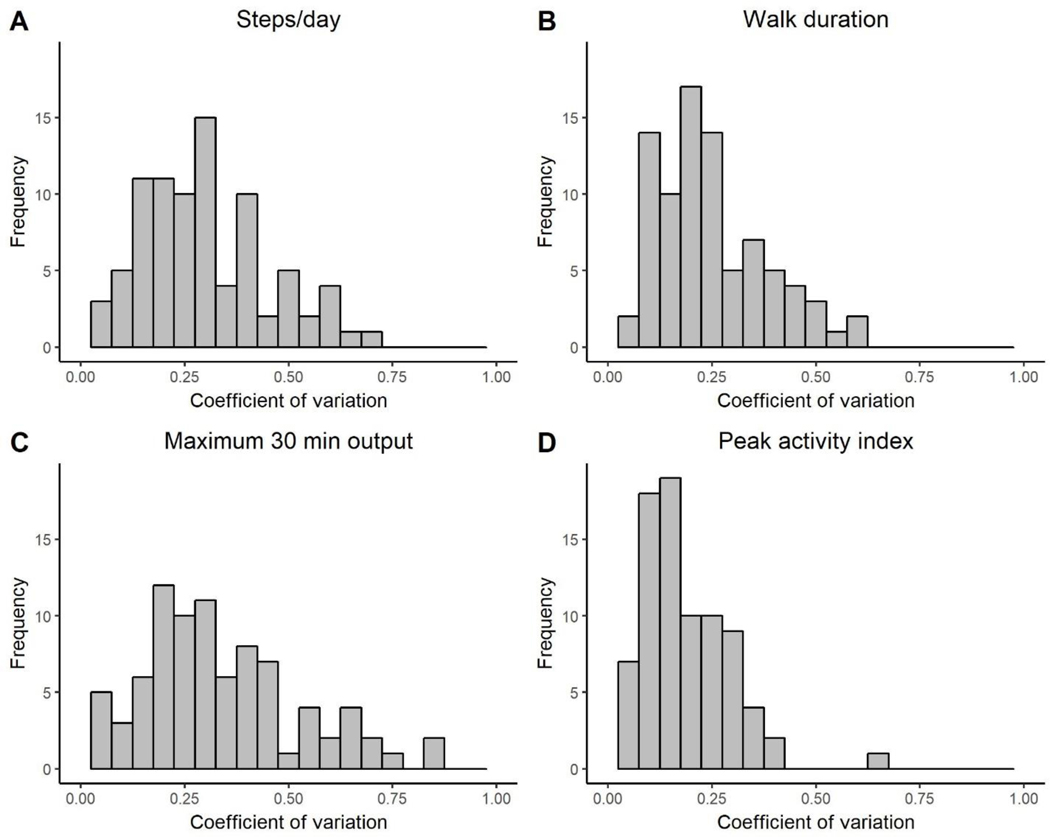
Coefficients of variation provide a standardized unit of variability that can be compared across variables that use different scales. To illustrate the range of day-to-day variability across the sample, Figure 3 shows histograms of the individual coefficients of variation for the four walking performance variables. Coefficients of variation averaged 0.30 ± 0.15, 0.25 ± 0.13, 0.34 ± 0.18, and 0.18 ± 0.11 for steps/day, walk duration, maximum 30 minute output, and peak activity index, respectively. The figure and these mean values demonstrate that for many participants, day-to-day variability was > 25% of their mean value, suggesting substantial variability over the course of the recording period.
Figure 3.
Histograms showing the individual standardized variability (coefficient of variation) of each gait performance measure. For most participants, the day-to-day variability of the measure is ≥ 25% of their mean value.
A series of regression models were used to determine the influence of multiple variables on the variability in walking performance. The primary variable predicting day-to-day variability in walking performance was the mean of that performance variable (steps per day, adjusted R2= 0.40; walk duration, adjusted R2= 0.18; max 30-min output, adjusted R2=0.26; peak activity index, adjusted R2=0.04; all p<0.001). Adding diagnosis (stroke or PD) and walking speed did not alter the model, signifying that neither medical diagnoses nor walking capacity provided additional predictive information about day-to-day variability in walking performance. The only other significant predictive variable was the ADI in the model predicting day-to-day variability of steps per day (adjusted R2=0.44, p<0.001). ADI was not significant for the other three walking performance variables. Interestingly, no relationships were found between ADI and steps per day alone (zero-order r = −0.18, p = 0.11 for means; r = 0.08, p = 0.47 for standard deviations). This means that, after controlling for the average amount of walking, participants that lived in areas that were more socio-economically deprived had more day-to-day variability in steps per day.
DISCUSSION
This study examined the day-to-day variability in walking performance for people post stroke or with PD who were receiving outpatient physical therapy to improve mobility. Greater amounts of walking were related to greater variability in walking performance as measured across steps per day, walking duration, maximum 30-minute output and peak activity index, regardless of medical diagnosis. Individual means of each performance variable were the primary predictors of variability of each measure, with socioeconomic disadvantage an additional influential factor on day-to-day variability of steps per day. Socioeconomic disadvantage may play a role in the amount of options a particular individual has to perform mobility, with a reduction of options for those who live in more deprived areas. Knowledge of the extent of variability and the factors that influence variability will facilitate research and clinical care alike. Results presented here inform how to capture walking performance across an episode of clinical care. Results can also be utilized when designing intervention studies to estimate the minimum size of anticipated changes and evaluating whether or not change has occurred post intervention.
Variability in steps per day and other walking performance measures is large, as can be seen by the spread of data points in Figure 1 and the coefficients of variation in Figure 2. The large day-to-day variability seen here strongly supports previous recommendations of measuring walking performance in individuals over at least three days18,20,22. Recording for only one day is unlikely to provide an accurate quantification of an individual’s walking performance. While collecting a minimum of three days of walking performance data is well-established in research studies7,22,25, the data presented here highlight the critical need to also collect at least three days of walking performance data in clinical practice. Further, steps per day may be the most tangible and salient measure to capture and address over the course of clinical care.
These data were collected as participants engaged in routine outpatient services at several clinics across the United States, making them highly generalizable. Without multiple measurement days, individuals undergoing rehabilitation services and their treating clinicians will not know if changes from one time point to the next are true changes, versus just part of that individual’s day-to-day variability. For example, Figure 1A shows multiple individuals who walk around 5000 steps per day on average. The range of day-to-day variability in those individuals extends from about 750 steps per day to over 2300 steps per day. Thus, the size of a true change from one time point to the next will vary per individual and could exceed 2300 steps per day or nearly 50% of the mean value. These numbers suggest that any minimal detectable change (MDC) and minimal clinical important difference (MCID) values for steps per day or other walking performance variables would likely be quite large and would need to be tailored to the individual.
Individuals seek out rehabilitation services to improve walking performance in their daily lives. The role of the therapist is to assist the patient in achieving these goals. Unfortunately, at the present time, measurement of walking performance is not regularly assessed clinically. The most obvious barriers to routine assessment is the lack of accurate, low-cost devices and quick access to the data. Given the rapid advance of technology, this barrier should fade with time. Other barriers are lie in common misperceptions that: 1) walking capacity (e.g. gait speed) accurately reflects walking performance (e.g. steps per day); and 2) that a change in walking capacity indicates a change in walking performance. Indeed, walking capacity and walking performance are moderately related, as shown in previous reports31 and in the data set here. For an individual patient however, it may be unwise to assume that a particular walking speed matches a particular number of steps per day. Multiple reports indicate that walking capacity accounts for 30–45% of the variance in daily walking performance after stroke, leaving up to 70% of the variance unexplained.32,33 To the second point above, there is emerging data showing a discrepancy in amount of change in capacity measured in the clinic compared to change in performance for both the upper and lower limbs.34–36 Both capacity and performance measures are informative activity-level measures that, while related, provide unique information from which therapists need to make clinical decisions. Capturing both capacity and performance measures provides a more comprehensive picture of walking for each patient independent of medical diagnosis.
Limitations
There are three main limitations that could influence the interpretation of these data. First, three of the walking performance variables (duration of walking activity, maximum 30 -minute output and peak activity index) were calculated in 1- minute epochs. As indicated in the Methods, there is the potential that the presented data from these variables are overestimates of the true values. However, it is unlikely that a general overestimate would change the relationships found here between amount and variability. Second, gait speed was selected as the measure of walking capacity because it is the most common and easiest to administer in clinical care.5 An alternative walking capacity measure would have been the six-minute walk test. Walking speed and the six-minute walk test are highly correlated to each other,37,38 and the six-minute walk test has a moderate to strong relationship with walking performance, as measured with steps per day.39 Despite its relationship being somewhat stronger than gait speed, the six- minute walk test is estimated to explain 38–54% of the variance in daily walking performance.40 Thus, relationships between walking capacity and performance might have been stronger if we had used the six- minute walk test, but it still would not be an adequate substitute for directly measuring walking performance. Third and finally, some unknown portion of the relationships shown here are likely due to mathematical relationships between amount and variability. Since neither amount nor variability can be a negative number, only a small range of variability is possible for a small average amount. Likewise, with large average amounts, a larger range of variability is possible. The mathematical relationship cannot account for the majority of the relationships found however, because the data in Figure 1 show many examples of persons with the same average amounts but with different variabilities and vice versa.
Conclusion
There is large variability in day-to-day walking performance, as seen across the four variables quantified. The amount of daily walking consistently influenced day-to-day variability of walking performance. For steps per day, socioeconomic disadvantage additively influenced variability. Knowledge of day-to-day variability will inform clinicians and researchers as they try to evaluate change in walking performance across clinical settings and research studies. Future work could explore day-to-day variability in larger samples that include other neurologic populations to expand the generalizability of these results and further evaluate the role of medical diagnoses on variability. Further studies could also explore individualized approaches to determining real change and individualized approaches to improving walking performance in daily life.
Supplementary Material
Acknowledgments
Funding: NIH R01HD068290
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2012(8):CD002817. [DOI] [PubMed] [Google Scholar]
- 2.Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9(2):e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice DB, McIntyre A, Mirkowski M, et al. Patient-Centered Goal Setting in a Hospital-Based Outpatient Stroke Rehabilitation Center. PM & R : the journal of injury, function, and rehabilitation. 2017;9(9):856–865. [DOI] [PubMed] [Google Scholar]
- 4.Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M, Practice Recommendations Development G. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord. 2007;22(4):451–460; quiz 600. [DOI] [PubMed] [Google Scholar]
- 5.Moore JL, Potter K, Blankshain K, Kaplan SL, O’Dwyer LC, Sullivan JE. A Core Set of Outcome Measures for Adults With Neurologic Conditions Undergoing Rehabilitation: A CLINICAL PRACTICE GUIDELINE. Journal of neurologic physical therapy : JNPT. 2018;42(3):174–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. 2010;41(1):129–135. [DOI] [PubMed] [Google Scholar]
- 7.Danks KA, Roos MA, McCoy D, Reisman DS. A step activity monitoring program improves real world walking activity post stroke. Disability and rehabilitation. 2014;36(26):2233–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlin JE, Storti KL, Brach JS. Using activity monitors to measure physical activity in free-living conditions. Physical therapy. 2006;86(8):1137–1145. [PubMed] [Google Scholar]
- 9.Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40(3):293–298. [DOI] [PubMed] [Google Scholar]
- 10.International classification of functioning, disability, and health : ICF [computer program]. Version 1.0. Geneva: : World Health Organization, [2001] ©2001; 2001. [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez-Roux AV, Kiefe CI, Jacobs DR Jr, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11(6):395–405. [DOI] [PubMed] [Google Scholar]
- 14.Durfey SNM, Kind AJH, Buckingham WR, DuGoff EH, Trivedi AN. Neighborhood disadvantage and chronic disease management. Health Serv Res. 2019;54 Suppl 1:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med. 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Kind AJH, Nerenz D. Area Deprivation Index Predicts Readmission Risk at an Urban Teaching Hospital. Am J Med Qual. 2018;33(5):493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisconsin DoMUo. Neighborhood Atlas. 2015; https://www.neighborhoodatlas.medicine.wisc.edu/. [Google Scholar]
- 18.Schmidt AL, Pennypacker ML, Thrush AH, Leiper CI, Craik RL. Validity of the StepWatch Step Activity Monitor: preliminary findings for use in persons with Parkinson disease and multiple sclerosis. J Geriatr Phys Ther. 2011;34(1):41–45. [DOI] [PubMed] [Google Scholar]
- 19.Macko RF, Haeuber E, Shaughnessy M, et al. Microprocessor-based ambulatory activity monitoring in stroke patients. Med Sci Sports Exerc. 2002;34(3):394–399. [DOI] [PubMed] [Google Scholar]
- 20.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Physical therapy. 2014;94(2):222–229. [DOI] [PubMed] [Google Scholar]
- 21.Paul SS, Ellis TD, Dibble LE, et al. Obtaining Reliable Estimates of Ambulatory Physical Activity in People with Parkinson’s Disease. J Parkinsons Dis. 2016;6(2):301–305. [DOI] [PubMed] [Google Scholar]
- 22.Roos MA, Rudolph KS, Reisman DS. The structure of walking activity in people after stroke compared with older adults without disability: a cross-sectional study. Physical therapy. 2012;92(9):1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornby TG, Holleran CL, Hennessy PW, et al. Variable Intensive Early Walking Poststroke (VIEWS): A Randomized Controlled Trial. Neurorehabil Neural Repair. 2016;30(5):440–450. [DOI] [PubMed] [Google Scholar]
- 24.Smith BA, Lang CE. Sensor Measures of Symmetry Quantify Upper Limb Movement in the Natural Environment Across the Lifespan. Archives of physical medicine and rehabilitation. 2019;100(6):1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Capturing ambulatory activity decline in Parkinson’s disease. J Neurol Phys Ther. 2012;36(2):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaffer SD, Holzapfel SD, Fulk G, Bosch PR. Step count accuracy and reliability of two activity tracking devices in people after stroke. Physiotherapy theory and practice. 2017;33(10):788–796. [DOI] [PubMed] [Google Scholar]
- 27.Dorsch AK, Thomas S, Xu X, Kaiser W, Dobkin BH, investigators S. SIRRACT: An International Randomized Clinical Trial of Activity Feedback During Inpatient Stroke Rehabilitation Enabled by Wireless Sensing. Neurorehabilitation and neural repair. 2015;29(5):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How Physically Active Are People Following Stroke? Systematic Review and Quantitative Synthesis. Phys Ther. 2017;97(7):707–717. [DOI] [PubMed] [Google Scholar]
- 29.Benka Wallen M, Franzen E, Nero H, Hagstromer M. Levels and Patterns of Physical Activity and Sedentary Behavior in Elderly People With Mild to Moderate Parkinson Disease. Physical therapy. 2015;95(8):1135–1141. [DOI] [PubMed] [Google Scholar]
- 30.Cavanaugh JT, Coleman KL, Gaines JM, Laing L, Morey MC. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. J Am Geriatr Soc. 2007;55(1):120–124. [DOI] [PubMed] [Google Scholar]
- 31.Middleton A, Fulk GD, Beets MW, Herter TM, Fritz SL. Self-Selected Walking Speed is Predictive of Daily Ambulatory Activity in Older Adults. J Aging Phys Act. 2016;24(2):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danks KA, Pohlig RT, Roos M, Wright TR, Reisman DS. Relationship Between Walking Capacity, Biopsychosocial Factors, Self-efficacy, and Walking Activity in Persons Poststroke. Journal of neurologic physical therapy : JNPT. 2016;40(4):232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thilarajah S, Mentiplay BF, Bower KJ, et al. Factors Associated With Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Archives of physical medicine and rehabilitation. 2018;99(9):1876–1889. [DOI] [PubMed] [Google Scholar]
- 34.Doman CA, Waddell KJ, Bailey RR, Moore JL, Lang CE. Changes in Upper-Extremity Functional Capacity and Daily Performance During Outpatient Occupational Therapy for People With Stroke. Am J Occup Ther. 2016;70(3):7003290040p7003290041–7003290040p7003290011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waddell KJ, Strube MJ, Bailey RR, et al. Does Task-Specific Training Improve Upper Limb Performance in Daily Life Poststroke? Neurorehabil Neural Repair. 2017;31(3):290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardestani MM, Henderson CE, Hornby TG. Improved walking function in laboratory does not guarantee increased community walking in stroke survivors: Potential role of gait biomechanics. J Biomech. 2019;91:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulk GD, Echternach JL, Nof L, O’Sullivan S. Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiotherapy theory and practice. 2008;24(3):195–204. [DOI] [PubMed] [Google Scholar]
- 38.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82. [DOI] [PubMed] [Google Scholar]
- 39.Mudge S, Stott NS. Timed walking tests correlate with daily step activity in persons with stroke. Archives of physical medicine and rehabilitation. 2009;90(2):296–301. [DOI] [PubMed] [Google Scholar]
- 40.Mudge S, Stott NS. Timed Walking Tests Correlate With Daily Step Activity In Persons With Stroke. Archives of physical medicine and rehabilitation. 2009;90(2):296–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.