Abstract
Background
Venovenous extracorporeal membrane oxygenation (ECMO) is increasingly being used for acute respiratory distress syndrome and as a bridge to lung transplantation. After initiation of venovenous ECMO, systemic anticoagulation therapy is traditionally administered and can cause bleeding diathesis. Here, we investigated whether venovenous ECMO can be administered without continuous systemic anticoagulation administration for patients with acute respiratory distress syndrome.
Methods
This is a retrospective review of an institutional ECMO database. We included consecutive patients from January 2015 through February 2019. Overall, 38 patients received low levels of continuous systemic anticoagulation (AC + ) whereas the subsequent 36 patients received standard venous thromboprophylaxis (AC −). Published Extracorporeal Life Support Organization guidelines were used for the definition of outcomes and complications.
Results
Overall, survival was not different between the two groups (P = .58). However, patients in the AC + group had higher rates of gastrointestinal bleeding (28.9%, vs AC− group 5.6%; P < .001). The events per patient-day of gastrointestinal bleeding was 0.00025 in the AC− group and 0.00064 in the AC+ group (P < .001). In addition, oxygenator dysfunction was increased in the AC+ group (28.9% and 0.00067 events per patient-day, vs AC− 11.1% and 0.00062 events per patient-day; P = .02). Furthermore, the AC+ group received more transfusions: packed red blood cells, AC+ group 94.7% vs AC− group 55.5% (P < .001); fresh frozen plasma, AC+ 60.5% vs AC− 16.6% (P = .001); and platelets, AC+ 84.2% vs AC− 27.7% (P < .001). There was no circuit thrombosis in either groups throughout the duration of ECMO support.
Conclusions
Our results suggest that venovenous ECMO can be safely administered without continuous systemic anticoagulation therapy. This approach may be associated with reduced bleeding diathesis and need for blood transfusions.
Venovenous (VV) extracorporeal membrane oxygenation (ECMO) is increasingly used in the management of severe acute respiratory distress syndrome and as a bridge to lung transplantation.1,2 However, patients placed on VV-ECMO traditionally receive continuous systemic anticoagulant therapy, which increases the risk of bleeding diathesis. For instance, in the recently published EOLIA trial, 46% of the patients had bleeding complications such as gastrointestinal bleeding,3 increasing their risk of mortality and morbidity while being supported on VV-ECMO. In patients being bridged to transplantation, bleeding results in blood transfusions that increase sensitization to histocompatibility antigens, posing immunologic challenges.
The anticoagulation management guidelines for VV-ECMO are largely extrapolated from cardiopulmonary bypass literature and personal experiences. Recent studies suggest that lower levels of systemic anticoagulation can be used to support patients with VV-ECMO.4,5 However, the feasibility and safety of VV-ECMO without continuous systemic anticoagulation is only published in the form of anecdotal reports.6–9 Advances in circuit technology, including heparin-coated tubing, newer polymethylpentene oxygenators, and centrifugal pumps, have reduced the thrombogenicity of ECMO.10–13 We postulated that these improvements may obviate the need for continuous systemic anticoagulation during VV-ECMO therapy. Accordingly, we adopted a programmatic approach of administering VV-ECMO without anticoagulation therapy, and we report our experience here. Specifically, the goal of our study was to determine the safety and feasibility of VV-ECMO without continuous systemic anticoagulant therapy. Our data suggest that prolonged VV-ECMO support can be safely performed without therapeutic doses of anticoagulant agents, thereby reducing bleeding complications as well as blood transfusions. Given that hemorrhagic complications significantly contribute to the morbidity and mortality associated with VV-ECMO, we postulate that our approach could significantly improve outcomes associated with mechanical life support.
Patients and Methods
Study Subjects
Patient data were collected prospectively in our ECMO database, and for the purposes of the study, a retrospective analysis was performed using the database. We included consecutive adult patients placed on VV-ECMO at our medical center between January 2015 and February 2019. A total of 23 patients were excluded from this study to avoid confounding effects. These included patients with a pre-ECMO condition such as deep vein thrombosis, pulmonary embolism, or a hypercoagulable state that would necessitate therapeutic anticoagulation. We also excluded the patients who required conversion to venoarterial ECMO or venoarterial-venous ECMO.
All patients received anticoagulant treatment (n = 38) with either heparin or bivalirudin drip, before our change in practice in June 2017. The target anticoagulation levels for these patients included activated clotting time of 160 to 180 seconds or activated partial thromboplastin time between 50 and 70 seconds, as suggested by conventional guidelines and recent publications.14–17 Patients managed without systemic anticoagulation (n = 36), after June 2017, did not receive continuous anticoagulation therapy and were not monitored with bleeding measurements such as activated clotting time or activated partial thromboplastin time. As part of standard intensive care unit management, patients not receiving continuous systemic anticoagulant agents only received unfractionated heparin (5000 U given subcutaneously every 8 hours) for deep venous thrombosis prophylaxis.
To avoid thrombotic complication in the ECMO circuit, flow was maintained at least 3 to 3.5 L/min, consistent with our recent reports demonstrating the feasibility of using VV-ECMO without anticoagulation.7,18 The ECMO flows are maintained at a speed to allow normal blood oxygenation and carbon dioxide levels while minimizing hemolysis. The typical flow rates were 3.5 to 4.5 L/min for both groups. For both groups, transfusion thresholds included platelets less than 50,000/mL, hemoglobin less than 7 g/dL, or hemodynamic instability in the setting of active blood loss. Different cannulation strategies—internal jugular vein to femoral vein cannulation vs ProtekDuo (CardiacAssist, Pittsburgh, PA) cannulation—were used in patients depending on surgeon preference. However, the anticoagulation strategy was not different between the cannulation strategies as they were managed using the same ECMO circuit. The VV-ECMO circuit included Quadrox iD adult (7.0) oxygenator (Maquet, Rastatt, Germany) and Rotaflow pump (Maquet). Components of the circuit including the tubing and oxygenator were heparin coated but the cannulas were not.
At our institution, patients with respiratory failure are considered for ECMO if they fail to achieve satisfactory gas exchange (Pao2 greater than 55 mm Hg, oxygen saturations greater than 88%, pH higher than 7.2, with plateau pressures less than 35) despite lung protective mechanical ventilation and recruitment maneuvers with neuromuscular blockade, consistent with our previous reports.18 The decision to cannulate is made by a multidisciplinary ECMO team including pulmonologists, thoracic surgeons, ECMO specialists, and intensivists, using a teleconference line. This study was approved by the Northwestern University Institutional Review Board (STU00207250). However, the need for patient consent for data collection was waived by the Board as this was a retrospective study.
Definitions of Complications
Postcannulation complications were defined as follows. (1) Neurologic dysfunction was a new neurologic deficit associated with abnormal neuroimaging findings; cases of neurologic dysfunction were classified as either ischemic or hemorrhagic based on imaging findings. (2) Gastrointestinal bleeding was one or more of the following: guaiac-positive stool, hematemesis, melena, active bleeding at the time of endoscopy or colonoscopy, or blood within the stomach at endoscopy or colonoscopy. (3) Infection was defined as bacteremia or pneumonia with a positive bronchoalveolar lavage culture. (4) Acute kidney injury was defined using the “risk, failure, loss of kidney function, and end-stage kidney disease” (RIFLE) classification.19 For the amount of blood transfusion, units of packed red blood cells, fresh frozen plasma, and platelets transfusion were collected between VV-ECMO initiation and decannulation. Daily inspection of oxygenators with special regard to thrombotic scaling was performed by a perfusionist. Postoxygenator blood gas analysis was performed every 24 hours, and indication for change of the oxygenator was postoxygenator Pao2/fraction of inspired oxygen less than 250 mm Hg with clinical deterioration of pulse oximetry measured oxygen saturation.
Statistical Analysis
Patient demographics, postoperative complications, and outcomes were compared between the systemic anticoagulation-free group and the systemic anticoagulation group. Continuous variables were compared using Student’s t test and reported as median (interquartile range). Categoric variables were compared using χ2 test and reported as number (percentage). The Kaplan-Meier method was used to estimate survival, and a log rank test was performed to compare survival between the two groups. Cox proportional hazards regression was used to derive hazard ratios and 95% confidence intervals. All P values less than 0.05 were accepted as statistically significant. To build our models, we performed a univariate analysis and included all predictors if the test had a P value of 0.2 or less. To assess the overall goodness of fit, we used Gronnesby and Borgan tests. Statistical analyses were performed using Stata/MP14 (StataCorp, College Station, TX).
Results
Study Population
During the study period, 74 patients were placed on VV-ECMO (Table 1, Supplemental Table 1). Table 1 shows pre VV-ECMO characteristics of the study cohort. Thirty-six patients were managed without systemic anticoagulation (AC−) and 38 patients received systemic anticoagulation (AC+). There were no significant differences in patient characteristics between the two groups, except for sex (P = .04). However, the AC+ group had a significantly longer duration of support (AC+ group 12.7 ± 20.3 days vs AC− group 4.6 ± 6.1 days, P < .001). The etiologies of lung failure are shown in Table 2 and were not significantly different between the two groups. The median follow-up since the initiation of ECMO for the entire cohort, AC+ group, and AC− group was, respectively, 116.0 ± 191 days, 97.5 ± 197 days, and 126 ± 184.2 days (P = .80).
Table 1.
Clinical Characteristics of Patients Before Initiation of Venovenous Extracorporeal Membrane Oxygenation
| Variable | Overall (n = 74) | Anticoagulation Free (n = 36) | Anticoagulation (n = 38) | P Value |
|---|---|---|---|---|
| Support days | 7.2 ± 11.4 | 4.6 ± 5 | 12.7 ± 16.9 | <.001 |
| Age, y | 47 ± 23.3 | 47 ± 21.9 | 48.1 ± 27.3 | .41 |
| Female | 35 (47.2) | 13 (36.1) | 22 (57.8) | .04 |
| Body mass index, kg/m2 | 27.6 ± 11.1 | 27.5 ± 11.2 | 27.7 ± 11.2 | .57 |
| COPD | 9 (12.1) | 5 (13.8) | 4 (10.5) | .72 |
| Chronic kidney disease | 14 (18.9) | 6 (16.7) | 8 (21) | .58 |
| Dialysis | 5 (6.7) | 2 (5.6) | 3 (7.8) | .67 |
| RESP score | 1 ± 4 | 1.6 ± 4 | 0 ± 3 | .12 |
| Cannulation site | ||||
| Internal jugular-femoral | 47 (63.6) | 27 (75) | 20 (52.6) | .06 |
| Protek | 27 (36.4) | 9 (25) | 18 (47.4) | |
| Ventilation, h | 67.7 ± 87.5 | 33.2 ± 8.5 | 90.2 ± 131.5 | .11 |
| Laboratory | ||||
| Hemoglobin, g/dL | 10.5 ± 3.5 | 10.7 ± 3.6 | 10.4 ± 2.8 | .79 |
| WBC, 1000/mm3 | 13 ± 7.9 | 12.1 ± 8.2 | 13.3 ± 6.9 | .19 |
| Platelets, 1000/mm3 | 195 ± 163.7 | 197.5 ± 150.7 | 192.0 ± 164.5 | .71 |
| Albumin, g/dL | 3 ± 1.1 | 3.0 ± 1.2 | 3.0 ± 0.8 | .43 |
| INR | 1.5 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.2 | .06 |
| PT | 14.1 ± 3.3 | 12.9 ± 3.4 | 1.3 ± 2.2 | .15 |
| PTT | 30 ± 7.7 | 27.8 ± 7.6 | 30.8 ± 5.9 | .96 |
Values are median ± interquartile range or n (%).
COPD, chronic obstructive pulmonary disease; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; RESP, Respiratory ECMO Survival Prediction; WBC, white blood cell.
Table 2.
Etiology of Lung Failure
| Variable | Overall (n = 74) | Anticoagulation Free (n = 36) | Anticoagulation (n = 38) | P Value |
|---|---|---|---|---|
| Bacterial pneumonia | 18 | 11 | 7 | .22 |
| Worsening of ILD | 14 | 6 | 8 | .63 |
| Lung transplant-related PGD | 13 | 4 | 9 | .15 |
| Viral pneumonia | 7 | 3 | 4 | .74 |
| Worsening of COPD | 2 | 1 | 1 | .96 |
| Pulmonary artery hypertension | 2 | 0 | 2 | .16 |
| Pulmonary embolism | 2 | 0 | 2 | .16 |
| Chest trauma | 2 | 1 | 1 | .96 |
| Othersa | 14 | 10 | 4 | .11 |
Including pancreatitis-associated respiratory failure, hepatitis-related respiratory failure, transfusion-related acute lung injury, and airway obstruction.
COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; PGD, primary graft dysfunction.
Complication Rates and Mortality
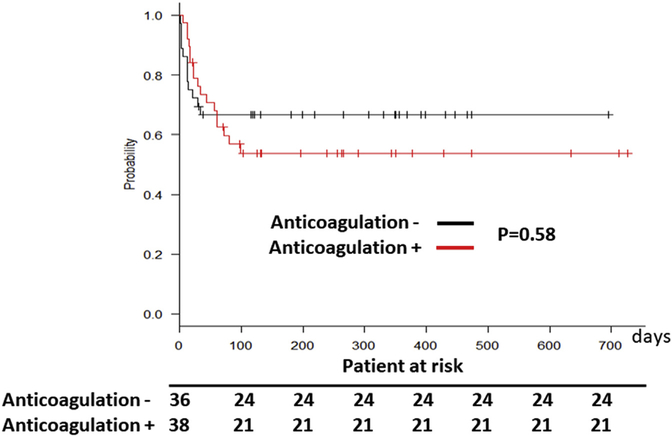
We compared postcannulation complications between patients managed without systemic anticoagulation and patients who received systemic anticoagulation. After VV-ECMO initiation, 13 patients had gastrointestinal bleeding: 2 AC− group (5.6%), and 11 AC+ group (28.9%; P < .001). The event per patient per day (EPPD) of gastrointestinal bleeding was 0.00025 in the AC− group and 0.00064 in the AC+ group (P < .001). Oxygenator dysfunction occurred 5 times (11.1%, 0.00062 EPPD) in the AC− group and 20 times (28.9%, 0.00067 EPPD) in the AC+ group (P = .02). There were no significant differences between the two groups in the incidence of acute kidney injury (33.3% vs 44.7%, P = .13), hemodialysis use (33.3% vs 31.5%, P = .86), and neurologic dysfunction (5.6% vs 10.4%, P = .42; Table 3). The mortality rates at 30, 90,180, and 365 days after VV-ECMO implantation were also not significantly different between the two groups (P = .58; Figure 1, Table 4).
Table 3.
Incidence of Adverse Events During Venovenous Extracorporeal Membrane Oxygenation Support
| Anticoagulation Free (n = 36; 225 days) |
Anticoagulation (n = 38; 780 days) |
||||||
|---|---|---|---|---|---|---|---|
| Adverse Event | Patientsa | Events | EPPD | Patientsa | Events | EPPD | P Value |
| Acute kidney injury | 11 (33.3) | ... | ... | 17 (44.7) | ... | ... | .13 |
| Dialysis | 11 (33.3) | ... | ... | 12 (31.5) | ... | ... | .86 |
| Neurologic dysfunction | 2 (5.6) | 2 | 0.00025 | 4 (10.4) | 4 | 0.00013 | .42 |
| IND | 0(0) | 0 | ... | 2 (5.2) | 2 | 0.00007 | .16 |
| HND | 2 (5.6) | 2 | 0.00025 | 2 (5.2) | 2 | 0.00007 | .97 |
| GI bleeding | 2 (5.6) | 2 | 0.00025 | 11 (28.9) | 19 | 0.00064 | <.001 |
| Oxygenator exchange | 4 (11.1) | 5 | 0.00062 | 12 (31.5) | 27 | 0.00091 | .03 |
Values are n (%).
EPPD, event per patient per day; GI, gastrointestinal; HND, hemorrhagic neurologic dysfunction; IND, ischemic neurologic dysfunction.
Figure 1.
Survival of patients undergoing venovenous extracorporeal membrane oxygenation with (+ [red line]) and without (− [black line]) systemic anticoagulation therapy.
Table 4.
Survival After Venovenous Extracorporeal Membrane Oxygenation Support
| Survival Days | Anticoagulation Free (n = 36) | Anticoagulation (n = 38) | P Value |
|---|---|---|---|
| 30 | 72.2 | 76.3 | .89 |
| 60 | 69.4 | 68.4 | .92 |
| 180 | 66.6 | 55.2 | .44 |
| 365 | 66.6 | 55.2 | .44 |
Values are percentages.
Blood Transfusion in Study Groups
The AC+ group received significantly more transfusions during VV-ECMO support: packed red blood cells, AC− group 20 (55.5%), AC+ group 36 (94.7%; P < .001); fresh frozen plasma, AC− group 6 (16.6%), AC+ group 23 (60.5%; P = .001); and platelets, AC− group 10 (27.7%), AC+ group 32 (84.2%; P < .001; Table 5).
Table 5.
Blood Transfusion During Venovenous Extracorporeal Membrane Oxygenation Support
| Anticoagulation Free (n = 36; 225 days) |
Anticoagulation (n = 38; 780 days) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Event | Patientsa | Units | UPP | UPPD | Patientsa | Units | UPP | UPPD | P value |
| pRBC | 20 (55.5) | 120 | 3.33 | 0.014 | 36 (94.7) | 955 | 25.1 | 0.051 | <.001 |
| FFP | 6 (16.6) | 12 | 0.33 | 0.0014 | 23 (60.5) | 198 | 5.21 | 0.008 | .001 |
| Plt | 10 (27.7) | 105 | 2.91 | 0.012 | 32 (84.2) | 366 | 9.63 | 0.018 | <.001 |
Values are n (%).
FFP, fresh frozen plasma; Plt, platelets; pRBC, packed red blood cells; UPP, unit per patient; UPPD, unit per patient per day.
Cox Multivariable Logistic Regression Analysis of Association Between VV-ECMO and Outcome
All variables were placed in a univariate analysis and included all predictors if the test had a P value of 0.2 or less for a multiple Cox proportional hazards model with postoperative survival as the outcome (Supplemental Table 2). We found that body surface area of the patient, Respiratory ECMO Survival Prediction score, and low platelet counts before initiation of ECMO were independent predictors of postoperative survival (Table 6). However, anticoagulation use, or its lack thereof, did not emerge as an independent predictor of mortality in this modeling.
Table 6.
Cox Multivariate Logistic Regression Analysis Predictors of Postoperative mortality
| Variable | HR | P value | 95% CI |
|---|---|---|---|
| Age, y | 1.02 | .12 | 0.992–1.061 |
| Body surface area, m2 | 0.11 | .01 | 0.022–0.629 |
| Chronic kidney disease | 1.28 | .23 | 0.847–1.937 |
| RESP score | 0.75 | <.001 | 0.639–0.891 |
| Cannulation site | 0.67 | .39 | 0.273–1.659 |
| Laboratory | |||
| Platelets, 1000/mm3 | 0.98 | <.001 | 0.984–0.998 |
| ALT, U/L | 1 | .63 | 0.997–1.004 |
| Albumin, g/dL | 0.73 | .39 | 0.365–1.492 |
ALT, alanine aminotransferase; CI, confidence interval; HR, hazard ratio; RESP, Respiratory ECMO Survival Prediction.
Comment
We found that adult patients supported with VV-ECMO can be managed without continuous systemic anticoagulant therapy for prolonged periods. These patients did not have higher rates of thrombotic complications or death, but had lower rates of gastrointestinal bleeding and blood transfusions. In addition, the oxygenator failure rates were surprisingly higher among patients receiving anticoagulation therapy, even though activated clotting times was maintained between 160 and 180 seconds and activated partial thromboplastin time was maintained between 50 and 70 seconds according to the Extracorporeal Life Support Organization guidelines.20 It is widely known that levels of anticoagulation vary, which might result in either increase or temporary withholding of anticoagulant agents.21 These fluctuations and cessation/re-initiation cycle of the anticoagulant can potentially lead to a prothrombotic state, which might have caused the higher incidence of oxygenator clots observed in the group receiving anticoagulation therapy. Alternatively, the increased rates of oxygenator failure might be related to the greater duration of ECMO support observed among patients receiving anticoagulant agents.
Data from our study are consistent with a prior report by Krueger and colleagues5 who reported that VV-ECMO with prophylactic dosage is feasible when applied in patients without thrombotic events. However, as that was an observational study with no controls, it was difficult to interpret the reported outcomes. Previous studies have demonstrated that bleeding diathesis, such as gastrointestinal bleeding and hemorrhage stroke, is high among ECMO patients, with incidence rates approaching 60%, and is independently associated with worse survival.22–25 Reports have also shown that the ECMO cannulation site is a common source of bleeding in patients undergoing ECMO,22,26,27 which can be worsened by systemic anticoagulation therapy. However, we did not encounter any cannula site bleeding, perhaps related to our practice of placing a pursestring suture around the cannulas, which might mitigate this complication.
Bleeding complications require blood transfusion that is associated with several adverse effects to patients. In our study, patients managed by anticoagulation-free VV-ECMO received significantly fewer blood transfusions compared with patients in the control cohort. Indeed, our patients managed without systemic anticoagulation also received many fewer transfusions compared with the historic controls in previously published literature.25,28–30 However, to achieve anticoagulation-free VV-ECMO, we believe that the flows should be kept at least 3 L/min to 3.5 L/min to reduce circuit clots. If there is consistent reduction in circuit flow due to hypovolemia, low-dose anticoagulation may be considered. Patients with an underlying prothrombotic state, such as heparin-induced thrombocytopenia, should not be considered for anticoagulation-free VV-ECMO. Moreover, patients with cardiac failure and intracardiac stasis should also not be managed using this approach. Finally, our findings cannot be generalized to ECMO circuits that do not have heparin coating.
Our study has some limitations. We studied a relatively small number of patients at a single-center, and that may limit the generalizability of our conclusions. Furthermore, our retrospective design is a potential source of bias. Finally, as our study is not a randomized trial, it can only demonstrate an association between systemic anticoagulation-free VV-ECMO and improved clinical outcomes. Nevertheless, our data suggest that prolonged VV-ECMO support can be safely performed without therapeutic doses of anticoagulant agents, thereby reducing bleeding complications and blood transfusions. Given that hemorrhagic complications significantly contribute to the morbidity and mortality associated with VV-ECMO, we postulate that our approach could significantly improve outcomes associated with mechanical life support.
Supplementary Material
Acknowledgments
AB is supported by National Institutes of Health HL125940, HL145478, HL147290, and HL147575. The authors would like to thank Ms Elena Susan for administrative assistance in the submission of this manuscript.
Footnotes
The Supplemental Tables can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2020.02.011] on http://www.annalsthoracicsurgery.org.
References
- 1.Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42:889–896. [DOI] [PubMed] [Google Scholar]
- 2.Lehr CJ, Zaas DW, Cheifetz IM, Turner DA. Ambulatory extracorporeal membrane oxygenation as a bridge to lung transplantation: walking while waiting. Chest. 2015;147:1213–1218. [DOI] [PubMed] [Google Scholar]
- 3.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. [DOI] [PubMed] [Google Scholar]
- 4.Aubron C, McQuilten Z, Bailey M, et al. Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: a pilot randomized trial. Crit Care Med. 2019;47:e563–e571. [DOI] [PubMed] [Google Scholar]
- 5.Krueger K, Schmutz A, Zieger B, Kalbhenn J. Venovenous extracorporeal membrane oxygenation with prophylactic subcutaneous anticoagulation only: an observational study in more than 60 patients. Artif Organs. 2017;41:186–192. [DOI] [PubMed] [Google Scholar]
- 6.Sklar MC, Sy E, Lequier L, Fan E, Kanji HD. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. a systematic review. Ann Am Thorac Soc. 2016;13:2242–2250. [DOI] [PubMed] [Google Scholar]
- 7.Tomasko J, Prasad SM, Dell DO, DeCamp MM, Bharat A. Therapeutic anticoagulation-free extracorporeal membrane oxygenation as a bridge to lung transplantation. J Heart Lung Transplant. 2016;35:947–948. [DOI] [PubMed] [Google Scholar]
- 8.Herbert DG, Buscher H, Nair P. Prolonged venovenous extracorporeal membrane oxygenation without anticoagulation: a case of Goodpasture syndrome-related pulmonary haemorrhage. Crit Care Resusc. 2014;16:69–72. [PubMed] [Google Scholar]
- 9.Wen PH, Chan WH, Chen YC, Chen YL, Chan CP, Lin PY. Non-heparinized ECMO serves a rescue method in a multitrauma patient combining pulmonary contusion and nonoperative internal bleeding: a case report and literature review. World J Emerg Surg. 2015;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Undar A, Wang S, Palanzo DA. Impact of polymethylpentene oxygenators on outcomes of all extracorporeal life support patients in the United States. Artif Organs. 2013;37:1080–1081. [DOI] [PubMed] [Google Scholar]
- 11.Palanzo DA, El-Banayosy A, Stephenson E, Brehm C, Kunselman A, Pae WE. Comparison of hemolysis between CentriMag and RotaFlow rotary blood pumps during extracorporeal membrane oxygenation. Artif Organs. 2013;37:E162–E166. [DOI] [PubMed] [Google Scholar]
- 12.Krawiec C, Wang S, Kunselman AR, Undar A. Impact of pulsatile flow on hemodynamic energy in a Medos Delta-stream DP3 pediatric extracorporeal life support system. Artif Organs. 2014;38:19–27. [DOI] [PubMed] [Google Scholar]
- 13.Qiu F, Khan S, Talor J, Kunselman A, Undar A. Evaluation of two pediatric polymethyl pentene membrane oxygenators with pulsatile and non-pulsatile perfusion. Perfusion. 2011;26: 229–237. [DOI] [PubMed] [Google Scholar]
- 14.Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl):e24S–e43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koster A, Ljajikj E, Faraoni D. Traditional and non-traditional anticoagulation management during extracorporeal membrane oxygenation. Ann Cardiothorac Surg. 2019;8:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med. 2013;14:e77–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl):e152S–e184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurihara C, Walter JM, Singer BD, et al. Extracorporeal membrane oxygenation can successfully support patients with severe acute respiratory distress syndrome in lieu of mechanical ventilation. Crit Care Med. 2018;46:e1070–e1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63:60–67. [DOI] [PubMed] [Google Scholar]
- 21.Kasirajan V, Smedira NG, McCarthy JF, Casselman F, Boparai N, McCarthy PM. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 1999;15:508–514. [DOI] [PubMed] [Google Scholar]
- 22.Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013;59:202–210. [DOI] [PubMed] [Google Scholar]
- 24.Marasco SF, Lukas G, McDonald M, McMillan J, Ihle B. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 2008;17(suppl 4):S41–S47. [DOI] [PubMed] [Google Scholar]
- 25.Sy E, Sklar MC, Lequier L, Fan E, Kanji HD. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care. 2017;39:87–96. [DOI] [PubMed] [Google Scholar]
- 26.Lubnow M, Philipp A, Foltan M, et al. Technical complications during venovenous extracorporeal membrane oxygenation and their relevance predicting a system-exchange-retrospective analysis of 265 cases. PLoS One. 2014;9:e112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–178. [PubMed] [Google Scholar]
- 28.Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal membrane oxygenation in the adult: a review of anticoagulation monitoring and transfusion. Anesth Analg. 2014;118:731–743. [DOI] [PubMed] [Google Scholar]
- 29.Ang AL, Teo D, Lim CH, Leou KK, Tien SL, Koh MB. Blood transfusion requirements and independent predictors of increased transfusion requirements among adult patients on extracorporeal membrane oxygenation—a single centre experience. Vox Sang. 2009;96:34–43. [DOI] [PubMed] [Google Scholar]
- 30.Rastan AJ, Lachmann N, Walther T, et al. Autopsy findings in patients on postcardiotomy extracorporeal membrane oxygenation (eCmO). Int J Artif Organs. 2006;29:1121–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.