Abstract
In human preterm newborns, caffeine increases brain activity and improves neurodevelopmental outcomes. In animal models of hypoxic ischemic (HI) brain injury, caffeine pretreatment reduces infarct volume. We studied the relationship between tissue neuroprotection and brain activity after injury to further understand caffeine neuroprotection. Rat dams received caffeine prior to birth or on postnatal day three (P3) through P16. Caffeine treated and untreated pups underwent the Vannucci procedure (unilateral carotid ligation, global hypoxia) at P2. A subset had EEG recordings. Brain hemispheric infarct volume was measured at P16. P2 HI results in histologic brain injury (average+/−standard deviation (SD) infarct volume 10.3+/−4.6%) and transient suppression of EEG activity. Caffeine pretreatment reduces brain injury (average+/−SD infarct volume 1.6+/−4.5%, P<0.001) and improves amplitude integrated EEG (aEEG) and EEG burst duration and amplitude. Caffeine treatment after HI does not reduce infarct volume (average+/−SD 8.3+/−4.1%, P=1.0). However, caffeine post-treatment was equally effective at restoring aEEG amplitude and EEG burst duration and amplitude. Thus, caffeine supports brain background electrical activity independent of tissue neuroprotection.
Keywords: activity dependent brain development, injury, neuroprotection
Introduction
Survival following extremely preterm birth has improved over the past two decades[1]. However, rates of neurodevelopmental impairment have largely remained the same, resulting in an increase in the population of children at risk of adverse school age outcomes[2]. Neurobiological substrates of impaired brain function after preterm birth are not completely understood but result from both acute injury and subsequent abnormal development characterized by arrest of normal developmental pathways, prolonged inflammation and disruption of neuronal connections[3]. This dysmaturation may be balanced by potential for repair and recovery through endogenous developmental mechanisms.
The substantial burden of brain injury and lifelong neurodevelopmental impairment following preterm birth, has prompted great interest in ‘neuroprotective’ treatments’[4]. However, success in clinical trials is rare. There are currently no accepted postnatal neuroprotective therapies specific to preterm neonates who have suffered or are at risk for developing brain injury. Several medications in routine clinical practice for other indications have some evidence for preterm neuroprotection including magnesium, corticosteroids, indomethacin and caffeine[5]. Some of these therapies (e.g. magnesium, corticosteroids) are administered antenatally to mothers who are at risk of preterm delivery, and the mechanisms of neuroprotection are not clear.
Electrophysiologic activity drives brain development through effects on progenitor cell proliferation, migration, differentiation[6], synapse formation[7], cortical circuit refinement[8] and myelination[9]. Cortical activity increases rapidly during the third trimester, appearing initially on EEG as discontinuous bursts with an abrupt transition to continuous activity around gestational week (GW) 34[10]. Immature bursts on EEG represent waves of correlated activity that sweep across all cortical areas[11] and function to refine nascent thalamocortical connections[8]. In human preterm newborns, a higher frequency of spontaneous activity transients correlates with faster brain growth and improved microstructural brain development[12,13]. Early HI brain injury transiently suppresses EEG activity and impairs activity dependent development of glutamate receptor expression, synapse formation, dendrite development and both early somatosensory[14] and late visual cortical plasticity[15]. Thus, activity may be an intrinsic injury, when suppressed, or neuroprotective mechanism promoting recovery and repair.
Caffeine treatment reduces brain tissue infarct in term animal models of hypoxia ischemia[16]. Caffeine treatment in human preterm newborns also increases brain activity measured by aEEG amplitude and continuity[17] and improves neurodevelopmental outcomes[18]. Thus, caffeine may improve neurodevelopmental outcome through multiple mechanisms including effects on activity. We sought to determine if caffeine (1) provides tissue neuroprotection in a preterm HI brain injury model, (2) can rescue suppressed activity and (3) whether effects on activity were associated with brain tissue preservation.
Materials and Methods
All animal research was approved by the University of California San Francisco Institutional Animal Care and Use Committee and performed in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. Time-mated Sprague Dawley dams were obtained from Charles River (Wilmington, MA). A total of 60 rat pups from 14 litters were used for these studies.
Hypoxia ischemia (HI).
HI was performed as described previously[14,15] using the Vannucci model of unilateral carotid ligation and subsequent temperature controlled hypoxia. To model very early preterm hypoxic ischemic brain injury the procedure is performed at postnatal day two (P2)[19]. Pups of either sex were anesthetized with isoflurane, and the right common carotid artery was electrocauterized. Pups were recovered with the mother for 1 h and then placed in temperature-controlled chambers to maintain a skin surface temperature of 36.5–37°C. Hypoxia was achieved with 5.6% oxygen gas[19]. Duration of hypoxia ranged between 2.5 and 3 h, with the hypoxia period terminated if any animal appeared premorbid or the mortality for any individual litter reached 20%. This method was used for all groups regardless of caffeine treatment and hypoxia duration was equal across groups. HI without caffeine treatment was performed in 16 animals distributed across 6 litters. All sixteen animals received EEG implants (described below). All animals were sacrificed and the brains analyzed for injury (described below).
Caffeine treatment.
Five litters were treated with caffeine via the dam’s drinking water at a concentration of 0.3 grams/liter (g/l) as described[20]. Caffeine treatments were started either at embryonic gestational day eight (E8, pretreatment) or on postnatal day three (P3, posttreatment after HI). Caffeine was continued through P16. Sixteen pups from caffeine pretreated dams and fourteen pups from caffeine post treatment dams underwent P2HI. Ten pups (caffeine pretreatment) and eight pups (caffeine posttreatment) also received EEG implants. All animals were sacrificed and the brains analyzed for injury. One animal from caffeine pretreatment group and three animals from caffeine posttreatment group lost head mounts and were euthanized without perfusion. Their brains were not available for measurement of infarct volume.
Determination of cortical injury severity.
After HI, injury severity was defined categorically based on histology as described[15] and confirmed with stereologic infarct volume measured on coronal sections stained with cresyl violet[21]. Hemispheric volume was measured using Cavalieri’s principal and Stereo Investigator software (MBF Bioscience). Infarct volume is derived from the difference between left (hypoxia alone) and right (hypoxia ischemia) hemispheres divided by the left hemisphere volume and expressed as a percentage. Injury categories displayed the following infarct volumes: (1) mild, 0–7%; (2) moderate, 8–15%; and (3) severe, ≥ 15%.
Miniature two channels wireless EEG head-mount surgery.
At P6 days of age, control littermates (no HI, N=9) and HI exposed (no caffeine, N=16; caffeine pretreatment, N=10, caffeine posttreatment, N=9) pups were anesthetized with isoflurane implantation of wireless EEG transmitter. For the surgery, the scalp was sterilized with betadine, and a midline incision was made to expose the skull for implanting the head mount (Epoch, Epitel Inc, UT) using an established protocol[22]. Holes were drilled at the following locations bilaterally: 2 mm posterior of bregma and at 2.5 mm lateral of the midsagittal suture in both hemispheres. A third hole was placed over cerebellum. The electrode wires of the transmitter system were trimmed to appropriate length and fed through the craniotomies with a target depth at the level of the dura. The transmitter was attached to the skull using a cyanoacrylate gel compound (Loctite 454) with accelerator (Loctite 7452). Additional cyanoacrylate was applied around the unit and the exposed areas of the skull to stabilize the implant. The skin was then sutured around the implant. The pups are allowed to recover and returned to the dam. Fourteen control rat pups (no hypoxia ischemia) were implanted with EEG transmitters.
EEG recording.
The wireless EEG systems was recorded through a differential preamplifier using LabChart 7 (ADINSTRUMENTS, CO) for data acquisition. Each Epoch EEG transmitter amplifies and transmits two channels of high-fidelity EEG data (0.1–120 Hz). EEG was recorded from each animal continuously for 1–2 h at P8, P11 and P14.
EEG data analysis.
EEG data analysis was performed as described previously[14]. Raw EEG recordings were imported into MATLAB (MathWorks) for processing. To derive the amplitude integrated EEG (aEEG), recordings were bandpass filtered (2–15 Hz), rectified, smoothed, and plotted with time compression (5 cm/h) to replicate the approach adapted for monitoring human newborns at term following birth asphyxia[23] and in preterm newborns[24]. The aEEG lower and upper margin amplitudes were quantified as described[14]. For detection of oscillatory events, unfiltered EEG was inspected for EEG deflections exceeding 5 times the baseline standard deviation (SD). Only events lasting >100 milliseconds and containing >3 cycles were considered for analysis. The events were analyzed in their peak-to-peak amplitude, duration, and occurrence. The time–frequency spectrogram plots of unfiltered EEG were performed using the MATLAB spectrogram function with a time window of 100 milliseconds and an overlap of 99 milliseconds. Minimal and maximal intensities in power were normalized to values between 0 and 1 and were displayed in dark blue and red, respectively. Fast Fourier transforms (FFTs) were performed to analyze EEG data in the frequency domain from 0 to 50 Hz. Time window of FFT is 3 sec, and the overlap time is 1 second. Power spectral densities (PSDs) were estimated from the FFT using the MATLAB software mtspecgramc command (Mathworks, Inc. USA), and transformed to dB with the transformation 20×log10 (FFT). Delta wave power (1–4Hz), theta wave power (8–13Hz), alpha wave power (13–30Hz), and gamma wave power (30–100Hz) are quantified.
Statistics.
Data are summarized as mean +/− standard deviation (SD) and plotted as combined scatter plus box plots to provide median, quartiles and range. Sample population difference testing was performed with unpaired, two-sided Student’s t-test. Comparison of more than two different groups was performed using one-way ANOVA tests, followed by multiple comparisons with Bonferroni correction. The association of infarct volume with EEG parameters was tested graphically with scatterplots and with Spearman’s Rank Correlation.
Results
Brain background electrical activity evolves from discontinuous to continuous over first two postnatal weeks.
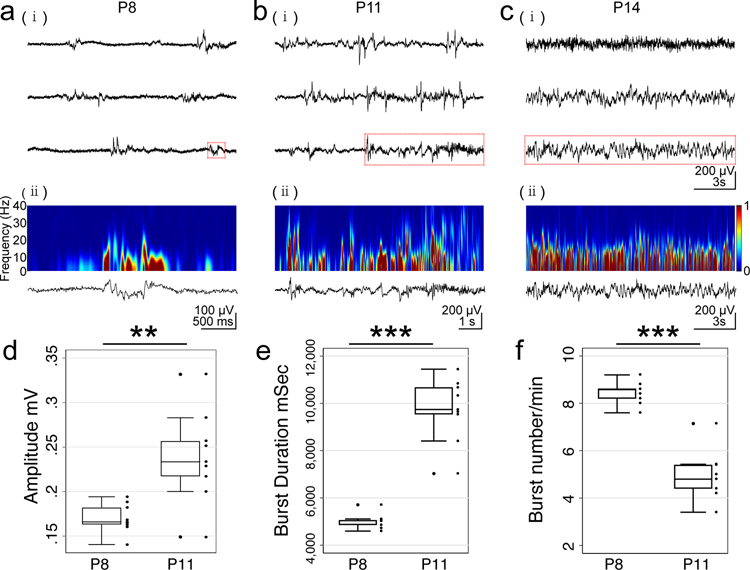
Normally developing Sprague Dawley rat pups were implanted with wireless EEG head-mounts at P6 and video-EEG recordings were made on P8, P11 and P14 to document normal development of background brain activity. Epochs of data were chosen for analyses when the animal was not moving. Initial background electrical activity is discontinuous and low amplitude (Figure 1ai). Bursts of activity contained nested low (0.3–2 Hz) and mid frequency (8–20 Hz) components characteristic of spindle bursts[25] (Figure 1aii spectrogram). Over the first two postnatal weeks, background activity increases (Figure 1bi, ii), becoming continuous by P14 (Figure 1ci, ii). Activity bursts increase in amplitude (Figure 1d P8 0.17 ± 0.01 mV vs P11 0.24± 0.02 mV, P = 0.003) and duration (Figure 1e, P8 5.02± 0.10 s vs P11 9.74± 0.45 s, P < 0.001), with a concomitant decrease in frequency (Figure 1f, P8 8.44±0.16 events per minute (epm) vs P11 4.95±0.34 epm P11, P < 0.001) before activity becomes continuous around P14.
Figure 1.
Developmental changes in EEG traces are shown for P8 (a), P11 (b) and P14 (c). Raw EEG (i) shows typical discontinuous activity with bursts followed by periods of no activity. Activity increases in both amplitude and burst frequency, becoming continuous around P14. Frequency spectrogram (ii) for a single burst of activity (highlighted in red in raw traces) is shown for each age. The activity includes both low frequency (0.5 – 2 Hz) activity and nested higher frequencies (8 – 20 Hz) characteristic of a spindle burst. Power is normalized from 0 to 1 (color scale). Quantification of developmental changes in EEG activity with increase in burst amplitude (d) and burst duration (e) with corresponding decrease in burst frequency (f) from P8 to P11. Bursts cannot be quantified by P14 as activity has become continuous.
Caffeine given before, but not after P2 hypoxia ischemia reduces brain injury.
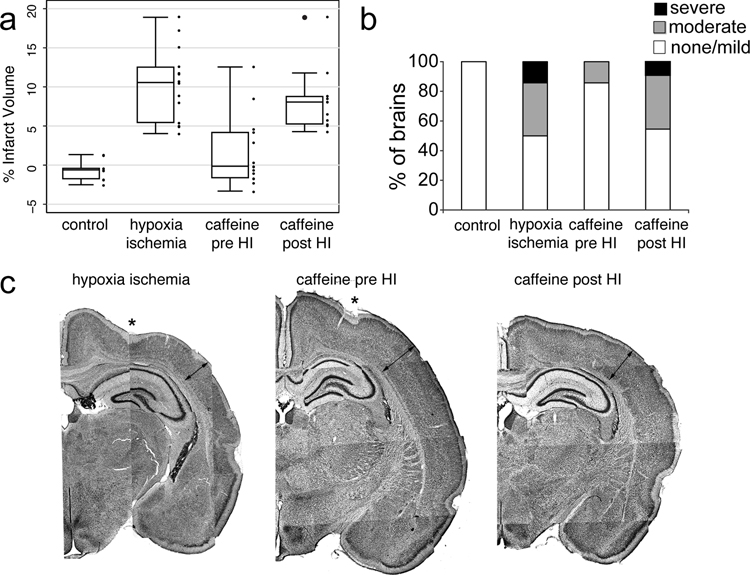
Rat pups received the Vannucci procedure (unilateral carotid ligation followed by temperature controlled global hypoxia) at P2, an age equivalent to 26 GW premature human newborn[26]. At P16, animals were sacrificed and brains processed for measurement of hemispheric volume[21]. P2 HI results in a range of brain injury (Figure 2a hypoxia ischemia, mean+/− SD infarct volume 10.3+/−4.6%) that is significantly different from control littermates (Figure 2a control, −0.6+/−1.3%, P<0.001 vs HI).
Figure 2.
Quantification of infarct volume after hypoxia ischemia at P2 (P2 HI) with and without caffeine treatment given before (pre HI) and after hypoxia ischemia (post HI) (a). Summary of qualitative brain injury grade after P2 HI with and without caffeine treatment (b). Representative low magnification montage images of injury pattern and extent are shown (c) for HI, caffeine pre HI and caffeine post HI treatments. Thinning of neocortex (lines with arrows) and disruption of layer V1b/subplate can be appreciated in the HI image but not caffeine pre HI image. Intermediate effects are seen in caffeine post HI image.
A separate group of pups from three litters were treated with caffeine (0.3 g/l added to the drinking water given to the rat dam beginning at E8) before P2HI. This treatment regimen has been shown to reduce brain injury following HI at P7, a model of near term human hypoxic ischemic encephalopathy (HIE)[20]. This dose produces a plasma concentration of caffeine in rat pups at P7 equivalent to target therapeutic levels in human preterm newborns treated for apnea of prematurity[27]. Caffeine treatment was continued through P16. Caffeine pretreatment significantly reduces infarct volume after P2 HI (Figure 2a caffeine pre HI, 1.6± 4.5% vs HI without treatment, P< 0.001).
Caffeine treatment is typically given to premature human newborns after birth to facilitate weaning from mechanical ventilation[27]. Thus, embryonic treatment does not replicate clinical practice. To more accurately model the human paradigm and to assess tissue protection when caffeine is given after injury, caffeine was given to rat dams in the drinking water beginning at P3, after P2 HI in two litters. Caffeine started after HI did not reduce infarct volume (Figure 2a caffeine post HI, 8.3+/−4.1% vs HI no treatment, P=1.0).
We also categorized HI injuries as mild, moderate or severe based upon histological criteria as described[15]. Caffeine pretreatment significantly reduced injury severity with more than 80% of caffeine treated pups having mild injury compared with only 50% of untreated pups (Figure 2b). Caffeine posttreatment did not effectively change the distribution of injury in comparison with hypoxia ischemia without treatment (Figure 2b).
P2 hypoxia-ischemia reduces EEG background activity
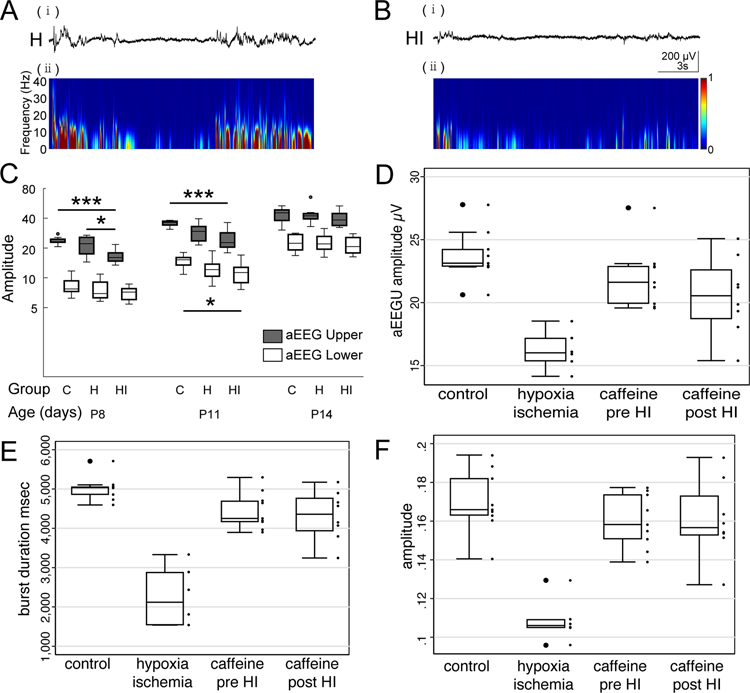
A subset of animals in the HI no caffeine group had EEG activity recorded. Moderate HI brain injury reduces background activity at P8, including spindle bursts, in the hypoxia ischemia hemisphere to a greater extent than in the hemisphere exposed to hypoxia alone (shown qualitatively in Figure 3a,b). Epochs of movement free recording were processed for amplitude integrated EEG (aEEG)[14]. aEEG is a method developed for clinical bedside monitoring of background EEG activity commonly used in the neonatal intensive care unit to determine brain maturity and to stratify injury severity[23,24]. Upper and lower aEEG margins were recorded for all groups. Both upper and lower aEEG margins increase over time from P8 through P14 with normal development (Figure 3c control). The upper aEEG margin is transiently reduced in the HI hemisphere at P8 and P11 (Figure 3c, P8 control 23.75± 0.67 μV vs HI 16.55± 0.93 μV, P < 0.001; P11 control 35.55± 0.76 μV vs HI 24.74± 1.74 μV, P < 0.001), before returning to normal at P14. The lower aEEG margin is reduced at P11 (Figure 3c, P11 control 15.59± 0.74 μV vs HI 11.93± 0.85 μV, P = 0.02). Moderate HI brain injury also reduces burst duration (Figure 3e) and burst amplitude (Figure 3f) at P8. No seizures were observed on any EEG recordings which were performed well after HI at P2.
Figure 3.
Early hypoxia ischemia results in decreased EEG bursts with reduced power across all frequency bands (a,b). Quantification of amplitude integrated EEG (aEEG) (c), in control, hypoxia and hypoxia ischemia hemispheres. HI results in a transient decrease in upper aEEG margin at P8 and P11 and a decrease in lower aEEG margin at P11. Activity returns to normal by P14. Caffeine pretreatment restores aEEG upper margins at P8 (d). Quantification of burst duration (e) and amplitude (f) in control animals compared with hypoxia ischemia and caffeine treated hypoxia ischemia hemispheres at P8. Hypoxia ischemia reduces burst duration and amplitude. Caffeine pre- and post-treatment completely restores burst duration and amplitude.
Caffeine restores background electrical activity after P2 HI
To determine the effects of caffeine treatment on background brain activity, a subset of pups from caffeine treated groups also received wireless EEG head mounts on P6 following HI at P2. To determine the effects of caffeine treatment before or after P2 HI, we compared aEEG margins at P8 and also measured burst duration and amplitude. P2 hypoxia ischemia significantly reduces aEEG upper amplitude at P8 (Figure 3d control vs HI, 23.7+/−2.0 vs 16.5+/−2.6 μV, P<0.001). Caffeine pretreatment preserves upper aEEG margin at P8 (Figure 3d, control vs caffeine pre HI, 23.7+/−2.0 vs 22.4+/−2.6 μV, P=1). Caffeine posttreatment was equally effective in preserving upper aEEG margin at P8 (Figure 3d, control vs caffeine post HI, 23.7+/−2.0 vs 20.5+/−3.1 μV, P=0.1). The effects of both HI and caffeine can also be measured in burst duration and amplitude at P8 (Figure 3e,f). Hypoxia ischemia significantly reduces burst duration (Figure 3e, control vs HI, 5017+/−312 msec vs 2280+/−663 msec, P < 0.001) and amplitude (Figure 3f, control vs HI, 0.17± 0.02 μV 0.11± 0.02 μV, P < 0.001). Both caffeine pretreatment and posttreatment completely restore burst duration and amplitude following HI (Figure 3e,f, all P<0.001 vs HI no treatment).
We examined the effects of P2 HI on EEG power spectrum to determine if EEG suppression was limited to specific frequencies. P2 HI suppresses EEG power in delta, alpha, theta and beta power spectrums at both P8 and P11 (supplemental Figure 1 and 2). Caffeine restores EEG power in the delta frequency spectrum (supplemental Figure 2) consistent with augmentation of spindle bursts. While global measures of EEG activity (aEEGU, burst duration and amplitude) is restored at P11, EEG activity specifically in alpha, gamma and beta frequency domains remain depressed even with caffeine treatment (supplemental Figure 2).
Relationship between brain tissue preservation and spontaneous activity.
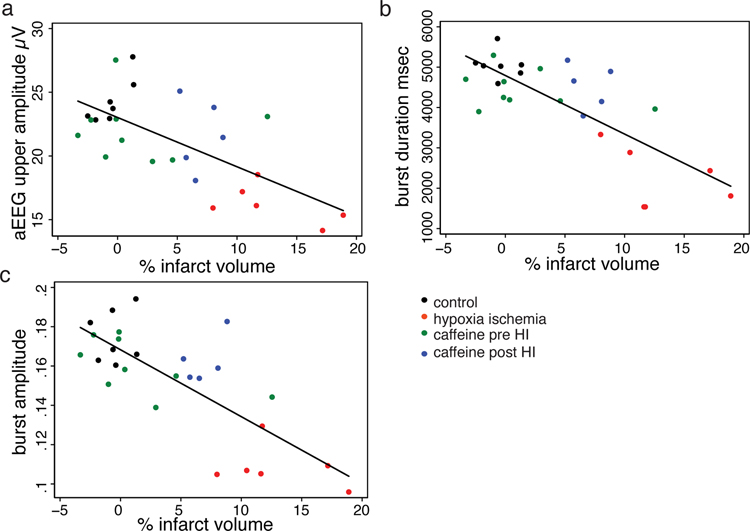
To explore the relationship of brain tissue loss and suppressed activity after P2 HI with and without caffeine treatment, we analyzed the association of infarct volume with EEG parameters at P8 in all rat pups that had both EEG recording and measurement of infarct volume. There is a significant negative association between infarct volume and P8 aEEG upper margin (Figure 4a, Spearman’s rho = −0.52, P=0.006). Even stronger associations exists between infarct volume and burst duration (Figure 4b, Spearman’s rho = −0.67, P<0.0001) or amplitude (Figure 4c, Spearman’s rho = −0.66, P<0.0002). Caffeine posttreatment did not preserve brain tissue in the overall sample (Figure 2). Consistent with this finding, most data points for the caffeine posttreatment group (Figure 4a,b,c blue dots) occur above the regression line indicating a stronger effect on brain activity than infarct volume. Consistent with this observation, when the caffeine posttreatment group is removed, the association between infarct volume and EEG parameters strengthens further (Burst amplitude infarct volume Spearman’s rho with caffeine pretreatment group, −0.67 vs without −0.73).
Figure 4.
Correlation between infarct volume (x-axis) and aEEG upper margin (a), burst duration (b) and burst amplitude (c). All groups are plotted by color coded symbol – control (black), hypoxia ischemia (red), caffeine before HI (green) and caffeine after HI (blue). A strong negative relationship exists between infarct volume and all measures of brain activity.
Discussion
These results confirm that caffeine pretreatment reduces brain infarct after hypoxia ischemia in a model of preterm brain injury as well as observed in term models[20]. Very early hypoxic ischemic injury transiently disrupts normal spontaneous background brain electrical activity[14] and pretreatment with low dose caffeine restores suppressed brain activity following HI. Caffeine started after HI does not provide tissue neuroprotection. However, treatment with caffeine after HI restores background brain activity to a similar extent as pretreatment. Finally, there is a strong inverse relationship between brain infarct volume and brain activity.
Evidence for caffeine neuroprotection in human clinical trials
Caffeine is commonly used in human preterm newborns to treat apnea of prematurity. The Caffeine for Apnea of Prematurity (CAP) trial enrolled infants with a birth weight of 500 to 1250 grams (extremely preterm), who were randomized to receive caffeine or placebo during the first 10 days of life to prevent apnea and facilitate extubation from mechanical ventilation[28]. This trial confirmed earlier extubation and reduced complications of prematurity (bronchopulmonary dysplasia, severe retinopathy), but no difference in brain injury on ultrasound. At 18 months however, caffeine treatment improved the rate of survival without neurodevelopmental disability, with a nearly two-fold reduction in cerebral palsy[29]. At five and eleven years of age, the combined outcome of death or disability was no longer different between groups, however caffeine treated children show a sustained improvement in motor function[18,30]. Neonatal caffeine therapy was also found to reduce the rate of developmental coordination disorder at 5 years of age[31]. Analysis of brain magnetic resonance diffusion imaging in a subgroup of infants enrolled in the CAP trial showed changes suggesting improved white matter development and myelination[32]. A subsequent pilot trial of early high-dose caffeine treatment did not replicate these findings and in fact showed increased cerebellar hemorrhage, worse motor function[33] and possibly increased seizure burden[34].
Timing of brain injury in human preterm newborns.
To determine the optimal window for a neuroprotective treatment in preterm newborns it is necessary to consider both injury timing and mechanism. However, exact timing of brain injury in human preterm newborns is unclear and mechanisms may be multifactorial. Preterm birth has been associated with both antenatal insults including chorioamnionitis, perinatal asphyxia and postnatal hypoxemia and hypotension resulting from cardiopulmonary immaturity. The model used in the present experiments involves hypoxia ischemia at P2. This gestational age is equivalent to a 26 week human[26]. The injury occurs shortly after birth and thus may not capture antenatal injuries in human. Neonatal hypoxia ischemia results in both early cell death as well as long lasting inflammation[3]. Prior studies evaluating caffeine neuroprotection employed pretreatment and were in term models[20]. We found that caffeine was equally effective for reducing infarct volume in a preterm model, when given before injury. In the CAP trial, caffeine is started after birth and thus most analogous to our posttreatment condition. Consistent with the CAP trial, we did not find that caffeine posttreatment prevents histologic brain injury. However, we did find that caffeine given before and after injury preserves brain electrical activity.
Caffeine signaling pathways
To consider potential neuroprotective pathways it is useful to review caffeine signaling. Caffeine has a variety of dose dependent effects. At very high doses, caffeine potentiates intracellular calcium release through the ryanodine receptor. Caffeine is a non-selective weak phosphodiesterase inhibitor and thus increases intracellular cyclic adenosine 3’,5’ monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). At therapeutic concentrations (~20 μM), caffeine is a nonselective antagonist for A1 and A2a adenosine receptors that are expressed presynaptically and affect neurotransmitter release. Through this mechanism, caffeine modulates many neurotransmitter systems including noradrenaline, dopamine, serotonin, acetylcholine, glutamine and gamma-aminobutyric acid (GABA). Modulation of neurotransmitter pathways accounts for caffeine’s physiologic effect to improve respiratory drive, including stimulation of respiratory pattern generators in the medulla and enhancement of peripheral chemoreceptors to carbon dioxide.
Caffeine tissue neuroprotection
The mechanism for caffeine tissue neuroprotection in hypoxic ischemic brain injury is unclear and controversial. Adenosine receptor signaling has direct neuroprotective effects in the mature brain through modulation of dopamine signaling and gene expression[35]. However, in the developing nervous system, adenosine may mediate brain injury. Adenosine receptor signaling results in ventriculomegaly through adverse effects on developing oligodendrocytes[36]. Caffeine is an adenosine receptor antagonist and caffeine treatment mitigates chronic hypoxia-induced white matter injury[37]. This effect would be consistent improved myelination on brain MRI in the CAP trial[32].
Adenosine signaling may also promote inflammation, an important mechanism of brain injury in preterm newborns[3]. Adenosine A2A receptors are expressed on immune cells including both T cells and macrophages. Caffeine’s overall effects on the immune system are suppressive, with reduction in inflammatory cytokine release and expression of innate and adaptive immune receptors including Toll-like receptors and major histocompatibility molecules[38]. In a mouse model of term HI, a single dose of caffeine given after P10 HI reduced infarct volume by 44%[39]. Treated animals showed reduced activated CD8 lymphocytes.
Finally, caffeine has antioxidant properties, limiting lipid peroxidation, reducing oxidative stress and preserving mitochondrial function. Thus, caffeine likely preserves brain tissue following hypoxia ischemia through reduction of both inflammation and oxidative stress, two well described neuroprotective strategies in the immature brain.
Caffeine, brain activity and activity dependent neurodevelopment
As reviewed, caffeine modulates many neurotransmitter systems including acetylcholine, glutamine and GABA with an overall effect to reduce inhibition and increase activity. In preterm infants receiving caffeine for treatment of apnea, caffeine was found to increase background brain electrical activity on aEEG[17].
EEG brain activity in premature newborns is characteristically discontinuous with bursts of activity followed by relative silence. Bursts represent spontaneous correlated waves of activity that sweep across cortex and serve to refine developing cortical circuits before the onset of sensory driven input[40]. Cortical activity abruptly transitions from bursting to precisely timed evoked potentials with maturation of local inhibition[10]. In rodents, this occurs just prior to eye opening (P11) and in humans between 34–36 gestational weeks. Thus, caffeine may promote cortical development directly through modulation of spontaneous activity. In human studies, spontaneous correlated brain activity in the form of slow activity transients are associated with both microstructural brain development[13] and macroscopic brain growth[12].
In the present study, we found that caffeine given before or after injury restores suppressed background EEG activity after HI. In prior studies, we found that P2 HI is associated with reduced expression of glutamate receptor subunits and synapses, simplified dendritic arbors and impaired somatosensory whisker barrel plasticity[14]. At later ages following P2 HI, we observed impaired ocular dominance plasticity and reduced response to an optimal visual stimulus[15]. Restoring background activity may help promote critical activity-dependent neuronal developmental mechanisms and facilitate repair.
Limitations
The findings we report have a number of important limitations. The Vannucci procedure is known to produce variable injury and injury severity may additionally vary across litters. By the nature of the study design where the caffeine treatment was administered to the dam, it was not possible to distribute treated and untreated pups across litters to balance litter size. Nor were we able to blind investigators to treatment. We did not measure pups plasma caffeine levels. Thus, we cannot exclude the possibility that post-treatment caffeine pups received a lower caffeine dose due to more brain injury impairing feeding. Additionally, these experiments included pretreatment with caffeine which is not representative of human clinical practice. Nevertheless, we find potent effects of caffeine to restore suppressed background EEG activity regardless of treatment timing or tissue neuroprotection. Given the essential developmental role of normal spontaneous activity for refinement of cortical circuits[41], rescue of suppressed background activity may represent a novel neuroprotective mechanism. Caffeine provides additional potential beneficial effects for pathophysiology associated with preterm birth including both anti-inflammatory and antioxidant properties. Caffeine started after birth would address episodes of hypoxia ischemia and infection associated with oxidative stress and inflammation. Future studies will be necessary to examine the effects of caffeine on specific activity dependent developmental pathways and their link to higher cortical functions. Given that high dose caffeine treatment may lower seizure thresholds[33,34], further studies will be needed to determine optimal dose of caffeine treatment to maximally promote recovery following early brain injury.
Supplementary Material
Supplementary Figure 1. Plots of EEG spectral power derived from unfiltered EEG recordings across all Frequency domains are shown for control, HI and caffeine pretreatment groups at P8 (A) and P11 (B).
Supplementary Figure 2. Power in specific frequency domains including delta (1–4Hz), theta (8–13Hz), alpha (13–30Hz), and gamma (30–50Hz) are plotted for control (C), hypoxia (H), hypoxia ischemia (HI) and caffeine pretreatment (Ca) groups at P8 through P14.
Acknowledgement:
We would like to acknowledge Armon Rahim and Sumudu Ranasinghe for assistance revising MATLAB routines used to analyze EEG data.
Statement of Financial Support: These studies were supported by NIH NINDS NS060896 (PSM).
Footnotes
Statement of Ethics: All animal research was approved by the University of California San Francisco Institutional Animal Care and Use Committee and performed in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used for each experiment.
Disclosure Statement: The authors declare no conflict of interest, nor financial support beyond grant funding listed below.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015. September;314(10):1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers EE, Hintz SR. Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol. 2016;40(8):497–509. [DOI] [PubMed] [Google Scholar]
- 3.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012. June;11(6):556–66. [DOI] [PubMed] [Google Scholar]
- 4.Parikh P, Juul SE. Neuroprotective Strategies in Neonatal Brain Injury. J Pediatr. 2018;192:22–32. [DOI] [PubMed] [Google Scholar]
- 5.Davis AS, Berger VK, Chock VY. Perinatal Neuroprotection for Extremely Preterm Infants. Am J Perinatol. 2016. February;33(3):290–6. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006. December;444(7120):707–12. [DOI] [PubMed] [Google Scholar]
- 7.West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol. 2011. June;3 10.1101/cshperspect.a005744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Ackman JB, Xu HP, Crair MC. Visual map development depends on the temporal pattern of binocular activity in mice. Nat Neurosci. 2012. February;15:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chorghay Z, Káradóttir RT, Ruthazer ES. White Matter Plasticity Keeps the Brain in Tune: Axons Conduct While Glia Wrap. Front Cell Neurosci. 2018;12:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colonnese MT, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, et al. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010. August;67:480–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. J Neurosci. 2010. March;30:4325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benders MJ, Palmu K, Menache C, Borradori-Tolsa C, Lazeyras F, Sizonenko S, et al. Early Brain Activity Relates to Subsequent Brain Growth in Premature Infants. Cereb Cortex N Y N 1991. 2015. September;25(9):3014–24. [DOI] [PubMed] [Google Scholar]
- 13.Tataranno ML, Claessens NHP, Moeskops P, Toet MC, Kersbergen KJ, Buonocore G, et al. Changes in brain morphology and microstructure in relation to early brain activity in extremely preterm infants. Pediatr Res. 2018;83(4):834–42. [DOI] [PubMed] [Google Scholar]
- 14.Ranasinghe S, Or G, Wang EY, Ievins A, McLean MA, Niell CM, et al. Reduced Cortical Activity Impairs Development and Plasticity after Neonatal Hypoxia Ischemia. J Neurosci Off J Soc Neurosci. 2015. August;35(34):11946–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Failor S, Nguyen V, Darcy DP, Cang J, Wendland MF, Stryker MP, et al. Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex. J Neurosci. 2010. January;30:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bona E, \AAdén U, Gilland E, Fredholm BB, Hagberg H. Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology. 1997;36(9):1327–1338. [DOI] [PubMed] [Google Scholar]
- 17.Supcun S, Kutz P, Pielemeier W, Roll C. Caffeine increases cerebral cortical activity in preterm infants. J Pediatr. 2010. March;156:490–1. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012. January;307:275–82. [DOI] [PubMed] [Google Scholar]
- 19.Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res Dev Brain Res. 1997;100:149–60. [DOI] [PubMed] [Google Scholar]
- 20.Bona E, Aden U, Fredholm BB, Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995. September;38:312–8. [DOI] [PubMed] [Google Scholar]
- 21.Mikhailova A, Sunkara N, McQuillen PS. Unbiased Quantification of Subplate Neuron Loss following Neonatal Hypoxia-Ischemia in a Rat Model. Dev Neurosci. 2017;39(1–4):171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zayachkivsky A, Lehmkuhle MJ, Fisher JH, Ekstrand JJ, Dudek FE. Recording EEG in immature rats with a novel miniature telemetry system. J Neurophysiol. 2013. February;109:900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toet MC, Hellström-Westas L, Groenendaal F, Eken P, Vries LS de. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic–ischaemic encephalopathy. Arch Dis Child - Fetal Neonatal Ed. 1999. July;81(1):F19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wikström S, Pupp IH, Rosén I, Norman E, Fellman V, Ley D, et al. Early single-channel aEEG/EEG predicts outcome in very preterm infants: Early aEEG/EEG predicts outcome in preterms. Acta Paediatr. 2012. July;101(7):719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J-W, Reyes-Puerta V, Kilb W, Luhmann HJ. Spindle Bursts in Neonatal Rat Cerebral Cortex. Neural Plast. 2016;2016:3467832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;0:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Hady H, Nasef N, Shabaan AE, Nour I. Caffeine therapy in preterm infants. World J Clin Pediatr. 2015. November;4(4):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006. May;354(20):2112–21. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007. November;357:1893–902. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt B, Roberts RS, Anderson PJ, Asztalos EV, Costantini L, Davis PG, et al. Academic Performance, Motor Function, and Behavior 11 Years After Neonatal Caffeine Citrate Therapy for Apnea of Prematurity: An 11-Year Follow-up of the CAP Randomized Clinical Trial. JAMA Pediatr. 2017. June;171(6):564–72. [DOI] [PubMed] [Google Scholar]
- 31.Doyle LW, Schmidt B, Anderson PJ, Davis PG, Moddemann D, Grunau RE, et al. Reduction in developmental coordination disorder with neonatal caffeine therapy. J Pediatr. 2014. August;165(2):356–359.e2. [DOI] [PubMed] [Google Scholar]
- 32.Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG, et al. Caffeine and brain development in very preterm infants. Ann Neurol. 2010. November;68:734–42. [DOI] [PubMed] [Google Scholar]
- 33.McPherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res. 2015. August;78(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vesoulis ZA, McPherson C, Neil JJ, Mathur AM, Inder TE. Early High-Dose Caffeine Increases Seizure Burden in Extremely Preterm Neonates: A Preliminary Study. J Caffeine Res. 2016. September;6(3):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredholm BB. Adenosine and neuroprotection. Int Rev Neurobiol. 1997;40:259–80. [PubMed] [Google Scholar]
- 36.Turner CP, Seli M, Ment L, Stewart W, Yan H, Johansson B, et al. A1 adenosine receptors mediate hypoxia-induced ventriculomegaly. Proc Natl Acad Sci. 2003. September;100(20):11718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, et al. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60(6):696–705. [DOI] [PubMed] [Google Scholar]
- 38.Al Reef T, Ghanem E. Caffeine: Well-known as psychotropic substance, but little as immunomodulator. Immunobiology. 2018. December;223(12):818–25. [DOI] [PubMed] [Google Scholar]
- 39.Winerdal M, Urmaliya V, Winerdal ME, Fredholm BB, Winqvist O, Ådén U. Single Dose Caffeine Protects the Neonatal Mouse Brain against Hypoxia Ischemia. PLoS ONE. 2017. January;12(1). 10.1371/journal.pone.0170545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006. July;29:414–8. [DOI] [PubMed] [Google Scholar]
- 41.Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012. October;490:219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Plots of EEG spectral power derived from unfiltered EEG recordings across all Frequency domains are shown for control, HI and caffeine pretreatment groups at P8 (A) and P11 (B).
Supplementary Figure 2. Power in specific frequency domains including delta (1–4Hz), theta (8–13Hz), alpha (13–30Hz), and gamma (30–50Hz) are plotted for control (C), hypoxia (H), hypoxia ischemia (HI) and caffeine pretreatment (Ca) groups at P8 through P14.